Abstract
Female rats express a conditioned place preference (CPP) for a context paired with mating. During a mating encounter, the female rat is exposed to several different types of stimuli, including, but not limited to, vaginocervical stimulation and social contact. The present experiment tested the hypothesis that two components of the mating interaction, vaginocervical stimulation or social contact, each induce a CPP in female rats. During conditioning rats received nonpaced mating, artificial vaginocervical stimulation, social interaction or a control treatment. Rats expressed a CPP for the context paired with nonpaced mating or artificial vaginocervical stimulation whereas social interaction and the control treatment did not induce a CPP. The present findings highlight the important role that vaginocervical stimulation plays in the reinforcing effects of mating in female rats.
Keywords: Sexual behavior, conditioned place preference, vaginocervical, mating
Introduction
Conditioned place preference (CPP) paradigms are often used to evaluate the reinforcing effects of drugs or behavior (Bardo and Bevins, 2000; Carlezon, 2003). Generally, rats express a preference for a context associated with a drug or behavior that is reinforcing (Carlezon, 2003). Female rats demonstrate a conditioned place preference for a context paired with mating. Both paced mating behavior, in which the female rat controls the timing of the mating interaction by approaching and withdrawing from the vicinity of the male, and nonpaced mating behavior, in which the female cannot escape from the male, induce a CPP in female rats (Oldenburger et al., 1992; Meerts and Clark, 2007; c.f., Paredes and Alonso, 1997; Paredes and Vasquez, 1999).
A female rat is exposed to a variety of stimuli from the male rat during a mating encounter including, but not limited to, vaginocervical stimulation and social contact. Under certain conditions vaginocervical stimulation alone is reinforcing to female rats; a single application of artificial vaginocervical stimulation (aVCS) can induce a CPP (Walker et al., 2002) and female rats will perform an operant response to receive aVCS (Ross et al., 1979). Social experiences can also be reinforcing events. Female rodents express a CPP for an environment paired with same-sex interactions (Douglas et al., 2004), for an aggressive interaction with an opposite-sex conspecific (Meisel and Joppa, 1994) and for an interaction with a sexually active male conspecific in which vaginocervical stimulation is prohibited by a vaginal mask (Kohlert and Olexa, 2005). Even though female rats show a CPP for a single application of aVCS, it is unknown whether female rats will express a CPP for a pattern of vaginocervical stimulation similar to that received during mating or for a social interaction with a gonadectomized (GDX) male rat.
Although several studies have reported that female rats only exhibit a mating-induced CPP when the female controls the mating interaction (Gans and Erskine, 2003; Martinez and Paredes, 2001; Paredes and Alonso, 1997; Paredes and Vazquez, 1999), more recent data from our laboratory and others (Jenkins and Becker, 2003; Meerts and Clark, 2007) have shown that control of the sexual encounter is not necessary for female rats to show a CPP for a context paired with mating. The present study demonstrates that female rats express a CPP for aVCS administered at a rate comparable to that experienced during mating, as well as nonpaced mating, and provides support for the notion that vaginocervical stimulation is an important aspect of the reinforcing effect of mating.
Methods
Subjects
Thirty-seven female Long-Evans rats weighing approximately 200 g were obtained from Harlan (Indianapolis, IN). Rats were housed individually in hanging metal cages in a light- (12:12, lights off at 1000 h) and temperature-controlled vivarium. Commercial rat pellets and water were available ad lib. All experimental rats were gonadectomized (GDX) under ketamine/xylazine anesthesia (50 mg/kg; Henry Schein, Indianapolis, IN) 7–10 days before behavioral testing. Experimental rats received 10 μg estradiol benzoate (Sigma Aldrich, St. Louis, MO) 48 h and 1 mg progesterone (Sigma) 4 h, prior to each reinforced conditioning session (Nonpaced Mating, aVCS, Social Interaction and Control treatment) but not before the baseline place preference test or post-conditioning test. Hormones were administered s.c. in a sesame oil vehicle. Sexually experienced male Long-Evans rats, aged 3–4 months, were used as stimulus rats in nonpaced mating tests. In addition, male rats that had been GDX at least 4–6 weeks previously and no longer showed mounting behavior when presented with a hormone-primed female rat were used as stimulus males for the social interaction group. The Institutional Animal Care and Use Committee at Dartmouth College approved the use of rats in these studies and all procedures were conducted in accordance with NIH guidelines. All behavioral testing occurred under dim red illumination in the same room as the place preference apparatus.
