Abstract
Aims
The aim of this study was to investigate whether the sera from chronic chagasic patients (CChPs) with beta-1 adrenergic activity (Ab-β) can modulate ventricular repolarization. Beta-adrenergic activity has been described in CChP. It increases the L-type calcium current and heart rate in isolated hearts, but its effects on ventricular repolarization has not been described.
Methods and results
In isolated rabbit hearts, under pacing condition, QT interval was measured under Ab-β perfusion. Beta-adrenergic activity was also tested in guinea pig ventricular M cells. Furthermore, the immunoglobulin fraction (IgG-β) of the Ab-β was tested on Ito, ICa, and Iks currents in rat, rabbit, and guinea pig myocytes, respectively. Beta-adrenergic activity shortened the QT interval. This effect was abolished in the presence of propranolol. In addition, sera from CChP without beta-adrenergic activity (Ab-β) did not modulate QT interval. The M cell action potential duration (APD) was reversibly shortened by Ab-β. Atenolol inhibited this effect of Ab-β, and Ab- did not modulate the AP of M cells. Ito was not modulated by isoproterenol nor by IgG-β. However, IgG-β increased ICa and IKs.
Conclusion
The shortening of the QT interval and APD in M cells and the increase of IKs and ICa induced by IgG-β contribute to repolarization changes that may trigger malignant ventricular arrhythmias observed in patients with chronic chagasic or idiopathic cardiomyopathy.
Keywords: Antibodies, Chagas’ disease, Electrophysiology, M cells, QT interval
Introduction
Circulating functional antibodies with beta ‘adrenergic-(Ab-β)-like’ activity have been described in chronic chagasic patients (CChP),1 as well as in patients with idiopathic dilated cardiomyopathy (DCM).2 Beta-adrenergic activity from CChPs increases heart rate in isolated rabbit hearts and decreases gap junction-mediated intercellular communication.3 In patients with DCM, the presence of IgG-β is a predictor of ventricular tachycardia and sudden death.4 In CChPs, the presence of functional IgG-β has been correlated with ventricular arrhythmias.5 Sudden death is the most important cause of death in CChP,6 and ventricular arrhythmias are the most probable cause for these deaths. The end of the T wave in the ECG has been associated with the end of repolarization of M cells. In addition, the modulation of the M cell action potential seems to influence directly the QT interval.7
The present work was designed to investigate the presence of Ab-β capable of modulating ventricular repolarization in sera from CChPs. In addition, the cellular and ionic effects of these antibodies were investigated in order to elucidate the mechanism responsible for this modulation.
Methods
Patients
In a longitudinal retrospective study, 17 CChPs with cardiomyopathy and positive serologic tests for Chagas’ disease using three distinct methods were enrolled.3 Beta-blockers, calcium channel blockers, and sotalol were transiently discontinued (five half-lives) before blood samples were drawn. Written consent was obtained from each patient, and World Health Organization and Helsinki Treaty regulations (1963) reviewed in Venice (1983) were followed. The study was approved by the Institutional Committee on Research.
Isolated heart and sera characterization
The characterization of Ab-β and non-beta-adrenergic (Ab-) sera was made by ECG recordings in isolated rabbit hearts.3 In this preparation, heart rate response to sera was assessed during spontaneous rhythm to dichotomize the sera of patients into two groups and to make sure that the antibodies present were functionally active. A given patient’s serum was characterized as Ab-β only when the serum was able to increase spontaneous heart rate by at least 10% within 30 min of initial perfusion of the isolated rabbit heart in the absence or presence of 1 μM atropine, and this effect was abolished by a beta-adrenergic antagonist (1 μM propranolol). Additionally, CChP’s serum was characterized as Ab- when the serum perfused alone or in the presence of propranolol (1 μM) or atropine (1 μM) and did not modulate the heart rate, excluding possible beta-adrenergic and muscarinic effects of these sera.
Sera from healthy orthopaedic surgery patients without Chagas’ or history of cardiac disease were also used (reference sera). Independent experiments were performed to test each serum using ECG recording, and the QT interval was measured under control condition and in the presence of total human serum (diluted 1:100 v/v), previously characterized as Ab-β or Ab- (discussed earlier). In addition, because the QT interval is rate-dependent, all the isolated rabbit heart experiments were performed under pacing condition (127 ± 12 bpm). Pacing was carried out through bipolar silver electrodes that were insulated except at the tips and placed upon the right atrium. A rectangular pulse was applied from an isolated stimulator (Digitimer, DS-2, Welwyn, Garden City, Hertfordshire, England) and controlled by a Digitimer D-4030 (Welwyn, Garden City, Hertfordshire, England). Two observers who were blinded to the sera used in the experiments performed analysis of the ECG recording.
Tissue preparation from guinea pig heart
Deep subendocardium and transmural strips were isolated from left ventricles and right deep layer of papillary muscle of hearts removed from male guinea pigs (300–400 g). Preparations were isolated using a dermatome (Davol Simon Dermatome, Cranston, RI, USA) to make sections parallel to the surface of the structures investigated.
The preparation was placed in a tissue bath and allowed to equilibrate for at least 2 h while superfused (5 mL/min) with an oxygenated (95% O2 and 5% CO2) Tyrode’s solution (37 ± 0.5°C and pH = 7.35). The composition of the Tyrode’s solution was (mM/L): NaCl, 129; KCl, 4; CaCl, 1.8; NaH2PO4, 0.9; MgSO4, 0.5; D glucose, 5.5; NaHCO3, 20; pH 7.4 adjusted with NaOH.
Action potential recordings
The tissue was stimulated at basic cycle lengths (BCLs) ranging from 300 to 2000 ms using field stimulation. Transmembrane potentials were recorded using glass microelectrodes filled with 2.7 M KCl (10–40 MΩ DC resistance) connected to a high input impedance amplification system (World Precision Instrument, Florida, USA). Amplified signals were displayed on Tektronix oscilloscope (Tektronix, model 5111 A, Beaverton, USA), digitized (Digidata 1200 interface AD/DA system, Axon Instrument, Inc., Sunnyvale, USA), analysed (Axotape, Clampfit 6 software, Axon Instrument, Inc., Sunnyvale, USA), and stored in a personal computer. The following action potential parameters were analysed: resting membrane potential (RMP), amplitude of phase 0, and action potential duration at 90% (APD90) and 30% (APD30) repolarization.
