Abstract
This report describes a whole-blood flow cytometric method for the determination of intracellular cytokines IFN-γ and IL-4 in canine T lymphocyte subpopulations. Conjugated anti-cytokine antibodies and commercially available reagents for cell fixation and permeabilization were used. Canine peripheral blood was cultured with a combination of phorbol-12-myristate-13-acetate (PMA) and ionomycin to promote cytokine synthesis in each cell, along with monensin to increase the sensitivity of the method by retaining IFN-γ and IL-4 within the cell to detectable levels. The optimum concentrations of PMA and ionomycin were determined. Maximum IFN-γ expression from both CD4+ and CD8+ T lymphocytes was detected after 6 h of incubation of cell culture, while maximum IL-4 production took 6 h from CD4+ cells and 4 h from CD8+ cells. This method is a simple immunologic technique for measuring intracellular cytokines which could be of value in the investigation of canine immunological response mainly in various intracellular and extracellular infections, since IFN-γ and IL-4 are considered key cytokines activating the cellular and humoral immunity, respectively.
Résumé
Cet article décrit une méthode de cytométrie en flux utilisant du sang entier pour la détermination des cytokines intracellulaires IFN-γ et IL-4 dans les sous-populations de lymphocytes T canins. Des anticorps conjugués anti-cytokines ainsi que des réactifs commerciaux pour la fixation et la perméabilisation cellulaire ont été utilisés. Du sang périphérique canin a été mis en culture avec une combinaison d’acétate de phorbol-12-myristate-13 (PMA) et de l’ionomycine afin de promouvoir la synthèse de cytokine dans chaque cellule, ainsi qu’avec du monensin afin d’augmenter la sensibilité de la méthode en maintenant l’IFN-γ et l’IL-4 dans les cellules à des seuils détectables. Les concentrations optimales de PMA et d’ionomycine ont été déterminées. L’expression maximale d’IFN-γ par les lymphocytes T CD4+ et CD8+ a été détectée après 6h d’incubation de la culture cellulaire, alors que la production maximale d’IL-4 à pris 6 h pour les cellules CD4+ et 4 h pour les cellules CD8+. Cette méthode est une technique immunologique simple pour mesurer les cytokines intracellulaires qui pourrait s’avérer intéressante dans l’étude de la réponse immunologique canine principalement lors d’infections intra- et extracellulaires variées, compte tenu du fait que IFN-γ et IL-4 sont considérés comme des cytokines importantes activant respectivement, l’immunité cellulaire et l’immunité humorale.
(Traduit par Docteur Serge Messier)
Introduction
Cytokines are important mediators during immune responses and are produced by a variety of activated cells including lymphocytes. IFN-γ and IL-4 are important cytokines implicated in both antimicrobial host defense and pathogenesis of diseases with an inflammatory component. IFN-γ is one of the main cytokines that preferentially activates the cellular immunity, whereas IL-4 is considered the main activator of humoral immunity in dogs (1). Although the production of these cytokines could be studied at both the protein and mRNA level, measuring the bulk production of cytokine protein or message following the isolation of lymphocytes or peripheral blood mononuclear cells, provides a useful but incomplete picture, since the relative contribution of individual cell subsets (such as, CD4+, CD8+) to cytokine production cannot be determined.
The advent of intracellular cytokine assessment by flow cytometry, through staining of permeabilized human cells with fluorochrome-conjugated anti-cytokine antibodies, permits large numbers of cells of known phenotype to be examined (2,3). This study reports a rapid, whole-blood flow cytometric assay for the determination of IFN-γ and IL-4 in canine CD4+ and CD8+ lymphocytes that requires small volumes of blood and 4 to 6 h culture time. The propensity of CD4+ and CD8+ lymphocytes from normal dogs to produce IFN-γ and IL-4 is given as an example.
Materials and methods
Animals
Peripheral blood from 7 healthy dogs of various breeds and ages was used. All animals were clinically healthy and seronegative for Leishmania infantum, Ehrlichia canis, and Dirofilaria immitis. Additionally, none of these dogs presented with clinical or laboratory evidence for concurrent infectious or noninfectious disease.
Cell culture and staining
Peripheral blood (5 mL) was collected from each dog into vials that contained lithium heparin and transported to the flow cytometry laboratory of National School of Public Health within 1 to 3 h for processing.
