Abstract
Human adenoviruses cause a significant number of acute respiratory, enteric and ocular infections, however they have also served as useful model systems for uncovering fundamental aspects of cell and molecular biology. In addition, replication-defective forms of adenovirus are being used in gene transfer and vaccine clinical trials. Over the past decade, steady advances in structural biology techniques have helped reveal important insights into the earliest events in the adenovirus life cycle as well as virus interactions with components of the host immune system. This review highlights the continuing use of structure-based approaches to uncover the molecular features of adenovirus—host interactions.
Keywords: Adenovirus, Receptors, Integrins, CAR, CD46, Factor X, Cryoelectron microscopy, Virus structure, Penton base, Adenovirus fiber, Adenovirus hexon
Adenovirus: a brief historical perspective
Human adenoviruses (HAdV), agents of acute respiratory, gastrointestinal, and ocular infections, were initially isolated more than fifty years ago by Hilleman and Rowe (Hilleman and Werner, 1954; Rowe et al., 1955). Currently, 51 different types of HAdV (genus Mastadenovirus) representing six different species (A—F) have been identified based on their DNA sequence similarity, hemagglutination patterns, and serologic profiles. Acute adenoviral infections are generally self-limiting however they can cause serious complications in immunosuppressed individuals (Bordigoni et al., 2001; Hoffman, 2006) and remain an impediment to the training of military recruits (Kajon et al., 2007). Early studies indicated that these nonenveloped, double-stranded DNA viruses have oncogenic potential in rodent cells (Trentin et al., 1962). However the initial excitement in this topic was later dampened by the discovery that the majority of primary human tumors lack HAdV DNA thus reducing the likelihood of the etiology of adenovirus in human cancers (Mackey et al., 1976). Nonetheless, HAdV has served as a particularly useful model system for uncovering fundamental aspects of molecular and cell biology including RNA splicing (Berget et al.,1977; Chow et al.,1977) and the regulation of the cell cycle (Chinnadurai, 1983; Yang et al., 1996). Further interest in HAdV biology has been rekindled by the use of replication-defective adenoviral vectors for gene transfer (Harvey et al., 2002). Despite the well-publicized setback in a single human gene therapy trial using an HAdV vector (Raper et al., 2003), these agents continue to be explored in a significant number of promising clinical applications (Henry et al., 2007). The recent discovery that HAdV vectors bind to certain blood coagulation factors resulting in substantial liver tropism in vivo (Kalyuzhniy et al., 2008; Waddington et al., 2008) may pave the way for improved use of HAdV vectors to treat a variety of human diseases.
HAdV structure
Increased understanding of HAdV pathogenesis as well as vector/vaccine development will likely require further elucidation of the precise mechanism(s) of HAdV interaction with host cells as well as its recognition by mediators of the immune system. Investigations of HAdV structure, including X-ray diffraction analysis of individual HAdV capsid proteins as well as cryoelectron microscopic (cryoEM) reconstruction of intact virus particles, have significantly bolstered the knowledge of Ad—host cell and immune interactions. These structural studies, combined with functional and biochemical information, have provided a valuable model of the HAdV capsid as well as the molecular basis of its association with cell receptors that promote virus attachment or internalization.
Adenovirus is one of the largest and most complex (∼900 Å dia., 150 MDa) nonenveloped viruses to be analyzed structurally. As is the case for many nonenveloped viruses, the HAdV protein capsid exhibits icosahedral symmetry. The virus is comprised of 11 different structural proteins (Fig. 1). In addition, 12–70 copies of the viral cysteine protease, a molecule that mediates the processing of capsid precursor proteins prior to virion assembly, are also incorporated inside the virus particle. The outer shell of HAdV is primarily formed by 240 trimers of the major capsid protein hexon. Each of the twelve vertices of the capsid also contains a penton base, a pentameric molecule that is non-covalently associated with the fiber protein. These vertex proteins serve crucial functions in cell attachment and entry. The capsid is decorated on the outer surface by the minor (cement) protein pIX. Three additional minor proteins pIIIa, pVI, and pVIII, are associated with the capsid. All three of these proteins are cleaved by the protease during viral maturation. The 36 KB viral DNA is located in the core of the virus and is tightly associated with numerous copies of proteins V, VII and Mu. The core proteins VII and Mu are also cleaved by the viral protease, presumably altering the structure of the viral core after immature virions have been assembled in the nucleus of the host cell. The linear HAdV chromosome contains a terminal protein covalently attached to each of its 5 ends, which facilitates replication of the viral nucleic acid.
