Abstract
We have previously shown that endoglin (CD105) is upregulated in prostatic fluid of men with large volume prostate cancer. We chose to assess endoglin levels in urine and serum from men with prostate cancer or at increased risk for the disease: Urine samples were collected after DRE from 99 men whose cancer status was confirmed by biopsy, and serum samples were collected from 20 men without prostate cancer at low risk for the disease, and from 69 men diagnosed with prostate cancer that subsequently underwent radical prostatectomy (30 pT2, 39 pT3). Endoglin levels were assessed by ELISA. Urinary endoglin was elevated in men with biopsy-positive prostate cancer compared to biopsy-negative men (p=0.0014). Urinary endoglin levels in men with prostate cancer correlated with radical prostatectomy tumor volume. The area under the receiver-operator characteristics (ROC) curve was 0.72 for urinary endoglin and 0.50 for serum prostate-specific antigen PSA (sensitivity for cancer detection 73%, specificity 63%). There were no differences in serum endoglin between normal and cancer cases, but there were increases in serum endoglin in non-organ confined (NOC, pT3+) vs. organ-confined (OC, pT2) cases (p=0.0004). The area under the ROC curve was 0.75 for serum endoglin and 0.63 for PSA for predicting NOC status, with a sensitivity of 67% and a specificity of 80%. In conclusion, elevations in post-DRE urinary endoglin suggest there may be value in further studying endoglin as a urinary biomarker of prostate cancer. Endoglin levels in both urine and serum may aid in prostate cancer detection and prognostication.
Keywords: Endoglin, CD105, Prostate Cancer, Biological Markers, Clinical Markers
Introduction
Prostate cancer is known to be clinically heterogeneous, with some cases presenting in an indolent fashion and others widely metastatic at diagnosis. Prostate-specific antigen (PSA), digital rectal examination (DRE) and biopsy Gleason score are the three clinical tools typically used to stratify newly diagnosed men into low, intermediate, or high-risk prognostic groups.1 No other marker in routine use significantly adds to either the diagnostic or prognostic power of these clinical parameters. Nevertheless, there is a need for additional markers of early or aggressive/advanced prostate cancer, and the search for these is ongoing and increasingly technology-driven.2,3, 4
We have previously used a human cytokine array to identify cytokines in expressed prostatic fluid associated with large volume prostate cancers. We found that a variety of growth factors, cytokines, and markers of angiogenesis were up-regulated in prostatic fluid from such cases.5 One of the 20 most-upregulated molecules (see ref. 5 Supplementary material) was endoglin (CD105), a type I homodimeric integral transmembrane glycoprotein and accessory TGF-β receptor; another was the endoglin ligand activin-A.6 Given these common pathway findings, we selected endoglin for further study.
Endoglin is primarily expressed in proliferating vascular endothelial and smooth muscle cells, and is highly expressed on endothelial cells during tumor angiogenesis and inflammation. It has weak or negative expression in normal tissues. Endoglin is expressed in prostate microvasculature in association with prostate cancer, and is increased in the serum of patients with colorectal, breast and lung cancer metastases.7,8 Immunohistochemical analysis has shown endoglin to be expressed not only by endothelium associated with prostate cancer, but also by some prostatic intraepithelial neoplasia (PIN) and prostate cancer epithelial cells and associated stromal components.9 Recently, soluble endoglin has been shown to be of independent prognostic value as a serum indicator of prostate cancer metastasis to pelvic lymph nodes and of biochemical recurrence after prostatectomy.10,11 Whether endoglin may serve as a marker for prostate cancer in locally-derived tissue (biopsies), or biofluids (expressed prostatic secretions, post-DRE urine) has been little studied.
We set out to assess whether endoglin levels could predict the presence of prostate cancer and/or correlate with advanced disease. Since endoglin is a local marker of vascular proliferation in response to injury and/or angiogenic stimulation, we felt that assessing endoglin levels from the prostatic microenvironment more directly might have merit: To this effect we assayed urine samples collected following DRE which is known to be enriched with prostatic secretions, from patients with and without prostate cancer. In addition, we assessed endoglin in archival serum samples from men with and without prostate cancer in order to assess its potential as a cancer biomarker.
Materials and Methods
Sample collection
Urine samples were collected in the Urology Clinic. Approval was obtained from our Institutional Review Board before initiating the study and all patients provided written informed consent prior to providing urine samples. Initial voided urine specimens (10 to 100ml) were prospectively collected from 99 men with known prostate cancer or with an indication for prostate biopsy, immediately following DRE during a single office visit. In all cases, the DRE was an approximately 30-second examination involving 3 finger strokes per prostate lobe and was done either 6 weeks or more after diagnostic biopsy, or just prior to diagnostic biopsy. In some men a urine sample was also collected prior to DRE. Voided urine specimens were kept at 4°C for up to 4 hours prior to centrifugation for 10min at 1000g to remove sediments and then urine supernatants were kept at −80°C until analysis. In addition, 89 archival serum samples were obtained from our biorepository and linked to information about patient prostate health status and other relevant demographic and pathologic data.