Immunoglobulin characterization and purification
Only IgG fractions from sera of Ab-β chagasic patients (IgG-β) were used in ion current experiments. Total IgG fraction from each patient was individually purified by the standard chromatographic procedure on DEAE–Sepharose (Amersham-Pharmacia Biotech, Buckinghamshire, UK), as described previously.3 Protein concentration in the fraction was determined by the Lowry method.8
Isolation of ventricular cardiomyocytes
Cells from rat, rabbit, or guinea pig ventricles were enzymatically dispersed, as described previously,9 to measure Ito, ICa, or IKs currents, respectively. M cells were obtained from deep subepicardium. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication no. 85-23, revised 1996).
Ionic current measurements
Voltage clamp was performed using the conventional whole-cell, patch–clamp technique to measure membrane potassium currents (Ito and IKs) and calcium current (ICa) in isolated myocytes. All experiments were performed at room temperature (22–25°C), and all tested drugs and IgG-β were added to the external bath solution, which was continuously perfused at 1 mL/min flow rate. Patch electrodes (4–9 MΩ) were prepared from borosilicate glass pipettes on a two-stage pipette puller (Sutter Instruments, P-97, Novato, USA). Cells were voltage-clamped using a patch–clamp amplifier Axopatch 200 (Axon Instrument, Inc., Sunnyvale, USA), and the signal was filtered through an 8-pole Bessel low-pass filter (Frequency Devices, Haverhill, USA) with 1 kHz cut-off frequency. Currents were sampled at a frequency of 2.5 kHz using an analogue-to-digital converter (DIGIDATA 1200, Axon Instrument, Inc., Sunnyvale, USA) connected to a PC compatible computer. Voltage-clamp protocols were generated by the pCLAMP software v6.03 (Axon Instrument, Inc., Sunnyvale, USA). To measure Ito, a protocol was applied evoking Ito every 6 s from the holding potential (−60 mV) to test potentials that varied from −50 to +60 mV, in 10 mV increments, for 500 ms after a short pre-pulse (80 ms) to −40 mV to inactivate the fast sodium current and T-type calcium current.
To measure ICa, a protocol was applied evoking ICa every 6 s from the holding potential (−40 mV) to test potentials that varied from −60 to +60 mV, in 10 mV increments, for 500 ms. To measure IKs, the protocol used applied every 6 s a depolarizing pulse from a holding potential of −50 mV to +40 mV for 3 s, followed by repolarization back to −40 mV. Each pulse was preceded by a short pre-pulse (80 ms) to −40 mV to inactivate fast Na+ current.
Solutions and reagents
Control Tyrode’s solution contained (in millimolar): 150.8 NaCl, 5.4 KCl, 1.8 CaCl2, 1.0 MgCl2, 11.0 D-glucose, 10.0 HEPES, and pH 7.4 adjusted with NaOH. IKs was recorded with an internal pipette solution containing (in millimolar): 15.0 KCl, 119 potassium glutamate, 3.75 MgCl2, 0.5 CaCl2, 5.0 EGTA, 5.0 HEPES, and 2.8 ATP; pH adjusted to 7.2 with KOH. External solution was Tyrode with nicardipine (1 μM), to block calcium current and E-4031 (5 μM) to block IKr. Ito was elicited in external Tyrode’ solution with nicardipine (1 μM) to block calcium current and tetraethylammonium chloride (TEA-Cl) to block Iks current. The internal solution contained (in millimolar): 50.0 KCl, 1.0 MgSO4, 10.0 EGTA, 80.0 L-aspartic acid, 10.0 KH2PO4, 5.0 HEPES, and 3.0 ATP (Na+); pH adjusted to 7.2 with KOH. To record the ICa, the internal solution contained (in millimolar): 110 CsCl, 30 TEA-Cl, 5 MgATP, 0.1 GTP, 10 HEPES, and 10 EGTA (pH adjusted to 7.1 with CsOH). To eliminate ion currents other than ICa L, once whole-cell recording was achieved, and the bath solution was switched to an extracellular solution (TEA-Cs solution), in which Na1 were substituted by TEA and K1 were replaced by Cs1 containing (in millimolar) 140 TEA-Cl, 6 CsCl, 1 MgCl2, 2 CaCl2, 11 glucose, and 10 HEPES (pH adjusted to 7.3 with TEA-OH).
Statistical analysis
All data are shown as mean ± SEM. Statistical significance was estimated by Student’s t-test or one-way analysis of variance (ANOVA) coupled with Newman and Keuls. Difference was considered significant at P < 0.05.
Results
Effects of chronic chagasic patients’ sera on QT interval in isolated rabbit hearts
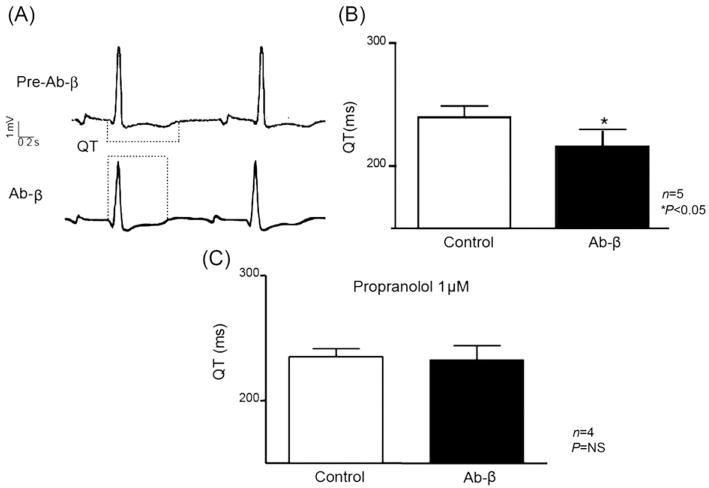
Serum from each CChP was tested in isolated rabbit hearts. Under paced conditions, adrenergic sera significantly shortened QT intervals from 240.0 ± 20 ms in control to 216.0 ± 26 ms in Ab-β (Student’s t-test, P < 0.05; n = 5; Figure 1A and B). The effect of Ab-β on QT interval was completely neutralized when propranolol (1 μM) was present in the perfusing solution, as shown in Figure 1C (QT Tyrode–propranolol = 235.0 ± 6.5 ms, QT Tyrode–propranolol–Ab-β = 232.8 ± 11.2 ms; Student’s t-test P > 0.05; n = 4; Figure 1C).
Figure 1.