Procedures
The CPP procedure was based on methods described previously (Meerts and Clark, 2007). The apparatus consisted of three distinct compartments (Med Associates, St. Albans, VT); the two outer compartments (28 × 21 × 21 cm high) were connected by a middle gray compartment (12 × 21 × 21 cm high) via manually controlled sliding guillotine doors. One side compartment had white walls, was illuminated and had a metal bar floor with aspen bedding in a waste pan beneath the floor. The other side compartment had black walls, metal grid flooring and was scented with a 2% glacial acetic acid solution. Photobeams spaced at 5-cm intervals were used to record the time spent in each compartment.
During the initial 10-min baseline (pre-conditioning) place preference test, rats began in the middle compartment and freely explored the entire apparatus; the compartment in which the rat spent the majority of time on the baseline test was designated the preferred compartment. Rats did not exhibit a significant preference for either side compartment on the pre-conditioning test (data not shown).
Rats were assigned to one of four groups by matching on the time spent in each compartment on the baseline test: Nonpaced Mating, Artificial Vaginocervical Stimulation (aVCS), Social Interaction or Control (n = 9/group). One rat was excluded from the experiment following the baseline test because the data from that rat deviated more than 2 standard deviations from the mean. Rats were habituated to the clear Plexiglas arenas (39.4 × 22.9 × 31.1 cm high) in which they would receive Nonpaced Mating, aVCS or Social Interaction for 5 min immediately prior to the treatment.
In the Nonpaced Mating group treatment began when a male rat was placed into the arena with the experimental rat and concluded when the female rat received 15 intromissions, including ejaculations (Meerts and Clark, 2007). If an ejaculation occurred before the 15th intromission, then the male rat was immediately replaced with a new male. The number and timing of mounts, intromissions, and ejaculations, proceptive and rejection behaviors as well as lordosis responses were recorded (Hardy and Debold, 1971; Madlafousek and Hlinak, 1977). The interintromission interval, defined as the mean length of time between intromissions, not including ejaculations, was calculated. The data from one rat that did not exhibit sexual receptivity on the first day of conditioning was excluded from the analysis. The remaining rats were fully receptive on all tests, so no further mention is made of these data.
In the aVCS group the treatment was administered according to the procedure described in Tetel and coworkers (1993). Stimulations were delivered with the rubber tipped end of the plunger from a 1cc syringe attached to a FDK Dial Force Gauge (Model: FDN-5, Wagner Instruments, Greenwich, CT). The experimenter gently grasped the rat by the tail and inserted the 1cc plunger into the vagina until it contacted the cervix, delivering aVCS with about 200 g of pressure. Fifteen stimulations were delivered, each for 2 s every 30 s. This timing was selected because in previous nonpaced mating experiments in our laboratory (Meerts and Clark, 2007) and others (Coopersmith et al., 1996; Gans and Erskine, 2003; Garcia Horsman and Paredes, 2004) the average interintromission interval was approximately 30 s and because such timing induces Fos-immunoreactivity in the brain in a similar pattern to that induced by mating (Bennett et al., 2001). The timing and amount of pressure (g) of each aVCS application was recorded along with the frequencies of proceptive and rejection behaviors (Madlafousek and Hlinak, 1977). In the present study the mean pressure applied was 207.4 ± 1.31 g across all conditioning sessions for the 9 rats receiving the aVCS treatment. The interstimulus interval, defined as mean length of time between aVCS applications was calculated.
In the Social Interaction group treatment consisted of placing a GDX male into the Plexiglas arena with the experimental rat for 7 min. A 7 min treatment was selected for the present study because that is the average length of time a female rat spends with the male during nonpaced mating tests to 15 intromissions in our laboratory (Meerts and Clark, unpublished observations).
