Abstract
L-asparaginase (ASNase) is a common chemotherapy agent for the treatment of lymphoid malignancies. L-asparaginase has been reported to cause clinical pancreatitis in both humans and canines. Canine pancreatic lipase immunoreactivity (cPLI) is now a common diagnostic tool for evaluating pancreatitis in dogs. A total of 52 dogs were enrolled into this study. Canine pancreatic lipase immunoreactivity (cPLI) concentrations were evaluated before and after administration of ASNase, vincristine, or both. All dogs enrolled in the study were evaluated for signs compatible with clinical pancreatitis. No dogs receiving ASNase alone showed evidence of clinical pancreatitis after administration. Also, there was no statistically significant change in cPLI concentrations before or after treatment. Fourteen percent of dogs that received both vincristine and ASNase concurrently had elevated concentrations of cPLI after treatment. Of the 11 dogs with clinical signs compatible with pancreatitis after any chemotherapy treatment, no dog had a cPLI concentration > 400 μg/dL. In conclusion, ASNase did not cause clinical pancreatitis in this cohort of dogs but larger sample sizes are required to further validate this data.
Résumé
La L-asparaginase (ASNase) est un agent de chimiothérapie usuel pour le traitement des cancers lymphoïdes. La L-asparaginase est rapportée comme causant des pancréatites cliniques chez les humains et les chiens. L’immunoréactivité de la lipase pancréatique canine (cPLI) est maintenant un outil diagnostique fréquent pour l’évaluation de pancréatite chez les chiens. Un total de 52 chiens a été inclus dans cette étude. Les concentrations de cPLI ont été évaluées avant et après administration d’ASNase, de vincristine, ou des deux. Tous les chiens inclus dans l’étude ont été évalués pour des signes compatibles avec une pancréatite clinique. Aucun des chiens ne recevant que de l’ASNase n’a montré d’évidence de pancréatite clinique après l’administration du produit. Également, il n’y avait aucun changement statistiquement significatif dans les concentrations de cPLI avant et après les traitements. Quatorze pourcent des chiens qui ont reçu de la vincristine et de l’ASNase simultanément avaient des concentrations élevées de cPLI après les traitements. Parmi les 11 chiens qui avaient des signes compatibles avec une pancréatite après n’importe lequel des traitements par chimiothérapie, aucun des chiens n’avaient une concentration de cPLI >400 μg/dL. En conclusion, l’ASNase n’a pas causé de pancréatite clinique dans cette cohorte de chiens mais une taille d’échantillons plus grande est requise pour valider ces résultats.
(Traduit par Docteur Serge Messier)
Introduction
The standard of care for the treatment of canine malignant lymphoma consists of multi-drug therapy (1). L-asparaginase (ASNase) is among the accepted drugs used for standard treatment in both the induction phase of chemotherapy for lymphoma and for rescue therapy (2–4). L-asparaginase, commonly derived from Escherichia coli, is a tumoricidal enzyme with a unique action mechanism (5). Neoplastic lymphocytes require the amino acid L-asparagine for development, but lack the ability to synthesize this amino acid due to a lack of L-asparagine synthetase (5). Consequently, lymphoma cells must acquire L-asparagine from the circulating blood supply (5). L-asparaginase hydrolyzes the body’s circulating L-asparagine, thus starving the neoplastic cells of this essential amino acid (5). However, L-asparagine is also an important component of many complex proteins, and its elimination results in inhibition of synthesis of albumin, coagulation factors, and insulin (5). Given the negative impact on protein synthesis by the liver and endocrine pancreas, it is reasonable to speculate that L-asparagine depletion could result in a similar reduction in protein synthesis by the exocrine pancreas.
In canines, ASNase has been linked to a variety of toxicities, including anaphylaxis, coagulation abnormalities, cerebral thrombosis, and pancreatitis (6–9). It remains unclear as to whether or not these side effects are a result of decreased protein synthesis or are due to the presence of an endotoxin associated with E. coli-derived ASNase. All of the aforementioned side effects in humans have been shown to be independent of dosing, but this has not been documented in canines (10,11).
In humans, the incidence of pancreatitis induced by ASNase administration ranges from 0.7% to 16.2% (12–14). Treatment of humans by administration of ASNase is often done intravenously over successive days. The concern about pancreatitis as a side effect in veterinary patients is based mostly on human data and on sporadic case reports in the veterinary literature (6,9). The incidence of pancreatitis secondary to ASNase administration has not been reported in the veterinary literature. In fact, pancreatitis has not been reported in studies regarding ASNase side effects (15).
Canine pancreatitis continues to be a diagnostic dilemma in veterinary medicine. Recently, a new assay for the diagnosis of pancreatitis in dogs, canine pancreatic lipase immunoreactivity (cPLI) concentration, has been developed and analytically validated (16). Many experienced practitioners considered this test to be the gold standard, in combination with findings from a physical examination, for non-invasive diagnostic testing for pancreatitis in canines (17). L-asparaginase-induced pancreatitis in humans is diagnosed using a variety of tests including physical examination, serum amylase and lipase elevations, abdominal sonography, and abdominal computed tomography (10,13,14,18). In some reports, serum concentrations of trypsin and elastase-1 have been used to diagnose subclinical pancreatitis secondary to ASNase administration (19).
The purpose of this study was to prospectively evaluate canine pancreatic lipase immunoreactivity (cPLI) in conjunction with clinical signs in an attempt to ascertain the incidence of clinical and subclinical pancreatitis in dogs diagnosed with lymphoma and treated with ASNase.
