SUMMARY
Most autoreactive B cells are normally counterselected during early B cell development. To determine whether Toll-like receptors (TLRs) regulate the removal of autoreactive B lymphocytes, we tested the reactivity of recombinant antibodies from single B cells isolated from patients deficient for IRAK-4, and MyD88, whose cells do not respond to TLRs except TLR3 and from UNC-93B-deficient patients whose cells are irresponsive to TLR3, TLR7, TLR8 and TLR9. All patients suffered from defective central and peripheral B cell tolerance checkpoints resulting in the accumulation of large numbers of autoreactive mature naïve B cells in their blood. Hence, TLR7, TLR8, and TLR9 may normally prevent the recruitment of developing autoreactive B cells in healthy donors. Paradoxically, IRAK-4-, MyD88- and UNC-93B-deficient patients do not display autoreactive antibodies in their serum nor develop autoimmune diseases revealing that IRAK-4/MyD88/UNC-93B pathways blockade is likely to thwart the development of autoimmunity in humans.
INTRODUCTION
Autoreactive B cells generated by random V(D)J immunoglobulin gene assembly are normally eliminated during their development by both central and peripheral B cell tolerance checkpoints (Wardemann et al., 2003). The mechanisms that ensure human central B cell tolerance are poorly characterized, but they are mostly controlled by intrinsic B cell factors that sense B cell receptors (BCRs) recognizing autoantigens (Goodnow, 1996; Nemazee et al., 2000; Samuels et al., 2005a). In addition to their BCRs, B cells also express germline encoded transmembrane receptors called Toll-like receptors (TLRs) that were originally described to bind microbial components but that are also able to recognize self-antigens (Marshak-Rothstein, 2006). Indeed, in addition to TLR1/10 complexes whose ligands are unknown, human B cells express TLR7 and TLR9 that bind RNA and DNA respectively, and could play a role in the removal of developing ANA-expressing B cells (Bernasconi et al., 2003; Bourke et al., 2003; Hasan et al., 2005). The regulation of the peripheral B cell tolerance checkpoint may involve other cell populations such as regulatory T (Treg) cells whose development and function may depend on some TLR expression (Hervé et al., 2007).
To assess whether the mechanisms that preside over the removal of developing autoreactive B cells involve TLRs, we analyzed B cell tolerance checkpoints in IL-1R-associated kinase (IRAK)-4-, myeloid differentiation factor 88 (MyD88)- and UNC-93B-deficient patients. All TLRs except TLR3 triggering induce the recruitment to their TIR domain of the adaptor protein MyD88/IRAK-4 kinase complex that is essential for mediating signaling of these receptors (Akira and Takeda, 2004; Beutler, 2004). In addition, it has been recently reported that the endoplasmic reticulum membrane protein UNC-93B interacted with, and was required for intracellular TLR3, 7, 8 and 9 trafficking (Brinkmann et al., 2007; Casrouge et al., 2006; Kim et al., 2008; Tabeta et al., 2006).
Consistent with the role of TLRs in innate immunity, mice lacking IRAK-4 or MyD88 showed severely impaired immunological responses to bacterial challenges but were resistant to a lethal dose of lipopolysaccharide (LPS) (Kawai et al., 1999; Suzuki et al., 2002; Takeuchi et al., 2000). The “triple d, 3d” mice, which show missense mutation in the Unc93b1 gene encoding UNC-93B, also suffer from hypersusceptibility to infection from mouse cytomegalovirus and other microbes (Tabeta et al., 2006). In humans, MyD88- and IRAK-4-deficient patients are susceptible to pyogenic Gram-positive bacterial infections due to the inability of their blood cells to produce proinflammatory cytokines such as IL-1β, IL-6, IL-12, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) in response to TLR and IL-1R ligation (Haraguchi et al., 1998; Ku et al., 2007; Medvedev et al., 2003; Picard et al., 2003). In contrast, human UNC-93B deficiency, like TLR3 deficiency, does not compromise the immunity to most pathogens, but elicits specific defects in clearing Herpes simplex viral infections resulting in recurrent encephalitis (Casrouge et al., 2006; Zhang et al., 2007). By studying the reactivity of recombinant antibodies from single B cells from a MyD88-, three IRAK-4- and two UNC-93B-deficient patients, we found a high proportion of autoreactive B cells in all patients, suggesting that TLR pathways may prevent these B lymphocytes to enter the mature naïve B cell compartment.
RESULTS
TLR7 and TLR9 requires UNC-93B expression to activate human B cells
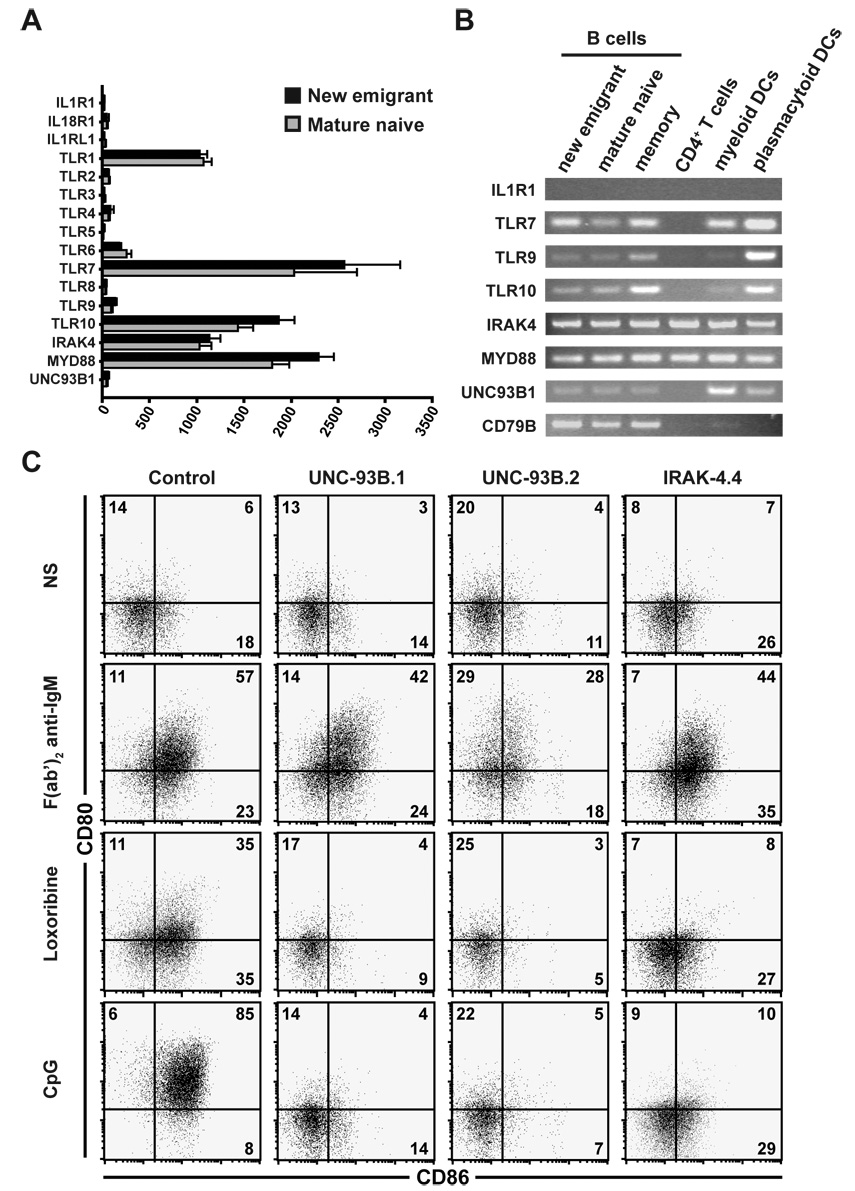
IRAK-4 and MyD88 are essential molecules required to mediate intracellular signaling generated upon triggering of IL-1R family members, including IL-1R1, IL-18R1, IL-1RL1 (also known as IL-33R) and most TLRs except TLR3 (Akira and Takeda, 2004; Beutler, 2004). Since the genes encoding IL-1R1, IL-18R1, and IL-1RL1 are not expressed in control new emigrant and mature naïve B cells, these molecules are not likely to play a direct role in the removal of developing autoreactive B cells (Figure 1A and 1B, and Genomics Institute of the Novartis Research Foundation expression anatomy database). In agreement with previous reports, we found that TLR1, TLR6, TLR7, TLR10, IRAK4, MYD88, and to a lower level TLR9 genes were expressed when analyzing gene expression profiles in both new emigrant and mature naïve B cells from healthy controls but TLR2, TLR3, TLR4, TLR5, and TLR8 were not (Figure 1A and Genomics Institute of the Novartis Research Foundation expression anatomy database) (Bernasconi et al., 2003; Bourke et al., 2003; Hasan et al., 2005). In addition, UNC93B1 did not seem to be expressed in human B cells whereas unc93b1 is highly expressed in mouse B cells (Figure 1A and Genomics Institute of the Novartis Research Foundation expression anatomy database). Because of the very low levels if any of UNC93B1 transcripts detected by microarray gene expression profile analysis, we analyzed by RT-PCR the expression of UNC93B1 and other TLR-related genes in new emigrant, mature naïve, and memory B cells as well as in CD4-positive T cells, myeloid and plasmacytoid dendritic cell (DC) enriched fractions (Figure 1B). CD79B(also known as Igβ) gene encodes a component of the BCR and its expression was used as a positive control for all B cell fractions. PCR analysis confirmed microarray gene expression data in that TLR7, TLR10, IRAK4, MYD88, and to a lesser extent TLR9 were expressed in all B cell fractions (Figure 1B). In addition, both TLR9 and TLR10 were expressed at a higher level in memory B cells than in new emigrant and mature naïve B cells (Figure 1B) (Bernasconi et al., 2003; Bourke et al., 2003). UNC93B1 transcripts were weakly detected in human B cells whereas they were more abundant in myeloid and plasmacytoid DC enriched fractions (Figure 1B and Genomics Institute of the Novartis Research Foundation expression anatomy database).