Aliquots of blood (0.5 mL) were added into 2 ventilation-capped, 5-mL polystyrene round-bottomed plastic tubes (Greiner Labortechnik, Dursley, Gloucestershire, United Kingdom) with 0.5 mL culture medium RPMI 1640 (Gibco-BRL, Gaithersburg, Maryland, USA). One tube (the resting population) contained the cells that were not to be activated, and the other tube contained cells to be activated. To increase the quantity of cytokine available for detection within each cell, 3 μL of monensin (Sigma, St. Louis, Missouri, USA) was added to all cultures at a concentration of 3 μmol/L. In tubes containing the cells to be activated, phorbol-12-myristate-13-acetate (PMA) (Applichem, Darmstadt, Germany) was added at a final concentration of 10 ng/mL, and 3 μL of ionomycin (Applichem, Darmstadt, Germany) at a final concentration of 2 μmol/L. Tubes were then incubated for 4 to 6 h at 37°C in a humid 5% CO2 atmosphere.
After incubation, and following the manufacturer’s instructions, 100-μL aliquots of cell suspension were added to the appropriate tubes. Monoclonal antibodies (20 μL) specific for canine CD4 and CD8 cell surface antigens (rat anti-canine CD4:FITC and rat anti-canine CD8:FITC) (Serotec, Kidlington, Oxford, United Kingdom) were added along with appropriate isotypic negative (rat IgG2a: FITC and rat IgG1: FITC) (Serotec) and positive (mouse anti-human CD69:RPE; Beckman Coulter, Miami, Florida, USA) controls; these tubes were incubated for 15 min at room temperature. The cells were then fixed by adding 100 μL Leucoperm-Reagent A (Serotec) to each tube and then incubating for 15 min. After this, the cells were washed twice with phosphate buffer saline (PBS) containing sodium azide 0.1% and bovine serum albumin 1%, and then permeabilized by adding 100 μL of Leucoperm-Reagent B (Serotec). Additionally, 20 μL of monoclonal antibodies specific for bovine IFN-γ and IL-4 (Serotec) along with appropriate isotypic negative controls (mouse IgG2a:RPE and mouse IgG1:RPE; Serotec), at a concentration of 100 μg/mL, were added and the cells incubated at room temperature for 20 min. Samples were then washed twice with PBS and analyzed with flow cytometry. All samples were stained in the dark.
Monoclonal antibodies
The monoclonal antibodies used in this study are shown in Table I.
Table I.
Monoclonal antibodies (MoAb) used in the study
| Subclass | MoAb | Conjugate |
|---|---|---|
| IgG1 | Rat anti-canine CD8a | FITC |
| IgG1 | Rat anti-canine CD8a | RPE |
| IgG2a | Rat anti-canine CD4a | FITC |
| IgG2a | Mouse anti-bovine IL-4a | RPE |
| IgG1 | Mouse anti-bovine IFN-γa | RPE |
| IgG2a | Rat immunoglobulina | FITC |
| IgG1 | Rat immunoglobulina | FITC |
| IgG1 | Mouse immunoglobulina | FITC |
| IgG2a | Mouse immunoglobulina | RPE |
| IgG1 | Mouse immunoglobulina | RPE |
| IgG1 | Mouse antihuman CD69b | RPE |
Serotec (Kidlington, Oxford, United Kingdom).
Beckman Coulter (Miami, Florida, USA).
Flow cytometry
A Coulter Epics-XL-MCL 4-color cytometer was used in this study. Listmode data from 20 000 events were acquired without color compensation. The latter was subsequently applied to the listmode data using the color compensation toolbox within the WinList Version 3.0 flow cytometry analysis software (Verity; Topsham, Maine, USA) using canine lymphocytes stained with the strongly positive single color MoAbs CD4:FITC, CD8:FITC, CD8:RPE, IFN-γ:RPE, and IL-4:RPE as standards. IFN-γ and IL-4 positive CD4+ or CD8+ lymphocytes were defined by setting regions with the lower limits for cytokine positivity determined from the unstimulated cultures. Results were expressed as the percentage of cytokine positive CD4+ or CD8+ cells within the gating area of lymphocytes.