Materials and methods
Canine patients admitted to the Texas A&M University (TAMU) Small Animal Clinic and to the Veterinary Specialty Center of Texas and diagnosed with large-cell lymphoma were considered for enrollment. Diagnosis of lymphoma was confirmed by cytology or histopathology. All dogs were staged based on the World Health Organization’s (WHO) canine lymphoma standard (20). Routine staging included a complete blood (cell) count (CBC), chemistry profile, urinalysis, thoracic radiographs, and abdominal radiographs or ultrasound. Immunocytochemistry for B-cell or T-cell typing was evaluated in some dogs. Dogs were considered substage “b” if they displayed systemic signs and were clinically ill at presentation. Dogs with no systemic symptoms were considered substage “a.” Dogs who were substage b typically received an abdominal ultrasound examination, while healthy dogs routinely had abdominal radiographs performed. Bone marrow aspirates were only performed if patients had CBCs that suggested bone marrow infiltration, with cytopenias or abnormal circulating lymphocytes. The project was reviewed and approved by the Clinical Research Review Committee (CRRC #05–36). Before enrollment into the study, all owners were required to sign an informed consent form. Fifty-two dogs were considered eligible and enrolled into the study.
The standard multi-drug regimen for canine large-cell lymphoma used at both referral centers consisted of ASNase (Elspar; Merck & Company, Whitehouse Station, New Jersey, USA) and vincristine (Vincristine; Mayne Pharma, Parmus, New Jersey, USA) administration in the 1st wk and daily oral prednisone (Prednisone; Qualitest Pharmaceuticals, Huntsville, Alabama, USA) at a dosage of 30 to 40 mg/m2. After the 1st wk, the protocol continued as a standard cyclophosphamide hydroxydaunorubicin (doxorubicin) Oncovin (vincristine) prednisone (CHOP) based protocol. The CHOP protocol at TAMU consists of the following weekly chemotherapy treatments: cyclophosphamide in week 2, vincristine again in week 3, and doxorubicin in week 4. No chemotherapy is administered in week 5 and the treatment is then repeated 4 times without further ASNase therapy. L-asparaginase was administered at a dose of 400 IU/kg given subcutaneously. Vincristine was administered intravenously at a dose of 0.5 to 0.7 mg/m2. The vincristine dosage was based primarily on clinician preference, size of the dog, and pretreatment CBC values. Because of the risk for potential synergistic neutropenia, ASNase and vincristine administration were often separated by 24 to 96 h (21). The decision for variation in time between treatments of 1 to 4 d depended on the dog’s CBC at the time of administration, the attending clinician’s clinical evaluation, and the accommodation of a normal work week as no treatments occurred on the weekends. Consequently, there were 2 initial test groups: those that received ASNase and vincristine concurrently (group 1), and those that received ASNase 24 to 96 h prior to vincristine (group 2). Some dogs in group 2 received only ASNase without further vincristine treatment. Those dogs that did not receive further treatment were lymphoma patients in “rescue” protocols or patients who were sent to the local veterinarian for the remainder of the treatment protocol. The study was not randomized, nor were the clinicians blinded to the drugs administered, as clinicians’ assessment determined the group into which each dog would be enrolled. Clinical signs of pancreatitis were defined as any or all of the following: decrease in appetite, vomiting, and abdominal pain on palpation. These signs were evaluated by multiple clinicians.
Based on preliminary data suggesting that the combination of vincristine and ASNase caused elevations in cPL, group 3 was added to the study (22). Group 3 was composed of dogs that were all in clinical remission and were only treated with vincristine, but were not treated with ASNase. These dogs had not had any chemotherapy for at least 7 d prior to vincristine treatment, but were on appropriate doses (30 to 40 mg/m2) of oral prednisone.
Sample collection
For all groups, the 1st serum sample was collected on the day of presentation immediately prior to administration of ASNase.
Group 1 — The 2nd serum sample was obtained 7 d after the administration of both drugs.
Group 2 — The 2nd sample was collected before the vincristine administration (24 to 96 h after the ASNase). A final sample was collected 7 d after the administration of both week 1 drugs (8 to 12 d after presentation).
Group 3 — Samples were collected prior to and 7 d after vincristine administration.
Samples in groups 2 and 3 were collected 7 d after administration based solely on the next appointment time. As clients of both clinics routinely travel extended lengths, it was deemed unnecessary to have clients return solely for the benefit of this study. Consequently, all samples were collected at the time of the next treatment within the CHOP protocol. Mailed samples from local veterinarians would have eliminated the standardized collection method that was designed to minimize degradation of Spec cPLI within the serum.
Serum samples were stored at −80°C until measurement of cPL concentration, and were batched at appropriate intervals to help eliminate testing error (23). A commercial immunoassay (Spec cPL; Idexx Laboratories, Portland, Maine, USA) was used to measure serum cPLI concentrations. This assay is a double-sandwich ELISA that utilizes 2 different monoclonal antibodies directed against canine pancreatic lipase. The reference range for the immunoassay is 0 to 200 μg/dL. A serum cPL concentration > 400 μg/dL is considered diagnostic for pancreatitis and a concentration of 200 to 400 μg/dL is considered equivocal.