Figure 1.
Schematic diagram of human adenovirus (HAdV) showing the eleven structural proteins in the virion. The major capsid proteins, hexon, penton base and fiber, form the preponderance of the icosahedral capsid. The capsid is stabilized on the outside by the minor protein pIX. The remaining minor capsid proteins, pIIIa, pVI and pVIII, have been localized in recent cryoEM structural studies to the inner surface of the capsid where they are accessible to the viral cysteine protease. Note that although only a subset of hexons in the diagram are shown associating with protein VI, a cryoEM analysis at 6Å resolution suggests that protein VI is associated with every hexon (Saban et al., 2006). The core proteins, terminal protein, Mu, V and VII, are associated with the double-stranded DNA genome in the interior of the virion. The core proteins and DNA are not packaged with icosahedral symmetry and thus their structural arrangement has not been characterized.
Due to its complexity and size, a high resolution crystal structure of the entire virus has not yet been attained. CryoEM structural analyses of Ad at 6–10 Å resolution, combined with the X-ray structures of individual capsid proteins, have been used to construct pseudo-atomic models of the virus (Fabry et al., 2005; Saban et al., 2006). At ∼6 Å resolution α-helices within hexon and penton base can be identified as rods within the cryoEM density. This level of resolution has been useful for interpreting the density from the additional capsid proteins for which we currently do not have atomic resolution structures. For example, secondary structure prediction algorithms indicate that of all the Ad capsid proteins, only pIX is expected to form a coiled coil. In fact a long 40 amino acid stretch in the C-terminal region of pIX is predicted to have a strong propensity for coiled coil formation. When a coiled coil was resolved between hexons on the outer capsid surface, it was possible to assign this region to pIX (Fig. 2)(Saban et al., 2006). This assignment implies that the remaining capsid proteins, pIIIa, pVI, and pVIII, are entirely internal and are not solvent exposed on the exterior surface of the mature HAdV capsid. Presumably programmed disassembly of the virion must take place before these internal Ad proteins can interact with the host cell (i.e. endosomal membranes) (Greber et al., 1994; Wiethoff et al., 2005).
Figure 2.
CryoEM structure and pseudo-atomic model for the Ad35f vector. (A) The vertex region of the 6Å resolution cryoEM structure (Saban et al., 2006). The penton base and protruding fiber are roughly in the center of the image and are surrounded by hexons. One region of coiled coil density assigned to the C-terminal region of pIX is indicated with a white oval. The surface is shown radially color coded (radius=380Å, blue; radius=460Å, red). (B) A similar region from the pseudo-atomic model. The Ad35f vector contains essentially the Ad5 capsid pseudotyped with the Ad35 fiber. The pseudo-atomic model was generated by positioning the Ad2 penton base with fiber peptide (PDB 1×9T) and Ad5 hexon crystal structures (PDB 1P30) within the cryoEM density. The penton base and most of the hexons are shown filtered to 6Å resolution. The fiber density is from the cryoEM structure. One coiled coil model with four 40-residue long α-helices is indicated with a white oval. (Panel B) Copyright American Society for Microbiology, Journal of Virology, Volume 80, Number 20, p. 12049-12059, cover image, 2006.
HAdV cell entry pathway
Given the complex structure of the HAdV capsid, it is not surprising that interactions of this virus with host cells are also complicated (Fig. 3). Initial attachment to host cells is mediated by a high affinity interaction of the fiber knob domain with a cell receptor. For nearly all HAdV types, the Coxsackie and Adenovirus receptor (CAR) (Bergelson et al., 1997) or CD46 (Gaggar et al., 2003) serve as the major primary HAdV receptors on most cell types. Subsequent Ad internalization via clathrin-mediated endocytosis is facilitated by secondary interactions of the penton base with αv integrins (Wickham et al., 1993). This event is accompanied by partial disassembly of the virus particle in which the vertex proteins (i.e. penton base, pIIIa, fiber, peripentonal hexons) are released in the acidified environment of the early or late endosome. The precise molecular events involved in HAdV disassembly are not very well defined but it is thought that the release of the internal capsid protein pVI at this stage facilitates endosomalysis (Wiethoff et al., 2005) allowing entry of the uncoated particle into the cytoplasm. The N-terminal region of pI contains a predicted amphipathic α-helical domain, a motif that is known to associate with cell membranes. Truncation of this ∼20 residue domain ablates membrane disruption (Wiethoff et al., 2005). Following endosomal escape, association of partially uncoated Ad particles with microtubule motors (i.e. dynein) allows vectorial transport to the nucleus along microtubules (Suomalainen et al., 1999). Cell entry culminates in the release of the viral nucleic acid from the virion and its transit through the nuclear pore complex. These early events set the stage for subsequent replication of HAdV DNA and production/assembly of progeny virions in the nucleus.
