Abstract
We investigated the mechanisms underlying neurocognitive dysfunction in patients with low-grade glioma (LGG) by relating functional connectivity revealed by magnetoencephalography to neurocognitive function. We administered a battery of standardized neurocognitive tests measuring six neurocognitive domains to a group of 17 LGG patients and 17 healthy controls, matched for age, sex, and educational level. Magnetoencephalography recordings were conducted during an eyes-closed “resting state,” and synchronization likelihood (a measure of statistical correlation between signals) was computed from the delta to gamma frequency bands to assess functional connectivity between different brain areas. We found that, compared with healthy controls, LGG patients performed more poorly in psychomotor function, attention, information processing, and working memory. LGG patients also had significantly higher long-distance synchronization scores in the delta, theta, and lower gamma frequency bands than did controls. In contrast, patients displayed a decline in synchronization likelihood in the lower alpha frequency band. Within the delta, theta, and lower and upper gamma bands, increasing short-and long-distance connectivity was associated with poorer neurocognitive functioning. In summary, LGG patients showed a complex overall pattern of differences in functional resting-state connectivity compared with healthy controls. The significant correlations between neurocognitive performance and functional connectivity in various frequencies and across multiple brain areas suggest that the observed neurocognitive deficits in these patients can possibly be attributed to differences in functional connectivity due to tumor and/or treatment.
Keywords: low-grade glioma, magnetoencephalography, neurocognition, resting-state functional connectivity
Low-grade gliomas (LGGs) constitute 20%–25% of all gliomas.1 The mean 5-year progression-free survival rate ranges from 46% to 73%,2–4 with median survival times ranging from 5 to 16.7 years.5
Epilepsy is the first symptom in at least two-thirds of all patients with LGG,6 and LGG is almost invariably accompanied by a loss of neurocognitive functioning.7,8 These neurocognitive deficits tend to be generalized and cannot be explained unequivocally by tumor localization alone.8,9 Bressler10 has suggested that higher neurocognitive functions depend on the integrated activity of several specialized brain areas. These interactions can be studied with functional imaging techniques such as functional MRI (fMRI), electroencephalography (EEG), and magnetoencephalography (MEG). Statistical correlations between time series of brain activity recorded over distinct regions are assumed to reflect interactions between the brain regions. This concept is referred to as “functional connectivity.”11 Local processing of information, or local synchrony, is reflected in MEG or EEG power and in the synchronization between the signals from pairs of channels at nearby sites (we refer to this as short-distance functional connectivity), and global integration (long-distance and interhemispheric functional connectivity) reflects the synchronization between channel pairs at spatially well separated sites; both are required for optimal functioning of the brain.12 EEG and MEG are distinct methods used to assess functional connectivity of the brain. EEG measures electrical activity generated by extracellular currents in the brain, whereas MEG detects magnetic fields related to intra-cellular currents. The skull and scalp do not distort the magnetic field patterns as they do electrical currents in EEG measurement. Also, MEG does not require the use of a reference electrode. Therefore, MEG seems to be more suitable than EEG for estimations of functional connectivity.13,14
In cognitive neuroscience, functional imaging techniques are used to measure task-specific changes in activity associated with mental activities. Recent fMRI research has shown that the no-task, resting state is stable and active15,16 and is characterized by activation of a “default” network.17 It has recently been shown in patients with Alzheimer’s disease that resting-state connectivity is a good indication of neuropsychological functioning.18 MEG signals can also be recorded during the resting state as a way to study baseline functional networks in the brain.19–21
Functional connectivity (as recorded with MEG) in brain tumor patients has been investigated and compared with that in healthy controls.22,23 Differences in resting-state functional connectivity were found in the patient population within several frequency bands when compared with those of healthy controls. Interestingly, these differences were not limited to the tumor area and were more notable in patients with a tumor in the left hemisphere. However, these studies did not evaluate whether these reported changes are only an epiphenomenon or are indeed associated with poorer neurocognitive functioning.
Neurocognitive functioning in glioma patients can be affected by the tumor and tumor treatment, as well as by tumor-related epilepsy and antiepileptic drugs, and can be assessed by neuropsychological assessments. The underlying pathophysiological mechanism in the brain responsible for these neurocognitive deficits has not yet been described. We hypothesize changes in functional connectivity to be the intermediate between the effect of tumor and tumor-related treatment, on the one hand (“input”), and these neurocognitive deficits (“output”), on the other hand, as shown in Fig. 1.
Fig. 1.
Flow diagram of the relationship between tumor-related factors, higher neurocognitive function, and functional connectivity in patients with low-grade glioma. Abbreviations: fMRI, functional MRI; EEG, electroencephalography; MEG, magnetoencephalography.
In the present study, we investigated functional connectivity and neurocognitive functioning in LGG patients and the correlation between these two variables. On the basis of our previous studies, we hypothesized that LGG patients show differences in resting-state functional connectivity of the brain, compared with healthy controls. More important, on the basis of the notion that resting-state connectivity is a good indication of neuropsychological functioning,18 we expected significant correlations between resting-state functional connectivity in the brain and neurocognitive functioning in LGG patients.
Materials and Methods
Patients
Twenty-three patients with LGG were approached to participate in this study. Patients were eligible if (1) they had a suspected or histologically confirmed LGG, (2) they had no radiological (confirmed by MR or CT scan) and/or clinical tumor progression in the previous 6 months, and (3) they did not use medication possibly interfering with neurocognitive function, other than antiepileptic drugs.
Patients were recruited from the VU University Medical Center and the Academic Medical Center, both tertiary referral centers in Amsterdam for brain tumor patients, after the study was approved by the institutional ethics review boards of both participating hospitals. Relatives of the patients were asked to participate as healthy controls. Healthy controls were eligible if they (1) did not have any neurological disease and (2) did not use any medication possibly influencing neurocognitive function. For patients who could not provide a healthy control participant, VU University Medical Center staff members served as controls.
