Abstract
Hypoxia inducible factor 1 (HIF-1) has been suggested to play a critical role in the fate of cells exposed to hypoxic stress. However, the mechanism of HIF-1-regulated cell survival is still not fully understood in ischemic conditions. Redox status is critical for decisions of cell survival, death and differentiation. We investigated the effects of inhibiting HIF-1 on cellular redox status in SH-SY5Y cells exposed to hypoxia or oxygen and glucose deprivation (OGD), coupled with cell death analyses. Our results demonstrated that inhibiting HIF-1α expression by HIF-1α specific siRNA transfection increased reactive oxygen species generation, and transformed the cells to more oxidizing environments (low GSH/GSSG ratio, low NADPH level) under either hypoxic or OGD exposure. Cell death increased dramatically in the siRNA transfected cells, compared to non-transfected cells after hypoxic/OGD exposures. In contrast, increasing HIF-1α expression by desferrioxamine, a metal chelator and hydroxylase inhibitor, induced a more reducing environment (high GSH/GSSG ratio, high NADPH level) and reduced cell death. Further studies showed that HIF-1 regulated not only glucose transporter-1 expression, but also the key enzymes of the pentose phosphate pathway such as glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase. These enzymes are important in maintaining cellular redox homeostasis by generating NADPH, the primary reducing agent in cells. Moreover, catalase significantly decreased cell death in the siRNA-transfected cells induced by hypoxia and OGD. These results suggest that maintenance of cellular redox status by HIF-1 protects cells from hypoxia and ischemia mediated injuries.
Keywords: HIF-1, cell death, redox status, PPP pathway, hypoxia, ischemia
Introduction
Hypoxia inducible factor 1 (HIF-1) has been identified as a master regulator in cellular responses to hypoxia. HIF-1 expression promotes cellular adaptation and cell survival in hypoxic conditions. It has been shown to potentially provide protection under ischemic conditions (Baranova et al. 2007, Siddiq et al. 2005, Siddiq et al. 2007, Hamrick et al. 2005, Bergeron et al. 1999, Zhou et al. 2008). A number of mechanisms have been proposed to account for this cytoprotective effect of HIF-1: 1) Expression of its downstream gene product erythropoietin (EPO) has been found to protect cells from hypoxic/ischemic injury (Ehrenreich et al. 2002, Bernaudin et al. 1999, Sakanaka et al. 1998, Siren et al. 2001, Zaman et al. 1999); 2) Vascular endothelial growth factor (VEGF) expression, another downstream gene of HIF-1, counteracts detrimental ischemic injuries (Marti et al. 2000, Jin et al. 2000); 3) HIF-1 may prevent apoptotic cell death through inhibition of cytochrome c release, caspase activation, and PARP cleavage (Piret et al. 2004, Sasabe et al. 2005); and 4) HIF-1 may also have the ability to suppress p53 activation and thereby maintains cell survival (Li et al. 2004).
In addition to the mechanisms discussed above, increased glucose transport and glycolytic flow as a result of HIF-1 activation by hypoxia has been linked to tissue viability and cell survival (Bergeron et al. 1999, Zaman et al. 1999, Freret et al. 2006, Lawrence et al. 1996, Prass et al. 2002, Hamrick et al. 2005). One important function of glucose metabolism is to sustain a cellular reducing environment by generating reducing agents through oxidative phosphorylation, glycolysis, and the pentose phosphate pathway (PPP). The PPP (also called the hexose-monophosphate shunt) is a main cellular source of NADPH, which is the major reducing compound in cells and essential in glutathione (GSH) regeneration from glutathione disulfide (GSSG) by glutathione reductase (Almeida et al. 2002, Kletzien et al. 1994, Delgado-Esteban et al. 2000). In some cancer cells, it has been reported that HIF-1 upregulates glucose-6-phosphate dehydrogenase (G6PD), a key enzyme involved in PPP (Bergeron et al. 1999, Semenza et al. 1994, Ebert et al. 1995). These previous results imply that HIF-1 may be critical in maintaining cellular redox status under low oxygen conditions. However, there is no direct study on the effect of HIF-1 on cellular redox status in the literature.
The cellular redox environment is primarily a consequence of the balance between the levels of oxidants such as reactive oxygen species (ROS) and endogenous reducing agents. It plays a critical role in cellular functions including proliferation and differentiation, as well as processes such as apoptosis (Kemp et al. 2008). For example, salsolinol and malonate cause cell death in SH-SY5Y cells by inducing an oxidizing environment (Wanpen et al. 2004, Fernandez-Gomez et al. 2005). Selective depletion of mitochondrial GSH results in an accelerated and enhanced ROS generation and subsequent cell death after hypoxic exposure (Lluis et al. 2007). In contrast, a reducing environment prevents programmed cell death in neurons (Vincent et al. 2002). Interestingly, cellular redox status seems to regulate the factors proposed for HIF-1’s cytoprotective effects such as EPO (Neumcke et al. 1999, Rondon et al. 1995), VEGF (Welsh et al. 2003), cytochrome c release (Kirkland & Franklin 2001), caspase (Kirkland & Franklin 2001), and p53 (Wu & Momand 1998).
We hypothesized that HIF-1 would play an important role in maintaining cellular redox status and affect cell survival through regulating cellular redox status in hypoxic/ischemic exposures. This hypothesis was tested by using SH-SY5Y cells transfected with HIF-1α specific small interfering RNA (siRNA), coupled with analyses of cell death and cellular redox status. Depletion of HIF-1α was found to induce a significantly more oxidizing environment, higher ROS levels, and higher cell death rates after hypoxic and OGD exposures. Increasing HIF-1α by inhibiting hydroxylase activity resulted in a significantly more reducing environment, indicated by higher GSH/GSSG ratio and higher NADPH levels. Our findings suggest that HIF-1 may play a critical role in regulating cellular redox status, which in turn affects cell death/survival under hypoxic/ischemic conditions.
Materials and Methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM), DMEM without glucose, desferrioxamine (DFO), 2′,7′-dichlorofluorescin diacetate (DCFH-DA), trypsin, penicillin, streptomycin, bovine serum albumin, catalase, Hoechst 33258, and protease inhibitor cocktail were purchased from Sigma (St. Louis, MO). Newborn calf serum was obtained from Hyclone (Logan, UT). HIF-1α siRNA and negative control siRNA labeled with Alexa Fluro 488 were from Qiagen (Valencia, CA). Mn(III)-tetrakis(1-methyl-4-pyridyl)porphyrin pentachloride (MnTMPyP) was obtained from A. G. Scientific Inc. (San Diego, CA).
Cell culture and siRNA transfection
The human SH-SY5Y neuroblastoma cell line (CRL-2266; American Type Culture Collection, Manassas, VA) was cultured in DMEM (25 mM glucose, supplemented with 10% (v/v) heat- inactivated new-born calf serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin) in a 5% CO2 humidified incubator at 37 °C according to a previously described protocol (Guo et al. 2005). Medium was changed every 2 3 days. The morphology and physiological functions of SH-SY5Y cells are very similar to normal neurons, possessing metuliform and obvious axons. The concentration of 25 mM was selected because it has widely been used in neuronal cultures and provides the highest rate of neuronal survival in culture (Brewer 1995). The concentration of 25 mM is considered a basal level of glucose for SH-SY5Y cells and higher concentrations than 25 mM are being used for high glucose treatments to SH-SY5Y cells (Leinninger et al. 2004).