Enzyme-Linked Immunosorbent Assay
Endoglin levels were measured by enzyme-linked immunosorbent assay (ELISA). A human Duo set (R&D Systems, Minneapolis, MN)) was used to detect endoglin in urine and serum. Briefly, 96-well microplates were coated with capture antibody and incubated overnight. After the blocking with 10%BSA in PBS for urine and 25% FBS in PBS for serum, samples were added (100µl/well) in duplicate for incubation for 2 hrs at room temperature. Detection antibodies were subsequently added (100µl/well)) and incubated for 2 hrs at room temperature. Incubation with streptavidin-horseradish-peroxidase (for 20 min) was followed by detection with 3,3V,5,5V-tetramethylbenzidine (TMB) for 20 min. The reaction was stopped by the addition of 1.5 M H2SO4. Plates were read at 450 nm wavelength on a microplate reader (PHERA star, BMG Labtech, Durham, NC). All reactions were done at room temperature. Serum samples were assayed at a 4-fold dilution.
ELISA data from urine samples were normalized by total urinary protein or urinary creatinine levels as measured by Dade Dimension RxL. Serum ELISA data were not normalized.
These data were analyzed by cancer grade on biopsy (Gleason score 6 vs.≥7) and, for the 36 radical prostatectomy cases, by pathologic stage, pathologic grade (Gleason score 6 vs. ≥7), and tumor volume (minimal-moderate vs. extensive). After assessing cancer diameters on all radical prostatectomy sections, specimens with minute foci of cancer with a maximum tumor area < 15 mm2 were termed “minimal” disease, while specimens with a maximum tumor area > 80mm2 were termed “extensive” disease; tumors of in-between sizes were termed “moderate” disease.5
Data Analysis and Statistics
Statistical analyses were done using GraphPad Prizm 4.0 for Windows. Mann-Whitney tests were used to analyze the difference of 2 categories. Power calculations were performed based on serum endoglin levels reported in the literature in order to detect a 5ng/ml difference between cancer and control patients (one standard deviation). 8, 10, 11 With a two-sided alpha = 0.05, and given a limited number of patients in the control group (20) due to specimen availability, power was >0.80 to detect a difference between groups. Biochemical and clinical prostate cancer recurrence data were available for 39 patients with non-organ confined disease. Kaplan-Meier recurrence curves were generated for cases with low (<median) and high (> median) serum endoglin levels, and Log-Rank tests were used to analyze the differences. Statistical significance was defined as a p value < 0.05.
Results
Endoglin Levels in Urine
ELISA was used to quantitate the levels of urinary endoglin in a 99-man cohort of men with known prostate cancer or at increased risk of prostate cancer. Of these 99 men, 67 had biopsy-positive prostate cancer (40 were biopsied 6 weeks or more prior to post-DRE urine collection and 27 were diagnosed after post-DRE urine collection) and 32 were biopsy-negative, for an overall rate of prostate cancer in this cohort of 67.7%. The men with and without biopsy-positive prostate cancer were well-matched by age, PSA and DRE findings (Table 1A). Preliminary experiments demonstrated that endoglin levels were significantly higher in matched urine samples collected post-DRE compared to pre-DRE (mean endoglin in voided urine: 27.1pg/ml±16, mean endoglin in post-DRE urine: 68.9pg/ml±39), therefore post-DRE urine samples were used for all subsequent urinary analyses.