Magnetoencephalography
MEG recordings were obtained using a 151-channel whole-head MEG system (CTF Systems Inc., Port Coquitlam, BC, Canada) while participants were seated inside a magnetically shielded room (Vacuumschmelze GmbH, Hanau, Germany). Magnetic fields were recorded during a no-task, eyes-closed resting state. Metal artifacts were avoided as much as possible. A third-order software gradient24 was used with a recording passband of 0.25–125 Hz and a sample frequency of 312.5 Hz. At the beginning, middle, and end of each recording, the head position relative to the coordinate system of the helmet was recorded by leading small alternating currents through three head position coils attached to the left and right preauricular points and the nasion on the subject’s head. Head position changes up to approximately 1.5 cm during a recording condition were accepted.
For this study, 149 of the 151 channels could be used. MEG recordings were converted to ASCII files. From these ASCII files, four artifact-free epochs of 13 s (4,096 samples) were carefully selected by visual analysis by one of the authors (I.B.).
Magnetic field frequencies ranging from 0.5 to 80 Hz were recorded. The signals were then filtered into seven frequency bands: delta (0.5–4 Hz), theta (4–8 Hz), lower alpha (8–10 Hz), upper alpha (10–13 Hz), beta (13–30 Hz), lower gamma (30–45 Hz), and upper gamma (55–80 Hz).18
The synchronization likelihood (SL) was used as a measure of statistical interdependencies between MEG time series.20 The SL is based on the concept of general synchronization.25 This concept states that independent time series of two separate systems (i.e., MEG channels) need not be linearly similar to act in a synchronous manner, as long as recurrent patterns of the first system coincide (in time) with recurrent patterns of the second system. Thus, general synchronization takes into account linear as well as nonlinear synchronicity.
The SL ranges from 0.01 (no synchronicity) to 1.00 (maximal synchronicity), and it has proven to measure the linear as well as the nonlinear component that MEG signals contain.26 The SL has recently been used to assess functional connectivity in patients with Alzheimer’s disease.18,27,28 The SL was computed off-line with DIGEEGXP software developed at the Department of Neurophysiology of the VU University Medical Center.18 The parameter setting of the SL computation in this study was lag L = 10, embedding dimension m = 10, and Pref = 0.01.
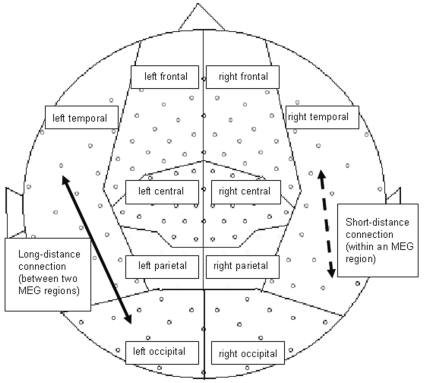
In the present study, SL values were calculated between all possible pairwise combinations of MEG sensors for all frequency bands separately. The MEG sensors were grouped according to their location above the hemisphere: central, frontal, occipital, parietal, and temporal areas (Fig. 2). Three types of SL averages were then calculated: (1) five between-area SLs in each hemisphere, (2) eight (four per hemisphere) long-distance intrahemispheric SLs (left and right frontotemporal, frontoparietal, parieto-occipital, and temporo-occipital), and (3) 10 (five per hemisphere) within-area local SLs. The first two SL measures we summarized as long-distance functional connectivity, and the within-area SLs represent short-distance functional connectivity (see Fig. 2).
Fig. 2.
Distribution of magnetoencephalography (MEG) regions and illustration of short-distance (dashed arrow) and long-distance (solid arrow) connections.
Neurocognitive Assessment
Participants were asked to complete a neurocognitive assessment (Table 1) after the MEG recording. The total duration of the assessment varied between 1 and 2 h.
Table 1.
Description of neuropsychological testing battery
| Test | Cognitive Abilities |
|---|---|
| Letter Digit Substitution Test29,30 | Psychomotor function relatively unaffected by intellectual ability |
| Concept Shifting Test31,32 | Executive (frontal) function, attention, visual scanning, and mental processing speed |
| Stroop Color Word Test33,34 | Executive (frontal) function, attention, mental speed, and mental control |
| Visual Verbal Learning Test35 | Various aspects of verbal learning, organization, and memory |
| Memory Comparison Test36 | Selective attention, mental concentration, memory, and information processing |
| Categoric Word Fluency37 | Frontal dysfunction and flexibility of verbal thought processes |
Individual patient test scores were converted to z-scores, using the means and standard deviations of the matched healthy controls as a reference.
To reduce data, individual scores on these tests were summarized into six neurocognitive domains: information processing speed, psychomotor function, attention, verbal memory, working memory, and executive functioning. Construction of these domains has been previously reported38 and was based on a principal component analysis using varimax rotation with Kaiser normalization performed on the z-scores of a large group of healthy controls.39 The domains found are commonly used in neurocognitive practice and research. An overall measure of cognition was also determined by calculating the mean of all test z-scores for each participant.
Statistical Analyses
Statistical analyses were performed using SPSS for Windows (version 14.0; SPSS Inc., Chicago, IL, USA). The nonparametric Mann-Whitney U test was used to investigate whether patients’ standardized z-scores on neurocognitive tests in the overall measure of cognition and in the aforementioned neurocognitive domains differed significantly from the z-scores of healthy controls.
To allow parametric statistical testing, we normalized the SL scores by means of the transformation Lg10[x/(1 − x)].40 To quantify differences in the SL scores between the patients and the controls, we used analysis of covariance (ANCOVA) testing with repeated measures for each frequency band. The repeated-measure factor had 8 levels for long-distance connections, 5 levels for interhemispheric connections, and 10 levels for short-distance connections. The between-subjects factor had two levels (LGG patients and controls), and age, sex, and education were used as covariates. In cases of significant group effects or interaction effects involving groups (Greenhouse-Geiser corrected p-value), subsequent post hoc analyses with regard to the regional differences in SL were performed between the patients and the controls. Again, age, sex, and education were used as determinants.