The sense, antisense and target DNA sequences of the human HIF-1α siRNA and negative control siRNA were as follows: HIF-1α, 5′-r(CCAUAUAGAGAUACUCAAA)dTdT-3′ (sense), 5′-r(UUUGAGUAUCUCUAUAUGG)dTdG-3′ (antisense), and 5′-CACCATATAGAGATACTCAAA- 3′ (target); negative control, 5′-UUCUCCGAACGUGUCACGUdTdT-3′ (sense), 5′-ACGUGA-CACGUUCGGAGAAdTdT-3′ (antisense), and 5′-AATTCTCCGAACGTGTCACGT-3′ (target). The siRNA was transfected into the SH-SY5Y cells using the HiPerFect Transfection Reagent (Qiagen) according to the manufacturer’s protocol. The siRNA was diluted in 100 μl culture medium without serum to a final concentration of 5 nM. HiPerFect Transfection Reagent was added to the diluted siRNA to form the transfection complexes. After transfection complexes were added, the cells were incubated under normal growth conditions. After 24 hr transfection, cells transfected with HIF-1α siRNA were incubated with Hoechst 33258 (final concentration, 2μg/ml in PBS) for 30 min at 37 °C, and washed three times in PBS. Cells were observed and photographed using an inverted Nikon TE-300 microscope with an epifluorescent attachment equipped with a Princeton Instrument Micromax camera. Images were acquired with Image Pro-plus software (Media Cybernetics, Silver Spring, MD). Alexa Fluro 488 was excited at 488 nm and observed at 520 nm. Hoechst 33258 was excited at 352 nm and observed at 461 nm. The transfection rates were the percentage of the number of siRNA positive cells to the total number of cells. To detect the dosage effect of HIF-1α siRNA, different concentrations of HIF-1α siRNA (0, 0.1, 1.0, 2.5, 5.0 nm) were transfected into cells. The transfection rate, cell death, HIF-1α expression, ROS level, and GSH/GSSG ratio were measured.
Hypoxic and OGD treatments
After cells were washed with pre-warmed PBS, the cells were treated with experimental media. For hypoxic exposures, the experimental media was DMEM. For oxygen and glucose deprivation (OGD) treatments, the experimental media were DMEM without glucose. All media were previously gassed with 95%N2/5%CO2 for 15 min. Cells were incubated in a polymer hypoxic glove box (Coy Laboratory Products Inc. Grass Lake, MI) with 1% oxygen for 3 hrs at 37°C. For control experiments, cells were cultured in DMEM in a normoxic (21% O2) environment at 37 °C. To increase HIF-1α levels, cells were treated with DFO (100μM) for 15 hrs. To test the roles of ROS on cellular injury induced by hypoxic and OGD treatments, specific antioxidants were studied: SOD mimic MnTMPyP for scavenging superoxide anion radical and catalase for degrading hydrogen peroxide. In these antioxidant experiments, cells were treated with the antioxidants 30 min before the onset of hypoxic or OGD exposures.
Western blot analysis
There was significant cell death of the siRNA transfected cells under OGD exposures, which reduced the number of cells we could collect significantly and made the measurements of of the protein levels of GLUT1, G6PD, and PGD very difficult under these conditions. Thus, the protein expressions were measured only under hypoxic conditions. Cells were lysed in RIPA buffer (150 mM NaCl, 100 mM Tris, pH8.0, 1% (v/v) Triton X-100, 0.1% (w/v) SDS, 5 mM EDTA, 10 mM NaF, 2 mM DTT, 0.8 mM PMSF, 1 mM Na3VO4, 1% proteinase inhibitor cocktail). Total proteins were loaded on SDS-PAGE, transferred to nitrocellulose membrane, blocked, and incubated with a primary antibody at 4 °C overnight (HIF-1α, 1:500, Novus; G6PD, 1:2000, Novus; GLUT1, 1:1000, Abcam; PGD, 1:500, Abcam; β-actin, 1:2000, Sigma). The membrane was then incubated with a secondary antibody (1:2000, Santa Cruz, Santa Cruz, CA) for 1 hr at room temperature. The signal development was carried out with an enhanced chemiluminescence (ECL) detection kit (Pierce, Rockford, IL). The intensity of immunoreactive bands was quantified using Image-J. Results were normalized to β actin levels.
Total HIF-1α assay by ELISA
The quantity of HIF-1α was measured by Duoset-enzyme-linked immunosorbent assay (DuoSet IC ELISA) following the manufacturer’s protocol (R&D Systems, Minneapolis, MN). Briefly, 96-well plates were coated with anti-human HIF-1α antibody overnight, and blocked with 200 μl PBS, 0.05% (v/v) Tween 20, and 5% (w/v) BSA for 2 hrs. Cells were lysed in lysis buffer (50 mM Tris-Cl, pH7.4, 300 mM NaCl, 10% (v/v) glycerol, 3 mM EDTA, 1 mM MgCl2, 20 mM β glycerophosphate, 25 mM NaF, 1% (v/v) Triton X-100, 25 μg/ml Leupeptin, 25 μg/ml Pepstatin, and 3 μg/ml Aprotinin). The lysates (100 μl) were added to the plates and incubated for 2 hrs. The samples were incubated with streptavidin-horseradish peroxidase for 20 min after which they were incubated with biotinylated anti-HIF-1α antibody for another 2 hrs. The reaction was started by adding 100 μl substrate solution (H2O2 and tetramethylbenzidine) for 30 min in dark, and stopped by adding 1 M H2SO4. The optical density of each well was then detected by a Model 680 Microplate Reader (Bio-Rad Laboratories Inc.) at 450 nm. All the steps were performed at room temperature (24 °C).
Active HIF-1α activity assay by ELISA
The activity of HIF-1α was measured by Duoset human active HIF-1α activity assay (DuoSet IC ELISA) following the manufacturer’s protocol (R&D Systems, Minneapolis, MN). Briefly, 96-well plates were coated with active HIF-1α capture antibody overnight, and blocked with 200 μl PBS, 0.05% Tween 20, 5% BSA for 2 hrs. Cells were lysed in lysis buffer A (10 mM HEPES, pH7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 0.1% NP-40, 2 mM Na3VO4, 5 mM NaF, 3 μg/ml Aprotinin, 25 μg/ml Leupeptin, 25 μg/ml Pepstatin, 25 μg/ml Chymostatin, and 0.2 mM PMSF). After centrifuge (16,000 g for 10 min 4 °C), the nuclear pellet was lysed in lysis buffer B (20 mM HEPES, pH7.9, 1.5 mM MgCl2, 420 mM NaCl, 0.5 mM DTT, 25% (v/v) glycerol, 2 mM Na3VO4, 5 mM NaF, 3 μg/ml Aprotinin, 25 μg/ml Leupeptin, 25 μg/ml Pepstatin, 25 μg/ml Chymostatin, and 0.2 mM PMSF). The lysates (50 μg) were incubated with 3 μl of biotin-labeled ds-oligonucleotide for 30 min. Then, the samples were mixed with 200 μl Reagent Diluent, and added to the plates and incubated for 2 hrs. The samples were incubated with streptavidin-horseradish peroxidase for 20 min. The reaction was started by adding 100 μl substrate solution (H2O2 and tetramethylbenzidine) for 30 min in dark, and stopped by adding 1 M H2SO4. The optical density of each well was detected by a Model 680 Microplate Reader (Bio-Rad Laboratories Inc.) at 540 nm. All the steps were performed at room temperature (24 °C).
Measurement of cell death by LDH (lactate dehydrogenase) release
The release of LDH was used to measure cytotoxicity in cells exposed to the different experimental conditions. Briefly, the cell-free culture medium (100 μl) was collected and incubated with 100 μl of the reaction mixture from the Cytotoxicity Detection Kit (Takara Bio Inc., Shiga, Japan) for 30 min at room temperature (24 °C). The optical density of the solution was measured at 490 nm (655 nm as reference) on a Bio-Rad 3350 microplate reader. Cell lysates with 1% (v/v) Triton X-100 was used as 100% cell death. Six sets of experiments were carried out, if not otherwise noted. In addition, cells were observed and photographed under an inverted Nikon TE-300 microscope after hypoxic or OGD treatments.
Measurement of intracellular ROS
ROS levels were monitored using the cell-permeable probe dichlorofluorescin diacetate (DCFH-DA) (Huang et al. 2004). Cells were incubated with 100 μM DCFH-DA (dissolved in DMSO) for 30 min at 37 °C. After the incubation, cells were washed three times with PBS and the relative levels of fluorescence were quantified with a fluoro-microplate reader (Ex: 485 nm and Em: 535 nm). The measured fluorescence values were expressed as a percentage of the fluorescence in control cells. In addition, DCF levels were also observed and photographed under the inverted Nikon TE-300 microscope with an epifluorescent attachment equipped with a Princeton Instrument Micromax camera (Ex: 485 nm, Em: 535 nm).