In all, each experimental rat underwent a total of 6 conditioning sessions: three reinforced and three nonreinforced sessions. All conditioning sessions lasted 30 min. On the first, third and fifth conditioning day rats received nonreinforced conditioning sessions and on the second, fourth and sixth conditioning day rats received reinforced conditioning sessions. For reinforced conditioning sessions rats were placed into the nonpreferred compartment of the CPP apparatus for 30 min immediately following the designated treatment. For nonreinforced conditioning sessions rats were placed directly from their home cages into the preferred compartment of the CPP apparatus for 30 min. Rats in the Control group were placed directly from their home cages into the preferred or nonpreferred compartment on nonreinforced or reinforced conditioning sessions, respectively.
Twenty-four h after the final conditioning session all rats received the post-conditioning preference test following the same procedures outlined above for the baseline test. The walls and waste pans were wiped with distilled water after each conditioning session.
Data analysis
We followed established procedures to assess the display of a CPP for the context paired with the three treatments (Coria-Avila et al., 2005; Dominguez-Salazar et al., 2005; Frye et al., 1998; Meerts and Clark, 2007; Paredes and Alonso, 1997; Paredes and Vazquez, 1999). The following measures were calculated: 1) a preference score, defined as time in reinforced compartment/(time in reinforced compartment + time in nonreinforced compartment), and 2) a difference score, defined as the time in the nonreinforced compartment − time in reinforced compartment (Paredes and Alonso, 1997). The analysis of these transformed measures, instead of the raw data, is useful because the transformed measures eliminate the potential confound of time spent in the middle gray compartment. In addition, the convention is to consider these two measures in parallel because an increase in the preference score could occur because of a decrease in the time spent in the gray compartment; a corresponding decrease in the difference score ameliorates that concern (Paredes and Alonso, 1997). Paired t-tests were used to evaluate the change in preference score and difference score from the pretest to the test (Dominguez-Salazar et al., 2005; Kohlert and Olexa, 2005; Meerts and Clark, 2007; Meisel and Joppa, 1994). The criteria for the induction of a CPP were a significant increase in the preference score in tandem with a significant decrease in the difference score (Meerts and Clark, 2007; Paredes and Alonso, 1997). Analysis of variance with repeated measures was used to evaluate the effect of group (Nonpaced Mating, aVCS) across conditioning session on test length, interintromission and interstimulus interval, proceptive and rejection behaviors. The alpha level was set at p < 0.05.
Results
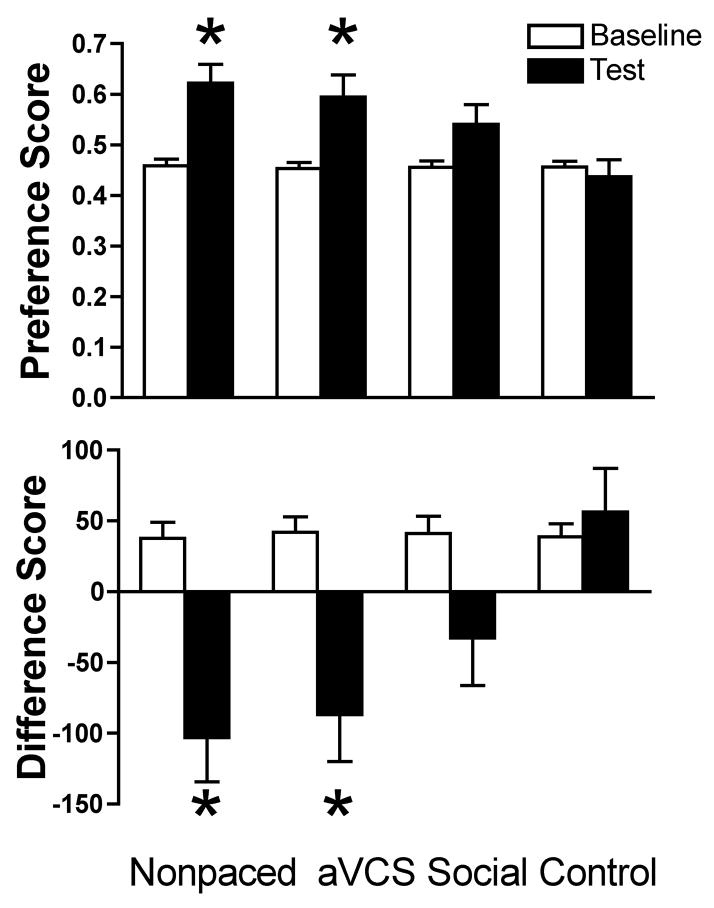
Rats that received Nonpaced Mating or aVCS as the treatment showed a robust CPP for the context paired with the treatment (Figure 1). Specifically, from the baseline to the postconditioning test, the preference score increased significantly in tandem with a significant decrease in the difference score (all t’s > 3.1, p < 0.05). Rats that received the Control treatment did not show a significant change in preference or difference score from the baseline to the test. Although the Social Interaction treatment was accompanied by an increase in the preference score and a decrease in the difference score, these changes did not attain statistical significance (t < 2.2, p > 0.05).