All analyses of the relation between higher neurocognitive function and SL scores within the patient population involved separate ANCOVAs with repeated measures for each frequency band. Again, the repeated-measure factor had 8 levels for long-distance connections, 5 for interhemispheric connections, and 10 for short-distance connections. Age, sex, education level, tumor lateralization, treatment modalities (radiotherapy, surgery), and epilepsy burden were used as covariates together with one of the neurocognitive domains. In cases of significant group or interaction effects (Greenhouse-Geiser corrected p-value), subsequent post hoc analyses with regard to the regional differences in SL were performed with one of the neurocognitive domains and the above-mentioned possible confounders as determinants.
Results
Sociodemographic and Clinical Characteristics
From the initial patient group, six patients were excluded: four because of metal artifacts on the MEG, one because of severe epileptic seizures, and one because of tumor progression. The four patients with metal artifacts had dental implants or amalgam fillings that had become magnetized as a result of previous MRI scans. The final analyses were performed on a sample of 17 patients and 17 matched healthy control participants.
Because of the matching procedure, there were no significant differences between patients and healthy controls in age (patients: mean = 42.7, SD = 11.2 years; healthy controls: mean = 42.6, SD = 12.7 years; p = 0.99) or educational level (patients: mean = 5.2, SD = 1.8; healthy controls: mean = 5.5, SD = 1.8; p = 0.64). The male-to-female ratio between the two groups did not differ significantly (chi squared = 0.47, p = 0.37).
Fourteen of the 17 LGG patients had a histologically confirmed LGG and were clinically and radiologically stable for more than 6 months before inclusion. Another three patients were suspected of having LGG and were stable for more than 6 months in the outpatient clinic; 1 year after the MEG registration, two of these three patients underwent surgery because of increasing epilepsy frequency.
Of the 16 patients with a histologically confirmed LGG, the pathological diagnosis was grade II astrocytoma in 10 patients, oligodendroglioma grade II in four patients, and oligoastrocytoma grade II in two patients.
The mean time in years between diagnosis and the MEG registration for the patient population in our study was 8 years, with a range of 1–19 years.
Seven of the 17 patients underwent radiotherapy, with prior chemotherapy in two patients (one patient with five cycles of procarbazine, lomustine, and vincristine [PCV] and one patient with two cycles of PCV and three cycles of temozolomide). Eleven patients had left hemisphere tumors, and six patients had right-side tumors. Table 2 shows the specific locations of the tumors.
Table 2.
Tumor lateralization and localization
| Tumor Location | No. Patients |
|---|---|
| Left hemisphere | |
| Left frontal | 4 |
| Left parietal | 3 |
| Left temporal | 3 |
| Left parieto-occipital | 1 |
| Total | 11 |
| Right hemisphere | |
| Right frontal | 2 |
| Right frontoparietal | 3 |
| Right insular region | 1 |
| Total | 6 |
We used T1-weighted MRI and defined tumor size or postoperative cavity as the product of the two largest perpendicular diameters.41 The mean tumor size or postoperative cavity in the patient group was 12 mm (range, 0.63–43 mm).
In the patient group, all but one patient used anti-epileptic mono-or polytherapy. Six of the 17 patients receiving antiepileptic drugs were free of seizures, and 10 patients were still having seizures while receiving antiepileptic mono-or polytherapy.
Differences between Patients and the Healthy Control Group
Neurocognitive functioning
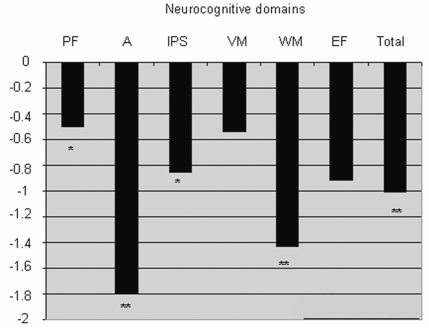
Six of the 17 patients had received neurocognitive assessments 1–9 months earlier and were clinically stable. These patients were not tested again, and the data from this last assessment were used, because their neurological status had not changed. Overall, patients performed more poorly than healthy controls on the neurocognitive test battery (controls mean ≈ 0.00): they had a significantly lower z-score on the overall measure of cognition (mean = −1.01, SD = 1.42) than did control participants (SD = 0.45, p = 0.009) (Fig. 3).
Fig. 3.
Patients’ z-scores on the six neurocognitive domains on total neurocognitive functioning. Abbreviations: PF, psychomotor functioning; A, attention; IPS, information processing speed; VM, verbal memory; WM, working memory; EF, executive functioning. Performance is relative to that of age-, sex-, and education-matched healthy controls (represented by the “0” line). A higher score (i.e., approaching 0) indicates better performance. *p ⩽ 0.05, **p ⩽ 0.01.
Furthermore, patients had significantly lower psychomotor function z-scores (mean = −0.50, p = 0.035), lower working memory z-scores (mean = −1.43, p = 0.003), slower information processing speed z-scores (mean = −0.85, p = 0.011), and lower attention z-scores (mean = −1.92, p = 0.003) relative to healthy controls. Patients’ performance in the verbal memory and executive function domains did not differ significantly from those of controls.
To determine whether the small sample group was representative of LGG patients in general, mean neuropsychological test scores were compared with those of a much larger group of patients (n = 195) from a previous study by Klein et al.8 The mean neuropsychological test scores of our patient group did not deviate more than 1 SD from those of the large group participating in that study. The SD seemed slightly higher in our group than in the sample of Klein and colleagues, most likely due to the small sample size of our patient group.
Because we are primarily interested in the relation between SL and impaired neurocognitive functioning, the remainder of the analyses focus on psychomotor function, working memory capacity, information processing speed, and attentional tasks.