Statistical analysis
Statistical analysis was performed using statistical software (GraphPad Prism, version 5.0; GraphPad Software, San Diego, California, USA). Clinical signs of pancreatitis were dichotomized. Signs of pancreatitis included anorexia, vomiting, and signs of nausea (as evidenced by a decreased appetite, smacking of lips, or both). Because of the equivocal range of cPLI from 200 μg/dL to 400 μg/dL, the data was analyzed twice. The 1st set of data considered a cPLI cut off value of > 200 μg/dL. The 2nd set, considered the more standard, used > 400 μg/dL as the cut off value for pancreatitis. A Fischer’s exact test with constructed contingency tables was used to evaluate cPL concentrations and clinical evidence of pancreatitis before and after specific therapies. Gaussian distribution was evaluated with the Shapiro-Wilk normality test for all 3 groups. As none of the data were normally distributed, a Wilcoxon signed rank test (groups 1 and 3) or non-parametric one-way ANOVA (group 2) was used for additional statistical analysis. A Mann-Whitney test was used for non-paired data comparisons. P-values of < 0.05 were considered statistically significant.
Results
Fifty-two dogs were enrolled into the study between July of 2005 and June of 2007. The median age of all enrolled patients was 8.0 y (range: 2.0 to 14.0 y). There were 23 spayed female, 1 intact female, 21 neutered male, and 7 intact male dogs. The following breeds were represented by more than 1 enrolled dog: mixed breed (n = 14), golden retriever (n = 6), basset hound (n = 4), boxer (n = 4), miniature schnauzer (n = 3), Labrador retriever (n = 3), and German shepherd (n = 2).
All dogs in the study were staged according to the World Health Organization (WHO) staging scheme. Two dogs were stage II, 24 dogs were stage III, 14 dogs were stage IV, and 12 dogs were stage V. Thirty-four dogs were substage a and 18 dogs were sub-stage b. The median serum total calcium concentration for all dogs was 10.3 mg/dL (range: 7.4 to 18.6 mg/dL); 7 dogs were hypercalcemic at the time of presentation. A total of 8 dogs had immuno cytochemistry results consistent with T-cell lymphoma, while 12 dogs were diagnosed with B-cell lymphoma. Two dogs had an equally mixed population of B and T cells and 8 dogs had inconclusive immunocytochemistry results. The remaining dogs did not have immunocytochemistry performed.
Group 1
Twenty-eight dogs received concurrent vincristine and ASNase treatment. Upon presentation, 1 dog was stage II, 16 dogs were stage III, 6 dogs were stage IV, and 5 dogs were stage V. Four of the 28 dogs were substage b and only 1 dog was hypercalcemic at presentation.
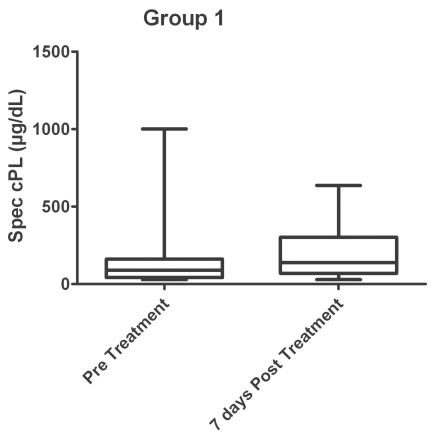
Four of these 28 dogs had clinical signs that could have been compatible with pancreatitis at presentation prior to receiving any chemotherapy. These signs included decreased appetite, vomiting, and/or abdominal pain on palpation. After concurrent administration of both ASNase and vincristine, 5 dogs had clinical signs compatible with pancreatitis. Two of these 5 dogs had clinical signs compatible with pancreatitis both before and after treatment. The median cPLI concentration before treatment was 88.5 μg/dL (range: 29.0 to 1001.0 μg/dL) and was 138.5 μg/dL (range: 29.0 to 637.0 μg/dL) 7 d after the combination therapy (Figure 1). While not significant (P = 0.058), the change in median serum cPL between the 2 groups approached a P-value of 0.05. However, there was no significant difference between the number of dogs with cPLI elevations > 200 μg/dL (P = 0.101), > 400 μg/dL (P = 0.670), or those with clinical signs compatible with pancreatitis (P = 1.000) before and after treatment. None of the dogs with clinical signs of pancreatitis after treatment had an increased serum cPLI concentration > 400 μg/dL. It is important to note that 14% of the dogs had a serum cPLI > 400 μg/dL after therapy, but had no clinical signs compatible with pancreatitis.
Figure 1.
Box and whiskers plot of group 1 measuring canine pancreatic lipase immunoreactivity (cPLI) concentrations before and after treatment with L-asparaginase (ASNase) and vincristine concurrently. P = 0.058
Group 2
Twenty-four dogs received ASNase alone and were reevaluated 24 to 96 h later. Upon presentation, 1 dog was classified as stage II, 8 dogs as stage III, 8 dogs as stage IV, and 7 dogs as stage V. Fourteen of the dogs were substage b and 6 dogs were hypercalcemic at presentation.
Ten of the 24 dogs presented with signs that could have been compatible with pancreatitis, with anorexia being the most common clinical finding (90%). After administration of ASNase, 5 dogs had clinical signs suggestive of pancreatitis. Three of these 5 dogs had signs compatible with pancreatitis prior to administration of the ASNase. The median pre-administration serum cPLI concentration for all 24 dogs was 63.5 μg/dL (range: 29.0 to 581.7 μg/dL). The median cPLI level after ASNase was 98.0 μg/dL (range: 29.0 to 851.1 μg/dL). Median serum cPLI concentrations did not change significantly after ASNase administration (P = 0.305). Only 1 of the dogs with clinical signs compatible with pancreatitis had a serum cPLI concentration > 400 μg/dL prior to ASNase administration. None of the dogs with clinical signs compatible with pancreatitis after administration of ASNase had a serum cPLI concentration > 400 mg/dL. Also, there was no statistically significant difference between the number of dogs with a cPLI concentration > 200 μg/dL (P = 1.00), > 400 μg/dL (P = 0.416), or between the number of dogs with clinical signs compatible with pancreatitis (P = 0.212) before and after treatment.