Table 1.
Patient Characteristics Respective to Analyzed Urine and Serum Samples
| A. | Urine samples | |||
|---|---|---|---|---|
| Negative biopsy |
Positive biopsy |
|||
| No. pts | 32 | 67 | ||
| Median age (range) | 62 (40–81) | 60 (45–84) | p=0.31 | |
| Median PSA (ng/ml) (range) |
5.4 (0.6–11.5) |
5.05 (1.7–20.5) |
p=0.98 | |
| Suspicious DRE (%) | 18.7 | 19.4 | ||
| Gleason Score | 6 | - | 43 (64%) | |
| 7 | - | 21 (31%) | ||
| 8 | - | 1 (2%) | ||
| 9 | - | 2 (3%) | ||
| B. | Serum samples | ||||||
|---|---|---|---|---|---|---|---|
| Controls | CaP Patients |
||||||
| All | pT2 | pT3+ | |||||
| No. pts | 20 | 69 | 30 | 39 | |||
| Median age (range) | 56 (46–66) | 61 (47–69) | p=0.10 | 57.5 (48–66) |
62 (47–69) |
p=0.01 | |
| Median PSA (ng/ml) (range) |
1.05 (0.3–1.9) |
5.29 (0.9–27.8) |
p<0.01 | 4.7 (2.9–13.4) |
6.5 (0.9–27.8) |
p=0.07 | |
| Suspicious DRE (%) | 0 | 24.3 | 13.3 | 32.5 | |||
| Gleason Score | 6 | - | 27 (39%) | 17 (57%) | 10 (26%) | ||
| 7 | - | 35 (51%) | 13 (43%) | 22 (56%) | |||
| 8 | - | 5 (7%) | 0 | 5 (13%) | |||
| 9 | - | 2 (3%) | 0 | 2 (5%) | |||
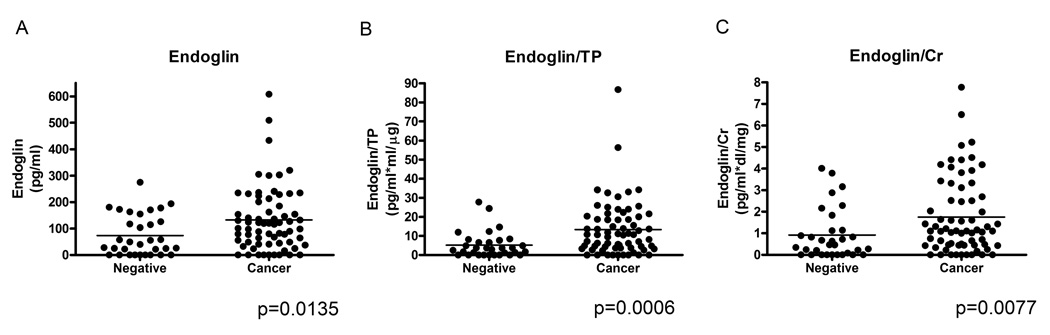
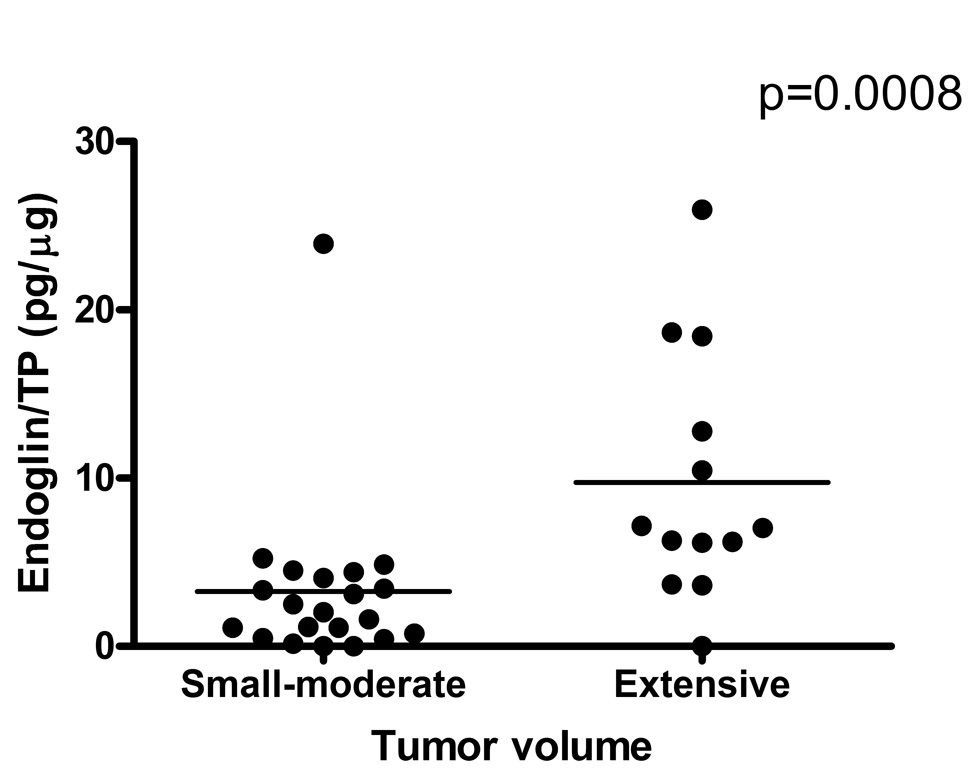
Endoglin levels were significantly higher in the urine of men with prostate cancer than in those without prostate cancer (Figure 1A). Endoglin levels were normalized both to total urinary protein (TP) (Figure 1B) and to urinary creatinine (Figure 1C), but remained significantly elevated in the cancer cases regardless of the method of normalization (though normalization to total urinary protein was most discriminating). In order to assess whether endoglin levels might confer prognostic information in patients diagnosed with prostate cancer, we stratified those who underwent radical prostatectomy (n=34) by stage (organ-confined (OC, pT2), non-organ confined (NOC, pT3+)), Gleason score (≤6, ≥7), and tumor volume (minimal-moderate, extensive). Urinary endoglin levels were significantly higher in cases with high tumor volume (extensive prostate cancer, mean endoglin level = 9.73pg/µg ±7.35, range 0 – 25.95) compared to cases with smaller tumor volume (minimal/moderate prostate cancer, mean endoglin level = 3.25 pg/µg ± 5.05, range 0 – 23.4) p=0.008 (Figure 2). Mean urinary endoglin in men without prostate cancer was 73.2pg/ml ± 77.0 (range 0 – 274.8), and in those with prostate cancer was 132.4pg/ml ± 121.4 (range 0 – 608.3) (p = 0.0135). Mean endoglin levels normalized by TP of men without prostate cancer were 5.18 pg/µg ± 6.8 (range 0 – 27.7), and those with prostate cancer were 13.4 pg/µg ± 14.4 (range 0 – 86.7) (p = 0.0006). Mean endoglin levels normalized by urinary creatinine of men without prostate cancer were 0.92 pg/ml*dl/mg ±1.17 (range 0 – 4.02), and those with prostate cancer were 1.75 pg/ml* dl/mg ±1.76 (range 0 – 7.78) (p = 0.0077). There were no significant differences in urinary endoglin levels by Gleason score or cancer stage, nor did men with benign biopsies but high urinary endoglin have an obvious increase in pre-neoplastic features such as PIN or atypia (data not shown). Urinary endoglin levels did not correlate with serum PSA or age.
Figure 1.