Figure 1. Human B cell activation by TLR7 and TLR9 requires UNC-93B.
(A) Human new emigrant and mature naive B cells do not express IL-1R family members. Histograms represent means ± s.e.m. of the expression of studied genes (IL1R1, IL18R1, IL1RL1, TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, IRAK4, MYD88, and UNC93B1) in human new emigrant (black bars, n=8) or mature naive (grey bars, n=4) B cells assessed by gene expression profiling using the Affimetrix Human Genome U133 Plus 2.0 Array.
(B) UNC93B1 gene is expressed in human DCs and to a lesser extent in B cells and not in T cells. IL1R, TLR7, TLR9, TLR10, IRAK4, MYD88, UNC93B1, and CD79B gene expression was analyzed by RT-PCR in CD19+CD10+IgM+CD27− new emigrant, CD19+CD10−IgM+CD27− mature naïve, CD19+CD10−CD27+ total memory B cells; TCRαβ+CD4+CD25− T cells; CD19−CD11c+HLA-DR+ myeloid DC enriched cells; and CD19−CD11c−HLA-DR+ plasmacytoid DC enriched cells from healthy controls.
(C) Human B cells responses to TLR7 and TLR9 agonists depend on UNC-93B expression. Dot plots show CD80 and CD86 expression on naive CD19+CD27− B cells from a healthy control, two UNC-93B-deficient patients and one IRAK-4-deficient patient that were either left unstimulated (NS) or stimulated with F(ab’)2 anti-IgM, CpG (TLR9 agonist) or Loxoribine (TLR7 agonist) for 48 hours.
To determine if UNC-93B expression was required by TLR7 and TLR9 to activate human B cells, we isolated naïve CD19+CD27− B cells from healthy controls, UNC-93B- and IRAK-4-deficient patients, and stimulated them by TLR agonists. Human naïve B cells from healthy controls stimulated with F(ab’)2 anti-IgM, Loxoribine (TLR7 agonist), or CpG (TLR9 agonist), upregulated the expression at their cell surface of costimulation molecules CD80 and CD86, and activation markers CD25 and CD69 (Figure 1C and Figure S1). Naïve B cells from UNC-93B- and IRAK-4-deficient patients showed responses to F(ab’)2 anti-IgM stimulation similar to control B cells (Figure 1C and Figure S1). However, UNC-93B-deficient B cells were similar to IRAK-4-deficient B cells and failed to upregulate CD80, CD86, CD25, and CD69 when stimulated by Loxoribine or CpG (Figure 1C and Figure S1) (Ku et al., 2007). We conclude that human B cells express TLR7 and TLR9 that bind RNA and DNA containing antigens respectively, and TLR10 that can associate with TLR1 and whose ligands are unknown. In addition, human B cells express IRAK-4/MyD88 complexes and UNC93B1, which is required for TLR7 and TLR9 to function.
Defective central B cell tolerance checkpoint in IRAK-4-, MyD88- and UNC-93B-deficient patients
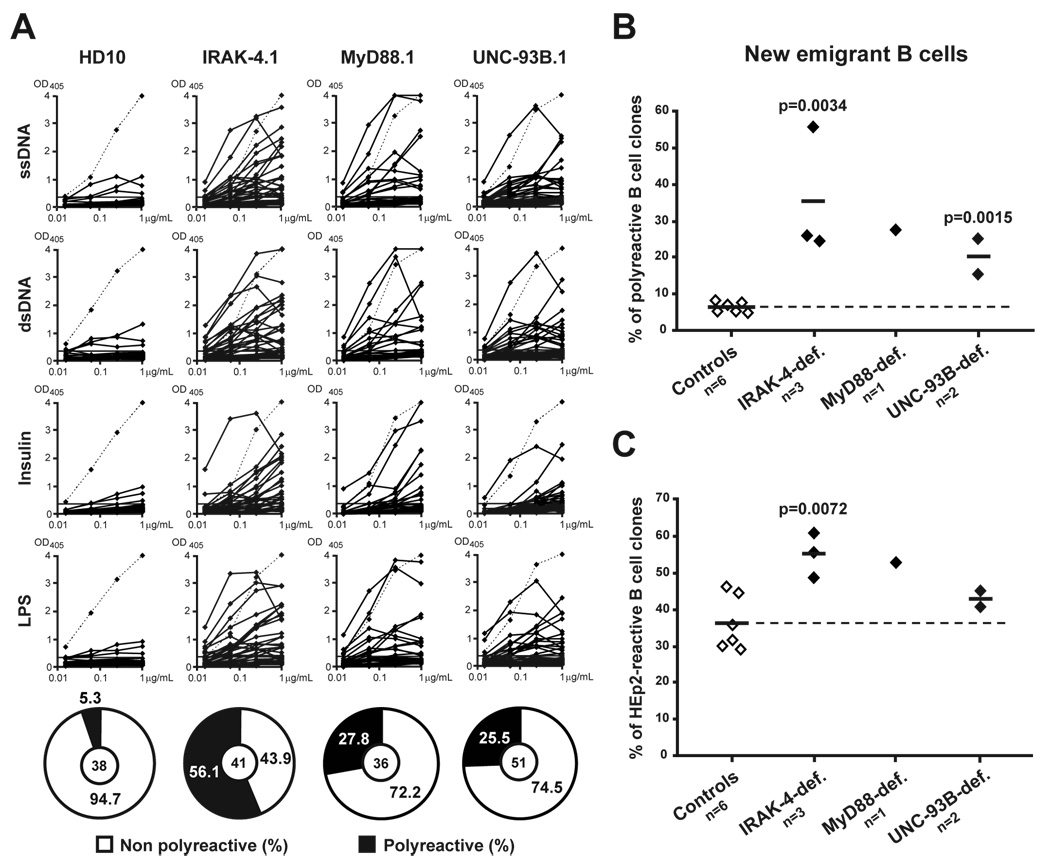
A central B cell tolerance checkpoint removes the vast majority of developing B cells that express highly polyreactive antibodies in the bone marrow of healthy donors and only a small fraction of clones with low levels of polyreactivity migrate to the periphery (Wardemann et al., 2003). To determine whether this checkpoint is affected by the absence of most TLR functions, we analyzed the reactivity of antibodies cloned from single IRAK-4-, MyD88-, and UNC-93B-deficient CD19+CD10+IgM+CD27− new emigrant B cells. Antibody reactivities were then compared to those of the corresponding B cell subpopulation from a healthy donor (HD10) and 5 previously reported controls (Figure 2) (Hervé et al., 2007; Ng et al., 2004; Tsuiji et al., 2006; Wardemann et al., 2003). We found that polyreactive new emigrant B cells were significantly increased in IRAK-4-, MyD88-, and UNC-93B-deficient patients in which they represented 15.6%–56.1% of the clones, whereas polyreactive new emigrant B cells represented only 5.0%–8.3% in healthy controls (Figures 2A and 2B). Using ELISA against human epithelial HEp-2 cell lysate, defects in central tolerance were further demonstrated in IRAK-4- and MyD88-deficient patients by the high frequency of HEp-2 reactive clones (Figure 2C and Figure S2). However, the frequency of HEp-2 reactive new emigrant B cells from UNC-93B-deficient patients was only slightly increased compared to controls and differences failed to reach statistical significance (Figure 2C and Figure S2). Thus, the removal of developing autoreactive B cells is severely defective in the absence of IRAK-4 and MyD88 expression, whereas the absence of UNC-93B partially affects the central B cell tolerance checkpoint.