Functional connectivity
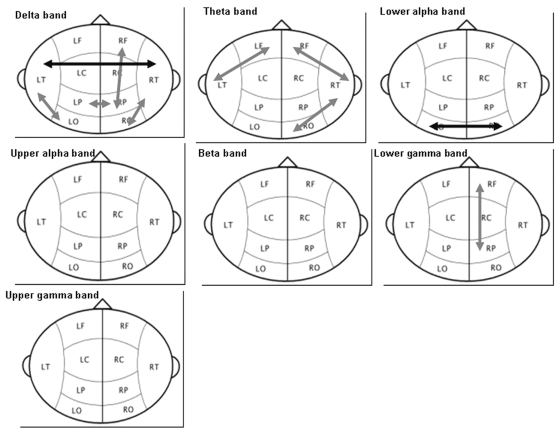
A significant group effect was seen in long-distance connectivity in the delta (ANCOVA with repeated measures; p = 0.027), theta (p = 0.004), lower alpha (p = 0.046), and lower gamma bands (p = 0.036). Post hoc regression analysis showed that long-distance functional connectivity was higher in the LGG patients than in the healthy controls, with only two exceptions: a significant decrease in functional connectivity in the patient population in the intertemporal region in the delta band and interoccipital region in the lower alpha band (Table 3, Fig. 4).
Table 3.
Significant differences in synchronization likelihood between patients and controls per frequency band and accompanying statistics
| Synchronization Likelihood (mean ± SD)a |
Model
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Frequency/Area | Patients (n= 17) | Controls (n= 17) | b | SEb | β | R2 | F-statistic | p value |
| Delta (0.5–4 Hz) | ||||||||
| Left parieto-occipital | 0.041 ± 0.009 | 0.035 ± 0.007 | −7.30E–02 | 0.030 | −0.398 | 0.234 | 2–32 | 0.018 |
| Right frontoparietal | 0.029 ± 0.005 | 0.024 ± 0.004 | −7.65E–02 | 0.025 | −0.487 | 0.237 | 1–32 | 0.004 |
| Right parieto-occipital | 0.049 ± 0.019 | 0.039 ± 0.008 | −8.52E–02 | 0.038 | −0.333 | 0.339 | 2–32 | 0.002 |
| Interparietal | 0.046 ± 0.038 | 0.038 ± 0.006 | −8.61E–02 | 0.032 | −0.434 | 0.189 | 1–32 | 0.012 |
| Intertemporal | 0.057 ± 0.016 | 0.080 ± 0.027 | 0.155 | 0.047 | 0.510 | 0.261 | 1–32 | 0.002 |
| Theta (4–8 Hz) | ||||||||
| Left frontotemporal | 0.023 ± 0.005 | 0.019 ± 0.003 | −7.02E–02 | 0.028 | −0.412 | 0.170 | 1–32 | 0.017 |
| Right frontotemporal | 0.026 ± 0.006 | 0.022 ± 0.005 | −7.10E–02 | 0.031 | −0.350 | 0.312 | 2–32 | 0.004 |
| Right temporo-occipital | 0.026 ± 0.005 | 0.023 ± 0.005 | −6.06E–02 | 0.028 | −0.357 | 0.128 | 1–32 | 0.041 |
| Lower alpha (8–10 Hz) | ||||||||
| Interoccipital | 0.039 ± 0.009 | 0.048 ± 0.010 | 9.40E–02 | 0.032 | 0.456 | 0.208 | 1–33 | 0.007 |
| Lower gamma (30–45 Hz) | ||||||||
| Right frontoparietal | 0.015 ± 0.000 | 0.015 ± 0.001 | −8.52E–03 | 0.004 | −0.299 | 0.390 | 2–33 | 0.000 |
Abbreviations: b, regression coefficient; SEb, standard error of b; R2, explained variance.
Significantly higher synchronization likelihood is shown in boldface.
Fig. 4.
Significant differences in long-distance connectivity between low-grade glioma patients and healthy controls in the different frequency bands. Gray arrows indicate significantly higher synchronization in the patient group. Black arrows indicate significantly lower synchronization in the patient group. Abbreviations: LF, left frontal; RF, right frontal; LT, left temporal; LC, left central; RC, right central; RT, right temporal; LP, left parietal; RP, right parietal; LO, left occipital; RO, right occipital.
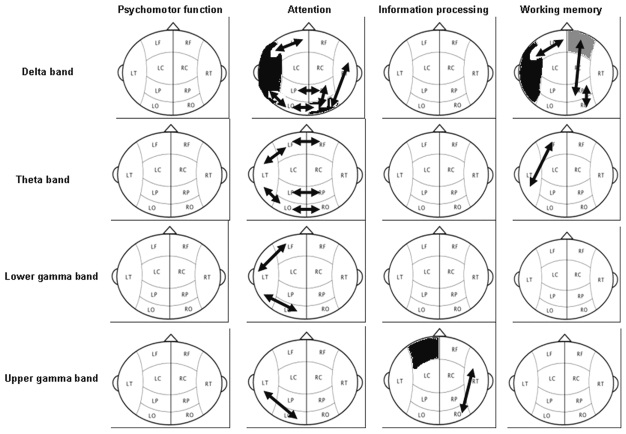
Associations between Functional Connectivity and Neurocognitive Functioning within the Patient Group
Table 4 and Fig. 5 show the significant associations between functional connectivity and neurocognitive functioning in the four disturbed neurocognitive domains. A significant group effect was seen in the delta band for long-and short-distance synchronization with working memory (ANCOVA with repeated measures: interhemispheric, p = 0.050; long distance, p = 0.032; short distance, p = 0.006). Post hoc regression analysis showed that an increase in synchronization was associated with a worsening of working memory in the left frontotemporal region (p = 0.003), right frontoparietal and right parieto-occipital regions (p = 0.007 and p = 0.018, respectively), and left temporal region (p = 0.007). An increase in synchronization in the right frontal region was associated with an improving working memory (p = 0.010).
Table 4.