Determination of optimal stimulant dose
Peripheral blood from 4 dogs was used as follows: a) ionomycin concentration (2 μmol/L) was kept stable and cells were cultured for 2 h with PMA at 5, 10, and 15 ng/mL concentrations; and b) PMA concentration (10 ng/mL) was kept stable and cells were cultured for 2 h with ionomycin at 1, 2, 5, and 10 μmol/L concentrations. The concentration of monensin was held constant at 3 μmol/L in all cases.
Determination of optimal culture time
Peripheral blood from 3 normal dogs was used as follows: cells were incubated at 37°C with monensin (3 μmol/L) with or without the addition of PMA and ionomycin at the optimal concentrations (as determined previously) for 2, 4, 6, and 8 h. The optimal culture time was estimated by the max cytokine production in either CD4+ or CD8+ lymphocytes.
Expression of CD4 and CD8 antigens with increasing culture time at optimal concentrations of cellular stimulants
The expression of CD4 and CD8 antigen was recorded during the process of optimal culture time determination (as described previously) in the blood of 3 normal dogs.
Results
Determination of the optimum stimulant dose
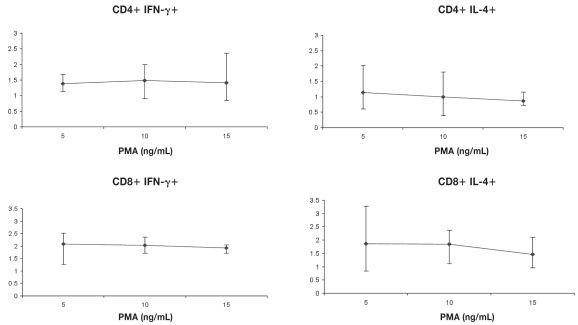
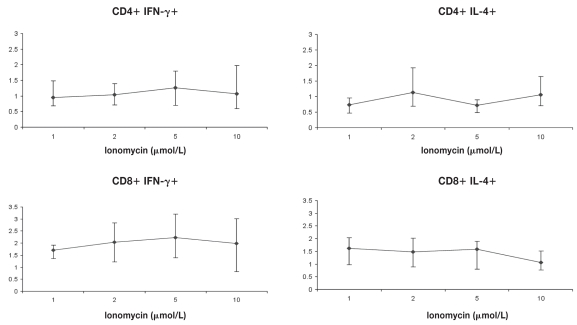
The optimum PMA concentration producing maximal IFN-γ expression was found to be 10 ng/mL for CD4+ lymphocytes and 5 ng/mL for CD8+ lymphocytes, while the optimum PMA concentration producing maximal IL-4 expression was found to be 5 ng/mL for both CD4+ and CD8+ lymphocytes when ionomycin was held constant at 2 μmol/L in both situations (Figure 1). When these concentrations of PMA were used with various concentrations of ionomycin (1, 2, 5, and 10 μmol/L), a 5 μmol/L concentration of ionomycin was found to be optimal for the maximum production of IFN-γ from both CD4+ and CD8+ lymphocytes, while the optimum ionomycin concentration producing maximal IL-4 expression was found to be 2 μmol/L for CD4+ lymphocytes and 1 μmol/L for CD8+ lymphocytes (Figure 2). In subsequent experiments, the above concentrations were used, along with monensin at a concentration of 3 μmol/L. The CD69 molecule (positive control) found to be markedly up-regulated on the cell surface of lymphocytes confirming cell activation.
Figure 1.
Determination of optimum stimulant concentrations. Ionomycin (2 μmol/L) and monensin (3 μmol/L) concentrations were held constant and cells were incubated for 2 h with PMA at 5, 10 and 15 ng/mL. Mean ± standard error of the mean (Sχ̄) of cytokine positive CD4+ and CD8+ cells is shown (n = 4).
Figure 2.
Determination of optimum stimulant concentrations. PMA (10 ng/mL) and monensin (3 μmol/L) concentrations were held constant and cells were incubated for 2 h with ionomycin at 1, 2, 5, and 10 μmol/L. Mean ± Sχ̄ of cytokine positive CD4+ and CD8+ cells is shown (n = 4).