Figure 2. Defective central B cell tolerance checkpoint in IRAK-4-, MyD88- and UNC-93B-deficient patients.
(A) Antibodies from new emigrant B cells from controls and patients were tested by ELISA for reactivity against single-stranded DNA (ssDNA), double-stranded DNA (dsDNA), insulin and lipopolysaccharide (LPS). Antibodies are considered polyreactive when they recognize at least 2 and usually all 4 antigens. Dotted lines show ED38-positive control (Wardemann et al., 2003). Horizontal lines show cut-off OD405 for positive reactivity. For each individual, the frequency of polyreactive and non polyreactive clones is summarized in pie charts with the number of antibodies tested indicated in the centers. The frequencies of polyreactive (B) and HEp-2-reactive (C) new emigrant B cells are compared between controls and patients, and statistically significant differences are indicated. Each diamond represents an individual, and the average is shown with a bar.
IRAK-4- and MyD88-dependent counterselection of ANA B cells does not require UNC-93B
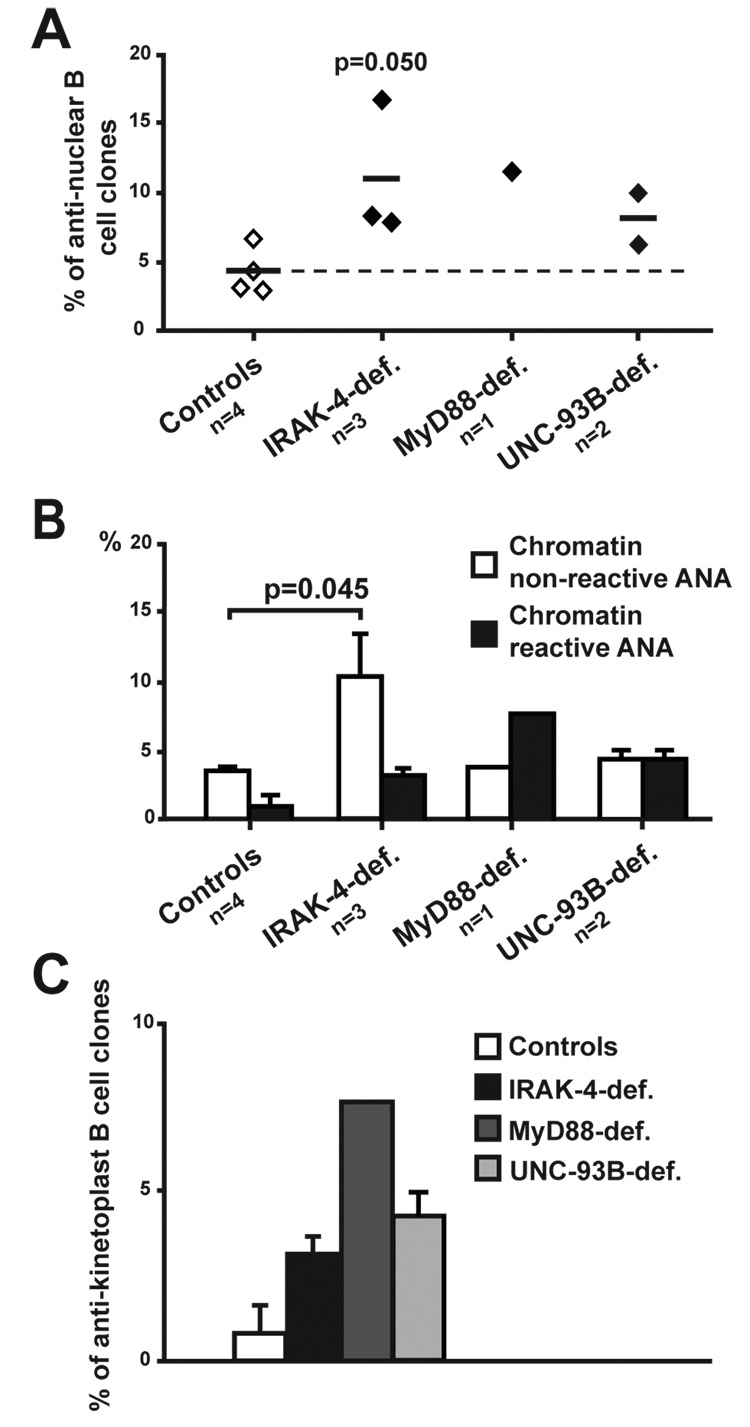
Central B cell tolerance is also responsible for the removal of developing B cells expressing ANAs as illustrated by the very low frequency of new emigrant B cells with such reactivity in healthy controls (Figure 3A) (Wardemann et al., 2003). In contrast, IRAK-4- and MyD88-deficient new emigrant B cells showed a high frequency of ANA clones that represented 34.1%, 18.5% and 15.4% in the 3 IRAK-4-deficient patients and 7.9% in the MyD88-deficient patient (Figure 3A). ANAs expressed by IRAK-4- and MyD88-deficient B cells could be divided into those that reacted or not with the condensed chromatin material in mitotic cells (Figures 3B and 3C). Chromatin non-reactive ANAs accounted for 10.9% and 5.3% of new emigrant B cells of IRAK-4- and MyD88-deficient patients, respectively, while chromatin reactive ANAs represented 10.7% and 2.6% of the clones (Figure 3B). Autoreactive antibodies expressed by IRAK-4- and MyD88-deficient B cells showed a large diversity of speckled, nucleolar, or homogeneous anti-nuclear staining patterns (Figure 3C). Paradoxically, UNC-93B-deficient patients were able to properly counterselect ANA-expressing developing B cells, thereby suggesting that ANA B cell removal did not seem to require UNC-93B (Figures 3A and 3B).
Figure 3. ANA expressing B cell removal requires IRAK-4 and MyD88 but not UNC-93B.
(A) The frequency of anti-nuclear new emigrant B cells in IRAK-4 and MyD88-deficient patients is higher than in controls and UNC-93B-deficient patients. Each diamond represents an individual, and the average is shown with a bar.
(B) The frequencies of chromatin non-reactive and reactive clones in IRAK-4- and MyD88-deficient patients are higher than in controls and UNC-93B-deficient patients. Histograms represent means ± s.e.m. of frequencies of chromatin non-reactive (open bars) or chromatin reactive (black bars) antibodies. Statistically significant differences between patients and controls are indicated.
(C) ANAs from IRAK-4- and MyD88-deficient new emigrant B cells show various patterns of anti-nuclear staining. White arrows indicate dividing cells that demonstrate negative (top row) or positive (bottom row) staining for the condensed chromatin material.
(D) Increased anti-kinetoplast antibodies in new emigrant B cells from IRAK-4- (black bars) and MyD88-deficient (dark grey bars) compared to controls (open bars) and UNC-93B-deficient patients (light grey bars). Histograms represent means ± s.e.m. of proportion of kinetoplast reactive clones. Statistically significant differences between patients and controls are indicated.
(E) IRAK-4- and MyD88-deficient clones express ANAs that can recognize the kinetoplast of C. Luciliae. White and red arrows indicate positively stained kinetoplasts and nucleus of C. Luciliae respectively.
(F) Kinetoplast reactive ANA-expressing B cells display the highest frequency of positively charged residues in their heavy chain CDR3s. New emigrant B cells from IRAK-4 and MyD88-deficient patients were grouped into non-autoreactive, autoreactive-non ANA (HEp-2-reactive and/or polyreactive), kinetoplast non-reactive ANA and kinetoplast reactive ANA clones. Pie charts show the proportion of heavy chain CDR3s with 0, 1, 2 and 3 or more positive charges. Absolute numbers of clones analyzed in each B cell fraction is indicated in the center. The average number of positively charged residues per CDR3 is indicated below each fraction. The numbers of positively charged residues in immunoglobulin heavy chain CDR3s were calculated excluding the arginin or lysin found at position 94 in most germ line VHs.