Measurement of GSH/GSSG ratio
A widely used indicator of cellular redox environment (status), the ratio of glutathione (GSH) to glutathione disulfide (GSSG), was used to determine the cellular redox status. The ratio was measured with a kit from Cayman (Cat# 703002, Ann Arbor, MI), which used a spectrophotometric recyclingassay to measure cellular levels of GSH and GSSG. Briefly, following treatments, cells were scrape-harvested in cold PBS on ice and centrifuged. The cell pellets were frozen at −80 °C. Cells were thawed and homogenized in cold MES buffer (0.2M 2-(N-morpholino) ethanesulphonic acid, 50mM phosphate, 1mM EDTA, pH 6.0), and centrifuged at 10,000g for 15 min at 4 °C. The supernatants were removed for assay according to the manufacturer’s instruction. All the determinations were normalizedto protein contents. Total protein concentrations were measured according to the method of Lowry et al (Lowry et al. 1951). The absorbance was recorded at 405 nm using a plate reader at 5 min intervals for 30 min.
Measurement of cellular NADPH
The total cellular NADPH content was determined using an assay kit (BioAssay Systems, Hayward, CA) according to the manufacturer’s instruction. The cells were homogenized in NADPH extraction buffer. After centrifugation (13,000g for 5 min), the supernatants were used for the assay. The working reagent was freshly prepared by mixing 50 μl assay buffer, 1 μl enzyme, 10 μl glucose, 14 μl PMS and 14 μl MTT for one sample, and 80 μl of the mixture was added per well. The OD value was read at 595nm on a Bio-Rad 3350 microplate reader, and ΔOD values between time 0 and 30 min were used to determine the sample NADPH concentration from the standard curve. Results were normalized to the protein concentration, and presented as percentages of normal control cells.
Statistical analysis
One-way ANOVA was used to determine overall significance followed by post hoc Tukey’s tests corrected for multiple comparisons. Data were presented as mean ± SEM, unless otherwise noted. Difference was considered significant at p<0.05.
Results
siRNA-mediated down-regulation of HIF-1α expression and activity in SH-SY5Y cells
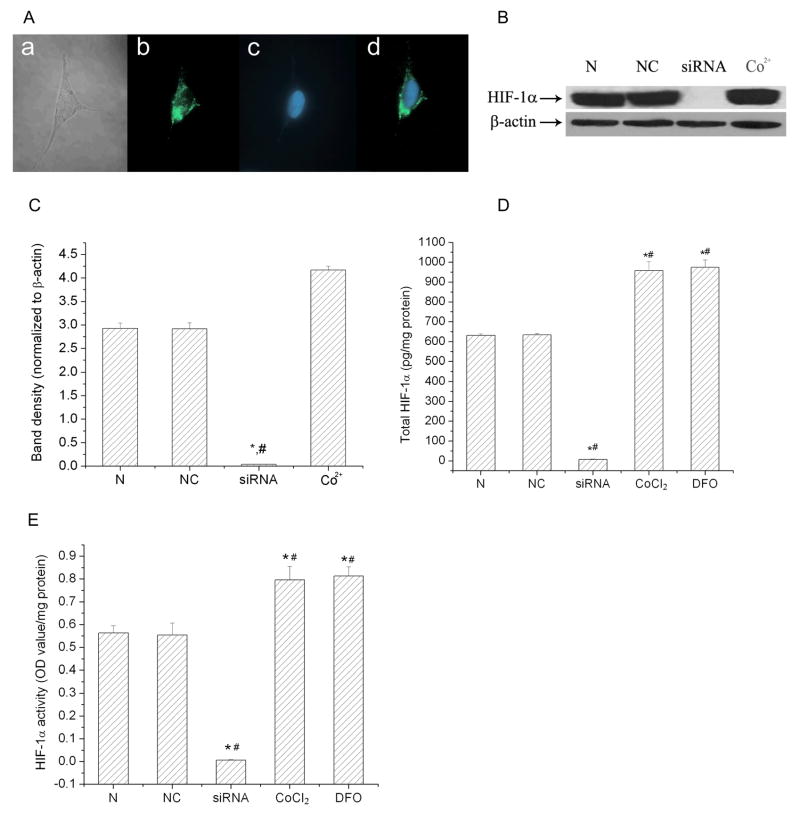
HIF-1 is composed of HIF-1α and HIF-1β protein subunits. HIF-1β is constitutively expressed and relatively stable. The HIF-1α level is dependent on oxygen levels and primarily determines HIF-1 activation. To selectively “knockdown” the expression of HIF-1α, we transfected SH-SY5Y cells with siRNA targeted to HIF-1α. The siRNA was tagged with Alexa Fluor 488 on the 3′ end of the sense strand in order to confirm the transfection. Fig. 1A shows that the HIF-1α siRNAs were successfully transfected into cells and that the majority of the siRNA was in the cytosol. The transfection rate was 90.5 ± 0.8% determined by the number of cells with the Alexa Fluor 488 fluorescence. We further determined the ability of this HIF-1α specific siRNA to knock down HIF-1α expression in the cells. The HIF-1α-siRNA- and negative control-transfected cells were exposed to a 3 hr hypoxic exposure (1% O2). The HIF-1α siRNA transfection efficiently blocked the protein expression and activity of HIF-1α as measured by both immunoblot and ELISA assays (Fig. 1B–E). Transfection with a negative control siRNA did not affect the expression of HIF-1α. The HIF-1α protein level in the cells transfected with the HIF-1α specific siRNA was less than 2% of that in negative control cells. As a positive control, cobalt and DFO increased HIF-1α protein expression and activity.
Fig. 1.
HIF-1α specific siRNA down-regulated HIF-1α expression in SH-SY5Y cells. A) The transfection of HIF-1α specific siRNA into SH-SY5Y cells. (a) A SH-SY5Y cell transfected with HIF-1α siRNA under light microscope. (b) SH-SY5Y cell transfected with HIF-1α siRNA under a fluorescence microscopy. The siRNA was labeled with Alexa Fluor 488 (green) on the 3′ end of the sense strand. (c) SH-SY5Y cell nuclear strained with Hoechst 33258 (blue). (d) Merged image of (b) and (c). Images were taken with a 40 objective. Images shown are representative of at least 10 fields of view. B) A representative of immunoblot of HIF-1α. C) Average of densitometric analyses of HIF-1α expression normalized to β-actin levels. D) The total HIF-1α level in SH-SY5Y cells detected by HIF-1α-Duoset-ELISA. E) The activity of HIF-1α in SH-SY5Y cells detected by HIF-1α activity-Duoset-ELISA. In B, C, D and E, cells were exposed to hypoxia (1%) for 3 hrs. N: normal cells; NC: cells transfected with negative control siRNA; siRNA: cells transfected with HIF-1α specific siRNA (5 nM). CoCl2 and DFO: cells treated with cobalt or DFO as positive HIF-1α control. Data are mean ± SEM, n=3. *p<0.01 v.s. normal cells; #p<0.01 v.s. negative control cells.
Inhibiting HIF-1α expression by siRNA transfection exaggerated cell death induced by hypoxic and OGD exposures
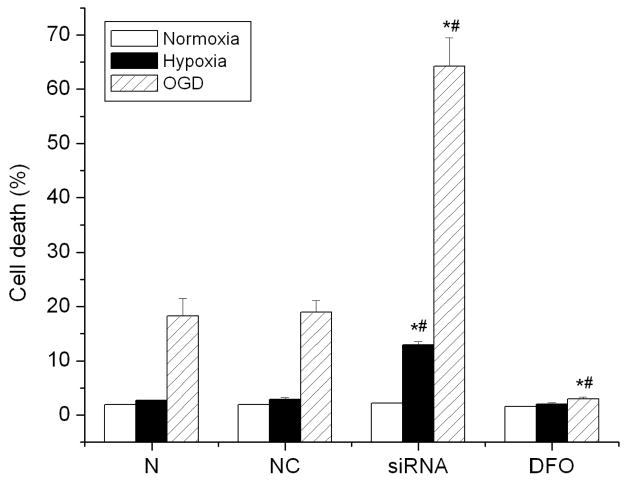
To determine the role of HIF-1 in cell death, HIF-1α specific siRNA transfected cells were treated with hypoxic and OGD exposures. Cell death was evaluated by LDH release. Specific knock-down of HIF-1α exaggerated cellular injury in SH-SY5Y cells following either hypoxic or OGD treatments, compared to control or negative control cells. After 3 hr OGD treatment, there were more siRNA transfected cells that rounded up, aggregated and detached from the bottom of the culture dish than the negative control cells (data not shown). As shown in Fig. 2, inhibiting HIF-1α increased cell death from 18.9 ± 2.2% in control cells to 64.3 ± 5.2% in the HIF-1α siRNA transfected cells (p<0.01) after a 3 hr OGD exposure. Cell death increased from 2.8 ± 0.4% in control cells to 12.9 ± 0.6% in the HIF-1α siRNA transfected cells after a 3 hr hypoxic exposure. The figure also reveals that OGD induced more cell death than hypoxic exposure in either control or HIF-1α siRNA transfected cells. In addition, the presence of DFO, which increased HIF-1α, significantly reduced cell death induced by OGD in control cells.