Figure 3.
The cell entry pathway of adenovirus. Step 1 involves attachment of the fiber to the primary receptor, which is on most cell types and for most HAdV types is either CAR or CD46. This is followed by clathrin-mediated endocytosis in step 2, which is facilitated by the penton base interaction with αv integrins. The virion begins to dissociate in the low pH environment of the early endosome (step 3), and releases the vertex proteins including pVI in the early or late endosome (step 4). Protein pVI has been implicated in disruption of the endosomal membrane, allowing the virion to escape from the endosome. The partially disassembled virion is transported by the microtubule motor dynein along microtubules to the nuclear pore complex (step 5). At the nuclear pore the viral DNA is imported into the nucleus (step 6).
Fiber—CAR association
A major determinant of HAdV infection of host cells involves interaction of the fiber protein with CAR, a member of the Ig superfamily whose normal function is in the formation of the tight junctions in polarized epithelial cells (Cohen et al., 2001; Walters et al., 2002). Structural studies of fiber—CAR association were made possible by the successful expression of a soluble form of recombinant HAdV type 5 fiber knob domain that retained the native homotrimeric organization (Henry et al., 1994). Crystallization and X-ray diffraction analysis of the fiber knob at 1.7 Å resolution (Xia et al., 1994) suggested that CAR association might occur at the central crevice formed by the three fiber subunits at the top of the fiber knob. However, subsequent X-ray diffraction analysis of the Ad12 type fiber knob in complex with the N-terminal Ig-like domain (D1) of CAR (Bewley et al., 1999) revealed an unexpected mode of interaction (Fig. 4). These studies showed that the surface loops on the side of the fiber knob, rather than in the central crevice at the top, constitute the sites for CAR association. This structural model predicts that a maximum of three CAR molecules can bind to each fiber knob. The CAR-binding surface loop sequences include amino acid residues in the AB loop which are conserved in the fibers of Ad serotypes that use this receptor for infection (i.e. species A and C) whereas they are lacking in the fibers of Ad types (e.g. species B) that use different receptors (i.e. CD46) (Law and Davidson, 2005; Roelvink et al., 1999). Kinetic binding analyses using purified recombinant proteins revealed that the affinity of a single CAR Ig-like domain for the fiber knob is relatively modest (KD=25 nM), while multivalent receptor interaction (i.e. 3 CAR molecules per fiber) produces a substantially higher affinity binding constant (Kd =1 nM) (Lortat-Jacob et al., 2001). Thus, it is likely that the Ad fiber engages multiple CAR molecules on host cells, creating high affinity binding interactions that allow subsequent virus internalization.
Figure 4.
Crystal structures of the HAdV fiber knob in complex with a CAR domain and a portion of the fiber shaft. (A) Top view of the Ad12 fiber knob and CAR domain 1 (D1) complex showing three CAR domains binding at the interfaces between fiber subunits (PDB 1KAC) (Bewley et al., 1999). The approximate fiber subunit interfaces are delineated by black lines. (B) Side view of the fiber knob CAR complex with the location of the fiber shaft indicated by a rectangle. (C) Side view of the Ad2 fiber knob with four triple β-spiral repeats of the fiber shaft (PDB 1QIU) (van Raaij et al., 1999).
As indicated by its moniker, CAR also serves as a receptor for another nonenveloped virus, group B coxsackieviruses (CVB). These picornaviruses are associated with meningitis and myocarditis. Following association with a receptor known as DAF (decay accelerating factor) and transport to tight junctions, subsequent CVB interactions with CAR results in a conformational change in the virus capsid that allows viral entry and release of its RNA genome into host cells (Coyne and Bergelson, 2006). CAR association with CVB occurs via the distal end of the Ig domain (D1) of the receptor which inserts into a prominent depression (canyon) on the surface of the virus particle (He et al., 2001).