Significant associations in patients between synchronization likelihood scores and neurocognitive function, per frequency band, and accompanying statistics
| Model
|
|||||||
|---|---|---|---|---|---|---|---|
| Frequency/Area | Neurocognitive Domain | b | SEb | β | R2 | F-statistic | p value |
| Delta (0.5–4 Hz) | |||||||
| Left frontotemporal | Working memory | −4.83E–02 | 0.010 | −0.852 | 0.652 | 3–16 | 0.003 |
| Right frontoparietal | Working memory | −2.40E–02 | 0.008 | −0.533 | 0.511 | 2–16 | 0.007 |
| Right parieto-occipital | Working memory | −5.04E–02 | 0.019 | −0.564 | 0.318 | 1–16 | 0.018 |
| Left temporal | Working memory | −2.52E–02 | 0.008 | −0.626 | 0.392 | 1–16 | 0.007 |
| Right frontal | Working memory | 2.72E–02 | 0.009 | 0.604 | 0.365 | 1–16 | 0.010 |
| Left frontotemporal | Attention | −0.16 | 0.005 | −0.679 | 0.497 | 2–16 | 0.008 |
| Left temporo-occipital | Attention | −0.022 | 0.006 | −0.621 | 0.548 | 2–16 | 0.004 |
| Right parieto-occipital | Attention | −0.027 | 0.007 | −0.736 | 0.541 | 1–16 | 0.001 |
| Right temporo-occipital | Attention | −0.029 | 0.008 | −0.689 | 0.475 | 1–16 | 0.002 |
| Interoccipital | Attention | −0.030 | 0.011 | −0.566 | 0.320 | 1–16 | 0.018 |
| Interparietal | Attention | −0.015 | 0.006 | −0.554 | 0.306 | 1–16 | 0.021 |
| Left temporal | Attention | −0.012 | 0.003 | −0.724 | 0.525 | 1–16 | 0.001 |
| Right occipital | Attention | −0.110 | 0.037 | −0.465 | 0.735 | 3–16 | 0.000 |
| Theta (4–8 Hz) | |||||||
| Left frontotemporal | Working memory | −4.06E–02 | 0.011 | −0.694 | 0.546 | 2–16 | 0.004 |
| Left frontotemporal | Attention | −0.018 | 0.004 | −0.749 | 0.592 | 2–16 | 0.002 |
| Left temporo-occipital | Attention | −0.022 | 0.006 | −0.686 | 0.471 | 1–16 | 0.002 |
| Interfrontal | Attention | −0.014 | 0.005 | −0.434 | 0.684 | 2–16 | 0.000 |
| Interoccipital | Attention | −0.022 | 0.006 | −0.707 | 0.499 | 1–16 | 0.002 |
| Interparietal | Attention | −0.015 | 0.003 | −0.687 | 0.876 | 5–16 | 0.000 |
| Lower gamma (30–45 Hz) | |||||||
| Left frontotemporal | Attention | −0.004 | 0.001 | −0.715 | 0.595 | 2–16 | 0.002 |
| Left temporo-occipital | Attention | −0.010 | 0.004 | −0.571 | 0.326 | 1–16 | 0.017 |
| Upper gamma (55–80 Hz) | |||||||
| Right temporo-occipital | Information processing | −1.81E–02 | 0.007 | −0.553 | 0.306 | 1–16 | 0.021 |
| Left frontal | Information processing | −5.65E–03 | 0.002 | −0.541 | 0.293 | 1–16 | 0.025 |
| Left temporo-occipital | Attention | −0.012 | 0.004 | −0.637 | 0.406 | 1–16 | 0.006 |
Abbreviations: b, regression coefficient; SEb, standard error of b; R2, explained variance.
Fig. 5.
Significant differences in the long-distance connectivity between synchronization likelihood scores in low-grade glioma patients and neurocognitive function in the different frequency bands. Black arrows and areas indicate higher synchronization associated with worsening in higher neurocognitive functioning. Gray arrows and areas indicate higher synchronization associated with improving higher neurocognitive functioning. Abbreviations: LF, left frontal; RF, right frontal; LT, left temporal; LC, left central; RC, right central; RT, right temporal; LP, left parietal; RP, right parietal; LO, left occipital; RO, right occipital.
A significant effect for group and interaction was seen in the delta band for long-and short-distance synchronization with attentional tasks (ANCOVA with repeated measures: interhemispheric, p = 0.015 [group] and p = 0.007 [interaction]; long distance, p = 0.002 [group] and p = 0.005 [interaction]; short distance, p = 0.018 [group] and p = 0.017 [interaction]). Post hoc regression analysis showed that an increase in synchronization was associated with a worsening in attentional tasks in the left frontotemporal and left temporo-occipital regions (p = 0.008 and p = 0.004, respectively), right parieto-occipital and temporo-occipital regions (p = 0.001 and p = 0.002, respectively), interoccipital and interparietal regions (p = 0.018 and p = 0.021, respectively), and left temporal and right occipital regions (p = 0.001 and p = 0.000, respectively).
A significant group effect was seen in the theta band for long-distance synchronization with working memory (ANCOVA with repeated measures; p = 0.030). Post hoc regression analysis showed that an increase in synchronization was associated with a worsening of working memory in the left frontotemporal region (p = 0.004).
A significant group effect was seen in the theta band for long-distance synchronization with attentional tasks (ANCOVA with repeated measures: interhemispheric, p = 0.017; long distance, p = 0.020). Post hoc regression analysis showed that an increase in synchronization was associated with a worsening in attentional tasks in the left frontotemporal and left temporo-occipital regions (both p = 0.002) and the interfrontal, interoccipital, and interparietal regions (p = 0.000, p = 0.002, and p = 0.000, respectively).
A significant interaction effect was seen in the lower gamma band for long-distance synchronization with attentional tasks (ANCOVA with repeated measures; p = 0.022). Post hoc regression analysis showed that an increase in synchronization was associated with a worsening in attentional tasks in the left frontotemporal and temporo-occipital regions (p = 0.002 and p = 0.017, respectively).
Also in the upper gamma band, a significant group effect was seen for long-and short-distance synchronization with information processing (ANCOVA with repeated measures: long distance, p = 0.014; short distance, p = 0.038). Post hoc regression analysis showed that an increase in synchronization was associated with a worsening of information processing in the right temporo-occipital region (p = 0.021) and left frontal region (p =0.025).
A significant interaction effect was seen for long-distance synchronization with attentional tasks (ANCOVA with repeated measures; p = 0.017). Post hoc regression analysis showed that an increase in synchronization in the left temporo-occipital region was associated with a worsening in attentional tasks in the left temporo-occipital region (p = 0.006).