Figure 1.
Mean ± SEM preference (top) and difference (bottom) scores on the baseline (preconditioning, open bars) and test (post-conditioning, black bars) are shown for female rats that received Nonpaced Mating, Artificial Vaginocervical Stimulation (aVCS), Social Interaction or the Control treatment. n = 8–9 rats per group. Rats receiving either Nonpaced Mating or aVCS displayed a CPP for the compartment associated with the treatment: a significant increase in preference score coincident with a significant decrease in difference score, *p < 0.05 vs. Baseline.
Test length did not differ significantly between the Nonpaced Mating, aVCS and Social Interaction groups (Table 1). The Nonpaced Mating and aVCS groups differed minimally in terms of behaviors exhibited during conditioning. The interintromission (Nonpaced Mating) and interstimulus (aVCS) intervals did not differ between groups or across conditioning sessions whereas the display of rejection behaviors decreased significantly across conditioning sessions in both the Nonpaced Mating and aVCS groups (F (2,30) = 11, p < 0.05). Across all conditioning sessions, significantly more proceptive behaviors were exhibited in the Nonpaced Mating than the aVCS group (F (1,15) = 10.3, p < 0.05).
Table 1.
Summary of behaviors displayed during conditioning sessions
| Treatment | Conditioning session | Test length (seconds) | Interintromission or interstimulus interval (seconds) | Proceptive behaviors (number) | Rejection behaviors (number) |
|---|---|---|---|---|---|
| Nonpaced Mating | 1 | 450.5 ± 93.7 | 29.5 ± 5.5 | 8.9 ± 3.4 | 13.6 ± 3.3 |
| 2 | 352.3 ± 25.9 | 23.0 ± 2.3 | 8.8 ± 1.8 | 6.0 ± 2 | |
| 3 | 409.8 ± 75.6 | 28.2 ± 5.8 | 10.3 ± 3.2 | 2.8 ± 1.2 | |
| Mean | 404.2 ± 44.6 | 26.9 ± 3.0 | 9.3 ± 2.2 | 7.5 ± 1.5 | |
| Artificial Vaginocervical Stimulation | 1 | 471.2 ± 6.7 | 32.9 ± 0.5 | 0 ± 0 | 12.8 ± 4.4 |
| 2 | 438.0 ± 5.4 | 30.5 ± 0.3 | 1.0 ± 0.7 | 9.1 ± 4.1 | |
| 3 | 449.1 ± 10.3 | 31.5 ± 0.7 | 4.8 ± 2.0 | 4.6 ± 3.6 | |
| Mean | 452.8 ± 6.5 | 31.6 ± 0.4 | 1.9 ± 0.9 * | 8.8 ± 3.8 | |
| Social Interaction | 1 | 420.0 ± 0 | |||
| 2 | 420.0 ± 0 | ||||
| 3 | 420.0 ± 0 | ||||
| Mean | 420.0 ± 0 |
Mean ± SEM are shown.
p < 0.05 vs. Nonpaced Mating.