Fiber—CD46 interactions
Based on the lack of sequence conservation in the CAR-binding regions of the fiber knob, species B adenoviruses were predicted to use a distinct primary cell receptor for infection. This prediction was subsequently verified by Gaggar et al. (2003) who reported that CD46, a member of the family of complement regulatory proteins, is the receptor for most species B adenoviruses as well as certain species D HAdVs including Ad37 (Wu et al., 2004). The ubiquitous expression of CD46 on disparate host tissues including hematopoietic cells, may partially explain its exploitation by diverse microbial pathogens as an attachment and/or entry receptor (Cattaneo, 2004). The extracellular domain of CD46 is comprised of four short consensus repeats (SCRs), each of which consists of 60–75 amino acids, followed by an O-linked glycan rich region (serine, threonine and proline-rich domain STP) and a small domain of unknown function proximal to the cell membrane. This receptor is anchored to the membrane via a single transmembrane spanning domain followed by a short cytoplasmic tail of 16 or 23 amino acids. Infectivity assays and mutagenesis analyses indicate that the N-terminal two SCR domains of CD46 comprise the HAdV fiber binding site (Fleischli et al., 2005; Gaggar et al., 2005). A major breakthrough in defining the molecular basis of CD46—Ad fiber interactions followed from the report of the co-crystal structure of the Ad11 fiber knob in complex with SCR1-2 of CD46 (Persson et al., 2007)(Fig. 5). As is the case for CAR interaction with Ad fibers, the sites for CD46 association are located on the lateral surface of the fiber knob domain in a diagonal orientation with respect to the axis of the fiber shaft. Both SCR-1 and SCR-2 are involved in fiber interactions and a glutamic acid residue in SCR-2 (E63) provides a critical interaction with arginine 280 of the fiber knob. Interestingly, the CD46 molecule bound to the HAdV fiber adopts a different conformation than the unbound receptor. In particular, the angle between SCR-1 and SCR-2 is significantly reduced in the fiber bound vs. unbound receptor. The Ad fiber knob contributes three separate CD46 interaction regions comprised of surface loops DG, HI, and IJ. Two key residues within the HI loop of Ad11 fiber knob, Arg279 and Arg280, appear to contribute a great deal to CD46 interactions based on mutagenesis and structural studies (Gustafsson et al., 2006; Pache et al., 2008). The overall association rate of CD46 binding to Ad11 or Ad35 fibers as determined by BIAcore analysis is in the low nanomolar range (5– 15 nM). In contrast, the measured affinity for CD46 association with the Ad16 fiber (KD =437 nM) is surprisingly low given that this HAdV type can effectively use this receptor on host cells (Pache et al., 2008). Additional structural, biochemical and functional analyses revealed that Ad16, a member of species B1 Ads, has two extra amino acids in the FG loop of its fiber compared to the corresponding DG loop of Ad11 and that this likely sterically hinders receptor binding. Nonetheless, despite its low intrinsic affinity, Ad16 and perhaps other B1 Ad types such as Ad3, can effectively use CD46 on host cells due to avidity effects. In particular, the twelve fiber knobs on a single virus particle, each with three CD46 binding sites, have the potential to engage the receptor via multivalent interactions. Thus, structural analyses of distinct HAdV fibers combined with functional studies have improved the knowledge of virus—CD46 interactions.
Figure 5.
Crystal structure of the HAdV fiber knob in complex with two CD46 domains. (A) Top view of the Ad11 fiber knob in complex with CD46 domains SCR-1 and SCR-2 showing three CD46 fragments binding at the interfaces between fiber subunits (PDB 2O39) and (Persson et al., 2007). The approximate fiber subunit interfaces are delineated by black lines. (B) Side view of the fiber knob CD46 complex with the location of the fiber shaft indicated by a rectangle and the direction of the rest of CD46 indicated by an arrow. (C) Comparison of the bound and unbound CD46 fragment structures. Unbound CD46 (red) exhibits a pronounced bend at the interface of the two SCR domains. Upon fiber knob association, the CD46 molecule assumes a straightened conformation (blue) that results in a continuous binding surface stretched along the glycan free surface of both SCR domains. The glycans of CD46 are shown in gray on unliganded CD46 (PDB 1CKL). The CD46 binding sites encompass the DG and HI loops of one Ad11 fiber knob subunit (dark green) and the IJ loop of a second subunit (light green).