Discussion
The aim of our study was to evaluate changes in functional connectivity in LGG patients compared with healthy controls and to study the correlation between impaired neurocognitive functioning and functional connectivity as a potential explanatory mechanism underlying cognitive deficits.
LGG patients’ overall neurocognitive performance and the neurocognitive domains of psychomotor function, working memory, information processing speed, and attention were impaired. These results corroborate previous studies indicating a decline in neurocognitive functioning in LGG patients.7,8,42–45
Consistent with our previous studies,22,23 we also found differences in resting-state functional connectivity in brain tumor patients compared with a healthy control population.
An increase in the lower-frequency bands was shown within the patient population compared with healthy controls. However, in contrast to our previous studies, we now report a strong increase in both the lower-and higher-frequency bands for long-distance functional connectivity, whereas in Bartolomei et al.23 we reported a strong increase in lower-frequency bands for short-distance connectivity and a decrease in higher-frequency bands for long-distance connectivity.
In a previous study,23 we found an increase in the alpha band, whereas in this study we found a decrease in the lower alpha band and an increase in the lower gamma band. It is not known whether these conflicting results are a result of differences in methodology in the two studies. Compared with the patients in our previous studies,22,23 the present patient population consisted of a homogeneous group of LGG patients instead of patients with a mixture of primary brain tumors. Furthermore, here we used a healthy control group that was better matched for age, education, and sex. As hypothesized, we found a high number of significant correlations between functional connectivity and neurocognitive performance in the patients. The associations between functional connectivity and cognition occurred mostly in the delta, theta, and lower and upper gamma bands, thus confirming earlier studies in which the delta band was linked to verbal memory28 and the theta band to working memory performance and attentional functions.46–50 The gamma band has been reported to correlate with learning and memory formation,51,52 selective attention and task complexity,53 and information processing.54–56
The present outcomes indicate an association between functional connectivity and neurocognitive functioning in LGG patients. It is possible that changes in resting-state synchronization are not merely an epiphenomenon. However, the causal relation of this association remains unclear because of the cross-sectional nature of this study.
Also, interpreting correlations between signals recorded at different MEG sensors as functional connectivity is difficult (i.e., the inverse problem). Volume conduction could have given rise to random correlations between the MEG channels, although this possibility can hardly explain all the reported patterns of correlation that we found. Moreover, long-distance connectivity, which we frequently found in this study, is much less likely to be subject to volume conduction.
The cause of the reported increase of functional connectivity, in particular, the lower frequencies, remains unknown. Evidence of increased functional connectivity has also been found in other patient groups, including those with Alzheimer’s disease, in which increased coherence was found in the delta and theta bands.57 Another study explored the relation between EEG synchronization and verbal memory in patients with mild cognitive impairments (MCIs).28 During the resting state, patients’ verbal memory scores correlated negatively with EEG recordings in the delta frequency band. The researchers propose a compensational mechanism in MCI patients: the increased synchronization in the lower alpha band could mean that the brain tries to adjust to the deleterious effect of synchronization on cognition. Patients suffering from full-blown Alzheimer’s disease do not show this increase, possibly because there is too much deterioration to allow compensation. Bookheimer et al.58 evaluated patterns of brain activation during fMRI scanning in healthy subjects, half of them carriers of the APOE ε4 allele, which has a dose-related effect on risk and age of onset of late-onset familial Alzheimer’s disease. They found a greater increase in signal intensity in brain regions necessary for tasks requiring memory among carriers of this allele compared with noncarriers. Bookheimer and colleagues suggest that in persons at risk for Alzheimer’s disease, such increased brain activity may effectively serve as a compensatory mechanism, wherein subjects use additional cognitive resources to bring memory-related performance to a normal level.58
Speculatively, the changes reported in the present study could be due to a compensation mechanism in the LGG patients, as well. The functional connectivity might increase because of the effect on the higher neurocognitive function, whereas the healthy controls do not have to compensate in this manner.
In our study, an increase in long-distance connectivity was found, especially in the low-frequency bands. Moreover, higher functional connectivity in these frequency bands was associated with poorer performance in working memory and attentional tasks, with the only exception for the right frontal region in the delta band, where an increase in the functional connectivity was associated with an improving working memory. In the lower and upper gamma band, an increase in short-and long-distance functional connectivity was associated with a worsening in information processing and attentional tasks. However, the negative correlation between functional connectivity and cognition implies that this presumed compensatory mechanism does not optimize neurocognitive function.
The short-distance functional connectivity measures were equal in patients and controls. Within the patient group, an increased short-distance connectivity in general went hand in hand with poorer neurocognitive performance.
In conclusion, LGG patients displayed changes in resting-state functional connectivity compared with healthy controls and showed impaired neurocognitive functioning. We found significant correlations between neurocognitive performance and functional connectivity in various frequencies and across multiple areas, suggesting that the changes in resting-state functional interactions may be relevant for the observed neurocognitive deficits in the LGG patients. Further research is needed, and a longitudinal study will start soon in our department. On the basis of the results of this study, we will evaluate the functional connectivity in brain tumor patients before and after treatment and correlate these results with higher neurocognitive function. In this way, we will be able to determine whether the changes in resting-state synchronization are merely an epiphenomenon.
Acknowledgments
We thank J. Verbunt, P.J. Ris, I. Manshanden, and G. de Vos for technical assistance, Els van Deventer for providing us with the relevant literature, and L. Stalpers, radiation oncologist at the Academic Medical Center, Amsterdam, for permission to recruit his patients. This study was supported by Schering Plough Nederland and the Kapteijnfonds.