Sera with beta-adrenergic activity from chronic chagasic patients shorten the QT interval in isolated rabbit hearts. Representative ECGs before and during Ab-β perfusion (Ab-β: 1/100 V/V) show shorter QT intervals under Ab-β perfusion (A). (B) Summary of the results. (C) The Ab-β effect on QT interval was abolished in the presence of propranolol (1 μM). Values are mean ± SEM. *P < 0.05.
Sera from normal subjects (QT during Tyrode perfusion 271.1 ± 12.1 vs. 275.7 ± 13.7 ms in sera from normal subjects, n = 5; P > 0.05) or from CChP but without beta-1-AR cardiac activity (QT during Tyrode perfusion, 249.1 ± 7.8 vs. 252.7 ± 9.8 ms in sera from CChP without cardiac activity, n = 13; P > 0.05) did not alter the QT interval in isolated rabbit heart ECGs.
M cells action potential
Typical guinea pig M cell action potential shape was recorded, as described previously by Sicouri et al.10 The action potential of M cells was longer than that of endocardial and epicardial cells. In addition, the guinea pig M cells were distinguished by the disproportionate prolongation of their action potential relative to the action potential of other ventricular myocardial cells in response to a slowing of rate (data not shown).
Adrenergic modulation of M cell action potential: the effects of the beta-1 adrenergic activity in the guinea pig M cells
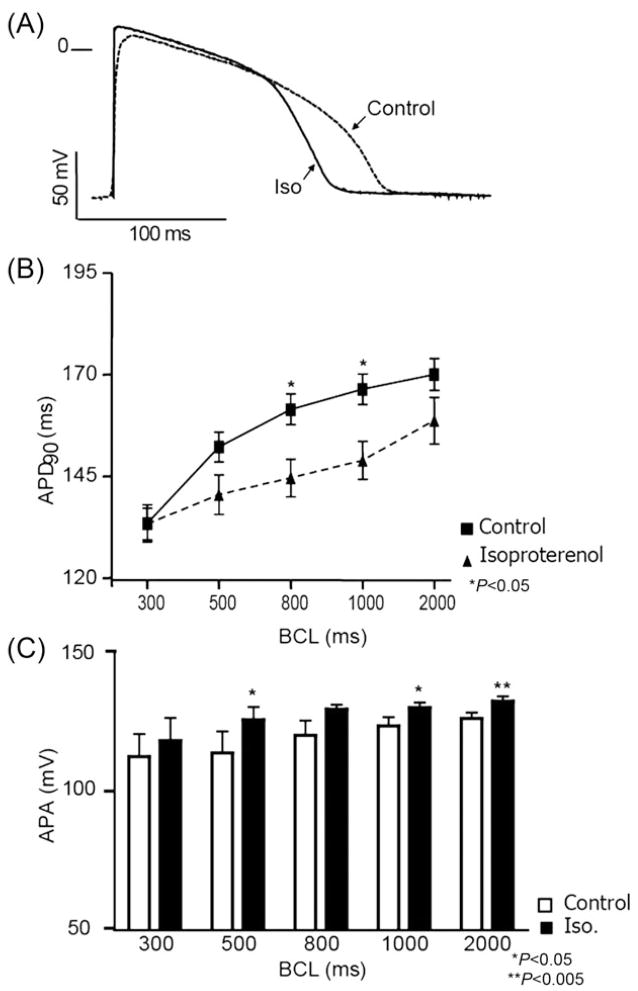
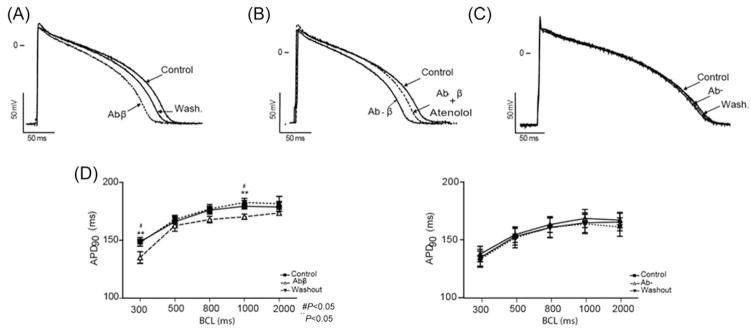
The modulation of M cells by adrenergic stimuli in guinea pig heart has not been previously described. As shown in Figure 2A, isoproterenol (1 μM) shortened action potential duration at 90% repolarization (APD90) at long and short BCLs. At a BCL of 1000 ms, isoproterenol shortened APD90 from 166.7 ± 7.4 to 149.3 ± 9.2 ms (n = 5; Student’s t-test, P < 0.05, Figure 2B) and modulated the amplitude of phase 0 of the AP upstroke (APA), as shown in Figure 2C. At a BCL of 2000 ms, isoproterenol increased APA from 126.3 ± 2.1 to 132.5 ± 1.8 mV (n = 5, P < 0.005). In the AP of Figure 2A, a change in phase 2 is observed; however, no statistical difference in APD measured at 30% repolarization (APD30) or RMP was observed at the distinct BCLs evaluated. The Ab-β reversibly shortened the APD90 (Figure 3A). This effect was statistically significant at BCLs of 1000 ms (Tyr: 179.4 ± 2.5, Ab-β: 170.3 ± 2.2, and wash: 182.5 ± 3.8; one-way ANOVA, P < 0.05; n = 7) and 300 ms (Tyr: 148.8 ± 3.8, Ab-β: 135.1 ± 5, and washout: 148.0 ± 3; one-way ANOVA, P < 0.0.5). The effect was abolished when atenolol (1 μM) was added in the presence of Ab-β (Figure 3B).
Figure 2.
Adrenergic response modulates guinea pig M cell action potential. At 1000 ms basic cycle length, isoproterenol (1 μM) shortens APD (A). (B) Action potential duration measured at 90% repolarization (APD90) under different BCLs: 300, 500, 800, 1000, and 2000 ms. Isoproterenol significantly decreased APD90 at 800 and 1000 ms. Phase 0 amplitude (APA) shows significant increment in the presence of isoproterenol (1 μM), under different BCLs (C). Values are mean ± SEM, n = 4. *P < 0.05 and **P < 0.005.
Figure 3.
Ab-β modulate M cell action potential by activating beta-adrenergic receptor. Transmembrane activity recorded from M cells isolated from the guinea pig left ventricle. Recordings were obtained at a BCL of 1000 ms under steady-state conditions, during Ab-β (Ab-β: 1/100 V/V) perfusion, and after washout (A). In (B), the presence of atenolol (1 μM) inhibited the effect mediated by Ab-β. Sera from chronic chagasic patients without adrenergic effect (Ab-: 1/100 V/V) did not modulate the APD of guinea pig M cells at a BCL of 1000 ms. (C) APD90 modulation by Ab- (n = 6) or Ab-β (n = 7) under different rates is summarized in (D). Values are mean ± SEM. *P < 0.05 and #P < 0.005.