We further analyzed the reactivity of recombinant antibodies from IRAK-4, MyD88, and UNC-93B-deficient patients and healthy donor controls by indirect immunofluorescence on Crithidia luciliae (Figures 3D and 3E). Antibody recognition of the kinetoplast of Crithidia luciliae, an organelle composed of dsDNA, is the most specific assay to identify anti-native dsDNA and is routinely used for the detection of these autoantibodies in SLE patients. Using this assay, we found that new emigrant B cells from controls and UNC-93B-deficient patients contained no anti-dsDNA clones, confirming the proper removal of ANA B cells in these individuals (Figure 3D). In contrast, 2.6%–17.1% of antibodies expressed by IRAK-4- and MyD88-deficient new emigrant B cells bound both the nucleus and the kinetoplast or only the kinetoplast of C. luciliae (Figures 3D and 3E, Tables S1–S14). In addition, we found that kinetoplast non-reactive ANAs and anti-kinetoplast antibodies display immunoglobulin heavy chain (IgH) complementarity determining regions 3 (CDR3) that contained the highest number of positively charged amino acids (aa) such as arginines previously shown to favor anti-DNA autoreactivity (Figure 3F) (Hervé et al., 2007; Radic et al., 1993; Wardemann et al., 2003). Hence, ANA clones including those reacting with the chromatin are enriched in the new emigrant B cell compartment when the MyD88/IRAK-4 signaling pathway is defective independently of UNC-93B.
Altered receptor editing regulation in developing IRAK-4- and MyD88-deficient B cells
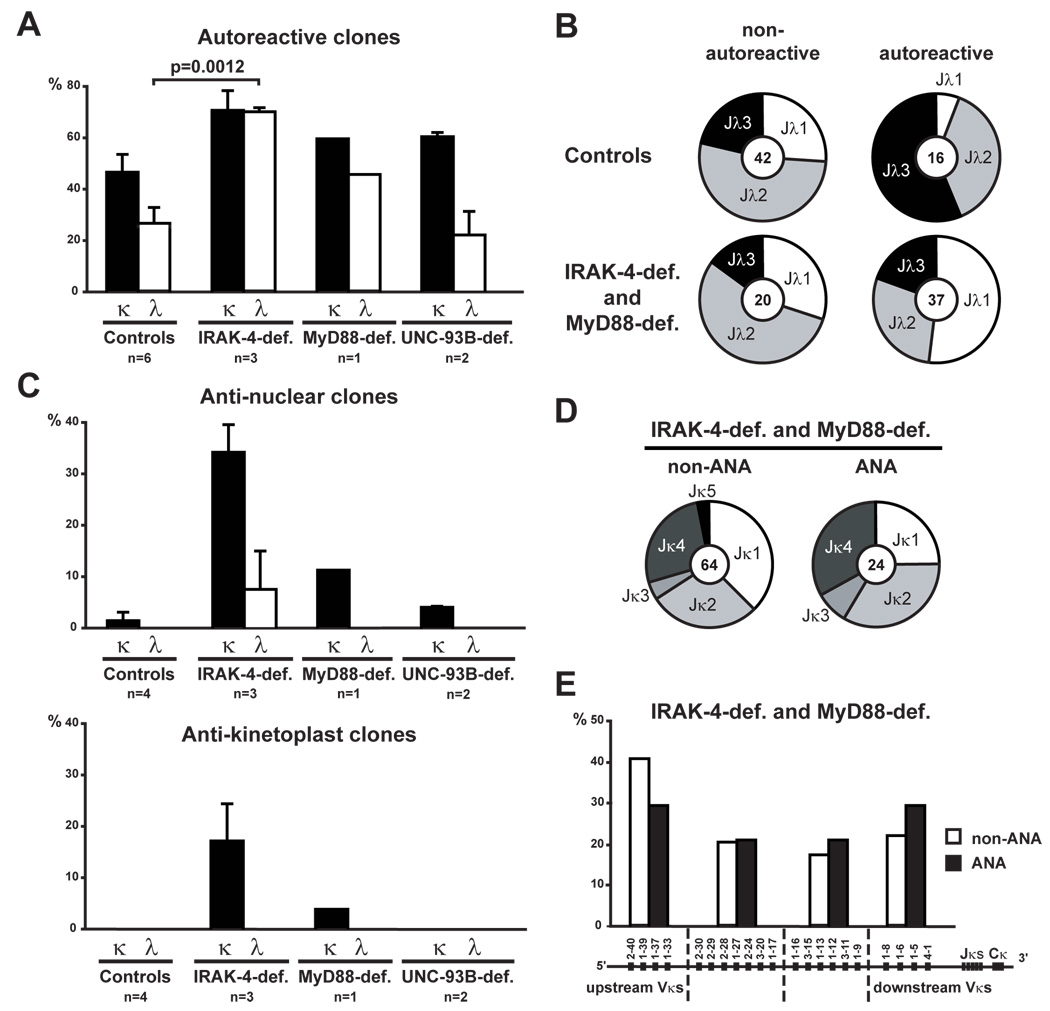
Receptor editing represents a major B cell tolerance mechanism by which developing autoreactive B cells can be silenced, especially those that express ANAs (Radic and Weigert, 1995). Secondary recombination events, first on the kappa locus and then on the lambda locus, provide attempts to edit autoreactive antibodies by substituting light chains until BCR autoreactivity is either abolished or diminished to levels that allow B cell development to proceed (Goodnow, 1996; Nemazee et al., 2000; Radic and Weigert, 1995). As a result, upstream variable (V) gene usage combined with downstream joining (J) segments is a signature for secondary recombination events. In humans, lambda chains are more efficient at silencing autoreactive/polyreactive antibodies than kappa chains as illustrated by the lower frequency of autoreactive clones in new emigrant B cells expressing lambda chains in healthy controls (Figure 4A) (Wardemann et al., 2004). In contrast, lambda chain usage in IRAK-4- and MyD88-deficient B cells did not diminish antibody autoreactivity compared to kappa chain expressing clones, which suggest defects in receptor editing in these patients (Figure 4A). In UNC-93B-deficient patients, lambda chain usage properly decreased autoreactivity suggesting differences with IRAK-4- and MyD88-deficient patients (Figure 4A). When lambda clones were separated into non-autoreactive vs autoreactive, we found that control and IRAK-4- and MyD88-deficient non-autoreactive B cells expressed a similar Jλ gene usage (Figure 4B). In contrast, IRAK-4- and MyD88-deficient autoreactive lambda new emigrant B cells were biased towards Jλ1 gene usage, which is the most upstream of all Jλs, suggesting that there was no attempt at editing these autoreactive antibodies compared to autoreactive lambda clones in controls that preferentially used downstream Jλ3 gene (Figure 4B). Despite the strong bias towards downstream Vλ gene usage in healthy controls, IRAK-4- and MyD88-deficient autoreactive lambda clones showed an increase in downstream Vλ gene usage, whereas there was no bias in Vλ gene usage between control and patient non-autoreactive lambda cells (Figure S3). Thus, downstream Vλ usage combined with highly increased upstream Jλ1 usage in IRAK-4- and MyD88-deficient autoreactive lambda new emigrant B cells reflect a dearth of secondary recombination on this locus when functional IRAK-4/MyD88 complexes could not be expressed.
Figure 4. Defective receptor editing induction in IRAK-4- and MyD88-deficient developing autoreactive B cells.
(A) New emigrant B cells from IRAK-4- and MyD88-deficient patients have an increased proportion of autoreactive lambda clones compared to controls and UNC-93B-deficient patients. Histograms represent means ± s.e.m. of proportion of κ (black bars) and λ (open bars) clones that are autoreactive (polyreactive and HEp-2-reactive combined).
(B) Autoreactive IRAK-4- and MyD88-deficient new emigrant B cells display a lambda repertoire suggesting defective receptor editing. Pie charts show the proportion of the different Jλ genes in non-autoreactive and autoreactive new emigrant B cells from healthy controls (top panel) or IRAK-4- and MyD88-deficient patients (bottom panel), with the number of analyzed sequences indicated in the centers.
(C) Most ANA and all kinetoplast-reactive B cells from IRAK-4 and MyD88-deficient patients express kappa chains. Histograms represent means ± s.e.m. of proportion of κ (black bars) and λ (open bars) clones that are anti-nuclear (top panel) and anti-kinetoplast (bottom panel).
(D) The ANA expressed by IRAK-4- and MyD88-deficient new emigrant B cells do not show any bias towards downstream Jκ3,4,5 usage compared to the non-ANA clones. Pie charts show the proportion of the different Jκ genes, with the number of analyzed sequences indicated in the centers.
(E) The ANA expressed in IRAK-4-and MyD88-deficient new emigrant B cells do not show any bias towards upstream Vκ gene usage compared to the non-ANA clones. The Vκ locus is shown below clustered into 4 clans of Vκ gene segments. Histograms represent the proportion of genes of each Vκ group for ANA (black bars) and non-ANA (open bars) expressed by new emigrant B cells from IRAK-4- and MyD88-deficient patients.