Fig. 2.
Effect of down-regulation of HIF-1α on cell death after hypoxia and OGD exposures. SH-SY5Y cells were incubated under normal condition (21% O2) (Normoxia), hypoxic condition (1% O2) (Hypoxia), and oxygen and glucose deprivation (OGD) for 3 hrs. DFO treatment was used to increase HIF-1 expression. Cell death was assessed by LDH release assay. Data are expressed as mean ± SEM, n=6. N: normal cells; NC: cells transfected with negative control siRNA; siRNA: cells transfected with HIF-1α specific siRNA (5 nM). *p<0.01 v.s. normal cells; #p<0.01 v.s. negative control cells.
Inhibiting HIF-1α expression by siRNA transfection increased intracellular ROS levels after hypoxic and OGD treatments
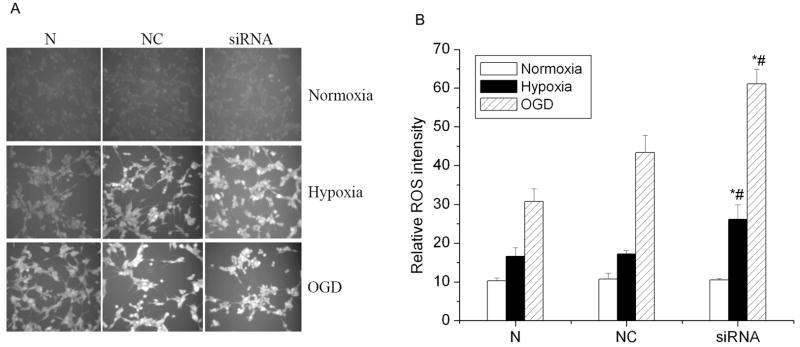
HIF-1 may promote glucose transport and glucose metabolism under hypoxic exposures, which are very important in maintaining cellular redox status and reducing ROS levels. We hypothesized that inhibiting HIF-1 expression would reduce cellular reducing potential and increase ROS generation, which, in turn, induce cell injury. Intracellular ROS levels were measured by using the cell-permeable probe DCFH-DA, which has been widely used to detect ROS and determine intracellular levels of oxidative stress. As shown in Fig. 3A, there was more oxidation of DCFH in HIF-1α siRNA transfected cells than in negative control cells after either the hypoxic or OGD exposure. Fig. 3B shows that the fluorescent value of HIF-1α siRNA transfected cells (61.1 ± 3.8) was significantly higher than that of the negative control cells (43.4 ± 4.4) after a 3 hr OGD treatment (p<0.01). After the hypoxic exposure, oxidation of DCFH in HIF-1α siRNA transfected cells (26.2 ± 3.7) was also higher than that in negative control cells (17.2 ± 0.8). There was no difference between normal control cells and negative control cells under these conditions (p>0.05). These results imply that inhibiting HIF-1α protein expression increased cellular ROS levels when cells were exposed to either hypoxia or OGD, but not under normal culture conditions.
Fig. 3.
Effects of HIF-1α siRNA on intracellular ROS levels after hypoxia and OGD treatments. A) Representatives of cell images with ROS fluorescence. Images were taken with a 10 objective. Images shown are representative of at least 10 fields of view. B) ROS level. The ROS level was measured with the cell-permeable probe dichlorofluorescin diacetate (DCFH-DA). Cells were incubated with 100 μM DCFH-DA (dissolved in DMSO) for 30 min at 37 °C. N: normal control cells; NC: negative control cells; siRNA: HIF-1α siRNA transfected cells (5 nM). All cells were incubated under normal condition (21% O2) (Normoxia), hypoxic condition (1% O2) (Hypoxia), and oxygen and glucose deprivation (OGD) for 3 hrs. The results were normalized by protein content. Data are the mean ± SEM. n=3. *p<0.01 v.s. normal control cells. #p<0.01 v.s. negative control cells.
HIF-1 regulated cellular redox status after hypoxic exposure
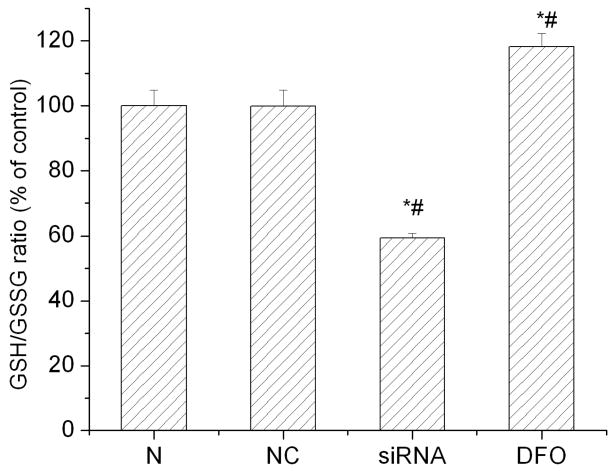
The above results indicate that inhibiting HIF-1α expression deteriorated cellular redox status after hypoxic and OGD exposures as shown by the increase of ROS level in HIF-1α siRNA transfected cells. We carried out experiments to measure the level of cellular GSH/GSSG ratio to further evaluate the effect of HIF-1α on the redox status in SH-SY5Y cells exposed to hypoxic stress. The ratio of GSH/GSSG has been widely used to indicate cellular redox status (Schafer & Buettner 2001). As shown in Fig. 4, HIF-1 inhibition by HIF-1α siRNA reduced the GSH/GSSG ratio to 59.4 ± 2.1% from 100% (control cells) after a hypoxic exposure. In contrast, DFO, a hydroxylase inhibitor and an inducer of HIF-1α, increased the GSH/GSSG ratio to 118.2 ± 7.1% in SH-SY5Y cells (p<0.05). These results indicate that HIF-1α can improve the redox environments and that in the absence of HIF-1, cellular redox status is deteriorated when cells are exposed to stresses such as hypoxia.
Fig. 4.
Effects of HIF-1α siRNA on cellular GSH/GSSG ratio after hypoxia treatments. All cells were incubated under hypoxic condition (1% O2) for 3 hrs. N: normal control cells; NC: negative control cells; siRNA: HIF-1α siRNA transfected cells (5 nM). DFO was used as a positive control. Data are expressed as mean ± SEM (n=6). *p<0.05 v.s. normal control cells; #p<0.05 v.s. negative control cells.
