Abstract
The purpose of this study was to determine any differences in pathogenicity when sheep are experimentally infected with different Histophilus somni isolates: a) 2336 bovine origin strain; b) an isolate from ram orchitis and epididymitis; c) an isolate from the brain of a sheep with neurological signs; d) an isolate from the vagina of a clinically healthy ewe. A total of 20 rams divided in groups of 5 animals each were inoculated in the epididymis with 1 × 107 CFU/mL of H. somni; a negative control group of 5 rams was used. All groups inoculated with H. somni showed some epididymitis, but the most pathology was caused by the epididymitis isolate, followed by the vaginal isolate. It was demonstrated that there is a difference in experimental infection capacity among isolates from different origins, as epididymitis occurred and the bacteria was recovered only from groups inoculated with isolates originating from epididymitis and vaginal exudate.
Résumé
Cette étude avait comme objectif d’établir s’il y avait une différence de pathogénicité lors d’infection expérimentale de moutons avec différents isolats d’Histophilus somni : a) la souche 2336 d’origine bovine; b) un isolat provenant d’un bélier avec une orchite et une épididymite; c) un isolat provenant du cerveau d’un mouton avec des signes neurologiques; d) un isolat provenant du vagin d’une brebis cliniquement en santé. Un total de 20 béliers ont été répartis dans quatre groupes de 5 animaux chacun, qui ont été inoculés dans l’épididyme avec 1 × 107 ufc/mL d’H. somni, et un groupe témoin de 5 béliers. Tous les groupes inoculés avec H. somni ont montré des signes d’épididymite, mais l’isolat provenant du cas d’épididymite a causé la pathologie la plus importante suivi de l’isolat provenant du vagin. Il a été démontré qu’il y avait une différence selon l’origine anatomique des isolats dans leur capacité à induire une infection expérimentale, tel que démontré par le fait qu’une épididymite s’est produite et que les bactéries n’ont été récupérées qu’à partir des groupes inoculés avec les isolats provenant du cas d’épididymite et de l’exsudat vaginal.
(Traduit par Docteur Serge Messier)
Histophilus somni (Haemophilus somnus) was isolated for the first time in 1956, as the cause of encephalitis in cattle. Histophilus somni is recognized as a cause of disease syndromes associated with the respiratory and reproductive tracts, having as well as septicemic and other miscellaneous forms (1).
Virulence of H. somni is controversial since in several studies it has been isolated in pathological processes, as well as from the mucosa, nasal cavity, prepuce or vagina of clinically health livestock; therefore, it has been considered as part of the normal flora (2,3).
The first report of the presence of H. somni in sheep was in a female with mastitis (4). Other reports occurred in 1977 when the bacterium was isolated from a ram with pneumonia, myocarditis, and lesions in the central nervous system (5). In South Africa, isolation was achieved from a lamb with epididymitis, while in Canada it was isolated from vaginal discharges (5,6). In Mexico, isolation was reported in 2005 from a lamb with epididymitis (7).
In a study carried out by Ward et al (8) an isolate of a bacterium with the same characteristics as H. somni was obtained from prepuce, vagina, and pneumonic lesions in bighorn sheep in the Rocky Mountains. The DNA restriction fragment length profiles of the bighorn-sheep isolates, however, had similarities not shared with the other isolates, suggesting distinct phylogenetic lines. All of the isolates had similar antimicrobial profiles, but the isolates from the bighorn sheep produced less pigment than those from the domestic livestock, and growth of the former was not enhanced by CO2 (8).
The purpose of this study was to establish if there is a difference in pathogenicity when sheep are experimentally infected with different Histophilus somni isolates.
A total of 4 isolates of H. somni were used: a) bovine pneumonic lung origin strain 2336 (8), that was previously inoculated in a sheep and subsequently recovered from its lung at necropsy (9); b) H. somni isolated from a case of epididymitis in a ram from a 3000 head feedlot experiencing an outbreak of 60 cases of orchitis and epididymitis (10); c) H. somni isolated from the brain of an ovine that presented neurological signs (10); d) H. somni isolated from the vaginal discharge of a clinically healthy young ewe (11).