The analysis of ANA expressing new emigrant B cells that escaped central tolerance in IRAK-4- and MyD88-deficient patients revealed that most ANA and all anti-kinetoplast clones utilized kappa chains, demonstrating that editing using lambda chains was not induced in these B cells (Figure 4C). In addition, IRAK-4- and MyD88-deficient new emigrant B cells that express ANAs did not show any evidence of secondary recombination on their kappa locus (Figures 4D and 4E). There was no bias towards downstream Jκ3,4,5 gene usage in IRAK-4- and MyD88-deficient ANA clones nor any bias towards upstream Vκ gene usage in ANA expressing B cells (Figures 4D and 4E). We conclude that kappa and lambda light chain receptor editing is not efficiently induced to thwart autoreactivity and silence autoreactive and ANA expressing B cell precursors in the absence of proper IRAK-4/MyD88 signaling.
Defective peripheral B cell tolerance checkpoint in IRAK-4-, MyD88- and UNC-93B-deficient patients
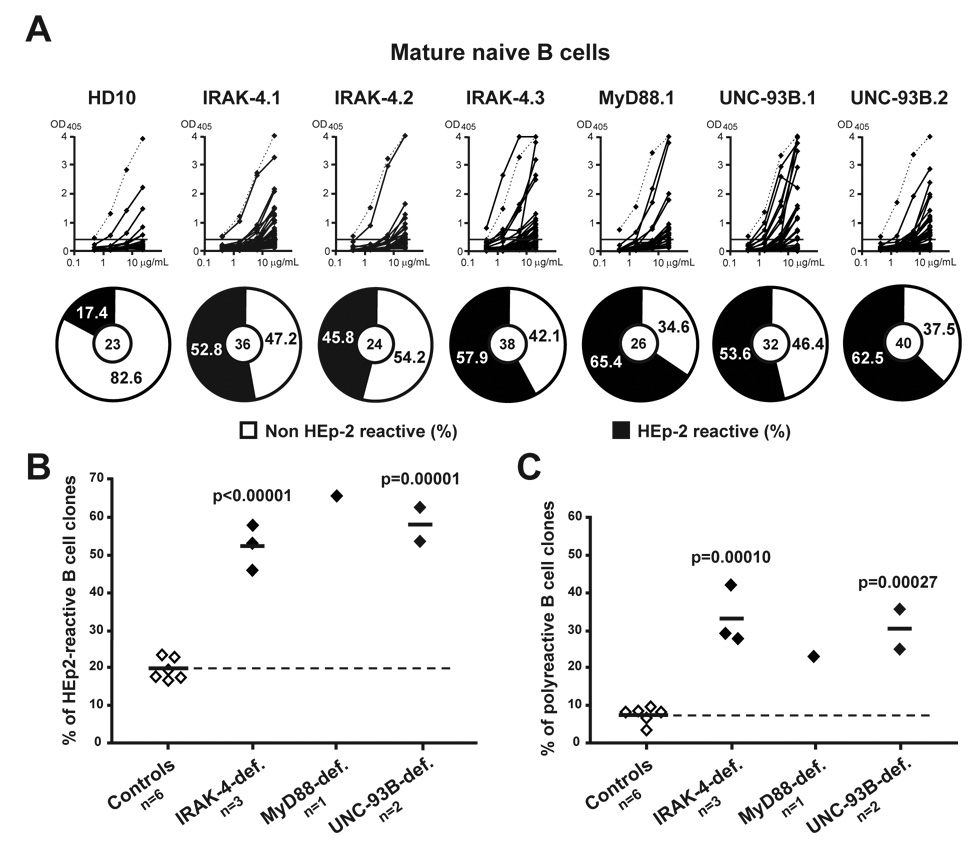
We previously demonstrated that a peripheral B cell tolerance checkpoint further counterselects autoreactive new emigrant B cells before they enter the CD19+CD10− IgM+CD27− mature naïve B cell compartment of healthy donors in which they represent 16.7%–23.3% of the clones (Figures 5A and 5B) (Wardemann et al., 2003). In contrast, we found that the frequency of HEp-2 reactive mature naive B cells was significantly increased and reached 45.8%–65.4% in IRAK-4-, MyD88- and UNC-93B-deficient patients (Figures 5A and 5B). The mature naïve B cell compartment of all patients was also significantly enriched in polyreactive clones compared to healthy controls (Figure 5C and Figure S4). In addition, the proportion of mature naïve B cells expressing ANAs reacting or not with the chromatin was slightly elevated in IRAK-4-, MyD88-, and UNC-93B-deficient patients when compared to healthy donors (Figures 6A and 6B). Correlating with the presence of chromatin-reactive antibodies, anti-kinetoplast B cells were also identified in the mature naïve B cell compartment of IRAK-4, MyD88, and UNC-93B-deficient patients (Figure 6C). We conclude that the peripheral B cell tolerance checkpoint requires IRAK-4, MyD88, and UNC-93B to be functional.
Figure 5. Defective peripheral B cell tolerance checkpoint in IRAK-4-, MyD88- and UNC-93B-deficient patients.
(A) Increased frequency of HEp-2-reactive antibodies in IRAK-4-, MyD88-, and UNC-93B-deficient mature naïve B cells. Antibodies from mature naïve B cells from one control (HD10), three IRAK-4-deficient patients, one MyD88-deficient, and two UNC-93B-deficient patients were tested in ELISA for reactivity with HEp-2 cell lysate. Dotted lines show ED38-positive control (Meffre et al., 2004; Wardemann et al., 2003). Horizontal lines show cut-off OD405 for positive reactivity. For each individual, the frequency of HEp-2-reactive (in black) and non HEp-2-reactive (in white) clones is summarized in pie charts with the number of antibodies tested indicated in the centers. The frequency of (B) HEp-2-reactive and (C) polyreactive (tested against single-stranded DNA, double-stranded DNA, insulin and lipopolysaccharide) clones in mature naive B cells of IRAK-4-, MyD88- and UNC-93B-deficient patients is higher than in controls. Each diamond represents an individual, and the average is shown with a bar. Statistically significant differences between patients and controls are indicated.
Figure 6. Identification of ANA-expressing mature naïve B cells in IRAK-4-, MyD88-, and UNC-93B-deficient patients.
(A) The frequency of anti-nuclear mature naive B cells in IRAK-4, MyD88-, and UNC-93B-deficient patients is higher than in controls. Each diamond represents an individual, and the average is shown with a bar.
(B) Chromatin non-reactive and reactive and (C) kinetoplast reactive clones are enriched in the mature naïve B cell compartment of IRAK-4-, MyD88- and UNC-93B-deficient patients compared to controls. Histograms represent means ± s.e.m. of proportion of (B) chromatin non-reactive (open bars) and chromatin reactive (black bars) clones and (C) kinetoplast reactive clones.
Human autoreactive B cells fail to secrete autoantibodies in the absence of IRAK-4, MyD88 and UNC-93B
Despite the accumulation of autoreactive B cells expressing ANAs and polyreactive antibodies in their blood, IRAK-4-, MyD88- and UNC-93B-deficient patients suffer from bacterial and viral infections, respectively, and not from autoimmune diseases (Casanova and Abel, 2007; Casrouge et al., 2006; von Bernuth et al., 2008). To investigate the role of IRAK-4-, MyD88- and UNC93-B-dependent TLRs on the activation of autoreactive B cells through the secretion of autoreactive antibodies, we tested 9 IRAK-4-, 3 MyD88-, and 3 UNC-93B-deficient patients for the presence of autoantibodies in their serum. We found that these patients displayed levels of ANAs or polyreactive IgG antibodies in their serum that were similar to those found in healthy controls (Figure 7 and Figure S5). Thus, autoreactive B cells in the blood of IRAK-4-, MyD88 and UNC-93B-deficient patients do not secrete ANAs and other polyreactive antibodies when TLR signaling and functions are altered in the absence of IRAK-4, MyD88, or UNC-93B expression.
Figure 7. Lack of serum ANAs in IRAK-4-, MyD88-, and UNC-93B-deficient patients.
The presence of IgG ANAs was assessed by ELISA in the serum of healthy controls, IRAK-4-, MyD88-, and UNC-93B-deficient patients. Each diamond represents an individual. Dashed lines indicate threshold between negative, low positive and positive individuals for serum IgG ANAs.
DISCUSSION
We reported herein that IRAK-4-, MyD88- and UNC-93B-deficient patients showed defects in the establishment of B cell tolerance. Indeed, IRAK-4-, MyD88- and UNC-93B-dependent pathways prevent the accumulation of large numbers of autoreactive B cells in the periphery of healthy donors. Developing autoreactive B cells are normally removed at two different checkpoints, first in the bone marrow and then in periphery. The central B cell tolerance checkpoint seems to be mainly controlled by intrinsic B cell molecules that regulate BCR signaling induced by binding to autoantigens at the immature B cell stage (Goodnow, 1996; Nemazee et al., 2000; Samuels et al., 2005a). Alterations of BCR signaling thresholds such as in B cells from X-linked agammaglobulinemia patients who carry mutations in their BTK gene result in defective central B cell tolerance checkpoint and in the release of autoreactive B cells in the periphery (Ng et al., 2004). In contrast, peripheral B cell tolerance checkpoint seems to rely on extrinsic molecular and cellular regulators, such as B cell-activating factor (BAFF) levels and Treg cells (Hervé et al., 2007). How does the absence of IRAK-4, MyD88 and UNC-93B affect mechanisms that normally mediate these counterselection steps?