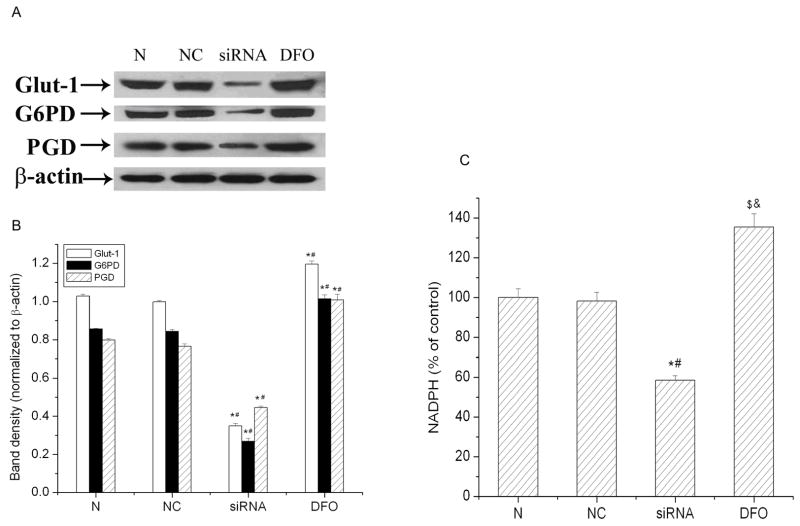
Effects of HIF-1α on glucose transport and the pentose phosphate pathway (PPP) after hypoxic treatment
Knockdown HIF-1 caused the disruption of redox homeostasis demonstrated by the above results. To determine the cause for the disruption, we studied the effect of HIF-1 on glucose transporters and key enzymes of PPP in SH-SY5Y cells under hypoxic exposures. The first step of glucose utilization is glucose transport, which is facilitated by a family of glucose transporters. Glucose transporter 1 (GLUT1) and GLUT3 are two main transporters in brain cells. It has been observed that GLUT1 is mainly expressed in SH-SY5Y cells (Russo et al. 2004). The PPP plays a central role in maintaining cellular redox homeostasis, mainly by generating NADPH. G6PD is the rate-limiting enzyme in the PPP (Chen et al. 2001, Gao et al. 2004). PGD is another enzyme of the pathway that is involved in NADPH production. As shown in Fig. 5A and B, all three proteins (GLUT1, G6PD, and PDG) were sharply down-regulated in HIF-1α siRNA transfected cells. DFO, which increased HIF-1α expression, elevated the expression of GLUT1, G6PD, and PGD in SH-SY5Y cells. Furthermore, we measured cellular NADPH levels after hypoxic exposures. As shown in Fig. 5C, there was no significant difference in NADPH levels between normal cells (100 ± 4.3%) and negative control cells (98 ± 4.5%) after a 3 hr hypoxic exposure. However, NADPH levels decreased to 59% of control cells in HIF-1α specific siRNA transfected cells. In contrast, DFO increased NADPH levels to 135% of control cells. These results suggest that HIF-1 plays an important role in maintaining redox homeostasis by regulating glucose transport and its metabolism.
Fig. 5.
The effect of HIF-1α knockdown on the expression of GLUT1, G6PD, PGD and the total cellular NADPH under hypoxic conditions in SH-SY5Y cells. A) Representative western blot of three independent experiments. β-actin serves as protein loading control. B) Average of densitometric analyses normalized to β-actin. C) The total cellular NADPH concentration. N: normal control cells; NC: negative control cells; siRNA: HIF-1α siRNA transfected cells; DFO was used as positive control. All cells were incubated under a hypoxic condition (1% O2) for 3 hrs. Data are expressed as mean ± SEM (n=3). $p<0.05, *p<0.01 v.s. normal control cells; & p<0.05, #p<0.01 v.s. negative control cells.
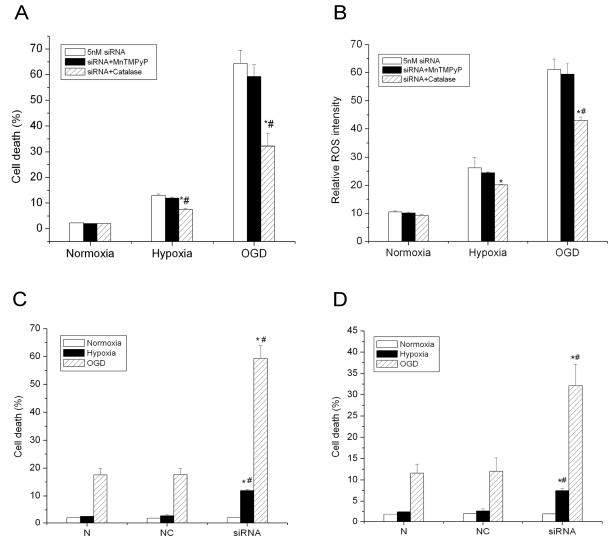
Effect of antioxidants on cell death induced by inhibiting HIF-1α after hypoxic and OGD treatments
If increasing ROS levels and deteriorating cellular redox status were responsible for cellular injury induced by inhibiting HIF-1, antioxidants should ameliorate cellular injury by reducing ROS and improving cellular redox status. We further investigated the effect of antioxidants on cell death of HIF-1α siRNA transfected cells under hypoxic/ischemic conditions. The cells were treated with either MnTMPyP (5 μM), a mimic of superoxide dismutase, or catalase (500 units/ml). MnTMPyP was used to dismutate superoxide anion radical, the primary ROS in cells. MnTMPyP readily permeates cell membranes achieving sufficiently high levels both inside and outside cells to effectively detoxify intracellular superoxide radical anion (Schlieve et al. 2006). Catalase was used to quench hydrogen peroxide, which can be generated from the dismutation of superoxide anion radical. We observed that MnTMPyP had no significant effect on cell death in HIF-1α siRNA transfected cells under either hypoxic or OGD exposures (Fig. 6A; 64.3 ± 5.2% for negative control cells v.s. 59.3 ± 4.7% for HIF-1α siRNA transfected cells under OGD condition; 12.9 ± 0.6% for negative control cells v.s. 11.8 ± 0.5% for HIF-1α siRNA transfected cells under hypoxia condition; p>0.05). MnTMPyP catalyzes the dismutation of superoxide anion radical to H2O2 that in turn induces oxidative stress and cell death. The generation of H2O2 from this reaction may account for the lack effectiveness of MnTMPyP on cell death. On the other hand, catalase significantly reduced cell death from 64.3 ± 5.2% to 32.1 ± 5.0% under OGD conditions and from 12.9 ± 0.6% to 7.5 ± 0.5% under hypoxic conditions compared to control cells (Fig. 6A, p<0.01). The significant effect of catalase suggests that H2O2 plays an important role in cytotoxicity induced by HIF-1α inhibition under hypoxic and OGD treatments. This result strongly supports the suggestion that HIF-1 promotes cell survival through reducing oxidative stress and maintaining redox status under hypoxic and OGD exposures.
Fig. 6.
Effects of SOD mimic MnTMPyP and catalase on cell death and ROS levels of HIF-1α siRNA transfected cells. A) Effect of MnTMPyP and catalase on cell death in HIF-1α siRNA transfected cells exposed to hypoxia and OGD. Cells were incubated under normal condition (21% O2) (Normoxia), hypoxic condition (1% O2) (Hypoxia), and oxygen and glucose deprivation (OGD) for 3 hrs. B) Effect of MnTMPyP and catalase on ROS levels in HIF-1α siRNA transfected cells exposed to hypoxia and OGD. C) Effect of MnTMPyP on cell death in control siRNA and HIF-1α siRNA transfected cells exposed to OGD. D) Effect of catalase on cell death in control siRNA and HIF-1α siRNA transfected cells exposed to OGD. MnTMPyP at 5 μM and catalase at 500 units/ml were used to treat cells. Cell death/viability was assessed by LDH release assay. Data are expressed as mean ± SEM (n=6). *p<0.01 v.s. control cells; #p<0.01 v.s. MnTMPyP treated cells.
In addition, we compared the effect of MnTMPyP (Fig. 6C) and catalase (Fig. 6D) on cell death of control siRNA and HIF-1α siRNA transfected cells exposed to hypoxic and OGD treatments. The data without treatments is shown in Fig. 2. MnTMPyP did not show any effect on cell death of control and HIF-1α siRNA transfected cells exposed to hypoxia and OGD. Catalase attenuated the OGD-induced cell death in cells transfected with a control siRNA from 18% (Fig. 2, NC +OGD) to 12% (Fig. 6D, NC+OGD), a 30% decrease. It reduced 50% cell death in HIF-1α siRNA transfected cells exposed to OGD, from 64% (Fig. 2, siRNA+OGD) to 32% (Fig. 6D, siRNA+OGD). There was a larger effect of catalase on cell death in HIF-1α siRNA transfected cells than in control siRNA transfected cell.