Determination of the optimum culture time
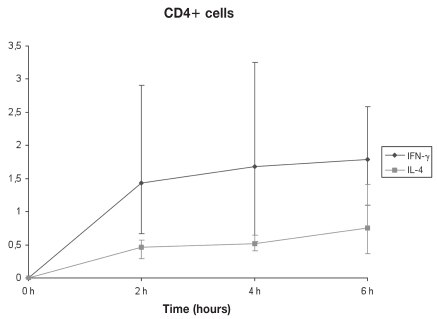
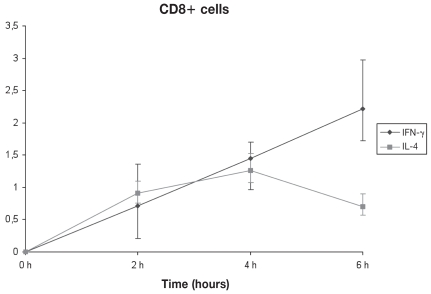
By using the optimum concentrations of PMA and ionomycin, the optimum stimulation time was assessed. For both CD4+ and CD8+ cells, a 6 h stimulus gave maximum IFN-γ production. The same time of culture (6 h) was also required for maximum IL-4 production by CD4+ cells, whereas for CD8+ cells, the corresponding time was found to be 4 h (Figures 3, 4, 5, and 6).
Figure 3.
Kinetics of IFN-γ and IL-4 production under optimal conditions by CD4+ cells. Stimulation for 6 h gave maximum production of both cytokines (n = 3). Error bars show the Sχ̄.
Figure 4.
Kinetics of IFN-γ and IL-4 production under optimal conditions by CD8+ cells. Stimulation for 4 h gave maximum production of IL-4, whereas 6 h stimulation yielded peak IFN-γ production (n = 3). Error bars show the Sχ̄.
Figure 5.
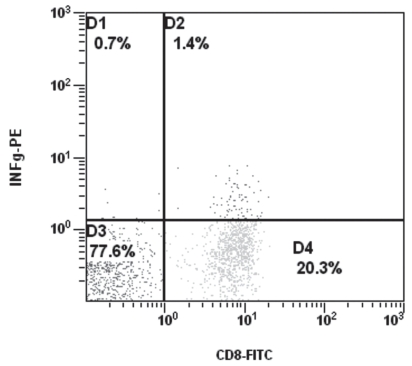
Dot plot and electronic cell separation of CD8+ IFN-γ+ cells at optimum culture time (6 h).
Figure 6.
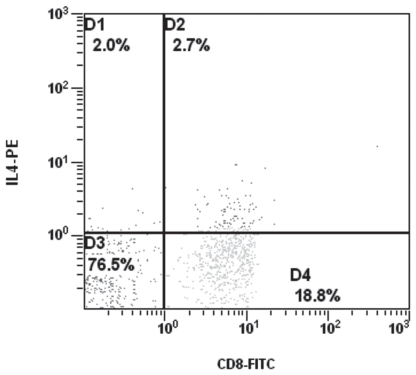
Dot plot and electronic cell separation of CD8+ IL-4+ cells at optimum culture time (4 h).
Expression of CD4 and CD8 antigens with increasing culture time at optimal concentrations of cellular stimulants
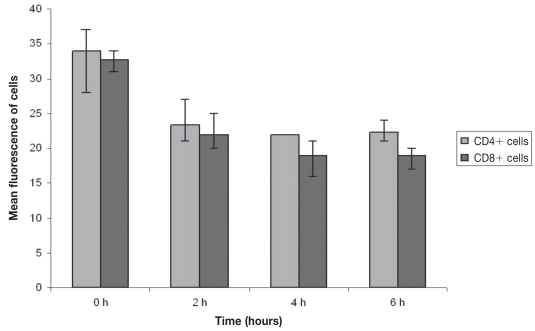
The expression of the canine surface CD4 molecule was observed to be down-regulated with increasing culture time. A down-regulation was observed at 2 h, whereas its expression thereafter (up to 6 h) remained approximately stable (Figure 7). As it is also shown in Figure 7, the down-regulation of canine surface CD8 molecule expression with increasing culture time is significant at 2 h and is continued up to 4 h, although with a lower rate, and then up to 6 h remains approximately stable. However, at 8 h culture time both CD4+ and CD8+ cell numbers were markedly reduced making the interpretation irrelevant.
Figure 7.