All H. somni strains were grown in chocolate agar that had 0.5% of yeast extract (Becton Dickinson, Rutherford, New Jersey, USA) and 7.5% of bovine blood, and incubated at 37°C for 48 h in a 5–10% CO2 atmosphere.
The work was carried out with 25 male 6- to 12-month-old intact rams. Samples of prepuce exudate were collected from all animals to try to obtain bacteriological isolation of H. somni.
Twenty-five rams were randomly assigned to one of 5 groups. The right epididymis of each ram was inoculated with 1 mL of either an isolate of H. somni 1 × 107 colony-forming units (CFU)/mL or 1 mL physiological saline solution (PSS) (control group only) (Table I).
Table I.
Groups of 5 ovines that were experimentally infected with Histophilus somni with strains isolated from different sources
| Group 1
H. somni isolated from the vaginal exudates of a clinically healthy ewe |
Group 2
H. somni isolated from the epididymis of a ram with orchitis and epididymitis |
Group 3
H. somni strain 2336, that was previously inoculated in a sheep and recovered from lung |
Group 4
H. somni isolated from the brain of a sheep with neurological signs |
Group 5
Negative control PSS |
|
|---|---|---|---|---|---|
| Percentage of animals with epididymitis within each group | 5/5
100% |
5/5
100% |
2/5
40% |
1/5
20% |
0/5
0% |
| Degree of macroscopic lesions at necropsy | +++ | ++++ | ++ | + | − |
Weekly clinical inspections were carried out during the entire study. One month after inoculation, all 25 animals were euthanized according to the determinations in the respective Mexican Official Standard (12) and approved by the animal care committee of INIFAP. Samples were collected from the tail and head of the epididymis, ampulla of the vas deferens, bulbourethral glands, seminal vesicles, urethra and testicles, observing macroscopic and microscopic lesions.
Samples from testicles, as well as from the head and tail of the epididymis were inoculated into chocolate agar in duplicate; all plates were incubated for 24–48 h at 37°C in a 5% to 10% CO2 atmosphere. Two independent repetitions of this process were carried out.
Samples from testicles, head and tail of the epididymis, vas deferens ampulla, bulbourethral glands, seminal vesicles and urethra were obtained and placed in glass jars containing 10% formalin solution to fixate them and sent to the pathology laboratory to carry out paraffin infiltration and staining with hematoxylin-eosin for histopathological analysis.
Identification was achieved through analysis of several aspects, including colony characteristics such as consistency and pigmentation, microscopic morphology, Gram stain (13) and species specific polymerase chain reaction (PCR) analysis (14,15). Histophilus somni 2336 type strain was used as a positive control in all analysis that were carried out.
Polymerase chain reaction was used to identify the H. somni isolations from ovine tissue using the following primers: 5′-GAAGGCGAT TAGTTTAAGAG-3′ and 5′-TTCGGGCACCAAGTATTCA-3′ (14). Bacterial samples were processed in a Gene Amp PCR System 2400 thermocycler with the following program: 35 cycles of denaturing at 94°C for 1 min, annealing at 42°C and extension at 72°C for 1 min. Polymerase chain reaction product detection was carried out with electrophoresis of 1% agarose gel (14,15). Control DNA was extracted from H. somni strain 2336. In order to determine the specificity of the test, DNA was extracted from other bacterial genus related to H. somni such as: A. seminis, Mannheimia haemolytica, P. multocida and Actinobacillus pleuropneumoniae, as well as Brucella ovis. All DNA was kept at −70°C until its use.
Fifteen days after experimental challenge, unilateral right epididymitis was observed in the 2 groups inoculated with H. somni originating from vaginal exudates and epididymitis extract (groups1 and 2, respectively). Animals in group 2 presented the most severe lesions, including unilateral increase of the epididymis and adhesions of the testicle to the scrotum. No clinical alterations could be found by palpation in groups 3, 4, and 5 (control).
At slaughter, 1 month after inoculation, unilateral right epididymitis was found in animals of the 4 groups experimentally infected with H. somni (Figures 1, 2), while the control group did not show any lesions (Figure 3). The percentage of ovines that suffered epididymitis in each group is listed in Table I.
Figure 1.