Discussion
Female rats exhibit a CPP for a context paired with nonpaced mating, but also for a context paired with aVCS, indicating that vaginocervical stimulation is reinforcing to female rats even in the absence of the social, tactile, auditory and olfactory stimulation from the male rat that normally accompanies mating. Walker and colleagues (2002) reported that a single application of vaginocervical stimulation is reinforcing to female rats. The results of the present study extend those findings to demonstrate that female rats display a CPP for aVCS delivered at the rate experienced during nonpaced mating (15 stimulations, each applied for 2 s and spaced 30 s apart over the course of 7–8 min). Furthermore, our data provide additional support for the view that the vaginocervical stimulation received at the interval experienced during nonpaced mating is an important component of a CPP for mating in female rats. Experiments varying the intensity, duration and specific intervals of aVCS are necessary to elucidate the specific characteristics of stimulation that contribute to the reinforcing effects of aVCS.
Although social interaction under some conditions induces a CPP (Douglas et al., 2004; Kohlert and Olexa, 2005; Meisel and Joppa, 1994), female rats exposed to a GDX male in the present study did not exhibit a CPP. One factor that varies across studies is the duration of social contact; the duration of social interaction ranges from 10–30 min (Douglas et al., 2004; Kohlert and Olexa, 2005; Meisel and Joppa, 1994). In the present study the 7 min interaction duration was chosen to coincide with the average length of time the female spends with the male rat during a nonpaced mating test (Table 1) and a more extensive exposure period may reveal a CPP for a context paired with social interaction with a GDX male. In addition, social interaction with a gonadally intact male rat, as opposed to a GDX male, could also elicit a CPP. One approach used successfully to allow opposite-sex social interaction while preventing vaginocervical stimulation has been to place a vaginal mask on the female during the test with a sexually active male. Specifically, sexually receptive hamsters with and without vaginal masks exhibited a CPP for the context paired with an interaction with a sexually active gonadally intact male hamster (Kohlert and Olexa, 2004). In a follow-up study, Kohlert and Cappellini (2007) reported that exposure to the sight and sound of a gonadally intact male hamster was not sufficient to induce a CPP in female hamsters. Additional studies in female rats are needed to examine the reinforcing value of conspecific interactions with a gonadally intact male in which a vaginal mask is used, or when visual, olfactory and tactile cues are restricted.
Isolating the contribution of vaginocervical cues to the reinforcing effects of mating provides a tool that allows researchers to further parse the role of the sensory versus motivational factors accompanying mating. For example, female rats with medial preoptic area lesions may not receive sufficient intromissions during a mating test to permit assessment of the reinforcing effects of mating (Guarraci and Clark, 2006). In such a case, aVCS could be used to test whether the reinforcing properties of vaginocervical stimulation are preserved despite the marked alterations in the pattern of mating behavior that accompany medial preoptic area lesions.
The neural responses to vaginocervical stimulation that are elicited by mating and aVCS may overlap but evoke different mechanisms to induce a CPP. Patterns of Fos-immunoreactivity in the brain induced by aVCS are similar to those induced by mating (Pfaus et al., 1993; Tetel et al., 1993). Research has shown that the systemic administration of the opioid receptor antagonist naloxone blocks a CPP for paced mating (Paredes and Martinez, 2001). Moreover, activation of dopamine receptors may also contribute to the reinforcing effects of mating (Coria-Avila et al., 2008a; Meisel et al., 1996; c.f., Garcia Horsman and Paredes, 2004; Paredes and Agmo, 2004). Recent studies have examined the effect of opiate and dopamine receptor blockade on the expression of a conditioned preference for paced vs. nonpaced mating (Coria-Avila et al., 2008a, 2008b). These studies show that the display of a conditioned partner preference for a scent or specific strain of male rat paired with paced mating is naloxone-sensitive (Coria-Avila et al., 2008b) whereas odor- but not strain-conditioned partner preference is disrupted by the dopamine receptor antagonist flupenthixol (Coria-Avila et al., 2008a). Analysis of the effects of antagonists to both opiate (Garcia Horsman and Paredes, 2004; Coria-Avila et al., 2008b) and dopamine receptors (Coria-Avila et al., 2008a; Quysner and Blaustein, 2001; Meisel et al., 1996) will advance our understanding of the neurochemical substrates for aVCS-induced CPP.
