It has long been suspected that brain norepinephrine (NE) plays important roles in addiction, but this view has been largely overshadowed in recent years by prominent attention to other brain systems involved in addiction such as dopamine and glutamate, and even more recently, orexin (1, 2). However, new studies have begun to reverse this trend by providing compelling evidence for the importance of NE in addiction, as exemplified by 2 articles in the current issue (Schank et al., Zachariou et al.).
Early studies of NE and addiction focused on opiates and opiate withdrawal. Opiates strongly inhibit impulse activity of NE locus coeruleus (LC) neurons, and opiate withdrawal strongly activates these cells, leading to the view that LC plays an important role in opiate abuse. However, lesions of LC or its projections have no effect on physical or aversive signs of acute opiate withdrawal (3, 4). Certainly, the highly elevated activity of LC neurons during withdrawal has consequences for behavior, but those consequences remain unclear.
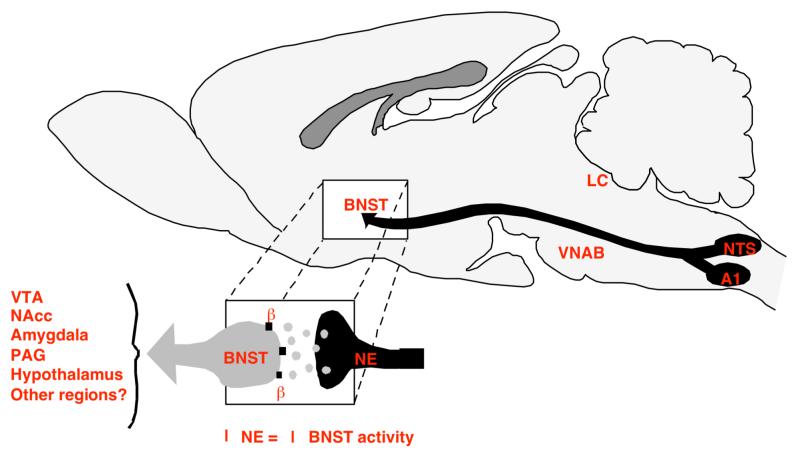
Recent studies have shown that NE neurons other than LC are important in opiate abuse. Thus, beta adrenoceptor stimulation in bed nucleus of the stria terminalis (BNST) is critical for the aversiveness of opiate withdrawal; this NE primarily originates from medullary A1 and A2 NE cells, not LC (4). This same NE input and receptor in BNST was found to be critical for stress-induced anxiety (5), suggesting that the aversiveness of opiate withdrawal may be related to withdrawal-induced anxiety (see figure 1 illustrating NE input to the BNST).
Figure 1.
Schematic illustrating NE projections to the BNST in rat brain, and subsequent outputs from the BNST to other regions of the limbic system. In the case of opioid withdrawal, activation of NE neurons in the NTS and A1 cell groups enhance NE release in the BNST. NE acting at β-NE receptors in the BNST activates neurons projecting to various limbic regions, such as the nucleus accumbens (NAcc), amygdala, periaqueductal gray (PAG), hypothalamus, and perhaps other regions. Adapted from Aston-Jones et al (1999).10
Fewer studies have examined brain NE involvement in stimulant abuse. One article in the present issue extends analysis of NE and addiction in psychostimulant abuse, and some of the same themes appear as for opiate abuse. Aside from its well-known rewarding/reinforcing effects, acute cocaine also acts as a potent anxiogenic agent (6). Schank et al. (pg * of this issue) uses the elegant, recently developed dopamine-beta-hydroxylase (DBH) knock-out mouse to show that central NE is necessary for such cocaine-induced anxiety. This animal model lacks the DBH enzyme, which is essential for making NE from dopamine. Using a standard anxiety test (elevated plus maze), the investigators found that DBH knockouts do not exhibit an anxiety response to an acute cocaine injection, whereas wild-type mice do. Importantly, anxiety in these DBH knockouts could be rescued by administration of DOPS, a compound that can be converted into NE without need for DBH and without altering dopamine levels. In an unusual but strong step in a knockout study, the investigators then confirmed the above results with pharmacological manipulations in wild type mice, showing that the DBH inhibitor disulfiram, or the beta adrenoceptor antagonist propranolol, but not alpha1 or alpha2 adrenoceptor antagonists, produced a similar behavioral result. Thus, these studies indicate that anxiogenic effects of cocaine are due, at least in part, to NE acting at beta adrenoceptors. These results are consistent with many previous findings that link elevated brain NE with anxiety. Importantly, these results also resemble findings that elevated anxiety during withdrawal from opiate or cocaine is dependent upon beta receptor stimulation (7). As the authors point out, this withdrawal-induced anxiety may be a point where their findings are most relevant to clinical treatment. Because avoidance of withdrawal responses is a driving factor in relapse during abstinence, treatments that limit NE-linked anxiety may prolong abstinence and reduce relapse. This conclusion is consistent with previous findings that stress-induced reinstatement of cocaine-seeking is also blocked by beta adrenoceptor antagonist treatments (8).
This relationship between the anxiety of acute cocaine and that of cocaine withdrawal is somewhat paradoxical at first — why would acute cocaine produce a response that resembles a response to cocaine withdrawal? Determining the cellular substrates of these noradrenergically-mediated anxiety responses will be important work for the future. Whatever the cellular mechanism underlying these anxiety responses, they are significant because they illustrate another paradoxical issue that may provide insights into important aspects of cocaine abuse. That is, in addition to its hedonic and reinforcing actions, acute cocaine produces anxiety, i.e., a withdrawal-like response. The desire to avoid withdrawal-associated anxiety may lead then lead to cocaine craving and intake, generating additional anxiety and again associated craving. Thus, similar anxieties produced by acute cocaine and cocaine withdrawal can produce a positive feedback cycle that may underlie some of the more insidious and clinically important aspects of cocaine abuse. This suggests that treating NE-driven anxiety associated with acute cocaine and cocaine withdrawal may be clinically beneficial.