Urinary endoglin collected after DRE in patients who had either a negative (n=32) or positive (n=67) biopsy for prostate cancer. A) Urinary endoglin B) Urinary endoglin/Urinary total protein (TP), C) Urinary endoglin/Urinary creatinine (Cr).
Figure 2.
Urinary endoglin/Urinary total protein in patients with prostate cancer who subsequently underwent radical prostatectomy and had tumor volume estimated.
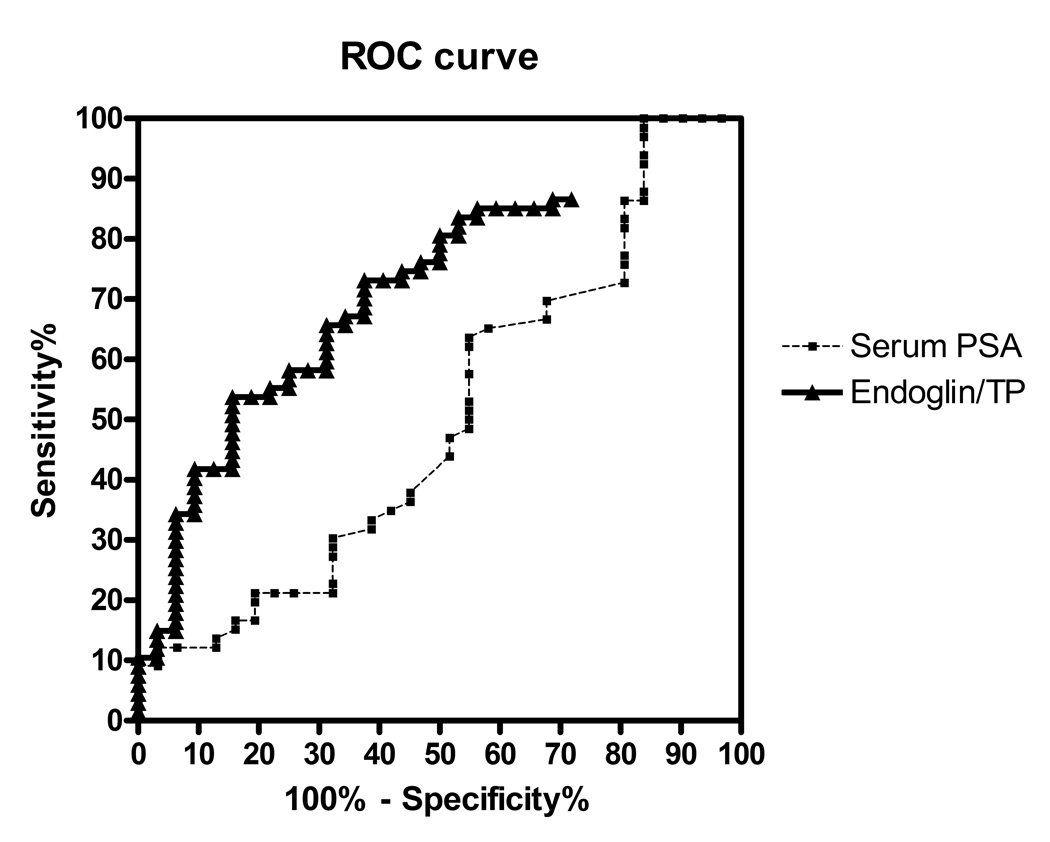
The area under the receiver-operator characteristics (ROC) curve (AUC) for urinary endoglin was 0.72 (95% CI 0.61 – 0.82), in contrast to an AUC for PSA of 0.50 (95% CI 0.37 – 0.63) (AUC comparison p<0.01) for cancer in our patient cohort (Figure 3). The sensitivity and specificity at different endoglin/urinary TP cutoffs are listed on Table 2.
Figure 3.
Receiver operating characteristic curves of urinary endoglin and serum PSA for the detection of cancer in our cohort.
Table 2.