Structure of the fiber shaft and its impact on cell receptor interactions
The central shaft domain of the fiber plays at least two important roles in cell tropism. First, it helps extend the receptor binding knob domain away from the surface of the virus capsid towards the cell membrane. Second, its length and flexibility facilitate receptor interactions on the lateral surface of the distal knob domain. The shaft domain of adenovirus is composed of a variable number of repeating motifs, ranging from 5.5 in Ad35 to 22.5 in Ad12 (Chroboczek et al., 1995). Each repeating motif contains ∼15 amino acids, some of which are highly conserved, that form an extended β-strand followed by a type 2 β-turn and then another β-strand. In the trimeric fiber the repeats, connected by variable-size loops, produce an axial rise of 13 Å per motif along the length of the fiber (van Raaij et al., 1999). The arrangement of the repeats is characterized by a clockwise displacement of approximately 50° between each subunit resulting in the formation of a spiral staircase-like structure with ∼7 repeats needed for each 36° turn. The intertwining of three of these polypeptides to form the highly stable trimeric fiber shaft domain constitutes a fold that has been termed a “triple-β-spiral” (van Raaij et al., 1999). This somewhat unusual fold is also found in the reovirus σ1protein (Chappell et al., 2002). Depending on the number of repeating units in the shaft, the overall length of the fiber is ∼340 Å in the case of the species A Ad12 or as short as ∼130 Å in the case of the species B Ad35.
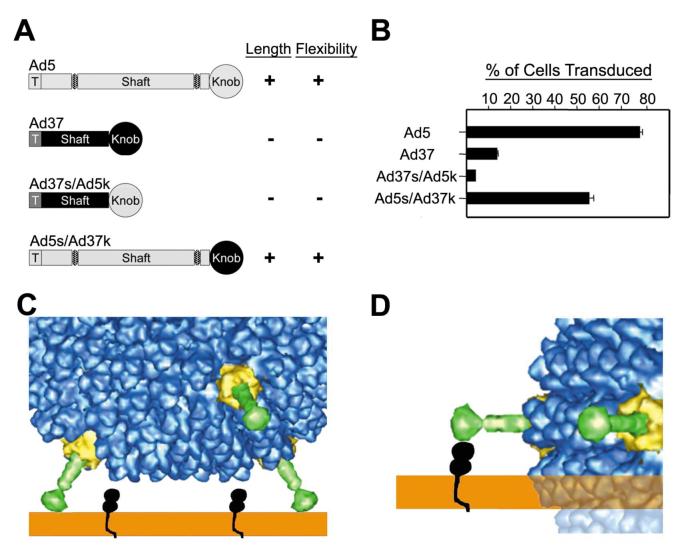
Previous studies indicated that the fiber is not a perfectly straight molecule and that a bend could be observed in negative-stain EM close to the penton base (Ruigrok et al., 1990). CryoEM structures of Ad2, Ad5 and Ad12 confirm that fibers of these types are flexible at a point approximately 60 to 100 Å above the penton base (Chiu et al., 1999; Von Seggern et al., 1999). The location of this bend or kink coincides with an irregularity of the amino acid sequence in the third repeat of the fiber shaft. The atypical third repeat contains an insertion of four additional residues in many HAdV species A, C, and E but not certain D HAdV types (i.e. Ad37). This divergent motif apparently cannot adopt a rigid triple β-spiral fold and this results in increased flexibility in this region of the shaft. A separate region of the fiber shaft located at the interface between the shaft and the knob domains also contains a putative flexibility motif sequence. Studies using Ad vectors displaying chimeric fiber proteins demonstrate that both the overall length of the fiber shaft as well as the presence of the flexibility motifs serve to modulate receptor interactions (Shayakhmetov and Lieber, 2000; Wu et al., 2003). Curiously the Ad37 fiber knob is able to bind CAR in solution, but Ad37 is unable to use CAR efficiently to infect host cells. While Ad5 readily infects A549 lung epithelial cells expressing CAR, Ad37 does not (Wu et al., 2003). In contrast a chimeric Ad5/Ad37 fiber protein comprised of the Ad5 shaft fused to the Ad37 fiber knob is able to utilize CAR for cell entry (Fig. 6). Thus it is not simply the receptor binding knob domain that governs cell tropism. A cryoEM structure of a pseudotyped vector with an Ad37 fiber shows that the Ad37 fiber is straight and rigid, as the full length of the fiber including the knob is reconstructed (Chiu et al., 2001). Modeling of the interaction between Ad37 and host cells indicates that the geometry of the short, rigid Ad37 fiber precludes the formation of a productive interaction between the Ad37 fiber knob and CAR. These combined structural, functional and mutagenesis studies together demonstrate that the architectural features of the entire fiber molecule govern cell tropism. This principle may also extend to other unrelated non-enveloped viruses. For example, the σ1 protein, a fiber-like molecule, protruding from the surface of reovirus, has a remarkably similar structure as the adenovirus fiber (Chappell et al., 2002). Moreover, the σ1 protein mediates attachment to JAM-1 (Barton et al., 2001), a member of the same family of Ig-like molecules as CAR that is also located in tight junctions of polarized epithelial cells. The close structural similarity of reovirus σ1 and the Ad fiber was exploited in recent gene delivery experiments. For these studies, the Ad5 fiber protein on an Ad5-based vector was replaced with a recombinant reovirus σ1 protein. This chimeric HAdV-σ1 vector exhibited altered cell tropism and increased transduction of human dendritic cells (Mercier et al., 2004).