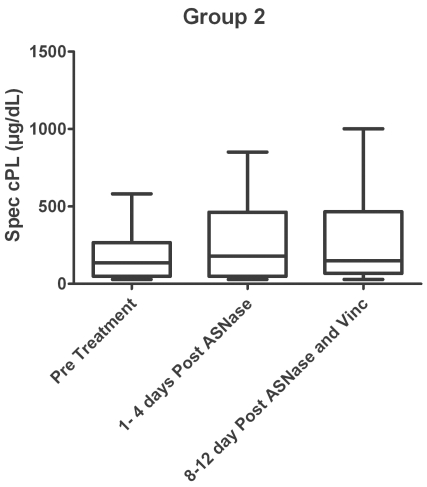
Eighteen of the dogs that initially received ASNase alone (group 2) were then treated with vincristine at their follow-up visit. The remaining 6 dogs continued with their therapy with their local veterinarian. The statistics were then recalculated with the remaining 18 dogs. Seven days after vincristine administration (8 to12 d after the initial ASNase treatment), 4 dogs had clinical signs compatible with pancreatitis. However, 2 of these dogs had initially presented with similar clinical signs even before vincristine was administered. The median serum cPLI concentration was 137 μg/dL (range: 29.0 to 581.7 μg/dL) before ASNase, 179 μg/dL (range: 29.0 to 851.0 μg/dL) after ASNase and immediately prior to vincristine therapy, and 149 μg/dL (range: 29.0 to 1001.0 μg/dL) 7 d after vincristine administration (Figure 2). There was no statistically significant difference in the median serum cPLI concentration between the 3 groups (P = 0.455). Also, there was no significant difference between the number of dogs with cPLI concentrations > 200 μg/dL (P = 0.927), or > 400 μg/dL (P = 0.320), or the number of dogs with clinical signs compatible with pancreatitis (P = 0.289) before or after treatment. None of the dogs with clinical signs compatible with pancreatitis after ASNase followed by vincristine therapy had a serum cPLI concentration > 400 μg/dL.
Figure 2.
Box and whiskers plot of the subpopulation of group 2 dogs (received vincristine 24 to 96 h after administration of ASNase) measuring canine pancreatic lipase immunoreactivity (cPLI) concentrations before chemotherapy, after ASNase, and after vincristine. P = 0.455
Group 3
A 3rd treatment group was later added to the study to evaluate response to vincristine therapy alone. These dogs were taken from both group 1 and group 2. All 13 dogs were in clinical remission at the time of presentation for an additional single dose of vincristine. None of these dogs had received any chemotherapy for at least 7 d prior to administration, but all of the dogs were on daily oral prednisone therapy (30 to 40 mg/m2).
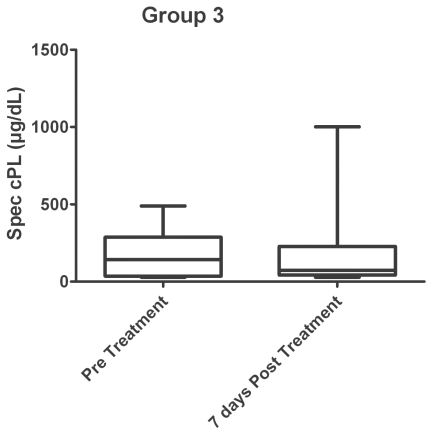
Three of the 13 dogs had clinical signs compatible with pancreatitis prior to administration of vincristine, but only 1 of these dogs had clinical signs compatible with pancreatitis 7 d after administration. The median serum cPLI concentration before treatment was 142 μg/dL (range: 29.0 to 489.0 μg/dL) and was 74 μg/dL (29.0 to 1001.0 μg/dL) 7 d after the treatment (Figure 3). There was no statistical difference (P = 0.424) in the median cPLI concentration between the 2 groups. Furthermore, there was no significant difference in the number of dogs with a cPLI concentration > 200 μg/dL (P = 1.000), > 400 μg/dL (P = 1.000), or those with clinical signs compatible with pancreatitis (P = 0.372) before and after treatment of vincristine. The only dog with clinical signs compatible with pancreatitis after vincristine therapy had a cPLI concentration of < 29 μg/dL.
Figure 3.
Box and whiskers plot of group 3 dogs (vincristine only) measuring canine pancreatic lipase immunoreactivity (cPLI) concentrations before and after vincristine chemotherapy. P = 0.424
Eight of the 52 dogs were presented to the 2 referral hospitals with a history of longterm corticosteroid use prior to their diagnosis of lymphoma. Longterm usage was defined as any steroid administration or abnormally high endogenous cortisol levels (such as, hyperadrenocorticism) for more than 7 d. One dog was previously diagnosed with hyperadrenocorticism and was included although it was not treated with exogenous corticosteroids. Only 1 of these dogs that had received long-term steroids had clinical signs compatible with pancreatitis prior to any chemotherapy treatment. None of the dogs had an increased serum cPLI concentration prior to treatment (median: 91.5 μg/dL, range: 29.0 to 177.0 μg/dL).
There was a marked difference in the percentage of dogs with substage b that received ASNase (50%) alone compared with the percentage that received both drugs concurrently (21%, P = 0.0010). Yet, systemic illness did not appear to have any effect on the outcome of therapy in regards to either clinical or subclinical pancreatitis. However, there were only a limited number of dogs with substage b in group 1 (concurrent ASNase and vincristine).