References
- 1.Shaw EG, Scheithauer BW, O’Fallon JR. Management of supratentorial low-grade gliomas. Oncology. 1993;7(7):97–104. doi: 10.1016/1053-4296(91)90006-s. [DOI] [PubMed] [Google Scholar]
- 2.Leighton C, Fisher B, Bauman G, et al. Supratentorial low-grade glioma in adults: an analysis of prognostic factors and timing of radiation. J Clin Oncol. 1997;15(4):1294–1301. doi: 10.1200/JCO.1997.15.4.1294. [DOI] [PubMed] [Google Scholar]
- 3.Zülch KJ. Histological Typing of Tumors of the Central Nervous System. Geneva: World Health Organization; 1979. [Google Scholar]
- 4.Shaw EG, Scheithauer BW, Dinapoli RP. Low-grade hemispheric astrocytomas. In: Black PM, Loeffler JS, editors. Cancer of the Nervous System. Cambridge: Blackwell Science; 1997. pp. 441–463. [Google Scholar]
- 5.Olson JD, Riedel E, DeAngelis LM. Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology. 2000;54(7):1442–1448. doi: 10.1212/wnl.54.7.1442. [DOI] [PubMed] [Google Scholar]
- 6.Wessels PH, Weber WE, Raven G, et al. Supratentorial grade II astrocytoma: biological features and clinical course. Lancet Neurol. 2003;7(2):395–403. doi: 10.1016/s1474-4422(03)00434-4. [DOI] [PubMed] [Google Scholar]
- 7.Taphoorn MJ, Heimans JJ, Snoek FJ, et al. Assessment of quality of life in patients treated for low-grade glioma: a preliminary report. J Neurol Neurosurg Psychiatry. 1992;55(5):372–376. doi: 10.1136/jnnp.55.5.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360(9343):1361–1368. doi: 10.1016/s0140-6736(02)11398-5. [DOI] [PubMed] [Google Scholar]
- 9.Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumors. Lancet Neurol. 2004;3(3):159–168. doi: 10.1016/S1474-4422(04)00680-5. [DOI] [PubMed] [Google Scholar]
- 10.Bressler S. Understanding cognition through large-scale networks. Curr Dir Psychol Sci. 2002;11:58–61. [Google Scholar]
- 11.Friston KJ. Brain function, nonlinear coupling, and neural transients. Neuroscientist. 2001;7(5):406–418. doi: 10.1177/107385840100700510. [DOI] [PubMed] [Google Scholar]
- 12.Tononi G, Edelman GM, Sporns O. Complexity and coherency: integrating information in the brain. Trends Cogn Sci. 1998;2:474–484. doi: 10.1016/s1364-6613(98)01259-5. [DOI] [PubMed] [Google Scholar]
- 13.Parra J, Kalitzin SN, da Silva FH. Magnetoencephalography: an investigational tool or a routine clinical technique? Epilepsy Behav. 2004;5(3):277–285. doi: 10.1016/j.yebeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Guevara R, Velazquez JL, Nenadovic V, et al. Phase synchronization measurements using electroencephalographic recordings: what can we really say about neuronal synchrony? Neuroinformatics. 2005;3(4):301–314. doi: 10.1385/NI:3:4:301. [DOI] [PubMed] [Google Scholar]
- 15.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 16.Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;12 103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laufs H, Krakow K, Sterzer P, et al. E lectroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci USA. 2003;100:11053–10058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stam CJ, Jones BF, Manshanden I, et al. Magnetoencephalographic evaluation of resting-state functional connectivity in Alzheimer’s disease. Neuroimage. 2006;32(3):1335–1344. doi: 10.1016/j.neuroimage.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 19.Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 20.Stam CJ, Dijk BW. Synchronization likelihood: an unbiased measure of generalized synchronization. Physica D. 2002;163:236–241. [Google Scholar]
- 21.Stam CJ. Nonlinear dynamical analysis of EEG and MEG: review of an emerging field. Clin Neurophysiol. 2005;116:2266–2301. doi: 10.1016/j.clinph.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Bartolomei F, Bosma I, Klein M, et al. How do brain tumors alter functional connectivity? a magnetoencephalography study. Ann Neurol. 2006;59(1):128–138. doi: 10.1002/ana.20710. [DOI] [PubMed] [Google Scholar]
- 23.Bartolomei F, Bosma I, Klein M, et al. Disturbed functional connectivity in brain tumor patients: evaluation by graph analysis of synchronization matrices. Clin Neurophysiol. 2006;117(9):2039–2049. doi: 10.1016/j.clinph.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 24.Vrba J, Anderson G, Betts K. 151-Channel whole-cortex MEG system for seated or supine positions. In: Yoshimoto T, Kotani M, Kuriki S, Karibe H, Nakasato N, editors. Recent Advances in Biomagnetism. Sendai, Japan: Tohoku University Press; 1999. pp. 93–96. [Google Scholar]
- 25.Rulkov NF, Sushchik MM, Tsimring LS, et al. Generalized synchronization of chaos in directionally coupled chaotic systems. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1995;51(2):980–994. doi: 10.1103/physreve.51.980. [DOI] [PubMed] [Google Scholar]
- 26.Stam CJ, Breakspear M, van Cappellen van Walsum AM, van Dijk BW. Nonlinear synchronization in EEG and whole-head MEG recordings of healthy subjects. Hum Brain Mapp. 2003;19(2):63–78. doi: 10.1002/hbm.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stam CJ, van der Made Y, Pijnenburg YA, Scheltens P. EEG synchronization in mild cognitive impairment and Alzheimer’s disease. Acta Neurol Scand. 2003;108(2):90–96. doi: 10.1034/j.1600-0404.2003.02067.x. [DOI] [PubMed] [Google Scholar]
- 28.Pijnenburg YA, van der Made Y, van Cappellen van Walsum AM, et al. EEG synchronization likelihood in mild cognitive impairment and Alzheimer’s disease during a working memory task. Clin Neurophysiol. 2004;115(6):1332–1339. doi: 10.1016/j.clinph.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 29.Jolles J, Houx PJ, Van Boxtel MPJ, Ponds RWHM, editors. Maastricht Aging Study: Determinants of Cognitive Aging. Maastricht: Neuropsych Publishers; 1995. [Google Scholar]