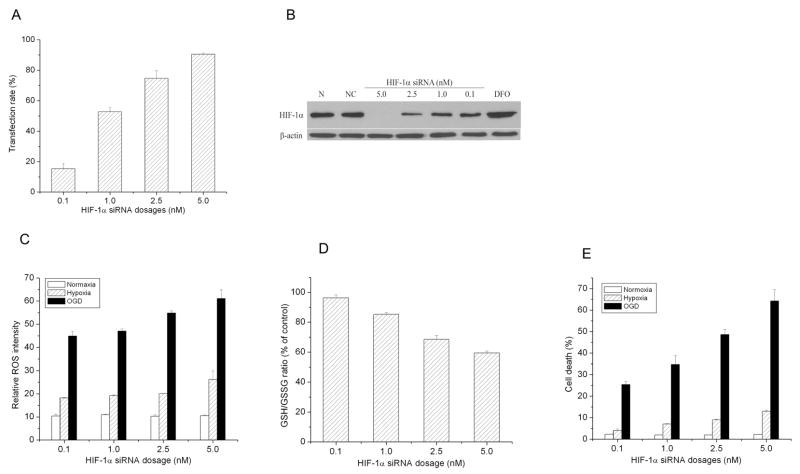
Dose dependent effect of HIF-1α siRNA on cell death, redox status, and ROS levels
Finally, multiple dosages of HIF-1α siRNA were transfected to cells to test the responsiveness of cells with different degrees of HIF-1α expression. As shown in Fig. 7, expression of HIF-1α was negatively proportional to the dose of HIF-1α siRNA. The figure also shows that the more HIF-1α siRNA, the higher ROS level and cell death rate and the lower GSH/GSSG ratio. These results of multidoses of HIF-1α siRNA confirmed that inhibiting HIF-1α deteriorates cellular redox status and induces cell death under hypoxic/ischemic exposures.
Fig. 7.
Dose-depended inhibition of HIF-1α by HIF-1α specific siRNA in SH-SY5Y cells. A) Concentration-dependent transfection rate of HIF-1α specific siRNA. B) A representative of immunoblot of HIF-1α expression in SY-SY5Y cells transfected with HIF-1α specific siRNA at various concentrations. C) Concentration-dependent effect of HIF-1α specific siRNA on ROS levels in the cells exposed to hypoxia and OGD. D) Concentration-dependent effect of HIF-1α specific siRNA on GSH/GSSG ratio in the cells exposed to hypoxia and OGD. E) Concentration -dependent effect of HIF-1α specific siRNA on cell death in the cells exposed to hypoxia and OGD. Cells were transfected with HIF-1α specific siRNA at the concentrations of 0, 0.1, 1.0, 2.5, and 5.0 nM. They were exposed to hypoxia (1%) or OGD for 3 hrs. N: normal cells; NC: cells transfected with negative control siRNA. DFO was used as a positive HIF-1α control. Data are mean ± SEM, n=3.
Discussion
The intracellular redox status regulates a milieu conducive for cellular growth and proliferation and has direct and indirect effects on the components of cell death/survival signaling pathways. In this study, we demonstrate that HIF-1α siRNA transfected cells are more sensitive to hypoxic and ischemic exposures, compared to normal cells. We find that silencing HIF-1α induces a more oxidizing environment, possibly through deceasing glucose transport and the activity of PPP. Furthermore, scavenging H2O2 inhibits cell death induced by hypoxic/ischemic exposures in HIF-1α specific siRNA transfected cells. The results provide evidence that maintaining cellular redox homeostasis is one important mechanism of HIF-1’s cytoprotection in response to hypoxic and ischemic exposures.
HIF-1 drives hypoxic gene expression required for cells to adapt a low oxygen environment. Increased glucose metabolism is necessary to produce energy when low oxygen will not support mitochondrial oxidative phosphorylation. Although glucose metabolism is regarded as being very important in supplying reducing agents in cells, the effect of HIF-1 on ROS generation and oxidative stress has been controversial in hypoxic cells. On one hand, from studies predominantly carried out in cancer cells, increasing glycolysis in hypoxia has been reported to reduce ROS generation and ameliorate oxidative injury. In an oral squamous cell carcinoma cell line, the level of ROS production decreased following transfection of HIF-1 expression vector (Sasabe et al. 2007). The reduction of mitochondrial glucose oxidation and transition to glycolysis likely prevents ROS production (Brand & Hermfisse 1997, Kim et al. 2006, Semenza 2007). On the other hand, it has been argued that reduced function of mitochondria by the activation of PDK1 induces ROS generation from complex III and results in oxidative stress (Guzy & Schumacker 2006, Chandel et al. 2000). These previous studies primarily focused on cellular ROS levels. In this study, we measured not only ROS levels but also the cellular redox status in a neuronal cell line. The redox status is an overall evaluation of cellular oxidative stress levels, which reflects the balance of ROS generation and ROS-scavenging ability. Our results clearly show that inhibiting HIF-1 increases oxidative stress and that upregulating HIF-1 by the hydroxylase inhibitor DFO decreases oxidative stress in SH-SY5Y cells exposed to hypoxia and OGD.
The PPP is an alternative to glycolysis. It is a major source for the rapid production of NADPH (Paglialunga et al. 2004). NADPH is the principal intracellular reductant and is critical in maintaining redox homeostasis. Previous studies have shown that up-regulating G6PD induces NADPH accumulation and offers remarkable resistance against oxidative stress-mediated cell death, whereas inhibiting G6PD depletes NADPH levels and exacerbates cellular vulnerability (Garcia-Nogales et al. 2003, Tian et al. 1999, Filosa et al. 2003). The results presented here show that HIF-1 actively regulates glucose transport in the cells, upregulates PPP enzymes such as G6PD and PGD, and increase cellular NADPH levels (Fig. 5). Knock-down HIF-1α significantly reduces the expression of these enzymes and decrease cellular NADPH levels. The result shown in Fig. 2 is evident that DFO, which elevates HIF-1α, GLUT1 and pentose shunt enzymes, increase the survival of cells. It should be pointed out that the results presented here do not establish a causal relationship between cell death induced by silencing HIF-1α and the level of the key enzymes (GLUT-1, G6PD, and PGD). However, the results do establish that cell death induced by inhibiting HIF-1α is strongly associated to the level of these enzymes. Future studies with both inhibiting HIF-1α and maintaining the level of the key enzymes will clarify the specific role of these enzymes in cell death induced by silencing HIF-1α.
The results show that the response of HIF-1α siRNA transfected cells to hypoxia is different from that to OGD exposure. Hypoxia did induce oxidative stress and cell death in the transfected cells. OGD treatments caused significantly higher oxidative stress and cell death rate than the hypoxic exposure. These results indicate that glucose is critical in maintaining redox status and cell survival in the HIF-1α siRNA transfected cells under low oxygen conditions, which is in agreement with our previous observations (Shi & Liu 2006, Guo et al. 2008). In fact, glucose is a main substrate required for producing the principalintracellular reductant NADPH by the pentose phosphate pathways (Averill-Bates & Przybytkowski 1994, Przybytkowski & Averill-Bates 1996). The results also suggest that HIF-1α knock down induces severe oxidative stress with the removal of glucose. In the absence of glucose, there is lack of the source of reducing agents such as NADPH and the regeneration of GSH from GSSG, which results in severe oxidative stress and thus cell death. It should be pointed out that although HIF-1α expression can be almost completely blocked by the siRNA transfection, the expression of GLUT1, G6PD, and PGD is inhibited to a lesser extent. This indicates that the enzymes are still partly functional in the siRNA-transfected cells, which may partly explain why OGD induces more oxidative stress and cell death than a hypoxia exposure. More importantly, the result suggests that HIF-1α is critical in preventing cell death from OGD exposures. The same OGD exposure induced 64% cell death in HIF-1α siRNA transfected cells, compared to only 19% cell death in control cells which had normal HIF-1 expression (Fig. 2).
Our results reveal that H2O2 seems to be responsible for the cell injury induced by HIF-1 inhibition. As shown in Fig. 6, catalase, but not the cell permeable SOD mimic MnTMPyP, significantly reduces cell death induced by HIF-1 inhibition following either hypoxic or OGD exposures. Besides catalase, glutathione peroxidase (GPx) mainly detoxifies H2O2 by using GSH as its substrate. Cellular GSH is mainly regenerated from GSSG by glutathione reductase, which uses NADPH as an electron donor (Ramos & Colquhoun 2003). The effectiveness of catalase implies that H2O2 detoxification by GPx in HIF-1α siRNA transfected cells is insufficient. Our finding demonstrates that HIF-1 inhibition reduces the GSH level and subsequently the cellular ability to detoxify H2O2. The excess amount of H2O2 induces cell death under hypoxic and ischemic exposures. As summarized in Scheme 1, the results strongly support the idea that cellular redox regulation mediated by HIF-1 may be critical in controlling cell’s survival process during ischemic exposures.