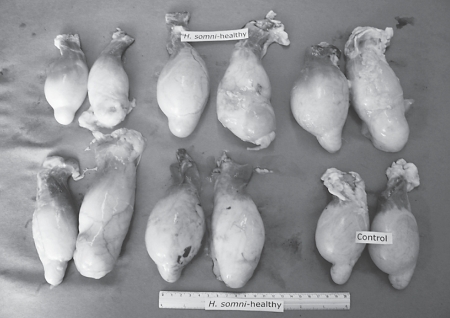
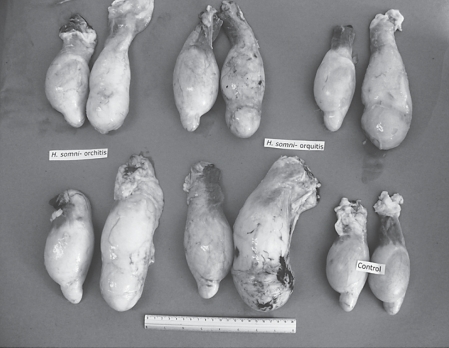
Testicles from 5 rams, slaughtered 1 month after being inoculated in the right epididymis with an H. somni strain isolated from vaginal exudates of a clinically healthy ewe (group 1) and testicles from group 5 (control).
Figure 2.
Testicles from 5 rams, slaughtered 1 month after being inoculated in the right epididymis with an H. somni strain isolated from the epididymis of a ram with orchitis and epididymitis (group 2) and testicles from group 5 (control).
Figure 3.
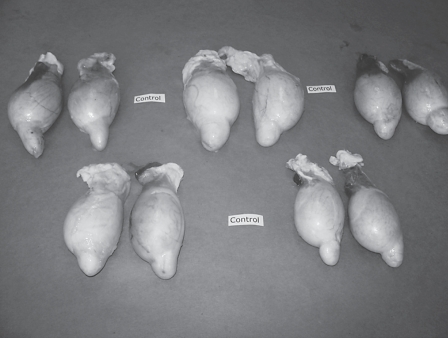
Testicles from 5 rams of control group (group 5) slaughtered 1 month after being inoculated in the right epididymis with saline solution.
The degree of epididymitis that was observed varied among the 4 groups; group 2 presented greater lesions; followed by group 1 (Figures 1, 2, 3); groups 3 and 4 had epididymitis to a lesser degree.
Macroscopic lesions that were observed in testicles and epididymis were: presence of adherence sites between the scrotum and dartos tunic, unilateral or bilateral increase in size and presence of abscesses at incision with purulent secretion.
Histopathological lesions were more pronounced in groups 1 and 2. The right epididymis tail sample had abundant disorganized fibrous connective tissue and cystic formations (dilated ducts) of different sizes composed of stratified squamous epithelium (epidermoid metaplasm) surrounded by blood vessels containing a large amount of inflammatory cells. These cells were predominantly neutrophils and to a lesser degree lymphocytes, monocytes, and macrophages together with abundant hyaline proteinaceous material. The ampulla had slight to moderate inflammatory cell infiltrate composed predominantly of neutrophils and smaller numbers of lymphocytes and macrophages. Other tissues did not demonstrate evidence of specific pathologic alterations.
In the bacteriological study of tissues and organs collected from animals within the 4 study groups, as well as from the control group, H. somni was isolated from testicles, as well as from the head and tail of the epididymis from inoculated animals within groups 1 and 2, but H. somni was not isolated from any ovine in the groups 3, 4, and 5.
Histophilus somni was identified by PCR in all isolations in groups 1 and 2, but not from the other groups (3, 4, and 5) without isolations. When determining PCR specificity with other different bacteria, DNA amplification was not achieved with A. seminis, P. multocida, A. pleuropneumoniae, and B. ovis.
It has been observed that although electrophoresis profiles of strains isolated from clinical cases in cattle and sheep were similar, with the exception of the major outer membrane proteins (16), an H. somni strain isolated from bovine pneumonia when experimentally inoculated into sheep does not cause the same disease (17). In a previous study in our group, strain 2336 that originated from a bovine was directly inoculated into the lung of a sheep causing only slight pneumonic lesions. When later isolated and inoculated into 15 animals through intratracheal or intraepididymal pathways the result was that no disease could be reproduced in any of the experimental groups (9). It is possible that the difference in origin of the strains used in this study is one of the reasons of not being able to infect sheep, apart from other factors including environment, handling, nutrition conditions, that could predispose an individual to the disease.