Discussion
It has previously been reported that 0.7% to 16.2% of humans have evidence of pancreatitis after intravenous administration of ASNase (12–14). Depending on the study, the diagnosis of pancreatitis was based on clinical signs of vomiting, abdominal pain, anorexia, abdominal ultrasound findings consistent with pancreatitis, and serum amylase and lipase activities (12–14). With the use of serum concentrations of trypsin and elastase-1, one study reported that 100% (P < 0.01) of human patients treated with ASNase showed evidence of subclinical pancreatitis (17).
Diagnosing clinical and subclinical pancreatitis, regardless of etiology, remains a challenge in veterinary medicine. Historical and physical examination findings in dogs are not specific for pancreatitis. However, when present they may be compatible with clinical disease. Serum amylase and lipase activities are not considered reliable for the diagnosis of canine pancreatitis (17,24). There are various sources of both amylase and lipase in the body, thus making these tests highly non-specific for pancreatitis (17,24). Furthermore, canine lymphoma has been shown to increase serum lipase activity, making this test even more unreliable for the purpose of this study (25). Abdominal sonography has been one of the best modalities for diagnosing pancreatitis in dogs, with a reported sensitivity of 68% (26). However, in humans, sonography was deemed ineffective at determining subclinical pancreatitis secondary to administration of ASNase (13).
Unlike human medicine, which may rely on trypsin and elastase-1 levels as more sensitive markers for pancreatitis, canine trypsin-like immunoreactivity has been found to have a suboptimal sensitivity and specificity for pancreatitis (17,19). However, it should also be noted that this diagnostic test is the gold standard for the diagnosis of exocrine pancreatic insufficiency in dogs (17). Canine pancreatic lipase immunoreactivity has recently been introduced as a non-invasive test for canine pancreatitis. When compared to clinical gastritis (a disease process with similar clinical signs), specificity of cPLI for canine pancreatitis was 96% (27). Recent unpublished data also show a significant improvement in sensitivity for the diagnosis of both clinical and subclinical pancreatitis (27). The specificity of this test to differentiate between clinical gastritis and pancreatitis makes it ideal for testing for chemotherapy-induced pancreatitis, as most chemotherapy agents (including vincristine) may be associated with clinical gastritis. However, the authors recognize that the sensitivity of cPLI is not scientifically documented and there may have been patients where ASNase induced low grade pancreatitis and clinical signs or elevations in cPLI were not apparent.
At some point during the study, vincristine, prednisone, or both were administered to all of the patients enrolled. While the incidence is highly debated, research in humans has documented pancreatitis secondary to long-term administration of glucocorticoids (28). It is unlikely that steroids would have played a role in the development of pancreatitis in the dogs in this study, as most of them were receiving prednisone at the beginning of the study and, therefore, had received prednisone for 7 d or less. Eight of the dogs in this study were either diagnosed with hyperadrenocorticism (n = 1) or received long-term steroids (more than 7 d of treatment; n = 7) for a variety of diseases including lymphoma. All of these dogs had serum cPLI concentrations within the reference range prior to therapy. These findings support previous data that showed that cPLI levels are not changed with chronic prednisone therapy (29). It appears unlikely that steroid therapy played any role in the results of this study.
There was no statistically significant change in cPLI concentrations before and after vincristine administration by itself (group 3). Only 1 dog had clinical signs compatible with pancreatitis after vincristine treatment. However, with a cPLI concentration of < 29 μg/dL, these clinical signs could easily be attributable to gastrointestinal toxicosis associated with vincristine. In mice, vinca alkaloids alone have been reported to cause autophagy and degeneration of acinar cells of the pancreas (30,31). However, as with prednisone, vincristine by itself does not appear to cause clinical or subclinical pancreatitis in dogs.
Eleven dogs from all 3 groups had clinical signs compatible with pancreatitis after their respective treatment protocol. One hundred percent of these dogs (11 of 11) had a serum cPLI concentration that measured < 400 μg/dL (median of 80 μg/dL, range: 29 to 367 μg/dL). This data suggests that the clinical signs observed in these dogs are more likely a result of primary gastrointestinal toxicosis and not drug induced pancreatitis (27). In our dogs, this would also suggest that ASNase did not result in clinical pancreatitis, which is dissimilar to reports in human medicine.
Elevations of serum cPL concentrations occurred in both groups of dogs receiving ASNase. These elevations ranged from minor increases within the reference range to substantial increases consistent with subclinical pancreatitis. While not statistically significant, there does appear to be an idiosyncratic rise in cPLI concentration associated with ASNase treatment. Thus, while the majority of dogs did not have a significant change in their serum cPLI concentrations after administration of ASNase, some dogs may be at risk for developing subclinical pancreatitis after ASNase treatment. Consequently, it is the authors’ opinion that the finding of subclinical pancreatitis (cPL > 400 μg/dL without clinical signs compatible with pancreatitis) after ASNase administration may justifiably result in an alteration of the therapeutic protocol, with ASNase being used only if absolutely necessary and not in combination with other pancreatitis inducing drugs.
Dogs in group 1 (administration of vincristine and ASNase at the same time) had a median serum cPLI concentration of 88.5 μg/dL prior to treatment and a median serum cPLI concentration of 138.5 μg/dL. Six of these dogs had serum cPLI concentrations within the reference range before therapy, but had a serum cPLI concentration in the questionable range (200 to 400 μg/dL) after treatment. The change in median cPLI concentrations was not statistically significant (P = 0.058). Perhaps a larger sample size of dogs may have shown a significant difference in median serum cPLI concentrations before and after treatment. However, based on this data, combination therapy with the 2 medications does not place dogs at an obvious increased risk for development of subclinical pancreatitis, among other toxicities.