One outstanding question in this study is where NE is acting to drive anxiety- or stress-related responses to cocaine. Previous work indicates that the extended amygdala is a good possibility, particularly BNST. Delfs et al (4) showed that beta adrenocceptor stimulation in BNST is critical for the aversiveness of acute opiate withdrawal, and the associated source of NE is medullary A1 and A2 neurons. Subsequent studies showed that this same pathway is necessary for stress-induced reinstatement of cocaine- or opiate-seeking (9). As noted above, this same pathway is also strongly associated with stress-induced anxiety responses (5). Thus, medullary NE projections into the extended amygdala are importantly involved in these anxiety/stress responses linked to drug relapse. One important extension of the Schank et al study would be to determine if this pathway is also involved in the anxiogenic effects of acute cocaine; such a finding would further establish links between anxiety associated with acute cocaine and withdrawal, and focus research on further elucidation of mechanisms and treatments for these anxiety responses.
As noted above, one important outstanding issue is how cocaine and opioids produce changes in NE neurons to alter associated behavioral responses to stress and anxiety. Stress and anxiety have a well-defined and broad association with drug addiction, ranging from precipitating relapse to being a vulnerability factor in developing addiction. Therefore, understanding the molecular adaptations produced by addictive drugs in NE neurons has the potential to reveal molecular targets for developing therapies to control addiction. In this volume, the paper by Zachariou et al (*) takes an important step in this direction. Based upon the well established relationship between changes in adenylyl cyclase (AC) activity, NE neuronal activity and the chronic morphine-induced withdrawal syndrome, these authors employed mice with genetic deletions in two of the three AC isoforms that are activated by calcium/calmodulin, AC1 and AC8. In addition, a mouse sustaining a double deletion of AC1 and AC8 was examined. Although findings with the AC1+AC8 knock out mice (KO) appeared partly confounded by compensatory changes, single deletion of each isozyme produced a consistent phenotype that was relatively resistant to the effects of chronic morphine, including reduced naloxone-precipitated behavioral withdrawal and decreased potentiation of basal firing rates and forskolin-induced firing of LC NE neurons. Furthermore, the development of tolerance in LC neurons to inhibition by mu opioids was attenuated in the AC1 and AC8 KO mice. Importantly, the acute analgesic effect of morphine and the development of tolerance to morphine-induced analgesia were intact in all three KO genotypes. These data nicely confirm the role of AC in regulating morphine-induced withdrawal syndrome, and the association between AC activity and morphine withdrawal.
Perhaps the most important aspect of the Zachariou et al. is in making the first attempt to screen gene expression patterns in the LC for potential correlates of morphine withdrawal. This is a daunting task for many reasons, including tissue and individual behavioral heterogeneity. However, comparing the profile of morphine induced changes in gene expression between genotypes that present distinct phenotypic behavioral and electrophysiological responses to chronic morphine might permit the identification of the morphine-induced changes associated with withdrawal. Unfortunately, while this first valiant attempt at such broad genetic profiling of LC neurons did identify interesting distinctions between the genotypes, many of which were in line with expectations based upon earlier studies, it did not reveal a small number of clear candidates to target in future studies. In retrospect, it is perhaps overly optimistic to expect even the impressive database generated in this report to contain sufficient information to identify critical common factors regulating a complex behavior such as opiate withdrawal; especially given that the genetic model used was a constitutive deletion of the gene encoding an AC isozyme and that the deletion was throughout the brain and not specific to the LC. The solution of course is that this report be an important first step in future studies using additional genetic approaches to further isolate and evaluate components of the genome that may strongly regulate the withdrawal phenotype induced by chronic opioids.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures — Both authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Weinshenker D, Schroeder JP. There and Back Again: A Tale of Norepinephrine and Drug Addiction. Neuropsychopharmacology. 2006;32:1433–1451. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- 2.Harris G, Aston-Jones G. Arousal and reward: A dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Christie MJ, Williams JT, Osborne PB, Bellchambers CE. Where is the locus in opioid withdrawal? Trends Pharmacol Sci. 1997;18:134–140. doi: 10.1016/s0165-6147(97)01045-6. [DOI] [PubMed] [Google Scholar]
- 4.Delfs J, Zhu Y, Druhan J, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- 5.Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 6.Paine TA, Jackman SL, Olmstead MC. Cocaine-induced anxiety: alleviation by diazepam, but not buspirone, dimenhydrinate or diphenhydramine. Behav Pharmacol. 2002;13:511–523. doi: 10.1097/00008877-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Harris G, Aston-Jones G. Beta-adrenergic antagonists attenuate withdrawal anxiety in cocaine and morphine dependent rats. Psychopharmacology. 1993;113:131–136. doi: 10.1007/BF02244345. [DOI] [PubMed] [Google Scholar]
- 8.Leri F, Flores J, Rodaros D, Stewart J. Blockade of Stress-Induced But Not Cocaine-Induced Reinstatement by Infusion of Noradrenergic Antagonists into the Bed Nucleus of the Stria Terminalis or the Central Nucleus of the Amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2000;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- 10.Aston-Jones G, Delfs J, Druhan J, Zhu Y. The bed nucleus of the stria terminalis: A target site for noradrenergic actions in opiate withdrawal. In: McGinty J, editor. Advancing from the Ventral Striatum to the Extended Amygdala: Implications for Neuropsychiatry and Drug Abuse. New York Academy of Sciences; New York: 1999. pp. 486–498. [DOI] [PubMed] [Google Scholar]