We demonstrated that signaling through IRAK-4/MyD88 complexes plays a major role in the establishment of central B cell tolerance in the bone marrow by counterselecting developing autoreactive B cells, including ANA-expressing clones. Since IL1R1/IL18R1/IL1RL1 genes are not expressed in control B cells, defects in central B cell tolerance checkpoint in IRAK-4 and MyD88-deficient patients are likely to result from the inability of TLRs to signal in the absence of functional IRAK-4/MyD88 complexes. TLR7 and TLR9, which bind RNA and DNA containing antigens respectively, were obvious candidates for the removal of ANA-expressing clones. UNC-93B has been reported to be required for these TLRs to reach endolysosomes where they bind ligands and initiate signaling (Kim et al., 2008; Tabeta et al., 2006). In agreement with this observation, we found that B cells from UNC-93B-deficient patients showed impaired responses to TLR7 and TLR9 triggering. The apparent removal of ANA-expressing B cells in UNC-93B-deficient patients seems however to argue against a role of TLR7 and TLR9 in this process. Nevertheless, UNC-93B-deficient patients displayed an increased frequency of polyreactive new emigrant B cells, suggesting that TLR7 and TLR9 play a partial role in the establishment of central B cell tolerance. In addition to TLR7 and TLR9, human B cells express the UNC-93B-independent TLR10, either as homodimers or as heterodimers with TLR1 (Hasan et al., 2005). TLR10 complexes do not have any known ligands and may be responsible for the counterselection of B cells expressing ANAs. Alternatively, other IRAK-4/MyD88-dependent receptors yet to be identified may be responsible for the removal of ANA-expressing B cell clones in the bone marrow. During their development, B cells may encounter nuclear self-antigens in blebs expressed at the surface of bone marrow cells dying by apoptosis, or from the enucleated material from millions of red blood cells (Cocca et al., 2002). The binding of DNA and RNA containing antigens to ANAs and other receptors may therefore generate synergistic signals that may mediate the silencing of these clones. Interestingly, we found that TLR10 and to some extend TLR9 cell surface expression were upregulated following either BCR triggering or BCR and TLR9 cotriggering (Figure S6). Thus, developing autoreactive B cells stimulated by self-antigens express increased cell surface TLR levels that may cosignal with BCRs and induce tolerance mechanisms. In the absence of functional IRAK-4/MyD88 expression, a lack of intracellular signaling may result in altered removal of ANA clones. In agreement with this hypothesis, we found that kappa and lambda light chain receptor editing was not properly induced in IRAK-4- and MyD88-deficient B cell precursors, which resulted in the appearance of a large population of kappa-expressing anti-nuclear B cells. Hence, the TLR/BCR coengagement paradigm may apply not only to B cell activation and proliferation but also to the selection of developing B cells in the bone marrow.
All IRAK-4-, MyD88- and UNC-93B-deficient patients displayed a similar defective peripheral B cell tolerance checkpoint, resulting in the accumulation of large numbers of autoreactive B cells in the blood of these patients. Since TLR3/7/8/9 are the only receptors that depend on UNC-93B to function, and because TLR3 signaling does not require IRAK-4/MyD88 complexes, it is likely that TLR7/8/9 are responsible for the removal of autoreactive B cells in the periphery. Little is known about the cells and molecules that regulate human peripheral B cell tolerance, and how TLR7/8/9 could be involved. In agreement with findings from mouse models, our recent data suggest that elevated BAFF levels may prevent the removal of autoreactive B cells probably by increasing their survival in the periphery (Hervé et al., 2007; Lesley et al., 2004; Mackay et al., 1999; Thien et al., 2004). However, BAFF does not account for the defective peripheral B cell tolerance checkpoint in IRAK-4-, MyD88-, and UNC-93B-deficient patients because they all showed normal BAFF levels in their serum (Figure S7). In addition to BAFF levels, Treg cells may also control the elimination of autoreactive B cells in the periphery (Hervé et al., 2007). Indeed, the decreased numbers of Treg cells in CD40L- and MHC class II-deficient patients who display a specific increase of autoreactive B cells in the mature naïve but not in the new emigrant B cell compartment suggest that these T cells may mediate peripheral B cell tolerance (Hervé et al., 2007). A similar hypothesis was previously proposed using transgenic mouse models, in which CD4+ T cells and the receptor/ligand pairs CD40L/CD40 and Fas/FasL have been shown to play an important role in removing tolerant hen egg lysozyme binding B cells (Rathmell et al., 1995; Rathmell et al., 1996). A direct involvement of TLR7/8/9 on human Treg cell development and functions is difficult to assess because TLR stimulations can both increase and decrease the regulatory properties of these T cells (Conroy et al., 2008). However, the roles of TLR7 and TLR9 on DCs have been well characterized, especially in plasmacytoid DCs (pDCs), which mostly express these TLRs (Kadowaki et al., 2001), Interestingly, pDCs have been reported to play an important role in the development of Treg cells when primed by TLR agonists (Ito et al., 2007; Moseman et al., 2004; Ouabed et al., 2008; Watanabe et al., 2005). We would therefore like to propose that in the absence of TLR7 and TLR9 signaling in IRAK-4, MyD88- and UNC-93B-deficient patients, pDCs may not be able to induce the development of Treg cells specific for a whole set of self-antigens. Moreover, DC-Treg cell interactions may also be affected because IRAK-4-deficient DCs show lower levels of CD40, CD80 and CD86 expression following stimulations by TLR agonist (Ku et al., 2007). Altogether, a failure to properly educate Treg cells may generate an incomplete Treg cell repertoire unable to counterselect some autoreactive B cells in the periphery. In addition, the inability of IRAK-4-, MyD88- and UNC-93B-deficient monocytes and DCs to properly secrete cytokines such as IFNs and TNF-α in response to TLR stimulation may also impact on the peripheral B cell tolerance checkpoint (Ku et al., 2007). In conclusion, altered Treg cell and/or DC functions in IRAK-4-, MyD88- and UNC-93B-deficient patients may lead to the defective peripheral B cell tolerance checkpoint observed in these patients. Despite the large number of peripheral autoreactive B cells in IRAK-4-, MyD88-, and UNC-93B-deficient patients, these individuals suffer from bacterial or viral infections and not from autoimmune diseases. We found that these patients did not display ANAs nor show any increase in polyreactive antibodies in their serum. Thus, the autoreactive/polyreactive B cells in their blood are not activated and do not secrete these antibodies when TLR signaling is altered. In agreement with this observation, many reports using mouse models demonstrated that TLR7 and TLR9 play an essential role in activating autoreactive B cells by transducing costimulatory signals to BCRs that bind DNA and RNA containing immune complexes (Baccala et al., 2007; Marshak-Rothstein, 2006). In addition, mouse models further support a role for MyD88 and TLR7 in the development of autoimmunity (Berland et al., 2006; Christensen et al., 2006; Ehlers et al., 2006; Pisitkun et al., 2006). TLR7 pathway was therefore proposed to represent a therapeutic target in diseases like SLE (Berland et al., 2006; Christensen et al., 2006; Pisitkun et al., 2006). Our data show that IRAK-4-, MyD88- and UNC-93B-deficient patients present frequencies of autoreactive mature naïve B cells similar to those from patients suffering from rheumatoid arthritis and systemic lupus erythematosus (Samuels et al., 2005b; Yurasov et al., 2005). However, in contrast to these patients, IRAK-4-, MyD88- and UNC-93B-deficient patients did not develop autoimmune diseases suggesting that blocking TLR pathways in humans is likely to thwart the development of autoimmunity.