Our results make clear that vaginal and/or cervical stimulation is reinforcing to female rats. During aVCS administration the vaginal walls are stretched, pressure is applied to the cervix and the clitoris may receive incidental contact. A better understanding of the role of sensory signals in the generation of a CPP for vaginocervical stimulation will help delineate the neural mechanisms subserving mating-induced CPP. Three main genitosensory nerves innervate the reproductive tract of the female rat, but the contributions of the nerves to the reinforcing effects of mating are unknown (Peters et al., 1987). The pelvic nerve is sensitive to stimulation of the vagina, the vaginal end of the cervix, and the perineal skin between the vagina and anus (Berkley et al., 1993; Komisaruk et al., 1972; Peters et al., 1987) whereas the sensory field of the pudendal nerve includes the perineal skin, the clitoral sheath and the inner thigh but not internal areas (Komisaruk et al., 1972; Peters et al., 1987). Finally, stimulation of the cervix and uterine horns activates the hypogastric nerve (Berkley et al., 1988; Berkley et al., 1993; Peters et al., 1987). Observations of responsiveness to electrical nerve stimulation or changes in the pattern of Fos-immunoreactivity following nerve transection within discrete brain regions supports a key role of signaling via one or more genitosensory nerves for the reinforcing effects of vaginocervical stimulation (Chadha and Hubscher, 2008; Hubscher, 2006; Pfaus et al., 2006; Rowe and Erskine, 1993; Wersinger et al., 1993). It will be important to test, in future experiments, the contributions of the pelvic, hypogastric and pudendal nerves to the reinforcing effects of vaginocervical stimulation.
Acknowledgments
We thank Eilish Boisvert, Ragavan Narayanan, Kimberly Quill and Kersti Spjut for invaluable technical assistance and Dr. Meg Kirkpatrick for guidance on the application of aVCS. We also thank Dr. Robert N. Leaton for his comments on the manuscript and Dr. George L. Wolford for statistical advice. This work was supported by HD050726 to A.S.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153(1):31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bennett AL, Blasberg ME, Blaustein JD. Sensory cues mediating mating-induced potentiation of sexual receptivity in female rats. Horm Behav. 2001;40(1):77–83. doi: 10.1006/hbeh.2001.1664. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Robbins A, Sato Y. Afferent fibers supplying the uterus in the rat. J Neurophysiol. 1988;59(1):142–163. doi: 10.1152/jn.1988.59.1.142. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Robbins A, Sato Y. Functional differences between afferent fibers in the hypogastric and pelvic nerves innervating female reproductive organs in the rat. J Neurophysiol. 1993;69(2):533–544. doi: 10.1152/jn.1993.69.2.533. [DOI] [PubMed] [Google Scholar]
- Carlezon WA., Jr Place conditioning to study drug reward and aversion. Methods Mol Med. 2003;84:243–249. doi: 10.1385/1-59259-379-8:243. [DOI] [PubMed] [Google Scholar]
- Chadha HK, Hubscher CH. Convergence of nociceptive information in the forebrain of female rats: Reproductive organ response variations with stage of estrus. Exp Neurol. 2008;210(2):375–387. doi: 10.1016/j.expneurol.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Coopersmith C, Candurra C, Erskine MS. Effects of paced mating and intromissive stimulation on feminine sexual behavior and estrus termination in the cycling rat. J Comp Psychol. 1996;110(2):176–186. doi: 10.1037/0735-7036.110.2.176. [DOI] [PubMed] [Google Scholar]
- Coria-Avila GA, Gavrila AM, Boulard B, Charron N, Stanley G, Pfaus JG. Neurochemical basis of conditioned partner preference in the female rat: II. Disruption by flupenthixol. Behav Neurosci. 2008a;122(2):396–406. doi: 10.1037/0735-7044.122.2.396. [DOI] [PubMed] [Google Scholar]