CD4 and CD8 expression with increasing culture time (n = 3). Error bars show the Sχ̄
Discussion
Flow cytometry allows both quantitative and statistical analysis of variable cell molecules expressed on the cellular membrane and intracellularly in each individual cell within a short time period (4). Intracellular cytokine staining is quite important in defining functional subsets in mixed T cell populations. The intracellular detection of IFN-γ and IL-4 in canine T lymphocytes allows the frequency of cells producing the respective cytokine to be estimated and thus, to some extent, the cellular or humoral immunity evaluated, respectively (1). This plays an important part in evaluating the immune status of dogs, specifically in cases of intracellular infections such as canine leishmaniasis (5,6).
The commercially available mouse anti-bovine IFN-γ and mouse anti-bovine IL-4 monoclonal antibodies, presenting cross-reaction with the respective canine cytokines, were used in this study (7,8). It has been shown that these cross-reacting antibodies can be used for flow cytometry in the dog (8). The method described in this study has already been applied successfully in humans (9). The advantages of a whole-blood method for the determination of intra-cellular cytokines include the small volume of blood required and the avoidance of lymphocyte separation procedures that may affect cell subpopulations, cytokine production, and activation status. The blood samples were processed immediately (within 2 to 4 h following collection), since it has been found, at least in humans, that irreproducible changes in cytokine production could occur if analysis was not initiated within approximately 4 h of sampling (9).
The results of this study showed that short culture times (from 4 to 6 h) for canine whole-blood samples are satisfactory for the maximum production of IFN-γ and IL-4. Longer times (8 h) resulted in marked reduction of CD4+ and CD8+ cells. The optimal conditions used are a compromise between maximizing cytokine production and minimizing reduction of CD4+ and CD8+ cells, which make the interpretation irrelevant. Despite the fact that apart from T lymphocytes, CD4 molecule is also expressed on canine neutrophils (10), there was no confusion during gating since neutrophils present different forward and side light scattering characteristics from lymphocytes during flow cytometry.
For these reasons, flow cytometry is now being used to assess single-cell cytokine production in both humans (11,2) and mice (12,13). In veterinary medicine, the application of flow cytometry for the determination of intracellular cytokines has been reported so far only in cattle (14). The cytokine monoclonal antibodies used in this study have been tested in a different flow cytometry method and on peripheral blood mononuclear cells isolated from peripheral blood, but not in whole blood (8).
Resting cells do not normally make cytokines, so intracellular cytokine staining requires cells to be stimulated in vitro in order for the respective cytokine genes to be activated. This is better achieved by using PMA and ionomycin (3,15), which is why these cellular stimulants were used in this study. Concurrently, with PMA and ionomycin, monensin was also used to prevent the intracellular transport of cytokines from Golgi apparatus and their secretion (11). The concentration of monensin that was used proved to be nontoxic to cells for up to a 6 h culture time, whereas at 8 h, significant cell damage was observed. Similar results have been reported in humans (11).
The optimal culture time maximizing IFN-γ and IL-4 production by canine lymphocytes was between 4 and 6 h. Similar results have been reported from a respective study in humans, in which PMA and ionomycin were also used as cellular stimulants (9).
In dogs, the down-regulation of CD4 and CD8 expression following cell stimulation seems to occur in a partially different way than in humans, in whom a continuous down-regulation of these molecules has been observed (16,17). In dogs, however, CD4 presents a decreased expression at a 2 h culture time without any further significant change up to 6 h, while CD8 expression is down-regulated at 4 h and then remains approximately stable up to 6 h. Additionally, the down-regulation of CD4 and CD8 at 6 h presents no problem in identification of respective lymphocyte subpopulations during flow cytometry (gating), as opposed to humans (9). However, at 8 h culture time, both CD4+ and CD8+ cell numbers were markedly reduced making the interpretation irrelevant. In mice, lymphocyte stimulation with PMA and ionomycin results in milder down-regulation of CD4 and CD8 antigens than in humans (18). In a similar study of bovine peripheral blood mononuclear cells, the expression of these antigens was not determined, thus it is unknown if there is a down regulation of their expression (14). Accordingly, canine lymphocytes immunophenotype, following stimulation, presents more similarities with mice than human lymphocytes. In human lymphocytes, it has been shown that, following primary stimulation, cytokines are produced in a successive manner as opposed to secondary stimulation (2). It is likely that this is also the case in dogs, since at least for CD8+ cells, IL-4 was preceded at 4 h versus IFN-γ production at 6 h.