- 30.Lezak MD. Neuropsychological Assessment. New York: Oxford University Press; 1995. [Google Scholar]
- 31.Eson ME, Yen YK, Bourke RS. Assessment of recovery from serious head injury. J Neurol Neurosurg Psychiatry. 1978;41:1036–1042. doi: 10.1136/jnnp.41.11.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houx PJ, Jolles J. Vulnerability factors for age-related cognitive decline. In: Jensen KF, editor. The Vulnerable Brain and Environmental Risks. Vol. 3. New York: Plenum Press; 1994. pp. 25–41. [Google Scholar]
- 33.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–62. [Google Scholar]
- 34.Perret E. The left frontal lobe of man and the suppression of habitual responses in verbal categorical behavior. Neuropsychologia. 1974;12:323–330. doi: 10.1016/0028-3932(74)90047-5. [DOI] [PubMed] [Google Scholar]
- 35.Brand N, Jolles J. Learning and retrieval rate of words presented auditorily and visually. J Genet Psychol. 1985;112:201–212. doi: 10.1080/00221309.1985.9711004. [DOI] [PubMed] [Google Scholar]
- 36.Sternberg S. Memory scanning: new findings and current controversies. Q J Exp Psychol. 1975;27:1–32. [Google Scholar]
- 37.Luteijn F, van der Ploeg FAE. Groningen Intelligence Test, Revised Manual. Lisse, The Netherlands: Swets and Zeitlinger; 1983. [Google Scholar]
- 38.Klein M, Postma TJ, Taphoorn MJ, et al. The prognostic value of cognitive functioning in the survival of patients with high-grade glioma. Neurology. 2003;61(12):1796–1798. doi: 10.1212/01.wnl.0000098892.33018.4c. [DOI] [PubMed] [Google Scholar]
- 39.Bosma H, van Boxtel MP, Ponds RW, et al. Pesticide exposure and risk of mild cognitive dysfunction. Lancet. 2000;356(9233):912–913. doi: 10.1016/s0140-6736(00)02685-4. [DOI] [PubMed] [Google Scholar]
- 40.Gasser T, Bächer P, Möcks J. Transformations towards the normal distribution of broad band spectral parameters of the EEG. Electroencephalogr Clin Neurophysiol. 1982;53:119–124. doi: 10.1016/0013-4694(82)90112-2. [DOI] [PubMed] [Google Scholar]
- 41.Vos MJ, Uitdehaag BM, Barkhof F, et al. Interobserver variability in the radiological assessment of response to chemotherapy in glioma. Neurology. 2003;61(12):826–830. doi: 10.1212/01.wnl.0000049467.54667.92. [DOI] [PubMed] [Google Scholar]
- 42.Hochberg FH, Slotnick B. Neuropsychologic impairment in astrocytoma survivors. Neurology. 1980;30(2):172–177. doi: 10.1212/wnl.30.2.172. [DOI] [PubMed] [Google Scholar]
- 43.Taphoorn MJ, Schiphorst AK, Snoek FJ, et al. Cognitive functions and quality of life in patients with low-grade gliomas: the impact of radiotherapy. Ann Neurol. 1994;36(1):48–54. doi: 10.1002/ana.410360111. [DOI] [PubMed] [Google Scholar]
- 44.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24(8):1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 45.Shaw EG, Rosdhal R, D’Agostino RB, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24(9):1415–1420. doi: 10.1200/JCO.2005.03.3001. [DOI] [PubMed] [Google Scholar]
- 46.Klimesch W, Schimke H, Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalogr Clin Neurophysiol. 1994;91(6):428–441. doi: 10.1016/0013-4694(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 47.Sarnthein J, Petsche H, Rappelsberger P, Shaw GL, von Stein A. Synchronization between prefrontal and posterior association cortex during human working memory. Proc Natl Acad Sci USA. 1998;95:7092–7096. doi: 10.1073/pnas.95.12.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stam CJ. Brain dynamics in the theta and alpha frequency bands and working memory performance. Neurosci Lett. 2000;286:115–118. doi: 10.1016/s0304-3940(00)01109-5. [DOI] [PubMed] [Google Scholar]
- 49.Stam CJ, van Cappellen van Walsum AM, Micheloyannis S. Variability of EE G synchronization during a working memory task in healthy subjects. Int J Psychophysiol. 2002;46:53–66. doi: 10.1016/s0167-8760(02)00041-7. [DOI] [PubMed] [Google Scholar]
- 50.Gootjes L, Bouma A, van Strien JW, Scheltens P, Stam CJ. Attention modulates hemispheric differences in functional connectivity: evidence from MEG recordings. Neuroimage. 2006;30:245–253. doi: 10.1016/j.neuroimage.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 51.Fell J, Klaver P, Lehnertz K, et al. Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nat Neurosci. 2001;4(12):1259–1264. doi: 10.1038/nn759. [DOI] [PubMed] [Google Scholar]
- 52.Kaiser J, Lutzenberger W. Induced gamma-band activity and human brain function. Neuroscientist. 2003;9(6):475–484. doi: 10.1177/1073858403259137. [DOI] [PubMed] [Google Scholar]
- 53.Micheloyannis S, Vourkas M, Bizas M, et al. Changes in linear and nonlinear EEG measures as a function of task complexity: evidence for local and distant signal synchronization. Brain Topogr. 2003;15(4):239–247. doi: 10.1023/a:1023962125598. [DOI] [PubMed] [Google Scholar]
- 54.Crone NE, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception. Clin Neurophysiol. 2001;112(4):565–582. doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- 55.Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci. 2001;5(1):16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- 56.Meador KJ, Ray PG, Echauz JR, et al. Gamma coherence and conscious perception. Neurology. 2002;59:847–854. doi: 10.1212/wnl.59.6.847. [DOI] [PubMed] [Google Scholar]
- 57.Locatelli T, Cursi M, Liberati D, et al. EEG coherence in Alzheimer’s disease. Electroencephalogr Clin Neurophysiol. 1998;106(3):229–237. doi: 10.1016/s0013-4694(97)00129-6. [DOI] [PubMed] [Google Scholar]
- 58.Bookheimer SY, Strojwas MH, Cohen MS, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]