Sera from CChP without adrenergic activity (Ab-) did not modulate the M cell AP (Figure 3C). The results of the effects of Ab-β and Ab- upon M cell are summarized in Figure 3D and Table 1.
Table 1.
Action potential results
| (ms) | 300 | 500 | 800 | 1000 | 2000 |
|---|---|---|---|---|---|
| RMP (-mV) | |||||
| Control | 79.2 ± 3.7 | 80.3 ± 3.3 | 80.3 ± 3.2 | 79.3 ± 3.2 | 79.4 ± 3.1 |
| Ab-β (n = 7) | 80.8 ± 3.9 | 81.1 ± 3.7 | 80.7 ± 3.6 | 80.2 ± 3.3 | 79.1 ± 3.1 |
| Phase 0 amplitude (mV) | |||||
| Control | 121.6 ± 1.5 | 122.5 ± 1.6 | 123.6 ± 1.3 | 124.0 ± 1.4 | 124.8 ± 1.5 |
| Ab-β | 120.1 ± 3.5 | 122.3 ± 2.7 | 123.0 ± 2.5 | 123.0 ± 2.6 | 123.2 ± 2.9 |
| APD90 (ms) | |||||
| Control | 148.8 ± 3.8* | 166.1 ± 4.2 | 176.0 ± 3.5 | 179.4 ± 2.5* | 178.7 ± 3.9 |
| Ab-β | 135.1 ± 3.1* | 162.6 ± 4.7 | 167.9 ± 2.8 | 170.3 ± 2.2* | 173.5 ± 1.6 |
| APD30 (ms) | |||||
| Control | 64.2 ± 4.9 | 76.2 ± 4.9 | 82.1 ± 3.4 | 89.1 ± 3.1 | 92.7 ± 3.1 |
| Ab-β | 56.9 ± 4.2 | 71.5 ± 3.5 | 82.5 ± 1.9 | 84.1 ± 3.2 | 90.6 ± 2.3 |
| RMP (-mV) | |||||
| Control | 86.3 ± 2.9 | 86.1 ± 3.0 | 86.1 ± 3.2 | 85.1 ± 3.2 | 83.4 ± 3.1 |
| Ab- (n = 8) | 85.3 ± 2.7 | 84.9 ± 2.8 | 85.0 ± 3.0 | 84.5 ± 2.8 | 85.2 ± 2.9 |
| Phase 0 amplitude (mV) | |||||
| Control | 121.1 ± 1.8 | 122.3 ± 1.7 | 123.4 ± 1.6 | 123.2 ± 1.7 | 124.6 ± 1.9 |
| Ab- | 121.3 ± 2.6 | 123.5 ± 2.5 | 124.7 ± 2.5 | 125.3 ± 2.6 | 126.5 ± 2.2 |
| APD90 (ms) | |||||
| Control | 136.7 ± 6.7 | 153.6 ± 6.8 | 162.3 ± 6.8 | 167.5 ± 7.8 | 166.1 ± 7.2 |
| Ab- | 133.5 ± 7.2 | 152.0 ± 7.5 | 159.6 ± 8.3 | 162.8 ± 8.3 | 160.2 ± 8.0 |
| APD30 (ms) | |||||
| Control | 63.9 ± 2.6 | 72.8 ± 3.6 | 85.7 ± 3.2 | 90.7 ± 3.9 | 92.9 ± 3.7 |
| Ab- | 58.3 ± 3.9 | 70.7 ± 4.6 | 79.7 ± 3.7 | 85.5 ± 3.4 | 90.1 ± 3.2 |
BCL, basic cycle lengths; RMP, resting membrane potential; APD90, action potential duration at 90% repolarization; APD30, action potential duration at 30% repolarization;
P < 0.05, control vs. Ab-β. All values are expressed as mean ± SEM.
Ionic mechanisms
To elucidate the ionic mechanisms involved in the Ab-β-induced reduction of the QT interval and APD of M cells, IgGs were purified from Ab-β sera of CChPs. The effect of these IgGs was then tested in potassium ion currents known to modulate action potential duration. The overload of calcium was previously demonstrated as a triggering factor in the genesis of ventricular arrhythmias.11–13 Because the adrenergic stimulus, mediated by IgG-β, could increase the intracellular calcium, the effect of IgG was tested in the L-type calcium current.
Transient outward potassium current
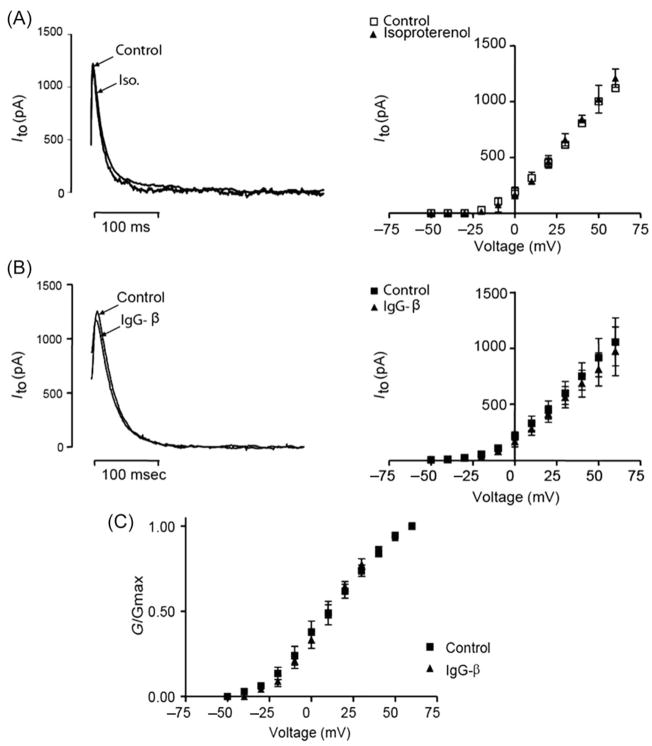
The Ito current was elicited in isolated rat myocytes. First, we tested the adrenergic agonist isoproterenol (1 μM). The drug was added to Tyrode’s solution, and no change in the current–voltage relationship was observed (Figure 4A). IgG-β (24 μg/mL) was also unable to modify the current–voltage relationship (Figure 4B). In addition, IgG-β did not modulate the kinetics nor voltage dependence of Ito channel activation (V50 = 6.4 ± 2.5 control vs. 7.1 ± 3.0 mV IgG-β; P > 0.05; slope = 11.2 ± 4.0 control vs. 8.3 ± 3.6 IgG-β; P > 0.05; Figure 4C).