In both humans and mice, it has been shown that IL-4 is detected with difficulty and transiently in a low number of cells following stimulation, as opposed to IFN-γ (18). However, the results of this study showed that in dogs, although IL-4 detected in a low number of lymphocytes, there seems to be no difficulty in its detection in either CD4+ or CD8+ cells. It is likely that the intracellular production of IL-4 is higher in dogs than in humans and mice under similar circumstances. Because both cytokines seem to be expressed at relatively low levels, it probably explains their appearance on dot plots as a continuous shoulder instead of being a well separated bi-modal distribution (Figures 5,6). Similar observations have been made in both human and mouse studies (18). Breed or age cannot be excluded as a cause of variation in IFN-γ and IL-4 production by CD4+ and CD8+ T lymphocytes observed in this study. Further research is needed to elucidate this issue.
Because activated and standard production IFN-γ and IL-4 canine lymphocytes were not available, the mouse anti-human CD69 monoclonal antibody was used as positive control. It has been shown that the expression of CD69 on T lymphocytes occurs following their activation both in humans (19,20) and dogs (21).
The results of this study show that IL-4 is detected not only in canine CD4+ but in CD8+ lymphocytes as well. IL-4 production from CD8+ cells has also been reported in mice and humans (22), leading to the proposal that 2 functionally different subpopulations of these cells are likely to exist (Tc1 and Tc2), proportionate to Th1 and Th2 subpopulations of CD4+ lymphocytes (22). However, in a similar study in cattle, CD8 + IL-4+ cells were not detected or more likely their number was too low to be detectable (14). The existence of CD8 + IL-4+ cells in dogs is quite important at least for intracellular infections, such as leishmaniasis (23). A study for the determination of intracellular cytokines IFN-γ and IL-4 in peripheral T lymphocytes in canine leishmaniasis is in progress in our laboratory.
The results presented demonstrate the potential of using flow cytometry to monitor the frequency of cells producing cytokines. Modification of the present assay will permit the measurement of IFN-γ and IL-4 or other cytokines within further subsets of canine lymphocytes studied (such as, CD45RA + CD4+). The production of directly conjugated MoAbs against additional canine cytokines and chemokines, with different fluorochromes, would enable the determination of multible inducible biologically relevant proteins within single cell. This would further permit the evaluation of immune response in various canine diseases, mainly those caused by intracellular infections with the potential for monitoring therapy.
Acknowledgments
The authors thank Dr. Matralis Dimitrios for providing blood samples and Mrs. Dactylidou Diamond for technical assistance. We also thank Dr. Paterakis for critical review of the manuscript.
References
- 1.Day M. Clinical Immunology of the Dog and Cat. London UK: Manson Publ; 1999. [Google Scholar]
- 2.Prussin C, Metcalfe D. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995;188:117–128. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 3.Schauer U, Jung T, Krug N, Frew A. Measurement of intracellular cytokines. Immunol Today. 1996;17:305–306. doi: 10.1016/0167-5699(96)30020-0. [DOI] [PubMed] [Google Scholar]
- 4.Papadogiannakis E, Kontos V, Tamamidou M, Roumeliotou A. Flow cytometry and its applications in veterinary medicine. Journal of the Hellenic Veterinary Medical Society. 2005;56:71–77. [Google Scholar]
- 5.Ferrer L, Solano-Gallego L, Arboix M, Alberola J. Evaluation of the specific immune response in dogs infected by Leishmania infantum. Vet Dermatol. 2000;11:7. [Google Scholar]
- 6.Papadogiannakis E. Canine leishmaniosis: A scientific monograph. Athens, Greece: Pashalidis Med Publ; 2007. [Google Scholar]