Urinary endoglin normalized to total urinary protein (TP) as a marker for prostate cancer in men at increased risk for prostate cancer (abnormal PSA &/or DRE)
| Sensitivity | Specificity | |||
|---|---|---|---|---|
| Urinary Endoglin/TP Cutoff | ||||
| % | 95% CI | % | 95% CI | |
| 14.8 | 34.3 | (23.1 – 46.9) | 93.7 | (79.1 – 99.2) |
| 8.9 | 53.7 | (41.1 – 66.0) | 84.3 | (67.2 – 94.7) |
| 4.0 | 73.1 | (60.9 – 83.2) | 62.5 | (43.6 – 78.9) |
| 3.1 | 80.6 | (69.1 – 89.2) | 50.0 | (31.8 – 68.1) |
| 1.9 | 85.0 | (74.2 – 92.6) | 43.8 | (26.3 – 62.3) |
Endoglin Levels in Serum
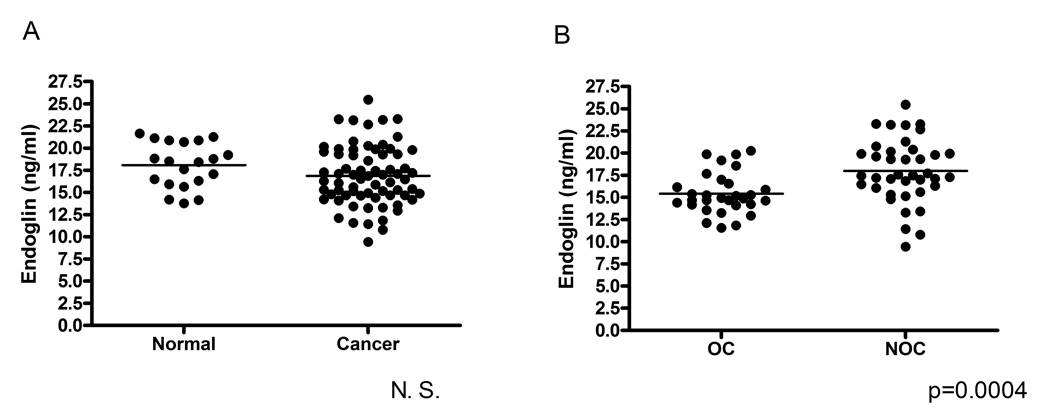
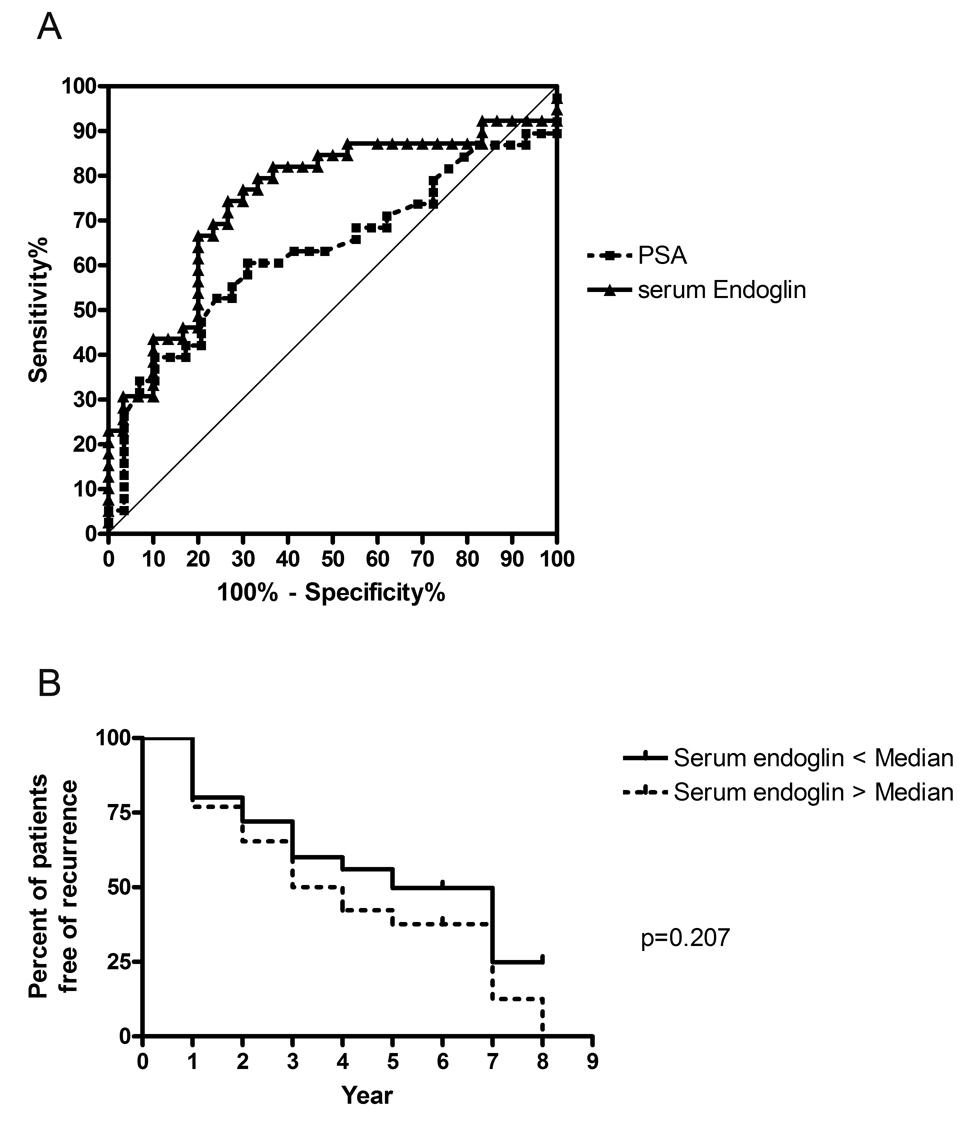
Serum samples in a separate cohort of 89 patients with and without prostate cancer were also assessed for endoglin levels by ELISA (Table 1B). There was no overall difference in serum endoglin levels in men with prostate cancer compared to men without prostate cancer (16.9ng/ml ± 2.6, range 9.4 – 25.5 vs. 18.1ng/ml ± 2.6, range 13.8 – 21.6, respectively) (Figure 4). However, among the 69 men with prostate cancer, endoglin levels were significantly higher in NOC (pT3+) (mean 18.0 ng/ml ± 3.6, range 9.4 – 25.5) vs. OC (pT2) disease (mean 15.4ng/ml ± 2.3, range 11.5 – 20.2) (p<0.01). The men with prostate cancer were typically older, had higher PSA, and had more abnormal DRE findings than the men who did not have prostate cancer (Table 1B), but in separate univariate analyses, no correlation was found between serum endoglin levels and age or Gleason score. The ROC curve for serum endoglin is compared to that for PSA to predict pT3+ disease (Figure 5A), with an AUC for endoglin of 0.75 (95% CI 0.63 – 0.87) in contrast to an AUC for PSA of 0.63 (95% CI 0.50 – 0.77) (AUC comparison p=0.10). The sensitivity was 67% and the specificity was 80% for the prediction of non-organ-confined disease with a serum endoglin cutoff of 17.0ng/ml.