Scheme 1.
HIF-1 may provide cytoprotection through maintaining cellular redox status under hypoxic/ischemic exposures.
Toxic effects of HIF-1 on cell survival have also been observed in ischemic conditions. For instance, HIF-1α may coordinate the activity of p53 in driving ischemia-induced delayed neuronal death (Halterman & Federoff 1999). The adverse effects of HIF-1 on ischemic injuries seem dependent on the duration and the degree of severity of ischemia. Using the same neuron-specific HIF-1α knock-out mice, Helton et al. and Baranova et al. reported distinct effects of HIF-1 on neuronal injuries following ischemia. Baranova et al. reported that neuron-specific knockdown of HIF-1α increased tissue damage and reduced survival rate of MCAO mice (Baranova et al. 2007) while Helton et al. observed that the knock-out of HIF-1α reduced hypoxic-ischemic damage (Helton et al. 2005). Interestingly, the ischemic model that Baranova et al. used was 30 min ischemia with unilateral common carotid artery occlusion while Helton’s model was 75 min ischemia with bilateral occlusion. The two observations support the notion that HIF-1α may induce cell death in a severe and prolonged ischemia. The concept of redox regulation mediated by HIF-1 may help to explain the discrepancy between the two observations. In the mild ischemic model, glucose supply is still available and not depleted in the affected region. HIF-1 mediated glycolysis may have enough substrate to maintain cellular redox status and promote cell survival. This would suggest that in mild ischemia, induction of anti-apoptotic pathways prevail as the cells could maintain a more reducing environment. However, in a severe ischemia, especially a bilateral occlusion, glucose could soon be depleted. The glycolysis pathway would have insufficient substrate to produce reducing agents. Thus, severe ischemia could induce a more oxidizing environment, which activates pro-apoptotic pathways and results in cell death.
In conclusion, knock-down of HIF-1α in SH-SY5Y cells down-regulates the key enzymes in glucose metabolism that are critical in production of important reducing agents, decreases the GSH/GSSG ratio, increases ROS levels, and induces cell death under hypoxic or OGD conditions. HIF-1 maintains cellular redox state and prevents ischemic injury mediated by ROS production, possibly through up-regulating the expression of key enzymes such as GLUT1, G6PD and PGD. The present study provides new evidence that HIF-1 protect cells from ischemic injury by maintaining the cellular redox status.
Acknowledgments
This work was partly supported by grants from NIH (P20 RR15636 and R01NS058807).
We thank Dr. William Shuttleworth of Department of Neurosciences, UNM, for editing of the manuscript.
References
- Almeida A, Delgado-Esteban M, Bolanos JP, Medina JM. Oxygen and glucose deprivation induces mitochondrial dysfunction and oxidative stress in neurones but not in astrocytes in primary culture. J Neurochem. 2002;81:207–217. doi: 10.1046/j.1471-4159.2002.00827.x. [DOI] [PubMed] [Google Scholar]
- Averill-Bates DA, Przybytkowski E. The role of glucose in cellular defences against cytotoxicity of hydrogen peroxide in Chinese hamster ovary cells. Arch Biochem Biophys. 1994;312:52–58. doi: 10.1006/abbi.1994.1279. [DOI] [PubMed] [Google Scholar]
- Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007;27:6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron M, Yu AY, Solway KE, Semenza GL, Sharp FR. Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischaemia in rat brain. Eur J Neurosci. 1999;11:4159–4170. doi: 10.1046/j.1460-9568.1999.00845.x. [DOI] [PubMed] [Google Scholar]
- Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, Petit E. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- Brand KA, Hermfisse U. Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB J. 1997;11:388–395. doi: 10.1096/fasebj.11.5.9141507. [DOI] [PubMed] [Google Scholar]
- Brewer GJ. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res. 1995;42:674–683. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol. 2000;165:1013–1021. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem. 2001;276:9519–9525. doi: 10.1074/jbc.M010144200. [DOI] [PubMed] [Google Scholar]
- Delgado-Esteban M, Almeida A, Bolanos JP. D-Glucose prevents glutathione oxidation and mitochondrial damage after glutamate receptor stimulation in rat cortical primary neurons. J Neurochem. 2000;75:1618–1624. doi: 10.1046/j.1471-4159.2000.0751618.x. [DOI] [PubMed] [Google Scholar]
- Ebert BL, Firth JD, Ratcliffe PJ. Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter-1 via distinct Cis-acting sequences. J Biol Chem. 1995;270:29083–29089. doi: 10.1074/jbc.270.49.29083. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Hasselblatt M, Dembowski C, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gomez FJ, Galindo MF, Gomez-Lazaro M, Yuste VJ, Comella JX, Aguirre N, Jordan J. Malonate induces cell death via mitochondrial potential collapse and delayed swelling through an ROS-dependent pathway. Br J Pharmacol. 2005;144:528–537. doi: 10.1038/sj.bjp.0706069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa S, Fico A, Paglialunga F, Balestrieri M, Crooke A, Verde P, Abrescia P, Bautista JM, Martini G. Failure to increase glucose consumption through the pentose-phosphate pathway results in the death of glucose-6-phosphate dehydrogenase gene-deleted mouse embryonic stem cells subjected to oxidative stress. Biochem J. 2003;370:935–943. doi: 10.1042/BJ20021614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freret T, Valable S, Chazalviel L, Saulnier R, Mackenzie ET, Petit E, Bernaudin M, Boulouard M, Schumann-Bard P. Delayed administration of deferoxamine reduces brain damage and promotes functional recovery after transient focal cerebral ischemia in the rat. Eur J Neurosci. 2006;23:1757–1765. doi: 10.1111/j.1460-9568.2006.04699.x. [DOI] [PubMed] [Google Scholar]
- Gao L, Mejias R, Echevarria M, Lopez-Barneo J. Induction of the glucose-6-phosphate dehydrogenase gene expression by chronic hypoxia in PC12 cells. FEBS Lett. 2004;569:256–260. doi: 10.1016/j.febslet.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Garcia-Nogales P, Almeida A, Bolanos JP. Peroxynitrite protects neurons against nitric oxide-mediated apoptosis. A key role for glucose-6-phosphate dehydrogenase activity in neuroprotection. J Biol Chem. 2003;278:864–874. doi: 10.1074/jbc.M206835200. [DOI] [PubMed] [Google Scholar]
- Guo S, Bezard E, Zhao B. Protective effect of green tea polyphenols on the SH-SY5Y cells against 6-OHDA induced apoptosis through ROS-NO pathway. Free Radic Biol Med. 2005;39:682–695. doi: 10.1016/j.freeradbiomed.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Guo S, Bragina O, Xu Y, et al. Glucose upregulates HIF-1alpha expression in primary cortical neurons in response to hypoxia through maintaining cellular redox status. J Neurochem. 2008;105:1849–1860. doi: 10.1111/j.1471-4159.2008.05287.x. [DOI] [PubMed] [Google Scholar]
- Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- Halterman MW, Federoff HJ. HIF-1alpha and p53 promote hypoxia-induced delayed neuronal death in models of CNS ischemia. Exp Neurol. 1999;159:65–72. doi: 10.1006/exnr.1999.7160. [DOI] [PubMed] [Google Scholar]
- Hamrick SE, McQuillen PS, Jiang X, Mu D, Madan A, Ferriero DM. A role for hypoxia-inducible factor-1alpha in desferoxamine neuroprotection. Neurosci Lett. 2005;379:96–100. doi: 10.1016/j.neulet.2004.12.080. [DOI] [PubMed] [Google Scholar]