EXPERIMENTAL PROCEDURES
Patients and healthy donor controls
The four IRAK-4-deficient patients belong to four unrelated families. They all suffered from recurrent pyogenic bacterial infections but were healthy when blood samples were obtained. They had no history of related autoimmune disorders. Patient 1 is a 23-yr-old woman who was born from unrelated parents (Kuhns et al., 1997; Medvedev et al., 2003). She suffers from two different mutations on her IRAK-4 gene alleles. The first mutation is a cytidine to thymidine at nucleotide 877, resulting in a stop codon instead of the wild-type glutamine in the IRAK-4 kinase domain (Medvedev et al., 2003). The second mutation is a two-nucleotide deletion at nucleotides 620–621 that results in a frameshift creating a stop codon 36–39 nucleotides downstream also in the kinase domain of IRAK-4 (Medvedev et al., 2003). Patient 2 is a 10-yr-old girl who was born to consanguineous parents (Day et al., 2004; Haraguchi et al., 1998). She displays the same cytidine to thymidine mutation from patient 1 on both of her IRAK-4 gene alleles (Picard et al., 2003). Patient 3 is a 8-yr-old boy who suffers from a homozygous deletion of the non-coding exon 1 of the IRAK-4 gene reported as P15 in (Ku et al., 2007). Patient 4 is a 16 month-old boy. He suffers from two different mutations on his IRAK-4 gene alleles. The first mutation is the same cytidine to thymidine mutation displayed by patients 1 and 2. The second mutation is a deletion in exon 5. The MyD88-deficient patient is a 2-yr-old Turkish girl born from non-consanguineous parents (von Bernuth et al., 2008). This patient displays compound heterozygous mutations in MYD88; L93P in exon 1 that encodes the Death Domain of MyD88 and R196C in exon 3 that is contained in the TIR Domain of the protein (von Bernuth et al., 2008). Both UNC-93B-deficient patients were teenagers born from first cousin parents (Casrouge et al., 2006). UNC-93B-deficient patient 1 is homozygous for a four-nucleotide deletion at position 1034–1037 in exon 8 while patient 2 is homozygous for a single-nucleotide substitution in exon 6 that prevents the splicing of this exon (Casrouge et al., 2006). Healthy donors include a 36 y.o. Caucasian male (HD10) and 5 previously reported controls (Hervé et al., 2007; Ng et al., 2004; Tsuiji et al., 2006; Wardemann et al., 2003). Overall, the ages of healthy controls are ranging from 5 years-old to 37 years-old and match the diverse ages of the patients enrolled in the study (Hervé et al., 2007; Ng et al., 2004; Tsuiji et al., 2006; Wardemann et al., 2003). All samples were collected after patients signed informed consent in accordance with institutional review board-reviewed protocols. Informed consent as approved by the institutional review board was obtained from the custodians of subjects younger than 18, and assent was signed by children and adolescents older than 8 years as well.
Cell sorting
Peripheral B cells were purified from the blood of patients and control donors by negative selection using RosetteSep™ procedure (StemCell Technologies, Vancouver, BC, Canada). Enriched B cells were stained with fluorescein isothiocyanate (FITC) anti-human CD27, phycoerythrin (PE) anti-human CD10, anti-human IgM biotin, and allophycocyanin (APC) anti-human CD19. Biotinylated antibodies were revealed using streptavidin-PECy7. Single CD19+CD10+IgM+CD27− new emigrant and CD19+CD10−IgM+CD27− peripheral mature naïve B cells from patients and control donors were sorted on a FACSVantage (Becton Deckinson) into 96-well PCR plates containing 4 µl of lysis solution (0.5× PBS containing 10 mM DTT, 8 U RNAsin (Promega), and 0.4 U 5’-3’ RNase Inhibitor (Eppendorf)) and immediately frozen on dry ice. All samples were stored at −70°C. B cell subpopulations as well as T cell and DC fractions were batch-sorted using antibodies described above and anti-TCRαβ-FITC, CD25-PE, CD4-PECy7, CD11c-PE and HLA-DR-APC. All antibodies were purchased from Pharmingen, Becton Dickinson.
B cell activation
Naïve B cells were enriched using the Human Naïve B Cell Isolation Kit II (Miltenyi). CD19+CD27− naïve B cells from control donors and patients were plated at 150,000–200,000 cells per well in a 96 well plate in RPMI 10% serum and 20 µg/mL polyclonal F(ab)’2 rabbit anti-human IgM (Jackson Immunoresearch), 1 µg/mL CpG (Invivogen), or 0.5 µM Loxoribine (Invivogen) for 48 hours. Flow cytometry analysis was performed using anti-CD86-FITC, CD25-FITC, CD10-APC (Biolegend), CD80-PE, CD69-PE, CD19-PECy7 (Pharmingen, Beckton Dickinson), TLR10-FITC, and TLR-9-PE (Imgenex).
RNA and RT-PCRs
Total RNA was extracted from 105 purified cells using Absolutely RNA microprep kit (Stratagene). RNA was reverse transcribed in 20 µl with Superscript II (Gibco BRL). For RT-PCR reactions, 2 µl of cDNA was amplified for 32 cycles of 30 sec at 94°C, 30 sec at 60°C (TLR7 or at 63°C (IL1R, TLR9, TLR10, IRAK4, MYD88, UNC93B1, and CD79b) and 30 sec at 72°C with a final 7-min extension at 72°C using Taq DNA polymerase (Roche) and the following primers:
IL1R1 sense 5'CATAGAGGGAACTATACTTGTCATG3';
IL1R 1antisense 5'CATTAGCTGGGCTCACAATCACAG3’;
TLR7 sense 5'TTTGGAAGAAGACTAAAAATGGTG3’;
TLR7 antisense 5'GAACATCCAGAGTGACATCACAG3';
TLR9 sense 5'CCGCCAGACCCTCTGGAGAAG3';
TLR9 antisense 5'ACTTCAGGAACAGCCAGTTGCAG3’;
TLR10 sense 5'CCCAAGAACAACTCAAGAGAAATG3';
TLR10 antisense 5'TGCTTTTGCCAGGGTCAAAGTAG3’
IRAK4 sense 5'CATATGTGCGCTGCCTCAATGTTG3';
IRAK 4antisense 5'CCAGTCAAACAGTAATTCAGAAGTG3’;
MYD88 sense 5'GACGACGTGCTGCTGGAGCTG3’;
MYD88 antisense 5'GATGAAGGCATCGAAACGCTCAG3';
UNC93B1 sense 5'GCGAGGTGAAGTATGGCAACATG3';
UNC93B1 antisense 5'GGCGTAGATGCCCACAGCGAG3’. CD79b primers were previously described (Meffre et al., 1998). RT-PCR products were analyzed on 2% agarose gels.
Microarray gene expression profile analysis
RNA was extracted from 105-3.105 batch sorted new emigrant and mature naïve B cells isolated from donors using the Absolutely RNA microprep kit (Stratagene). 100–200 ng of RNA was obtained per sample, and the quality of the purified RNA was assessed by the Bioanalyzer from Agilent. Using the Ovation biotin system kit from Nugen, 30–50ng of RNA was amplified and labeled to produce cDNA. Labeled cDNA was hybridized on chips containing the whole human genome (Human Genome U133 2.0 from Affymetrix).
cDNA, RT-PCR, antibody production and purification
RNA from single cells was reverse-transcribed in the original 96 well plate in 12.5µl reactions containing 100U of Superscript II RT (Gibco BRL) for 45 min at 37°C. RT-PCR reactions, primer sequences, cloning strategy, expression vectors, antibody expression, and purification were as described (Meffre et al., 2004; Wardemann et al., 2003). Immunoglobulin sequences were analyzed by Ig BLAST comparison with GenBank. Heavy chain CDR3 was defined as the interval between the conserved cysteine at position 92 in the VH framework 3 and the conserved tryptophan at position 103 in JH segments.
ELISAs and IFAs
Antibody concentrations, reactivity against specific antigens, and indirect immunofluorescence were as described (Meffre et al., 2004; Wardemann et al., 2003). Highly polyreactive ED38 was used as positive control in HEp-2-reactivity and polyreactivity ELISAs (Meffre et al., 2004; Wardemann et al., 2003). Antibodies were considered polyreactive when they recognized at least 2 and usually all of the 4 analyzed antigens that include single-stranded DNA (ssDNA), double-stranded DNA (dsDNA), insulin and lipopolysaccharide (LPS). Serum ANA titers of total IgG from patients and healthy controls were determined using INOVA QUANTA Lite ™ ANA ELISA according to the manufacturer’s instructions.
For indirect immunofluorescence assays, HEp-2 cell coated slides (Bion Enterprises, LTD) and Crithidia luciliae coated slides (Antibodies Inc.) were incubated in a moist chamber at room temperature with purified recombinant antibodies at 50–100 µg/mL and 100–200 µg/mL, respectively. FITC-conjugated goat anti-human IgG was used as detection reagent.
Statistics
Differences were analyzed for statistical significance with unpaired Student’s t-tests, using SigmaPlot software (Systat). A P value of less than 0.05 was considered significant.
Supplementary Material
Seven figures and fourteen tables are available.