Neutropenia is a well-documented side effect of concurrent vincristine and ASNase administration and appears to be a result of synergistic toxicity between the 2 drugs (19). Synergistic pancreatitis has also been reported in humans treated with vinca alkaloids and a variety of other chemotherapy agents (32,33). It is possible that a second synergistic toxicity, subclinical pancreatitis, could also be caused by the combination of ASNase and vincristine. In fact, the combination of vinca alkaloids and ASNase therapy has been linked to pancreatitis in humans (34). Therefore, it has been recommended by manufacturing pharmaceutical companies and independent research that ASNase be administered at least 12 h after vincristine administration, because it may interfere with hepatic clearance of the vinca alkaloid (Elspar; Merck & Company, Whitehouse Station, New Jersey, USA; Vincristine; Mayne Pharma, Pannus, New Jersey, USA) (34). Our study did not follow those guidelines, as we opted to administer ASNase simultaneously or before vincristine. Consequently, a cPLI increase noted in some dogs may have been a result of delayed hepatic clearance of vincristine. Unfortunately, liver function was never evaluated in the dogs in this study after the initial blood work prior to administration of chemotherapy.
Based on the low percentage of dogs with clinical signs compatible with pancreatitis after treatment, evaluation of serum cPLI concentration for all dogs with newly-diagnosed lymphoma does not appear to be clinically necessary. The significance of subclinical pancreatitis remains unclear in veterinary medicine. It is the authors’ recommendation, until further studies can more accurately rule out a reaction, that substage b dogs or dogs with predisposing factors for pancreatitis not be treated with ASNase and vincristine concurrently as this may potentiate an episode of pancreatitis.
Several limitations of the study should be noted. First, ASNase can result in clinical pancreatitis up to 10 wk after administration in humans with lymphoid malignancy (14). This has never been reported in veterinary medicine. However, our study’s lack of longterm follow-up eliminates a more accurate assessment of this phenomenon. The variation in sample collection times after the initial ASNase dose represents a study flaw. In theory, serum cPLI concentrations peak very early after ASNase, as the half life of this drug in humans is 30 h, but not documented in the canine to date (5). Because of the lack of weekend and holiday treatments, combined with sometimes substantial client commutes, sampling of all patients 24 h after the ASNase treatment was not possible. While this design flaw may have altered the cPLI levels, it should not change the incidence of clinical pancreatitis, as thorough histories from owners were obtained regarding the time interval between treatments.
Abdominal ultrasound was not preformed in all patients during staging, nor performed by the same radiologist, and never performed after administration of ASNase. Consequently, this study is unable to further validate the cPLI measurements with ultrasonographic findings in relation to identification of subclinical pancreatitis. Elevations in cPLI concentration combined with abdominal ultrasound findings consistent with pancreatic inflammation would have further substantiated our findings. Due to the lack of validity surrounding cPLI levels and pancreatitis, further studies regarding drug induced pancreatitis should incorporate abdominal ultrasound of the pancreas.
While an incidence of lymphoma in the pancreas at the time of diagnosis has not been reported, the authors recognize that lymphoma within the pancreas does exist. Pancreatic lymphoma could have explained the elevations in cPLI prior to treatment. Furthermore, cPLI concentrations may have declined post treatment due to the elimination of tumor while the dog experiences a low grade, undetectable subclinical pancreatitis.
The authors acknowledge the lack of a control arm. However, the ethical dilemma in treating healthy animals with chemotherapeutic agents prevented us from setting up such a control group, and only animals diagnosed with lymphoma were included in the study. Animals with elevated cPLI and evidence of clinical pancreatitis were also enrolled because removal of these 2 groups of dogs would be detrimental to the clinical relevance of the manuscript. First, the dogs with clinical signs of pancreatitis may or may not have actually had pancreatitis and, in all likelihood, had some level of lymphoma induced gastritis. Removal of this population would not account for a significant number of dogs that present to the audience in substage “b” which could safely receive ASNase. Furthermore, prior to beginning the investigation the hypothesis was made that ASNase dose not cause pancreatitis. Based on that hypothesis, it was important to investigate whether or not dogs with an elevated cPLI could still safely receive ASNase. Finally, removal of the dogs that fall under either category would have eliminated 16 dogs and further weakened the power of the study.
Finally, human patients have an increased risk of anaphylactoid reactions with repeated dosing of ASNase, especially when given intravenously (11,35). Our study did not look at the risk or incidence of pancreatitis with multiple dosings or when given via an alternative route other than subcutaneous administration. In fact, the discrepancy between the incidence of pancreatitis in humans versus the incidence in dogs may be a result of the intravenous administration of ASNase over successive days, as is done in many human treatment protocols (35).
In conclusion, administration of ASNase alone or in conjunction with vincristine did not cause clinical pancreatitis in the dogs enrolled in this study. This is in contrast with an incidence of up to 16% of clinical pancreatitis in humans treated with ASNase. It must be noted that this study was underpowered and results should be evaluated appropriately. When given as a sole chemotherapeutic agent, ASNase did not cause a significant change in serum cPLI concentrations after treatment. However, individual dogs did show increases in serum cPLI concentrations without developing signs compatible with pancreatitis, suggesting subclinical pancreatitis. There was a percentage of dogs that showed an increase in serum cPLI above the reference range when ASNase was administered concurrently with vincristine. Further studies are needed to prove or disprove the notion that concurrent treatment may predispose rare individuals to subclinical or clinical pancreatitis.