Figure 4.
Serum endoglin levels in patients A) without prostate cancer (Normal, n=20) and with prostate cancer (Cancer, n=69), and B) with organ-confined prostate cancer (pT2, n=30), and with non-organ confined prostate cancer (pT3+, n=39).
Figure 5.
A) ROC curve of serum endoglin and serum PSA for the prediction of non-organ confined disease in patients with prostate cancer on biopsy. B) Kaplan-Meier recurrence curves for cases with low (< median) and high (> median) serum endoglin levels for 39 cases with documented non-organ confined disease.
A subset of patients with NOC disease with (20) and without (19) postoperative PSA recurrence was compared by preoperative serum endoglin level, and no difference was found (18.6 vs. 17.3 ng/mL). Log Rank analysis for post-prostatectomy biochemical recurrence showed no significant difference between men in this subset with low versus high endoglin levels (<50%ile vs. >50%ile endoglin, p = 0.21) (Figure 5B).
Discussion
We hypothesized that biomarkers associated with the development of prostate cancer and/or of its dedifferentiation can be measured from the prostatic microenvironment. Prostatic stroma and epithelium are known to be rich sources of cytokines and growth factors involved in the regulation of prostatic development, hypertrophy, and neoplasia, as well as of inflammation and local immunity.12 In previous experiments, we assayed prostatic fluid for cancer-associated proteins: In addition to increased amounts of cytokines such as HGF and IL18 binding protein-a, we noted increased CD105/endoglin and increased amounts of one of its ligands (activin-A) in expressed prostatic fluid collected from radical prostatectomy specimens with large volume cancers.5 In the present study, we show that endoglin is increased in urine collected after DRE from men with prostate cancer on biopsy compared to men without prostate cancer, and that post-DRE urinary endoglin levels are predictive of prostate cancer in a cohort of men at increased risk by PSA and DRE criteria (Figure 3). This is the first assessment of the ability of endoglin to distinguish between benign and malignant prostate disease. In addition, endoglin levels measured from serum were predictive of non-organ confined prostate cancer using an archival set of serum samples from men with and without prostate cancer.
Endoglin was assayed in the urine after DRE in order to directly (but minimally-invasively) assess its presence in the prostatic microenvironment in vivo. An attentive DRE exerts pressure on much of the prostate, and at least in theory allows for a sampling of secretions from the entire gland, unlike a prostate biopsy. It is known that initial voided urine obtained after DRE is enriched in prostatic proteins.13 We did not specifically assess whether the urinary endoglin we detected was a result of circulating and filtered endoglin or a result of local prostatic endoglin. However, given that the assays were performed after prostatic manipulation, that we have previously found endoglin in expressed prostatic secretions, that only initial urine was collected as it coursed through the prostate after prostatic examination (“Voided bladder 3” samples, per Meares-Stamey),13 and that we normalized to total protein in the urine samples (which mostly comes from prostatic sources after a DRE), we surmise that the endoglin we assayed was predominantly of prostatic origin. Urine is likely to become an increasingly powerful source of prostate-specific biomarkers,2,4 but until quantitative detection methods improve it may be reasonable to sample urine enriched in prostatic secretions rather than urine that is prostate secretion-poor (such as mid-stream urinalysis).
Serum PSA is an extremely powerful marker of prostatic disease, with tremendous diagnostic and prognostic utility, but it is not cancer-specific.14 Nevertheless, PSA and its isoforms are the sole prostatic serum markers in clinical use today, and PSA testing alone has changed the epidemiology of prostate cancer dramatically since its introduction in the 1980s.15 Our cohort of men who provided post-DRE urine samples had mean PSA levels between 5 and 5.5 ng/ml (i.e. elevated), and almost 20% had abnormal DRE findings (Table 1). These men could be characterized as being at elevated risk for prostate cancer primarily based on PSA criteria. Our urinary endoglin test demonstrated better performance characteristics than PSA in this cohort of high-risk men; however, it is unclear how urinary endoglin would perform in a more generalizable male population, where PSA has significant clinical utility. Another caveat is that the cohort under study included men with known untreated prostate cancer as well as men at elevated risk for prostate cancer undergoing biopsy. Our overall cohort was thus weighted 2:1 with men harboring prostate cancer, in contrast to routine clinical settings in which biopsies yield a 1:2 prostate cancer detection rate. Therefore, while the performance of urinary endoglin in such a cohort was better than PSA, it remains to be tested in more routine clinical scenarios and on a larger scale.