- 7.Collins RA, Howard CJ, Duggan SE, Werling D. Bovine interleukin-12 and modulation of IFN-γ production. Vet Immunol Immunopathol. 1999;68:193–207. doi: 10.1016/s0165-2427(99)00020-3. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen L, Castelruiz Y, Jacobsen S, Aaasted B. Identification of monoclonal antibodies that cross-react with cytokines from different animal species. Vet Immunol Immunopathol. 2002;88:111–122. doi: 10.1016/s0165-2427(02)00139-3. [DOI] [PubMed] [Google Scholar]
- 9.Sewell WAC, North ME, Webster ADB, Farrant J. Determination of intracellular cytokines by flow-cytometry following whole-blood culture. J Immunol Methods. 1997;209:67–74. doi: 10.1016/s0022-1759(97)00150-6. [DOI] [PubMed] [Google Scholar]
- 10.Moore PF, Affolter VK, Olivry T, Schrenzel MD. The use of immunological reagents in defining the pathogenesis of canine skin diseases involving proliferation of leukocytes. In: Kwochka KW, Willemse T, Tscharner CV, editors. Advances in Veterinary Dermatology. Vol. 3. Oxford, UK: Butterworth Heinemann; 1998. pp. 77–94. [Google Scholar]
- 11.Jung T, Schauer U, Heusser C, et al. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 12.Aseenmacher M, Schmitz J, Radbruch A. Flow cytometric determination of cytokines in activated murine T helper lymphocytes: Expression of IL-10 in interferon-γ and in IL-4 expressing cells. Eur J Immunol. 1994;24:1097–1101. doi: 10.1002/eji.1830240513. [DOI] [PubMed] [Google Scholar]
- 13.Openshaw P, Murphy E, Hosken N, et al. Heterogeneity of intra-cellular cytokine synthesis at the single cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sopp P, Howard C. IFN-γ and IL-4 production by CD4, CD8 and WC1 γδ TCR+ T cells from cattle lymph nodes and blood. Vet Immunol Immunopathol. 2001;81:85–96. doi: 10.1016/s0165-2427(01)00334-8. [DOI] [PubMed] [Google Scholar]
- 15.Prussin C. Cytokine flow cytometry: Understanding cytokine biology at the single-cell level. J Clin Immunol. 1997;17:195–204. doi: 10.1023/a:1027350226435. [DOI] [PubMed] [Google Scholar]
- 16.Nakayama K, Nakauchi H. Cyclosporin A inhibits the decrease of CD4/CD8 expression induced by protein kinase C activation. Int Immunol. 1993;5:419–426. doi: 10.1093/intimm/5.4.419. [DOI] [PubMed] [Google Scholar]
- 17.Pelchen MA, Parsons IJ, Marsh M. Phorbol ester-induced down-regulation of CD4 is a multistep process involving dissociation from p56lck, increased association with clathrin-coared pits and altered endosomal sorting. J Exp Med. 1993;178:1209–1222. doi: 10.1084/jem.178.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pala P, Hussell T, Openshaw P. Flow cytometric measurement of intracellular cytokines. J Immunol Methods. 2000;243:107–124. doi: 10.1016/s0022-1759(00)00230-1. [DOI] [PubMed] [Google Scholar]
- 19.Marzio R, Manuel J, Betz-Corradin S. CD69 and regulation of the immune function. Immunopharmacol Immunotoxicol. 1999;21:565–582. doi: 10.3109/08923979909007126. [DOI] [PubMed] [Google Scholar]
- 20.Ziegler S, Ramsdell F, Alderson M. The activation antigen CD69. Stem Cells. 1994;12:456–465. doi: 10.1002/stem.5530120502. [DOI] [PubMed] [Google Scholar]
- 21.Bank I, Hardan I, Lokshin E, et al. Parenteral administration of an activating monoclonal antibody to the α1β1 integrin in dogs. Immunobiol. 2000;202:239–253. doi: 10.1016/s0171-2985(00)80031-5. [DOI] [PubMed] [Google Scholar]
- 22.Carter LL, Dutton RW. Type 1 and type 2: A fundamental dichotomy for all T cell subsets. Curr Opin Immunol. 1996;8:336–342. doi: 10.1016/s0952-7915(96)80122-1. [DOI] [PubMed] [Google Scholar]
- 23.Papadogiannakis E, Koutinas A, Saridomichelakis M, et al. Cellular immunophenotyping of exfoliative dermatitis in canine leishmaniosis (Leishmania infantum) Vet Immunol Immunopathol. 2005;104:227–237. doi: 10.1016/j.vetimm.2004.12.001. [DOI] [PubMed] [Google Scholar]