- Coria-Avila GA, Ouimet AJ, Pacheco P, Manzo J, Pfaus JG. Olfactory conditioned partner preference in the female rat. Behav Neurosci. 2005;119(3):716–725. doi: 10.1037/0735-7044.119.3.716. [DOI] [PubMed] [Google Scholar]
- Coria-Avila GA, Solomon CE, Vargas EB, Lemme I, Ryan R, Menard S, Gavrila AM, Pfaus JG. Neurochemical basis of conditioned partner preference in the female rat: I. Disruption by naloxone. Behav Neurosci. 2008b;122(2):385–395. doi: 10.1037/0735-7044.122.2.385. [DOI] [PubMed] [Google Scholar]
- Dominguez-Salazar E, Camacho FJ, Paredes RG. Prenatal blockade of androgen receptors reduces the number of intromissions needed to induce conditioned place preference after paced mating in female rats. Pharmacol Biochem Behav. 2005;81(4):871–878. doi: 10.1016/j.pbb.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45(3):153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE, Pursnani NK, Purdy RH. The neurosteroids, progesterone and 3alpha,5alpha-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 1998;808(1):72–83. doi: 10.1016/s0006-8993(98)00764-1. [DOI] [PubMed] [Google Scholar]
- Gans S, Erskine MS. Effects of neonatal testosterone treatment on pacing behaviors and development of a conditioned place preference. Horm Behav. 2003;44(4):354–364. doi: 10.1016/s0018-506x(03)00157-0. [DOI] [PubMed] [Google Scholar]
- Garcia Horsman P, Paredes RG. Dopamine antagonists do not block conditioned place preference induced by paced mating behavior in female rats. Behav Neurosci. 2004;118(2):356–364. doi: 10.1037/0735-7044.118.2.356. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Clark AS. Ibotenic acid lesions of the medial preoptic area disrupt the expression of partner preference in sexually receptive female rats. Brain Res. 2006;1076(1):163–170. doi: 10.1016/j.brainres.2005.12.120. [DOI] [PubMed] [Google Scholar]
- Hardy DF, Debold JF. Effects of mounts without intromission upon the behavior of female rats during the onset of estrogen-induced heat. Physiol Behav. 1971;7(4):643–645. doi: 10.1016/0031-9384(71)90120-x. [DOI] [PubMed] [Google Scholar]
- Hubscher CH. Estradiol-associated variation in responses of rostral medullary neurons to somatovisceral stimulation. Exp Neurol. 2006;200(1):227–239. doi: 10.1016/j.expneurol.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB. Female rats develop conditioned place preferences for sex at their preferred interval. Horm Behav. 2003;43(4):503–507. doi: 10.1016/s0018-506x(03)00031-x. [DOI] [PubMed] [Google Scholar]
- Kohlert JG, Cappellini C. Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2007. Female Syrian hamsters failed to condition a place preference to the sight and smell of a male hamster. Program No. 938.3. Online. [Google Scholar]
- Kohlert JG, Olexa N. The role of vaginal stimulation for the acquisition of conditioned place preference in female Syrian hamsters. Physiol Behav. 2005;84(1):135–139. doi: 10.1016/j.physbeh.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Komisaruk BR, Adler NT, Hutchison J. Genital sensory field: enlargement by estrogen treatment in female rats. Science. 1972;178(67):1295–1298. doi: 10.1126/science.178.4067.1295. [DOI] [PubMed] [Google Scholar]
- Madlafousek J, Hlinak Z. Sexual behavior of the female laboratory rat: inventory, patterning and measurement. Behavior. 1977;63:129–174. [Google Scholar]
- Martinez I, Paredes RG. Only self-paced mating is rewarding in rats of both sexes. Horm Behav. 2001;40(4):510–517. doi: 10.1006/hbeh.2001.1712. [DOI] [PubMed] [Google Scholar]