We studied endoglin levels from archival serum samples in a separate cohort of men with and without prostate cancer. We did not find a significant difference in serum endoglin between men with and without prostate cancer, but a study with greater power might elucidate any difference that may have been missed. On the other hand, serum endoglin levels in men with pathologic stage III (NOC) disease were significantly greater than those in men with pathologic stage II (OC) disease. This statistically significant finding may not easily translate into a clinically useful pretreatment counseling tool because of the small differences in absolute levels.
Endoglin has recently gained attention in prostate cancer prognostication by work from the group from the University of Texas Southwestern that has had a longstanding interest in TGF-β related proteins and prostate cancer. They analyzed endoglin levels in archival serum from a large cohort of prostatectomy patients and showed an independent association between increased plasma endoglin and the presence of lymph node metastasis and biochemical recurrence after prostatectomy, suggesting this molecule may be a marker of and/or facilitate extraprostatic spread.10,11 Interestingly, our two groups have come upon endoglin in distinct manners, one from scientific analysis of TGF-β related pathways, and the other from cytokine profiling of prostatic fluid; consistently, both have demonstrated associations between endoglin and aggressive prostate cancer.
Since endoglin is a marker of pan-endothelial damage and angiogenesis, it is unlikely that circulating endoglin levels would be significantly affected by localized prostatic disease states - indeed, serum endoglin levels are affected by cardiovascular disease status, cholesteremia, and cirrhosis.16,17 However, circulating endoglin is increased in metastatic disease states.8,10, 11 Presumably, the angiogenic cascade necessary for metastasis is associated with systemic dysregulation of the TGF-β superfamily that results in an increase in detectable serum endoglin. Our finding of increased serum endoglin in non-organ confined prostate cancer states is consistent with the notion of endoglin as a marker of advanced disease and supports the dramatic associations found between endoglin and metastatic disease by the U.T. Southwestern group. However, we were unable to show increased endoglin levels in patients with prostate cancer compared to patients without it. In addition, serum endoglin levels in our study patients differed from those in the other studies, which were somewhat higher even in localized disease states (20–40ng/ml).10, 11 Levels in our cohort ranged between 7.5 and 27.5 ng/ml (Figure 4), while in the cardiovascular literature, levels in normal controls and in patients with familial atherosclerosis and/or in the setting of myocardial infarction average between 3 and 8 ng/ml.16, 17 There is no standard assay for endoglin, but a variety of kits and antibodies are commercially available; it is possible that the specific ELISA used may be responsible for the range of levels reported in these different studies. Alternate explanations are that endoglin levels in serum and plasma may differ, and that endoglin levels may be affected by time of archival storage.
Endoglin’s molecular role if any in prostate carcinogenesis and metastasis is unknown. Endoglin is known to be strongly up-regulated in the endothelium of various tumors compared with normal tissues, suggesting that endoglin plays a significant role in tumor angiogenesis.18 Hypoxia transcriptionally induces endoglin expression via HIF-1, expression which is enhanced in the hypoxic setting by TGF-β.19 In turn, endoglin antagonizes the inhibitory effects of TGF-β1 on human vascular endothelial cells; indeed normal cellular levels of endoglin/CD105 are required for the formation of new blood vessels.20 Future work is required to determine the specific source of the endoglin detectable in the urine of prostate cancer patients, if it is bioactive, and what are its most important downstream targets with respect to prostate oncogenesis and prostate cancer progression.
Conclusions
Endoglin is an accessory TGF-β receptor transmembrane glycoprotein associated with angiogenesis and prostatic neoplasia that is present in prostatic fluid. Urinary levels of endoglin are increased in men with prostate cancer compared to levels in men without prostate cancer, and serum endoglin levels appear to correlate with increasing prostate cancer stage. Further studies are necessary to validate these initial observations.
Acknowledgments
Funding Sources: NIDDK 1K23DK071262, DOD W81XWH-05-1-0167, NCI 5 P50 CA58236, NCI 5U01 CA86323-08
Abbreviations
- DRE
digital rectal examination
- ELISA
enzyme-linked immunosorbent assay
- NOC
non-organ confined
- OC
organ-confined
- PIN
prostatic intraepithelial neoplasia
- PSA
prostate-specific antigen
- ROC
receiver-operator characteristics
- TGF
transforming growth factor
- TP
total protein
Footnotes
Brief statements of novelty and impact: Endoglin is a promising serum biomarker for prostate cancer prognostication; we demonstrate in this study that it may also have utility as a urinary marker for prostate cancer diagnosis, and confirm its potential as a serum marker of advanced prostate cancer. Endoglin was significantly increased in urine collected after digital rectal examination from men with prostate cancer compared to men without it, and serum endoglin was increased in men with stage III compared to stage II disease.