Several strains of H. somni have been isolated in the bovine including strain 2336 isolated from pneumonia, strain 649 isolated from abortions, strain 8025 isolated from the encephalon, and strain 129Pt isolated from the prepuce. When animals were challenged with strain 8025 pneumonia did not appear; furthermore, when enzyme-linked immunosorbent assay (ELISA) tests were carried out with serum of susceptible animals several variants were found among the strains, demonstrating that in order to develop pneumonia in cattle, virulent strains isolated from lung are needed, such as strain 2336 (18).
Differences in pathogenicity observed in the present study is confirmed by the severity of lesions produced in epididymides and testes in animals from groups 1 and 2, both inoculated with strains isolated from ovine reproductive tract; in contrast with animals inoculated with strains from respiratory tract or nervous system. It is accepted that there is a direct relationship between tissue damage and bacterial virulence as demonstrated in the present work.
The results that were observed in the histopathology study correspond to an active chronic suppurative inflammatory process, which suggests bacterial infection. Ward et al (16) hypothesized that strains isolated from cattle would not infect sheep and vice versa, based on the results obtained when analyzing ribotype and biotype of different H. somni strains isolated from ovine and bovines. Therefore, in order to develop an infection in sheep, it is possible that a strain isolated from sheep is required, and it will only affect the region or anatomical site from where the strain was obtained.
In contrast, bacteria such as Actinobacillus seminis, which only affects sheep, and has an affinity for the epididymis when inoculated directly into the epididymis in sheep, invariably cause lesions within the reproductive tract (19), and when inoculated into young rams through nongenital pathways such as: conjunctival, intranasal, oral, intravenous, intramuscular, as well as the genital pathways such as epididymis, vas deferens, urethral or prepuce, in all but conjunctival and intranasal resulted in epididymitis, mainly in the tail of the epididymis (20). It was possible to confirm the isolation of H. somni from the epididymis of experimentally infected sheep using PCR. According to Angen et al (14), when using the primers used in this study, specificity of the test is adequate as bacteria such as Actinobacillus seminis or Pasteurella multocida, which are closely related to H. somni, did not amplify. Polymerase chain reaction combined with bacteriological studies to demonstrate the presence of H. somni is an excellent option due to high sensitivity.
Recommended management practices to avoid early infections in young animals as well as orchitis and epididymitis outbreaks are the separation of rams, in order to avoid sexual contact among them. Other measures to consider are frequent clinical inspections to search for abnormalities in testes, and the isolation of sick animals at the beginning of disease. Finally, it is of importance to send clinical samples to a diagnostic laboratory to determine the etiology of illness.
It was demonstrated that there is a difference in the capacity to experimentally infect sheep among H. somni isolates that originate from different sources, as epididymitis was reproduced but the degree of lesions varied among the inoculated groups. Furthermore, it was only possible to recover the bacterium through bacteriological study of sheep inoculated with H. somni isolates originating from an outbreak of epididymitis and from the vaginal exudates.
Footnotes
This work was partially funded by project 11928 CONACyT, México.