- Meerts SH, Clark AS. Female rats exhibit a conditioned place preference for nonpaced mating. Horm Behav. 2007;51(1):89–94. doi: 10.1016/j.yhbeh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Joppa MA. Conditioned place preference in female hamsters following aggressive or sexual encounters. Physiol Behav. 1994;56(5):1115–1118. doi: 10.1016/0031-9384(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Joppa MA, Rowe RK. Dopamine receptor antagonists attenuate conditioned place preference following sexual behavior in female Syrian hamsters. Eur J Pharmacol. 1996;309(1):21–24. doi: 10.1016/0014-2999(96)00389-5. [DOI] [PubMed] [Google Scholar]
- Oldenburger WP, Everitt BJ, de Jonge FH. Conditioned place preference induced by sexual interaction in female rats. Horm Behav. 1992;26(2):214–228. doi: 10.1016/0018-506x(92)90043-u. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Agmo A. Has dopamine a physiological role in the control of sexual behavior? A critical review of the evidence. Prog Neurobiol. 2004;73(3):179–226. doi: 10.1016/j.pneurobio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Alonso A. Sexual behavior regulated (paced) by the female induces conditioned place preference. Behav Neurosci. 1997;111(1):123–128. doi: 10.1037//0735-7044.111.1.123. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Martinez I. Naloxone blocks place preference conditioning after paced mating in female rats. Behav Neurosci. 2001;115(6):1363–1367. [PubMed] [Google Scholar]
- Paredes RG, Vazquez B. What do female rats like about sex? Paced mating Behav Brain Res. 1999;105(1):117–127. doi: 10.1016/s0166-4328(99)00087-x. [DOI] [PubMed] [Google Scholar]
- Peters LC, Kristal MB, Komisaruk BR. Sensory innervation of the external and internal genitalia of the female rat. Brain Res. 1987;408(1–2):199–204. doi: 10.1016/0006-8993(87)90372-6. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Kleopoulos SP, Mobbs CV, Gibbs RB, Pfaff DW. Sexual stimulation activates c-fos within estrogen-concentrating regions of the female rat forebrain. Brain Res. 1993;624(1–2):253–267. doi: 10.1016/0006-8993(93)90085-2. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Manitt C, Coopersmith CB. Effects of pelvic, pudendal, or hypogastric nerve cuts on Fos induction in the rat brain following vaginocervical stimulation. Physiol Behav. 2006;89(5):627–636. doi: 10.1016/j.physbeh.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Quysner A, Blaustein JD. A dopamine antagonist blocks vaginocervical stimulation-induced neuronal responses in the rat forebrain. Brain Res. 2001;921(1–2):173–182. doi: 10.1016/s0006-8993(01)03116-x. [DOI] [PubMed] [Google Scholar]
- Ross EL, Komisaruk BR, O’Donnell D. Evidence that probing the vaginal cervix is analgesic in rats, using an operant paradigm. J Comp Physiol Psychol. 1979;93(2):330–336. doi: 10.1037/h0077555. [DOI] [PubMed] [Google Scholar]
- Rowe DW, Erskine MS. c-Fos proto-oncogene activity induced by mating in the preoptic area, hypothalamus and amygdala in the female rat: role of afferent input via the pelvic nerve. Brain Res. 1993;621(1):25–34. doi: 10.1016/0006-8993(93)90294-w. [DOI] [PubMed] [Google Scholar]
- Tetel MJ, Getzinger MJ, Blaustein JD. Fos expression in the rat brain following vaginal-cervical stimulation by mating and manual probing. J Neuroendocrinol. 1993;5(4):397–404. doi: 10.1111/j.1365-2826.1993.tb00500.x. [DOI] [PubMed] [Google Scholar]
- Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73(4):743–752. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Baum MJ, Erskine MS. Mating-induced FOS-like immunoreactivity in the rat forebrain: a sex comparison and a dimorphic effect of pelvic nerve transection. J Neuroendocrinol. 1993;5(5):557–568. doi: 10.1111/j.1365-2826.1993.tb00522.x. [DOI] [PubMed] [Google Scholar]