References
- 1.D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, Wein A. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. Jama. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 2.Laxman B, Morris DS, Yu J, Siddiqui J, Cao J, Mehra R, Lonigro RJ, Tsodikov A, Wei JT, Tomlins SA, Chinnaiyan AM. A first-generation multiplex biomarker analysis of urine for the early detection of prostate cancer. Cancer Res. 2008;68:645–649. doi: 10.1158/0008-5472.CAN-07-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng SL, Sun J, Wiklund F, Smith S, Stattin P, Li G, Adami HO, Hsu FC, Zhu Y, Balter K, Kader AK, Turner AR, et al. Cumulative association of five genetic variants with prostate cancer. N Engl J Med. 2008;358:910–919. doi: 10.1056/NEJMoa075819. [DOI] [PubMed] [Google Scholar]
- 4.Wright JL, Lange PH. Newer potential biomarkers in prostate cancer. Rev Urol. 2007;9:207–213. [PMC free article] [PubMed] [Google Scholar]
- 5.Fujita K, Ewing CM, Sokoll LJ, Elliott DJ, Cunningham M, De Marzo AM, Isaacs WB, Pavlovich CP. Cytokine profiling of prostatic fluid from cancerous prostate glands identifies cytokines associated with extent of tumor and inflammation. Prostate. 2008;68:872–882. doi: 10.1002/pros.20755. Supplementary Table available at http://www.interscience.wiley.com/jpages/0270-4137/suppmat/index.html. [DOI] [PMC free article] [PubMed]
- 6.Bernabeu C, Conley BA, Vary CP. Novel biochemical pathways of endoglin in vascular cell physiology. J Cell Biochem. 2007;102:1375–1388. doi: 10.1002/jcb.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Gohary YM, Silverman JF, Olson PR, Liu YL, Cohen JK, Miller R, Saad RS. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in prostatic adenocarcinoma. Am J Clin Pathol. 2007;127:572–579. doi: 10.1309/X6NXYE57DLUE2NQ8. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi N, Kawanishi-Tabata R, Haba A, Tabata M, Haruta Y, Tsai H, Seon BK. Association of serum endoglin with metastasis in patients with colorectal, breast, and other solid tumors, and suppressive effect of chemotherapy on the serum endoglin. Clin Cancer Res. 2001;7:524–532. [PubMed] [Google Scholar]
- 9.Kassouf W, Ismail HR, Aprikian AG, Chevalier S. Whole-mount prostate sections reveal differential endoglin expression in stromal, epithelial, and endothelial cells with the development of prostate cancer. Prostate Cancer Prostatic Dis. 2004;7:105–110. doi: 10.1038/sj.pcan.4500716. [DOI] [PubMed] [Google Scholar]
- 10.Karam JA, Svatek RS, Karakiewicz PI, Gallina A, Roehrborn CG, Slawin KM, Shariat SF. Use of preoperative plasma endoglin for prediction of lymph node metastasis in patients with clinically localized prostate cancer. Clin Cancer Res. 2008;14:1418–1422. doi: 10.1158/1078-0432.CCR-07-0901. [DOI] [PubMed] [Google Scholar]
- 11.Svatek RS, Karam JA, Roehrborn CG, Karakiewicz PI, Slawin KM, Shariat SF. Preoperative plasma endoglin levels predict biochemical progression after radical prostatectomy. Clin Cancer Res. 2008;14:3362–3366. doi: 10.1158/1078-0432.CCR-07-4707. [DOI] [PubMed] [Google Scholar]
- 12.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 13.Nickel JC, Shoskes D, Wang Y, Alexander RB, Fowler JE, Jr, Zeitlin S, O'Leary MP, Pontari MA, Schaeffer AJ, Landis JR, Nyberg L, Kusek JW, et al. How does the pre-massage and post-massage 2-glass test compare to the Meares-Stamey 4-glass test in men with chronic prostatitis/chronic pelvic pain syndrome? J Urol. 2006;176:119–124. doi: 10.1016/S0022-5347(06)00498-8. [DOI] [PubMed] [Google Scholar]
- 14.Schroder FH, Carter HB, Wolters T, van den Bergh RC, Gosselaar C, Bangma CH, Roobol MJ. Early Detection of Prostate Cancer in 2007 Part 1: PSA and PSA Kinetics. Eur Urol. 2008;53:468–477. doi: 10.1016/j.eururo.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 15.Oesterling JE. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991;145:907–923. doi: 10.1016/s0022-5347(17)38491-4. [DOI] [PubMed] [Google Scholar]
- 16.Cruz-Gonzalez I, Pabon P, Rodriguez-Barbero A, Martin-Moreiras J, Pericacho M, Sanchez PL, Ramirez V, Sanchez-Ledesma M, Martin-Herrero F, Jimenez-Candil J, Maree AO, Sanchez-Rodriguez A, et al. Identification of serum endoglin as a novel prognostic marker after acute myocardial infarction. J Cell Mol Med. 2007 doi: 10.1111/j.1582-4934.2008.00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blaha M, Cermanova M, Blaha V, Jarolim P, Andrys C, Blazek M, Maly J, Smolej L, Zajic J, Masin V, Zimova R, Rehacek V. Elevated serum soluble endoglin (sCD105) decreased during extracorporeal elimination therapy for familial hypercholesterolemia. Atherosclerosis. 2008;197:264–270. doi: 10.1016/j.atherosclerosis.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 18.Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. Faseb J. 2003;17:984–992. doi: 10.1096/fj.02-0634rev. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Elsner T, Botella LM, Velasco B, Langa C, Bernabeu C. Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor-beta pathways. J Biol Chem. 2002;277:43799–43808. doi: 10.1074/jbc.M207160200. [DOI] [PubMed] [Google Scholar]
- 20.Li C, Hampson IN, Hampson L, Kumar P, Bernabeu C, Kumar S. CD105 antagonizes the inhibitory signaling of transforming growth factor beta1 on human vascular endothelial cells. Faseb J. 2000;14:55–64. doi: 10.1096/fasebj.14.1.55. [DOI] [PubMed] [Google Scholar]