Figure 4.
IgG-β does not modulate Ito. Representative current traces of Ito under control and isoproterenol (1 μM) perfusion (A) and under control and IgG-β (24μg/mL) perfusion (B) are superimposed. Current–voltage curves show that neither isoproterenol (P > 0.05 and n = 5) nor IgG-β (P > 0.05 and n = 7) modulated Ito current (right panels). IgG-β did not modulate the kinetics or voltage dependence of Ito channel activation (C). Values are mean ± SEM.
Slowly activating delayed rectifier potassium current
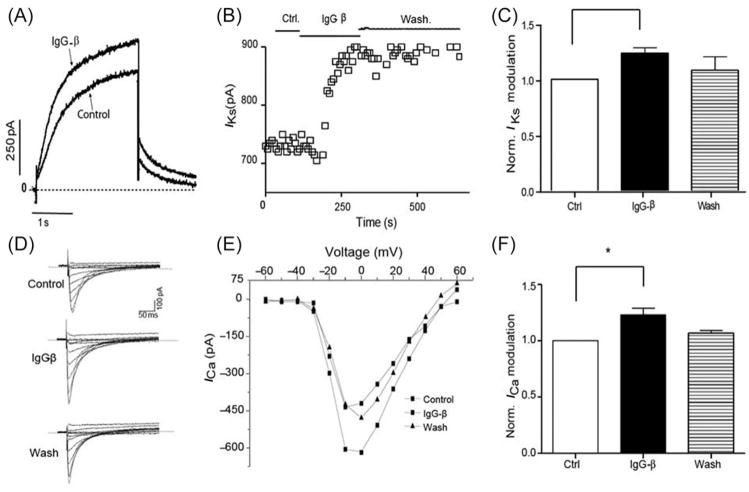
The IKs current was elicited from guinea pig ventricular myocytes and measured at the end of the depolarizing pulse (3 s). IgG-β (24 μg/mL) increased IKs from 643.3 ± 76.7 pA, under control conditions, to 793.3 ± 92.3 pA (n = 4; P < 0.05). The effect did not totally reverse after 5 min washout, with Iks reaching 708.3 ± 43.2 pA (control and IgG-β vs. wash, P > 0.05; Figure 5).
Figure 5.
IgG-β increases IKs and ICa current. (A) Representative trace of IKs before and during IgG-β perfusion. (B) Time course of the effect with bars indicating different conditions. (D) Typical trace of ICa under: control, IgG-β perfusion, and washout condition. In (E), I–V relationship from the same patient is plotted. (C) and (F) Normalized IKs and ICa values (values were normalized by the control condition—pre-IgG-β-perfusion-data in each cell) under control, IgG-β, and washout conditions (control vs. IgG-β *P < 0.05). Values are mean ± SEM; n = 4; *P < 0.05.
L-type calcium current
ICa current was elicited from rabbit ventricular myocytes and measured when it reaches the peak. IgG-β (24 μg/mL) increased ICa from 900 ± 167.1 pA, under control conditions, to 1075.2 ± 170.2 pA (n = 4; P < 0.05). The effect was reversed after a 5 min washout, with ICa reaching 932.3 ± 43.2 pA (control and IgG-β vs. wash, P > 0.05; Figure 5).
Discussion
Our results show that IgGs with β-adrenergic activity present in the sera of CChPs modulate the ventricular repolarization process. The observed increase of IKs by IgG-β can explain, at least in part, this modulation. Also, the L-type calcium current was increased by IgG-β. In addition, this is the first work to show adrenergic modulation of AP repolarization in guinea pig M cells either by IgG-β or by the adrenergic agonist isoproterenol.
Beta-1 adrenergic activity shortened the QT interval and M cell action potential duration by activating beta-1 adrenergic receptor
Changes in the QT interval duration were indicated as a predictor of sudden death in CChP.14 Adrenergic stimulation has been shown to shorten the QT interval and increase the transmural dispersion of repolarization in canine left ventricular wedge preparations.15 In our experiments, Ab-β shortened the QT interval, and simultaneous perfusion of Ab-β with propranolol prevented this effect, suggesting that the action of Ab-β is mediated by the activation of the beta-1-AR. In fact, we have previously shown that the sera from CChP but without Ab-β do not modulate QT interval duration in isolated rabbit hearts.16 These data reinforce the hypothesis that beta-activity is responsible for the shortening of the QT interval in the presence of Ab-β.
The QT interval is determined by the APD of cells of the ventricular wall. M cell AP has been proposed as responsible for the termination of the QT interval in the wedge preparation.7 Thus, because the M cells play an important role in ventricular repolarization, we examined the effect of Ab-β on these cells. In addition, the adrenergic characterization was performed. Both isoproterenol and Ab-β reversibly shortened the APD of guinea pig M cells. This finding is in agreement with the previous studies in M cells made in others species.15 In contrast to our results, IgG fraction with Ab-β (IgG-β) has been shown to prolong the AP of human atrial and rat ventricular myocytes.17 This discrepancy could be due to the different tissues or techniques used. The experiments describing a prolongation of the AP were carried out by patch–clamp technique under whole-cell configuration in isolated myocytes, whereas our experiments used intracellular microelectrodes in multicellular preparations targeting the M cells.
Another important difference relates to the animal model, as we used guinea pig M cells that have strong modulation of APD by the two components of the repolarizing potassium currents (IKs/IKr) as previously demonstrated, 10 whereas Christ et al. used rat ventricle preparation, known to be lacking these currents. Indeed, in cells that have strong modulation by IKs and IKr, it has been shown that adrenergic stimulation shortens APD, as described in humans,18 dogs,19 rabbits,20 and guinea pig21 ventricular cells.
Ionic mechanisms
As only sera with the Ab-β modulated the ventricular repolarization, shortening QT interval, and APD, we looked for the ionic currents known to be modified by the activation of the beta-1-AR. In ventricular myocytes, the phosphorylation mediated by the beta-1-AR activation of L-type calcium channel22 or IKr potassium channel23 leads to an increase of APD and could thus not explain the shortening of the QT interval. However, the increase in the calcium current could explain, in part, the ventricular arrhythmic episodes described in CChP with Ab-β.5 Therefore, Ito, ICa, and Iks ion currents were selected as possible targets of Ab-β.