There has been recent debate over the role of ASNase in the treatment of canine lymphoma (36,37). While evidence suggests that it does not improve survival time when incorporated into a standard multi-drug induction protocol, it still clearly has a place among chemotherapy agents as a safe and effective rescue treatment option. Further studies are needed to evaluate the incidence of pancreatitis in dogs after combination therapy with vincristine and ASNase, as well as the risks of pancreatitis after repeated exposure to ASNase.
Acknowledgments
The authors thank D. Green and K. Schneider for their help in sample collection. A special thanks to the Gastrointestinal Laboratory at Texas A&M University for funding this project and to its technical staff for running the assays.
Footnotes
The research samples were collected at both the Texas A&M University (TAMU) Small Animal Clinic and Veterinary Specialty Center of Texas. Samples were processed and the data analyzed at TAMU Small Animal Clinic and the GI Laboratory of TAMU.
Preliminary data was presented in abstract form and published as such in the annual proceedings of the 26th Annual Veterinary Cancer Society in Pine Mountain, Georgia, USA.
Funding was provided by the GI Lab of TAMU.
References
- 1.Vail DM, Young KM. Hematopoetic malignancies. In: Withrow SJ, Vail DM, editors. Small Animal Clinical Oncology. 4th ed. St. Louis, Missouri: Elsevier; 2006. pp. 699–733. [Google Scholar]
- 2.Zemann BI, Moore AS, Rand WM, et al. A combination chemotherapy protocol (VELCAP-L) for dogs with lymphoma. J Vet Intern Med. 1998;12:465–470. doi: 10.1111/j.1939-1676.1998.tb02151.x. [DOI] [PubMed] [Google Scholar]
- 3.Myers NC, Moore AS, Rand WM, Gliatto J, Cotter SM. Evaluation of a multidrug chemotherapy protocol (ACOPA III) in dogs with lymphoma. J Vet Intern Med. 1997;11:333–339. doi: 10.1111/j.1939-1676.1997.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 4.Saba CF, Thamm DH, Vail DM. Combination chemotherapy with L-asparaginase, lomustine, and prednisone for relapsed or refractory canine lymphoma. J Vet Intern Med. 2007;21:127–132. doi: 10.1892/0891-6640(2007)21[127:ccwlla]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Chabner BA, Friedmann AM. Asparaginase. In: Chabner BA, Longo DL, editors. Cancer Chemotherapy and Biotherapy. 4th ed. Philadelphia, Pennsylvania: Lippincott Williams and Wilkins; 2006. pp. 476–483. [Google Scholar]
- 6.Hansen JF, Carpenter RH. Fatal acute systemic-anaphylaxis and hemorrhagic-pancreatitis following asparaginase treatment in a dog. J Am Anim Hosp Assoc. 1983;19:977–980. [Google Scholar]
- 7.Rogers KS, Barton CL, Benson PA, Green RA. Effects of single-dose L-asparaginase on coagulation values in healthy dogs and dogs with lymphoma. Am J Vet Res. 1992;53:580–584. [PubMed] [Google Scholar]
- 8.Swanson JF, Morgan S, Green RA, et al. Cerebral thrombosis and hemorrhage in association with L-asparaginase administration. J Am Anim Hosp Assoc. 1986;22:749–755. [Google Scholar]
- 9.Teske E, Rutteman GR, Van Heerde P, Misdorp W. Polyethylene glycol-L-asparaginase versus native L-asparaginase in canine non-Hodgkins-lymphoma. Eur J Cancer. 1990;26:891–895. doi: 10.1016/0277-5379(90)90193-w. [DOI] [PubMed] [Google Scholar]
- 10.Sahu S, Saika S, Pai SK, Advani SH. L-asparaginase (Leunase) induced pancreatitis in childhood acute lymphoblastic leukemia. Pediatr Hematol Oncol. 1998;15:533–538. doi: 10.3109/08880019809018315. [DOI] [PubMed] [Google Scholar]
- 11.Haskell CM, Canellos GP, Leventha BG, et al. L-asparaginase: therapeutic and toxic effects in patients with neoplastic disease. N Engl J Med. 1969;281:1028–1034. doi: 10.1056/NEJM196911062811902. [DOI] [PubMed] [Google Scholar]
- 12.Cetin M, Yetgin S, Kara A, et al. Hyperglycemia, ketoacidosis and other complications of L-asparaginase in children with acute lymphoblastic-leukemia. J Med. 1994;25:219–229. [PubMed] [Google Scholar]
- 13.Nguyen DL, Wilson DA, Engelman ED, Sexauer CL, Nitschke R. Serial sonograms to detect pancreatitis in children receiving L-asparaginase. South Med J. 1987;80:1133–1136. doi: 10.1097/00007611-198708090-00015. [DOI] [PubMed] [Google Scholar]
- 14.Weetman RM, Baehner RL. Latent onset of clinical pancreatitis in children receiving L-asparaginase therapy. Cancer. 1974;34:780–785. doi: 10.1002/1097-0142(197409)34:3<780::aid-cncr2820340338>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 15.Ogilvie GK, Atwater SW, Ciekot PA, et al. Prevalence of anaphylaxis associated with the intramuscular administration of L-asparaginase to 81 dogs with cancer — 1989–1991. J Am Anim Hosp Assoc. 1994;30:62–65. [Google Scholar]