- Helton R, Cui J, Scheel JR, et al. Brain-specific knock-out of hypoxia-inducible factor-1alpha reduces rather than increases hypoxic-ischemic damage. J Neurosci. 2005;25:4099–4107. doi: 10.1523/JNEUROSCI.4555-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HM, Zhang H, Ou HC, Chen HL, Gibson GE. alpha-keto-beta-methyl-n-valeric acid diminishes reactive oxygen species and alters endoplasmic reticulum Ca2+ stores. Free Radic Biol Med. 2004;37:1779–1789. doi: 10.1016/j.freeradbiomed.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci USA. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M, Go YM, Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic Biol Med. 2008;44:921–937. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kirkland RA, Franklin JL. Evidence for redox regulation of cytochrome C release during programmed neuronal death: antioxidant effects of protein synthesis and caspase inhibition. J Neurosci. 2001;21:1949–1963. doi: 10.1523/JNEUROSCI.21-06-01949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien RF, Harris PK, Foellmi LA. Glucose-6-phosphate dehydrogenase: a “housekeeping” enzyme subject to tissue-specific regulation by hormones, nutrients, and oxidant stress. FASEB J. 1994;8:174–181. doi: 10.1096/fasebj.8.2.8119488. [DOI] [PubMed] [Google Scholar]
- Lawrence MS, Sun GH, Kunis DM, Saydam TC, Dash R, Ho DY, Sapolsky RM, Steinberg GK. Overexpression of the glucose transporter gene with a herpes simplex viral vector protects striatal neurons against stroke. J Cereb Blood Flow Metab. 1996;16:181–185. doi: 10.1097/00004647-199603000-00001. [DOI] [PubMed] [Google Scholar]
- Leinninger GM, Russell JW, van Golen CM, Berent A, Feldman EL. Insulin-like growth factor-I regulates glucose-induced mitochondrial depolarization and apoptosis in human neuroblastoma. Cell Death Differ. 2004;11:885–896. doi: 10.1038/sj.cdd.4401429. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang X, Sejas DP, Bagby GC, Pang Q. Hypoxia-induced nucleophosmin protects cell death through inhibition of p53. J Biol Chem. 2004;279:41275–41279. doi: 10.1074/jbc.C400297200. [DOI] [PubMed] [Google Scholar]
- Lluis JM, Buricchi F, Chiarugi P, Morales A, Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-{kappa}B via c-SRC and oxidant-dependent cell death. Cancer Res. 2007;67:7368–7377. doi: 10.1158/0008-5472.CAN-07-0515. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, Risau W. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156:965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumcke I, Schneider B, Fandrey J, Pagel H. Effects of pro- and antioxidative compounds on renal production of erythropoietin. Endocrinology. 1999;140:641–645. doi: 10.1210/endo.140.2.6529. [DOI] [PubMed] [Google Scholar]
- Paglialunga F, Fico A, Iaccarino I, Notaro R, Luzzatto L, Martini G, Filosa S. G6PD is indispensable for erythropoiesis after the embryonic-adult hemoglobin switch. Blood. 2004;104:3148–3152. doi: 10.1182/blood-2004-03-0835. [DOI] [PubMed] [Google Scholar]
- Piret JP, Lecocq C, Toffoli S, Ninane N, Raes M, Michiels C. Hypoxia and CoCl2 protect HepG2 cells against serum deprivation- and t-BHP-induced apoptosis: a possible anti-apoptotic role for HIF-1. Exp Cell Res. 2004;295:340–349. doi: 10.1016/j.yexcr.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Prass K, Ruscher K, Karsch M, Isaev N, Megow D, Priller J, Scharff A, Dirnagl U, Meisel A. Desferrioxamine induces delayed tolerance against cerebral ischemia in vivo and in vitro. J Cereb Blood Flow Metab. 2002;22:520–525. doi: 10.1097/00004647-200205000-00003. [DOI] [PubMed] [Google Scholar]
- Przybytkowski E, Averill-Bates DA. Correlation between glutathione and stimulation of the pentose phosphate cycle in situ in Chinese hamster ovary cells exposed to hydrogen peroxide. Arch Biochem Biophys. 1996;325:91–98. doi: 10.1006/abbi.1996.0011. [DOI] [PubMed] [Google Scholar]
- Ramos KL, Colquhoun A. Protective role of glucose-6-phosphate dehydrogenase activity in the metabolic response of C6 rat glioma cells to polyunsaturated fatty acid exposure. Glia. 2003;43:149–166. doi: 10.1002/glia.10246. [DOI] [PubMed] [Google Scholar]
- Rondon IJ, Scandurro AB, Wilson RB, Beckman BS. Changes in redox affect the activity of erythropoietin RNA binding protein. FEBS Lett. 1995;359:267–270. doi: 10.1016/0014-5793(95)00066-i. [DOI] [PubMed] [Google Scholar]
- Russo VC, Kobayashi K, Najdovska S, Baker NL, Werther GA. Neuronal protection from glucose deprivation via modulation of glucose transport and inhibition of apoptosis: a role for the insulin-like growth factor system. Brain Res. 2004;1009:40–53. doi: 10.1016/j.brainres.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci USA. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabe E, Tatemoto Y, Li D, Yamamoto T, Osaki T. Mechanism of HIF-1alpha- dependent suppression of hypoxia-induced apoptosis in squamous cell carcinoma cells. Cancer Sci. 2005;96:394–402. doi: 10.1111/j.1349-7006.2005.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabe E, Zhou X, Li D, Oku N, Yamamoto T, Osaki T. The involvement of hypoxia-inducible factor-1alpha in the susceptibility to gamma-rays and chemotherapeutic drugs of oral squamous cell carcinoma cells. Int J Cancer. 2007;120:268–277. doi: 10.1002/ijc.22294. [DOI] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Schlieve CR, Lieven CJ, Levin LA. Biochemical activity of reactive oxygen species scavengers do not predict retinal ganglion cell survival. Invest Ophthalmol Vis Sci. 2006;47:3878–3886. doi: 10.1167/iovs.05-1010. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Oxygen-dependent regulation of mitochondrial respiration by hypoxia- inducible factor 1. Biochem J. 2007;405:1–9. doi: 10.1042/BJ20070389. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- Shi H, Liu K. Effects of glucose concentration on redox status in rat primary cortical neurons under hypoxia. Neurosci Lett. 2006;410:57–61. doi: 10.1016/j.neulet.2006.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiq A, Aminova LR, Ratan RR. Hypoxia inducible factor prolyl 4-hydroxylase enzymes: center stage in the battle against hypoxia, metabolic compromise and oxidative stress. Neurochem Res. 2007;32:931–946. doi: 10.1007/s11064-006-9268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiq A, Ayoub IA, Chavez JC, et al. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system. J Biol Chem. 2005;280:41732–41743. doi: 10.1074/jbc.M504963200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siren AL, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian WN, Braunstein LD, Apse K, Pang J, Rose M, Tian X, Stanton RC. Importance of glucose-6-phosphate dehydrogenase activity in cell death. Am J Physiol. 1999;276:C1121–1131. doi: 10.1152/ajpcell.1999.276.5.C1121. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Brownlee M, Russell JW. Oxidative stress and programmed cell death in diabetic neuropathy. Ann N Y Acad Sci. 2002;959:368–383. doi: 10.1111/j.1749-6632.2002.tb02108.x. [DOI] [PubMed] [Google Scholar]
- Wanpen S, Govitrapong P, Shavali S, Sangchot P, Ebadi M. Salsolinol, a dopamine-derived tetrahydroisoquinoline, induces cell death by causing oxidative stress in dopaminergic SH-SY5Y cells, and the said effect is attenuated by metallothionein. Brain Res. 2004;1005:67–76. doi: 10.1016/j.brainres.2004.01.054. [DOI] [PubMed] [Google Scholar]
- Welsh SJ, Williams RR, Birmingham A, Newman DJ, Kirkpatrick DL, Powis G. The thioredoxin redox inhibitors 1-methylpropyl 2-imidazolyl disulfide and pleurotin inhibit hypoxia-induced factor 1alpha and vascular endothelial growth factor formation. Mol Cancer Ther. 2003;2:235–243. [PubMed] [Google Scholar]
- Wu HH, Momand J. Pyrrolidine dithiocarbamate prevents p53 activation and promotes p53 cysteine residue oxidation. J Biol Chem. 1998;273:18898–18905. doi: 10.1074/jbc.273.30.18898. [DOI] [PubMed] [Google Scholar]
- Zaman K, Ryu H, Hall D, et al. Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J Neurosci. 1999;19:9821–9830. doi: 10.1523/JNEUROSCI.19-22-09821.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Matchett GA, Jadhav V, Dach N, Zhang JH. The effect of 2-methoxyestradiol, a HIF-1alpha inhibitor, in global cerebral ischemia in rats. Neurol Res. 2008;30:268–271. doi: 10.1179/016164107X229920. [DOI] [PMC free article] [PubMed] [Google Scholar]