In humans, Ito is a major contributor in shaping the early phase of the cardiac AP. However, Ito current is absent in the ventricular guinea pig.24 Thus, the effect on Ito was investigated in rat ventricular myocytes. The adrenergic agonist isoproterenol did not modulate Ito in agreement with the previous work in rat25 and canine26 ventricular myocytes. As expected, IgG-β, from CChP, also did not modulate Ito. Interestingly, not only the macroscopic current, but also the kinetic parameters were not modulated by IgG-β, suggesting that this current is not involved in the IgG-β-induced QT and AP shortening.
The other current studied, IKs, is a major contributor to the repolarization of the human cardiac AP. Adrenergic stimulation, which increases phosphorylate Iks,27 is responsible for AP shortening.28 Here, we demonstrated that IgG-β increased IKs in agreement with its proposed activation of the beta-1-AR. This increment of IKs accelerates phase 3 of the AP and consequently shortens APD. The short QT interval is, therefore, a consequence of these pathophysiological changes induced by the antibodies.
Finally, the other current studied here was ICa, which was increased in the presence of IgG-β. These data confirm the previous results published in the literature, in which the IgG-β also increased ICa, but the antibodies were obtained from patients with DCM but without Chagas’ heart disease.17,29,30
Clinical implications
The presence of functional Ab-β in patients with DCM and Chagas’ disease has been proposed as capable of inducing myocardial damage.31 Thus, recently, antibodies present in the serum of patients with DCM were reported to activate the extracellular signal-regulated kinase in murine cardiomyocytes. This kinase plays an important role in hypertrophy and heart failure.32 In this regard, Jane-wit et al.33 demonstrated that IgG-β from mice immunized with the peptide corresponding to the second extracellular loop of the beta-1-AR activated caspase-3 through the calcium- and cAMP-dependent protein kinase A pathways in non-immunized hosts. As a result of this activation, IgG-β induced DCM by cardiomyocyte apoptosis. In addition, Ab-β from patients with DCM submitted an immunoadsorption procedure, which shows cytotoxicity and induced apoptosis in rat cardiomyocyte culture.34
In contrast, we have shown a direct modulation of ionic currents and AP morphology by IgG-β present in the sera of CChP, leading to shortening of the QT interval. The increment of IKs could justify this result. Therefore, if the present result is observed in isolation, we may conclude that the shortened action potential mediated by antibodies has a beneficial effect by diminishing the transmural dispersion of repolarization. However, the antibodies used in this study came from CChP, which have DCM. In DCM, a downregulation of IKs current was described.35,36 In fact, the increment of adrenergic drive could evoke long action potential duration when IKs is blocked under slow pacing rate, as shown by Volders et al.37 In another elegant work, in a chronic-AV block dog model, the same group found the downregulation of IKs component, KCNQ1, and consequently, the adrenergic stimulus induced early afterdepolarizations in 6 of 13 cells, either under short or long cycle length (500, 1000, and 2000 ms).38 The mechanism that could explain this phenomenon is IKs accumulation in the presence of beta-adrenergic stimulation.28,39 In CChP that have DCM, it is possible to hypothesize that IKs might be downregulated, and therefore, adrenergic stimulation mediated by IgG-β may contribute to enhanced malignant ventricular arrhythmias.
Antibodies also increased the calcium current. The calcium overload was indicated as responsible for early and delayed afterdepolarizations11–13; however, in the present study, these were not observed under antibody perfusion on action potential recording. In addition, no electrical disorder was observed in the ECGs obtained in isolated rabbit hearts in the presence of Ab-β. Nevertheless, these data do not exclude the possibility that the antibodies may trigger a ventricular electrical disturbance in CChP such as ventricular fibrillation that could finish with a sudden cardiac death event.
As sudden death is one of the main causes of death in Chagas’ disease and DCM, and disturbances of the repolarization process are intimately associated with fatal ventricular arrhythmias, we suggest that the Ab-β may contribute to the genesis of sudden cardiac death in CChP with DCM.
This hypothesis predicts that the immunoglobulin adsorption therapy in patients with Ab-β, besides improving cardiovascular function, as already demonstrated,33,40 may also decrease sudden death episodes.
Study limitations
One limitation of the present study relates to the small number of patient’s sera with Ab-β studied, but the effects described were present in all sera screened as positive for Ab-β. Trials with immunoadsorption of these antibodies shall critically test their role in the pathophysiology of Chagas’ disease and in the induction of sudden death. Another limitation was the different animal species used (rat, rabbit, and guinea pig). However, as described earlier, previous studies have shown the interspecies homology between a second extracellular loop of the beta-1AR and the effect of antibodies in these species.3,41–43 At any rate, the effects of Ab-β or isoproterenol on guinea pig M cells’ action potentials are in agreement with the effect of adrenergic stimulation in the human ventricular wall,18 thus justifying, in part, the extrapolation of the present findings to the clinical setting. Finally, the Ito channel is formed by distinct subunits in rabbit and rat and absent in guinea pig. Consequently, the results observed in repolarization parameters in rabbit and guinea pig need to be extrapolated carefully.
Acknowledgments
The authors gratefully acknowledge the excellent technical assistance of Daisy Avanzi.
Funding The present work was supported by grants from the National Research Council (CNPq-Brasil) and the Rio de Janeiro State Research Agency (FAPERJ) and Fundación de Investigaciones Cardiológicas Eindhoven.
Footnotes
Conflict of interest: none declared.