ACKNOWLEDGMENTS
We thank Dr. G. Charvin for help with microscopy analysis, Dr. N. Tangsinmankong for information on patient 2, Dr. M. C. Nussenzweig, Dr. S. Yurasov, and Dr. M. Tsuiji for sharing recombinant antibodies, Drs. M. Hervé, L. Ménard, and D. Saadoun for helpful discussions, and Drs. L. Abel, M. Tardieu, F. Rozenberg, V. Sancho-Shimizu and L. Lazaro, M. Chrabieh, and C. L. Ku for various support. We are very much indebted to the patients and their families. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06-RR12538-01 from the National Center for Research Resources, NIH. This publication was made possible by Grant Number AI061093 from NIH-NIAID (to E. M.) and a grant from Fondation pour la Recherche Médicale (to I. I.). The authors declare that they have no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- Berland R, Fernandez L, Kari E, Han JH, Lomakin I, Akira S, Wortis HH, Kearney JF, Ucci AA, Imanishi-Kari T. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity. 2006;25:429–440. doi: 10.1016/j.immuni.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–963. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- Brinkmann MM, Spooner E, Hoebe K, Beutler B, Ploegh HL, Kim YM. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J Cell Biol. 2007;177:265–275. doi: 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova JL, Abel L. Primary immunodeficiencies: a field in its infancy. Science. 2007;317:617–619. doi: 10.1126/science.1142963. [DOI] [PubMed] [Google Scholar]
- Casrouge A, Zhang SY, Eidenschenk C, Jouanguy E, Puel A, Yang K, Alcais A, Picard C, Mahfoufi N, Nicolas N, et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314:308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Cocca BA, Cline AM, Radic MZ. Blebs and apoptotic bodies are B cell autoantigens. J Immunol. 2002;169:159–166. doi: 10.4049/jimmunol.169.1.159. [DOI] [PubMed] [Google Scholar]
- Conroy H, Marshall NA, Mills KH. TLR ligand suppression or enhancement of Treg cells? A double-edged sword in immunity to tumours. Oncogene. 2008;27:168–180. doi: 10.1038/sj.onc.1210910. [DOI] [PubMed] [Google Scholar]
- Day N, Tangsinmankong N, Ochs H, Rucker R, Picard C, Casanova JL, Haraguchi S, Good R. Interleukin receptor-associated kinase (IRAK-4) deficiency associated with bacterial infections and failure to sustain antibody responses. J Pediatr. 2004;144:524–526. doi: 10.1016/j.jpeds.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med. 2006;203:553–561. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow CC. Balancing immunity and tolerance: deleting and tuning lymphocyte repertoires. Proc Natl Acad Sci USA. 1996;93:2264–2271. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi S, Day NK, Nelson RPJ, Emmanuel P, Duplantier JE, Christodoulou CS, Good RA. Interleukin 12 deficiency associated with recurrent infections. Proc Natl Acad Sci USA. 1998;95:13125–13129. doi: 10.1073/pnas.95.22.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan U, Chaffois C, Gaillard C, Saulnier V, Merck E, Tancredi S, Guiet C, Briere F, Vlach J, Lebecque S, et al. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J Immunol. 2005;174:2942–2950. doi: 10.4049/jimmunol.174.5.2942. [DOI] [PubMed] [Google Scholar]
- Hervé M, Isnardi I, Ng YS, Bussel JB, Ochs HD, Cunningham-Rundles C, Meffre E. CD40 ligand and MHC class II expression are essential for human peripheral B cell tolerance. J Exp Med. 2007;204:1583–1593. doi: 10.1084/jem.20062287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- Ku CL, von Bernuth H, Picard C, Zhang SY, Chang HH, Yang K, Chrabieh M, Issekutz AC, Cunningham CK, Gallin J, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J Exp Med. 2007;204:2407–2422. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhns DB, Long Priel DA, Gallin JI. Endotoxin and IL-1 hyporesponsiveness in a patient with recurrent bacterial infections. J Immunol. 1997;158:3959–3964. [PubMed] [Google Scholar]
- Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu H-B, Cyster JG. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1690–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev AE, Lentschat A, Kuhns DB, Blanco JCG, Salkowski C, Zhang S, Arditi M, Gallin JI, Vogel SN. Distinct mutations in IRAK-4 confer hyporesponsiveness to lipopolysaccharide and interleukin-1 in a patient with recurrent bacterial infections. J Exp Med. 2003;198:521–531. doi: 10.1084/jem.20030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffre E, Papavasiliou F, Cohen P, de Bouteiller O, Bell D, Karasuyama H, Schiff C, Banchereau J, Liu YJ, Nussenzweig MC. Antigen receptor engagement turns off the V(D)J recombination machinery in human tonsil B cells. J Exp Med. 1998;188:765–772. doi: 10.1084/jem.188.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffre E, Schaefer A, Wardemann H, Wilson P, Davis E, Nussenzweig MC. Surrogate Light Chain Expressing Human Peripheral B Cells Produce Self-Reactive Antibodies. J Exp Med. 2004;199:145–150. doi: 10.1084/jem.20031550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, Blazar BR, Chen W. Human plasmacytoid dendritic cells activateed by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- Nemazee D, Kouskoff V, Hertz M, Lang J, Melamed D, Pape K, Retter M. B-cell-receptor-dependent positive and negative selection in immature B cells. Curr Top Microbiol Immunol. 2000;245:57–71. doi: 10.1007/978-3-642-59641-4_3. [DOI] [PubMed] [Google Scholar]
- Ng Y-S, Wardemann H, Chelnis J, Cunningham-Rundles C, Meffre E. Bruton’s tyrosine kinase (Btk) is essential for human B cell tolerance. J Exp Med. 2004;200:927–934. doi: 10.1084/jem.20040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouabed A, Hubert FX, Chabannes D, Gautreau L, Heslan M, Josien R. Differential control of T regulatory cell proliferation and suppressive activity by mature plasmacytoid versus conventional spleen dendritic cells. J Immunol. 2008;180:5862–5870. doi: 10.4049/jimmunol.180.9.5862. [DOI] [PubMed] [Google Scholar]
- Picard C, Puel A, Bonnet M, Ku C-L, Bustamante J, Yang K, Soudais C, Dupuis S, Feinberg J, Fieschi C, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- Radic MZ, Mackle J, Erikson J, Mol C, Anderson WF, Weigert M. Residues that mediate DNA binding of autoimmune antibodies. J Immunol. 1993;150:4966–4977. [PubMed] [Google Scholar]
- Radic MZ, Weigert M. Origins of anti-DNA antibodies and their implications for B-cell tolerance. Ann N Y Acad Sci. 1995;764:384–396. doi: 10.1111/j.1749-6632.1995.tb55853.x. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Cooke MP, Ho WY, Grein J, Townsend SE, Davis MM, Goodnow CC. CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature. 1995;376:181–184. doi: 10.1038/376181a0. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Townsend SE, Xu JC, Flavell RA, Goodnow CC. Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95)-ligands modulated by the B cell antigen receptor. Cell. 1996;87:319–329. doi: 10.1016/s0092-8674(00)81349-5. [DOI] [PubMed] [Google Scholar]
- Samuels J, Ng Y-S, Coupillaud C, Paget D, Meffre E. Human B cell tolerance and its failure in rheumatoid arthritis. Ann N Y Acad Sci. 2005a;1062:116–126. doi: 10.1196/annals.1358.014. [DOI] [PubMed] [Google Scholar]
- Samuels J, Ng Y-S, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp Med. 2005b;201:1659–1667. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Suzuki S, Duncan GS, Millar DG, Wada T, Mirtsos C, Takada H, Wakeham A, Itie A, Li S, et al. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 2002;416:750–754. doi: 10.1038/nature736. [DOI] [PubMed] [Google Scholar]
- Tabeta K, Hoebe K, Janssen EM, Du X, Georgel P, Crozat K, Mudd S, Mann N, Sovath S, Goode J, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Tsuiji M, Yurasov S, Velinzon K, Thomas S, Nussenzweig MC, Wardemann H. A checkpoint for autoreactivity in human IgM+ memory B cell development. J Exp Med. 2006;203:393–400. doi: 10.1084/jem.20052033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Ben Mustapha I, Camcioglu Y, Vasconcelos J, et al. Human MyD88 deficiency. Science. 2008 In press. [Google Scholar]
- Wardemann H, Hammersen J, Nussenzweig MC. Human autoantibody silencing by immunoglobulin light chains. J Exp Med. 2004;200:191–199. doi: 10.1084/jem.20040818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Wang YH, Lee HK, Ito T, Cao W, Liu YJ. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, et al. TLR3 Deficiency in Patients with Herpes Simplex Encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Seven figures and fourteen tables are available.