- 16.Steiner JM, Williams DA. Development and validation of a radioimmunoassay for the measurement of canine pancreatic lipase immunoreactivity in serum of dogs. Am J Vet Res. 2003;64:1237–1241. doi: 10.2460/ajvr.2003.64.1237. [DOI] [PubMed] [Google Scholar]
- 17.Steiner JM. Diagnosis of pancreatitis. Veterinary Clinics of North America: Small Animal Practice. 2003;33:1181–1195. doi: 10.1016/s0195-5616(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 18.Turner MA. The role of US and CT in pancreatitis. Gastrointest Endosc. 2002;56:S241–245. doi: 10.1067/mge.2002.129019. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu T, Yamashiro Y, Igarashi J, Fujita H, Ishimoto K. Increased serum trypsin and elastase-1 levels in patients undergoing L-asparaginase therapy. Eur J Pediatr. 1998;157:561–563. doi: 10.1007/s004310050878. [DOI] [PubMed] [Google Scholar]
- 20.Owen LN. TNM Classification of Tumours in Domestic Animals. Geneva: World Health Organization; 1980. p. 53. [Google Scholar]
- 21.Northrup NC, Rassnick KM, Snyder LA, et al. Neutropenia associated with vincristine and L-asparaginase induction chemotherapy for canine lymphoma. J Vet Intern Med. 2002;16:570–575. doi: 10.1892/0891-6640(2002)016<0570:nawval>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Wright ZM, Rogers KS, Steiner JM. Incidence of subclinical pancreatitis after L-asparaginase administration in dogs with lymphoma. Proc Annu Meet Vet Canc Soc. 2006;4 [Google Scholar]
- 23.Gumminger SR, Steiner JM, Ruaux CG, et al. Stability of serum canine pancreatic lipase (cPLI) concentration and comparison of cPLI concentrations in serum and plasma. Proc Annu Meet Amer Coll Vet Intern Med. 2002;382 [Google Scholar]
- 24.Ruaux CG. Diagnostic approaches to acute pancreatitis. Clin Tech Small Anim Pract. 2003;18:245–249. doi: 10.1016/S1096-2867(03)00072-0. [DOI] [PubMed] [Google Scholar]
- 25.Strombeck DR, Farver T, Kaneko JJ. Serum amylase and lipase activities in the diagnosis of pancreatitis in dogs. Am J Vet Res. 1981;42:1966–1970. [PubMed] [Google Scholar]
- 26.Hess RS, Saunders HM, Van Winkle TJ, et al. Clinical, clinico-pathologic, radiographic, and ultrasonographic abnormalities in dogs with fatal acute pancreatitis: 70 cases (1986–1995) J Am Vet Med Assoc. 1998;213:665–670. [PubMed] [Google Scholar]
- 27.Steiner JM, Broussard J, Teague SR, et al. Serum canine pancreatic lipase immunoreactivity (cPLI) concentrations in dogs with gastritis. Proc Annu Meet Euro Coll Vet Intern Med. 2003 [Google Scholar]
- 28.Badalov N, Baradarian R, Iswara K, Li J, Steinberg W, Tenner S. Drug-induced acute pancreatitis: An evidence-based review. Clin Gastroenterol Hepatol. 2007;5:648–661. doi: 10.1016/j.cgh.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 29.Steiner JM, Lees GE, Willard MD. Serum canine pancreatic lipase immunoreactivity (cPLI) concentration is not altered by oral prednisone administration. J Vet Intern Med. 2003;17:444. [Google Scholar]
- 30.Nevalainen TJ. Cytotoxicity of vinblastine and vincristine to pancreatic acinar cells. Virchows Arch B Cell Pathol. 1975;18:119–127. doi: 10.1007/BF02889240. [DOI] [PubMed] [Google Scholar]
- 31.Riemenschneider TA, Wilson JF, Vernier RL. Glucocorticoid-induced pancreatitis in children. Pediatrics. 1968;41:428–437. [PubMed] [Google Scholar]
- 32.Newman CE, Ellis DJ. Pancreatitis during combination chemotherapy. Clin Oncol. 1979;5:83–84. [PubMed] [Google Scholar]
- 33.Socinski MA, Garnick MB. Acute-pancreatitis associated with chemotherapy for germ-cell tumors in 2 patients. Ann Intern Med. 1988;108:567–568. doi: 10.7326/0003-4819-108-4-567. [DOI] [PubMed] [Google Scholar]
- 34.Schuler D, Koós R, Révész T, Virág I, Gálfi I. L-asparaginase therapy and its complications in acute lymphoid leukaemia and generalized lymphosarcoma. Haematologia (Budap) 1976;10:205–211. [PubMed] [Google Scholar]
- 35.Copur MS, Rose MG, Chu E. Miscellaneous chemotherapeutic agents. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 7th ed. Philadelphia, Pennsylvania: Lippincott Williams and Wilkins; 2005. pp. 416–422. [Google Scholar]
- 36.Jeffreys AB, Knapp DW, Carlton WW, et al. Influence of asparaginase on a combination chemotherapy protocol for canine multi-centric lymphoma. J Am Anim Hosp Assoc. 2005;41:221–226. doi: 10.5326/0410221. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald VS, Thamm DH, Kurzman ID, Turek MM, Vail DM. Does L-asparaginase influence efficacy or toxicity when added to a standard CHOP protocol for dogs with lymphoma? J Vet Intern Med. 2005;19:732–736. doi: 10.1892/0891-6640(2005)19[732:dlieot]2.0.co;2. [DOI] [PubMed] [Google Scholar]