References
- 1.Borda E, Pascual J, Cossio P, De La Vega M, Arana R, Sterin-Borda L. A circulating IgG in Chagas’ disease which binds to beta-adrenoceptors of myocardium and modulates their activity. Clin Exp Immunol. 1984;57:679–86. [PMC free article] [PubMed] [Google Scholar]
- 2.Limas CJ, Goldenberg IF, Limas C. Autoantibodies against beta-adrenoceptors in human idiopathic dilated cardiomyopathy. Circ Res. 1989;64:97–103. doi: 10.1161/01.res.64.1.97. [DOI] [PubMed] [Google Scholar]
- 3.Costa PC, Fortes FS, Machado AB, Almeida NA, Olivares EL, Cabral PR, et al. Sera from chronic chagasic patients depress cardiac electrogenesis and conduction. Braz J Med Biol Res. 2000;33:439–46. doi: 10.1590/s0100-879x2000000400010. [DOI] [PubMed] [Google Scholar]
- 4.Iwata M, Yoshikawa T, Baba A, Anzai T, Mitamura H, Ogawa S. Autoantibodies against the second extracellular loop of beta1-adrenergic receptors predict ventricular tachycardia and sudden death in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2001;37:418–24. doi: 10.1016/s0735-1097(00)01109-8. [DOI] [PubMed] [Google Scholar]
- 5.Chiale PA, Rosenbaum MB, Elizari MV, Hjalmarson A, Magnusson Y, Wallukat G, et al. High prevalence of antibodies against beta 1- and beta 2-adrenoceptors in patients with primary electrical cardiac abnormalities. J Am Coll Cardiol. 1995;26:864–9. doi: 10.1016/0735-1097(95)00262-2. [DOI] [PubMed] [Google Scholar]
- 6.Rassi A, Jr, Rassi SG, Rassi A. Sudden death in Chagas’ disease. Arq Bras Cardiol. 2001;76:75–96. doi: 10.1590/s0066-782x2001000100008. [DOI] [PubMed] [Google Scholar]
- 7.Yan GX, Antzelevitch C. Cellular basis for the normal Twave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998;98:1928–36. doi: 10.1161/01.cir.98.18.1928. [DOI] [PubMed] [Google Scholar]
- 8.Lowry OH, Rosebrough NJ, Farr AL, Randall RL. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:267–75. [PubMed] [Google Scholar]
- 9.Masuda MO, Engel GM, Moreira APB. Characterization of isolated ventricular myocytes: two levels of resting potential. J Mol Cell Cardiol. 1987;19:831–9. doi: 10.1016/s0022-2828(87)80612-0. [DOI] [PubMed] [Google Scholar]
- 10.Sicouri S, Quist M, Antzelevitch C. Evidence for the presence of M cells in the guinea pig ventricle. J Cardiovasc Electrophysiol. 1996;7:503–11. doi: 10.1111/j.1540-8167.1996.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 11.Fozzard HA. Afterdepolarisations and triggered activity. Basic Res Cardiol. 1992;87:105–13. doi: 10.1007/978-3-642-72477-0_10. [DOI] [PubMed] [Google Scholar]
- 12.Priori SG, Corr Pb. Mechanisms underlying early and delayed afterdepolarisations induced by sarcocatecholamines. Am J Physiol. 1990;258:h1796–805. doi: 10.1152/ajpheart.1990.258.6.H1796. [DOI] [PubMed] [Google Scholar]
- 13.Tweedie D, Harding SE, MacLeod KT. Sarcoplasmic reticulum Ca content, sarcolemmal Ca influx and the genesis of arrhythmias in isolated guinea-pig cardiomyocytes. J Mol Cell Cardiol. 2000;32:261–72. doi: 10.1006/jmcc.1999.1070. [DOI] [PubMed] [Google Scholar]
- 14.Salles G, Xavier S, Sousa A, Hasslocher-Moreno A, Cardoso C. Prognostic value of QT interval parameters for mortality risk stratification in Chagas’ disease: results of a long-term follow-up study. Circulation. 2003;108:305–12. doi: 10.1161/01.CIR.0000079174.13444.9C. [DOI] [PubMed] [Google Scholar]
- 15.Extramiana F, Antzelevitch C. Amplified transmural dispersion of repolarization as the basis for arrhythmogenesis in a canine ventricular-wedge model of short-QT syndrome. Circulation. 2004;110:3661–6. doi: 10.1161/01.CIR.0000143078.48699.0C. [DOI] [PubMed] [Google Scholar]
- 16.Medei E, Pedrosa RC, Benchimol Barbosa PR, Costa PC, Hernandez CC, Chaves EA, et al. Human antibodies with muscarinic activity modulate ventricular repolarization: basis for electrical disturbance. Int J Cardiol. 2007;115:373–80. doi: 10.1016/j.ijcard.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Christ T, Wettwer E, Dobrev D, Adolph E, Knaut M, Wallukat G, et al. Autoantibodies against the beta1 adrenoceptor from patients with dilated cardiomyopathy prolong action potential duration and enhance contractility in isolated cardiomyocytes. J Mol Cell Cardiol. 2001;33:1515–25. doi: 10.1006/jmcc.2001.1414. [DOI] [PubMed] [Google Scholar]
- 18.Dorian P, Dunnmon P, Elstun L, Newman D. The effect of isoproterenol on the class III effect of azimilide in humans. J Cardiovasc Pharmacol Ther. 2002;7:211–7. doi: 10.1177/107424840200700403. [DOI] [PubMed] [Google Scholar]
- 19.Litovsky SH, Antzelevitch C. Differences in the electrophysiological response of canine ventricular subendocardium and subepicardium to acetylcholine and isoproterenol: a direct effect of acetylcholine in ventricular myocardium. Circ Res. 1990;67:615–27. doi: 10.1161/01.res.67.3.615. [DOI] [PubMed] [Google Scholar]
- 20.Lu HR, Vlaminckx E, Van De Water A, Gallacher DJ. Both beta-adrenergic receptor stimulation and cardiac tissue type have important roles in elucidating the functional effects of I(Ks) channel blockers in vitro. J Pharmacol Toxicol Methods. 2005;51:81–90. doi: 10.1016/j.vascn.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Salata JJ, Jurkiewicz NK, Wang J, Evans BE, Orme HT, Sanguinetti MC. A novel benzodiazepine that activates cardiac slow delayed rectifier K+ currents. Mol Pharmacol. 1998;54:220–30. doi: 10.1124/mol.54.1.220. [DOI] [PubMed] [Google Scholar]
- 22.Song Y, Shryock JC, Belardinelli L. Potentiating effect of acetylcholine on stimulation by isoproterenol of L-type Ca2+ current and arrhythmogenic triggered activity in guinea pig ventricular myocytes. J Cardiovasc Electrophysiol. 1998;9:718–26. doi: 10.1111/j.1540-8167.1998.tb00959.x. [DOI] [PubMed] [Google Scholar]