References
- 1.Humphrey JD, Stephens LR. Haemophilus somnus: A review. Vet Bull. 1983;53:987–1004. [Google Scholar]
- 2.Kwiecien JM, Little PB. Haemophilus somnus and reproductive disease in the cow: A review. Can J Vet Res. 1991;32:595–601. [PMC free article] [PubMed] [Google Scholar]
- 3.Miller RB, Lein DH, Hall CE, Shin S. Haemophilus somnus infection of the reproductive tract of cattle: A review. J Am Vet Med Assoc. 1983;182:1390–1392. [PubMed] [Google Scholar]
- 4.Roberts DS. A new pathogen from an ewe with mastitis. Aust Vet J. 1956;32:330–332. [Google Scholar]
- 5.Stephens LR, Humphrey JD, Little PB, Barnum DA. Morphological, biochemical, antigenic, and cytochemical relationships among Haemophilus somnus, Haemophilus agni, Haemophilus haemoglobinophilus, Histophilus ovis and Actinobacillus seminis. J Clin Microbiol. 1983;17:728–737. doi: 10.1128/jcm.17.5.728-737.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker RL, LeaMaster BR, Biberstein EL, Stellflug JN. Serodiagnosis of Histophilus ovis-associated epididymitis in rams. Am J Vet Res. 1988;49:208–212. [PubMed] [Google Scholar]
- 7.Palomares RG, Aguilar RF, Hernández L, Acosta JP, Herrera LE, Tenorio GV. Isolation and characterization of Histophilus somni (Haemophilus somnus) in semen samples of rams with epidymitis. Small Rum Res. 2005;60:221–225. [Google Scholar]
- 8.Ward AC, Weiser GC, Anderson BC, Cummings PJ, Arnold KF, Corbeil LB. Haemophilus somnus (Histophilus somni) in bighorn sheep. Can J Vet Res. 2006;70:34–42. [PMC free article] [PubMed] [Google Scholar]
- 9.García CL, Girela AJ, Arellano RB, et al. Humoral immune response assessment in sheep experimentally infected with Histophilus somni and previously inoculated with Parainfluenza 3 virus. J Anim Vet Adv. 2007;6:681–685. [Google Scholar]
- 10.Enríquez VI, Tenorio GV, Morales AJF, Díaz AE, Nieto LS, Aguilar RF. Orchitis and epididymitis in sheep from slaughterhouse, caused by Histophilus somni. Proceedings: Congreso Nacional de Buiatría, Acapulco; México. 2007. pp. 198–201. [Google Scholar]
- 11.Sosa EE. Amecameca, México: Universidad Autónoma del Estado de México; 2005. Isolation of Histophilus somni from the reproductive tract in clinically healthy ewes (Veterinary thesis) pp. 30–36. [Google Scholar]
- 12.Norma Oficial Mexicana, Humanitarian slaughter of domestic and wild animals. (Sacrificio humanitario de los animales domésticos y silvestres) NOM-033-ZOO-1995) Published on July 7th, 1995 en el Diario Oficial de la Federación, Mexico. http://www.sagarpa.gob.mx/Dgg/NOM/033zoo.pdf
- 13.Garcia-Delgado GA, Little PB, Barnum DA. A comparison of various Haemophilus somnus strains. Can J Comp Med. 1977;41:380–388. [PMC free article] [PubMed] [Google Scholar]
- 14.Angen O, Ahrens P, Tegtmeier C. Development of PCR test identification of Haemophilus somnus in pure and mixed cultures. Vet Microbiol. 1998;63:39–48. doi: 10.1016/s0378-1135(98)00222-3. [DOI] [PubMed] [Google Scholar]
- 15.Tegtmeier C, Angen O, Ahrens P. Comparison of bacterial cultivation, PCR in situ hybridization and immunohistochemistry as tools for diagnosis of Haemophilus somnus pneumonia in cattle. Vet Microbiol. 2000;76:385–394. doi: 10.1016/s0378-1135(00)00259-5. [DOI] [PubMed] [Google Scholar]
- 16.Ward ACS, Jaworski MD, Eddow JM, Corbeil LB. A comparative study of bovine and ovine Haemophilus somnus isolates. Can J Vet Res. 1995;59:173–178. [PMC free article] [PubMed] [Google Scholar]
- 17.Lees VW, Yates WD, Corbeil LB. Ovine Haemophilus somnus: Experimental intracisternal infection and antigenic comparison with bovine Haemophilus somnus. Can J Vet Res. 1994;58:202–210. [PMC free article] [PubMed] [Google Scholar]
- 18.Groom SC, Little PB, Rosendal S. Virulence differences among three strains of Haemophilus somnus following intratracheal inoculation of calves. Can J Vet Res. 1988;52:349–354. [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Katib WA, Dennis SM. Epididymal and testicular lesions in rams following experimental infection with Actinobacillus seminis. N Z Vet J. 2007;55:125–129. doi: 10.1080/00480169.2007.36754. [DOI] [PubMed] [Google Scholar]
- 20.Acosta DJ, Díaz AE, Tenorio GVR, Suárez GF, Tórtora PJ. Determination of pathological changes in the reproductive tract IgG, IgM and IgA antibodies in blood, seminal plasma and smegma of rams inoculated with Actinobacillus seminis. J Anim Vet Adv. 2007;6:105–113. [Google Scholar]