- 23.Thomas D, Zhang W, Karle CA, Kathöfer S, Schöls W, Kübler W, et al. Deletion of protein kinase A phosphorylation sites in the HERG potassium channel inhibits activation shift by protein kinase A. J Biol Chem. 1999;274:27457–62. doi: 10.1074/jbc.274.39.27457. [DOI] [PubMed] [Google Scholar]
- 24.Findlay I. Is there an A-type K+ current in guinea pig ventricular myocytes? Am J Physiol Heart Circ Physiol. 2003;284:H598–604. doi: 10.1152/ajpheart.00687.2002. [DOI] [PubMed] [Google Scholar]
- 25.Gallego M, Setien R, Puebla L, Boyano-Adanez M del C, Arilla E, Casis O. alpha1-Adrenoceptors stimulate a Galphas protein and reduce the transient outward K+ current via a cAMP/PKA-mediated pathway in the rat heart. Am J Physiol Cell Physiol. 2005;288:C577–85. doi: 10.1152/ajpcell.00124.2004. [DOI] [PubMed] [Google Scholar]
- 26.Pacioretty LM, Gilmour RF., Jr Restoration of transient outward current by norepinephrine in cultured canine cardiac myocytes. Am J Physiol. 1998;275:H1599–605. doi: 10.1152/ajpheart.1998.275.5.H1599. [DOI] [PubMed] [Google Scholar]
- 27.Lopes MB, Remon J, Matavel A, Jin LS, Keselman I, Medei E, et al. Protein + kinase A modulates PLC-dependent regulation and PIP2-sensitivity of K channels. Channels. 2007;2:124–34. doi: 10.4161/chan.4322. [DOI] [PubMed] [Google Scholar]
- 28.Terrenoire C, Clancy CE, Cormier JW, Sampson KJ, Kass RS. Autonomic control of cardiac action potentials: role of potassium channel kinetics in response to sympathetic stimulation. Circ Res. 2005;96:e25–34. doi: 10.1161/01.RES.0000160555.58046.9a. [DOI] [PubMed] [Google Scholar]
- 29.Christ T, Schindelhauer S, Wettwer E, Wallukat G, Ravens U. Interaction between autoantibodies against the beta1-adrenoceptor and isoprenaline in enhancing L-type Ca2+ current in rat ventricular myocytes. J Mol Cell Cardiol. 2006;41:716–23. doi: 10.1016/j.yjmcc.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Christ T, Adolph E, Schindelhauer S, Wettwer E, Dobrev D, Wallukat G, et al. Effects of immunoglobulin G from patients with dilated cardiomyopathy on rat cardiomyocytes. Basic Clin Pharmacol Toxicol. 2005;96:445–52. doi: 10.1111/j.1742-7843.2005.pto_07.x. [DOI] [PubMed] [Google Scholar]
- 31.Jahns R, Boivin V, Hein L, Triebel S, Angermann CE, Ertl G, et al. Direct evidence for a beta 1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest. 2004;113:1419–29. doi: 10.1172/JCI20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tutor AS, Penela P, Mayor F., Jr Anti-beta(1)-adrenergic receptor autoantibodies are potent stimulators of the ERK1/2 pathway in cardiac cells. Cardiovasc Res. 2007;76:51–60. doi: 10.1016/j.cardiores.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Jane-wit D, Altuntas CZ, Johnson JM, Yong S, Wickley PJ, Clark P, et al. Beta 1-adrenergic receptor autoantibodies mediate dilated cardiomyopathy by agonistically inducing cardiomyocyte apoptosis. Circulation. 2007;116:399–410. doi: 10.1161/CIRCULATIONAHA.106.683193. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Larsson L, Haugen E, Fedorkova O, Angwald E, Waagstein F, et al. Effects of autoantibodies removed by immunoadsorption from patients with dilated cardiomyopathy on neonatal rat cardiomyocytes. Eur J Heart Fail. 2006;8:460–7. doi: 10.1016/j.ejheart.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 35.Tsui Y, Opthof T, Kamiya K, Yasui K, Liu W, Lu Z, et al. Pacing-induced heart failure causes a reduction of delayed rectifier potassium currents along with decreases in calcium and transient outward currents in rabbit ventricle. Cardiovasc Res. 2000;48:300–9. doi: 10.1016/s0008-6363(00)00180-2. [DOI] [PubMed] [Google Scholar]
- 36.Li GR, Lau CP, Ducharme A, Tardif JC, Nattel S. Transmural action potential and ionic current remodeling in ventricles of failing canine hearts. Am J Physiol. 2002;283:H1031–41. doi: 10.1152/ajpheart.00105.2002. [DOI] [PubMed] [Google Scholar]
- 37.Volders PG, Stengl M, van Opstal JM, Gerlach U, Spätjens RL, Beekman JD, et al. Probing the contribution of IKs to canine ventricular repolarization: key role for beta-adrenergic receptor stimulation. Circulation. 2003;107:2753–60. doi: 10.1161/01.CIR.0000068344.54010.B3. [DOI] [PubMed] [Google Scholar]
- 38.Stengl M, Ramakers C, Donker DW, Nabar A, Rybin AV, Spätjens RL, et al. Temporal patterns of electrical remodeling in canine ventricular hypertrophy: focus on IKs downregulation blunted beta-adrenergic activation. Cardiovasc Res. 2006;72:90–100. doi: 10.1016/j.cardiores.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 39.Stengl M, Volders PG, Thomsen MB, Spätjens RL, Sipido KR, Vos MA. Accumulation of slowly activating delayed rectifier potassium current (IKs) in canine ventricular myocytes. J Physiol. 2003;551:777–86. doi: 10.1113/jphysiol.2003.044040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Felix SB, Staudt A, Dörffel WV, Stangl V, Merkel K, Pohl M, et al. Hemodynamic effects of immunoadsorption and subsequent immunoglobulin substitution in dilated cardiomyopathy: three-month results from a randomized study. J Am Coll Cardiol. 2000;35:1590–8. doi: 10.1016/s0735-1097(00)00568-4. [DOI] [PubMed] [Google Scholar]
- 41.Labovsky V, Smulski CR, Gomez K, Levy G, Levin MJ. Anti-beta1-adrenergic receptor autoantibodies in patients with chronic Chagas heart disease. Clin Exp Immunol. 2007;148:440–9. doi: 10.1111/j.1365-2249.2007.03381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu K, Liao YH, Wang ZH, Li SL, Wang M, Zeng LL, et al. Effects of autoantibodies against beta(1)-adrenoceptor in hepatitis virus myocarditis on action potential and L-type Ca(2+) currents. World J Gastroenterol. 2004;10:1171–5. doi: 10.3748/wjg.v10.i8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machida CA, Bunzow JR, Searles RP, Van Tol H, Tester B, Neve KA, et al. Molecular cloning and expression of the rat beta 1-adrenergic receptor gene. J Biol Chem. 1990;265:12960–5. [PubMed] [Google Scholar]