Figure 6.
Importance of the fiber architecture for HAdV cell tropism. (A) Chimeric fibers with Ad5 regions shown in light gray, Ad37 regions in black, and putative flexibility repeats in striped ovals. Pluses and minuses indicate the relative fiber length or flexibility. (B) Ad-mediated gene delivery by fiber-pseudotyped Ad. A549 lung epithelial cells were infected with Ad particles encoding GFP and analyzed for GFP expression 48h postinfection. (C) An enlarged view of a cryoEM structure of a pseudotyped vector with an Ad37 fiber shown together with a schematic representation of CAR on the host cell surface. The geometry of Ad37 with short, rigid fibers prevents the side of the fiber knob from contacting the appropriate surface of CAR. (D) If the side of the fiber knob on Ad37 were to bind CAR, there would be steric hindrance between the viral capsid and the host cell membrane. The clash is indicated by transparent density. (A, B) Copyright American Society for Microbiology, Journal of Virology, Volume 77, Number 13, p. 7225-7235, 2003. (C, D) Copyright American Society for Microbiology, Journal of Virology, Volume 75, Number 11, p. 5375-5380, 2001.
Species D HAdV fiber association with sialic acid
Cell surface glyco-conjugates containing α(2,3) or α(2,6) linked sialic acid residues have also been reported to serve as receptors for certain HAdV types including the species D Ad37 (Arnberg et al., 2000; Burmeister et al., 2004). The equilibrium binding constant (KD) of sialyl lactose binding to the Ad37 fiber knob was estimated to be 5 mM, similar to binding properties of other viruses that use sialic acid such as influenza (Sauter et al., 1989). Given the relatively low intrinsic sialic acid binding activity of Ad37, it is likely that multiple fiber—sialic acid interactions generate higher avidity needed for stable virus—cell association. A crystal structure of the Ad37 fiber knob with bound sialyl lactose has provided further insights into this receptor interaction (Burmeister et al., 2004). The top surface of the positively charged Ad37 fiber (calculated isoelectric point ∼9.4) (Nepomuceno et al., 2007) contains three potential sites for sialic acid binding. The galactose residues of the sialyl lactose are pointed away from the binding site, thus facilitating the binding of multiple residues (Fig. 7). The cationic properties of the Ad37 fiber knob have also been exploited to improve Ad-mediated gene delivery to hematopoietic cells that are normally difficult to transduce with Ad vectors alone (Nepomuceno et al., 2007). In this application, recombinant Ad37 fiber knob appears to mediate electrostatic (bridging) interactions between sialic acid residues on the cell surface and the negatively charged residues located on the hexon of the virus capsid (Li et al., 1997).
Figure 7.

Crystal structure of the Ad37 fiber knob with bound sialyl lactose. Top view of the Ad37 fiber knob trimer (gray) with three sialyl lactose molecules (red) bound close to the threefold axis. While the sialic acid residues make contact with the fiber knob, the galactose residues are pointed away from the binding site, thus allowing sialic acid binding independent of adjacently attached residues. The Figure is based on the structure of the Ad37 fiber knob in complex with sialyl lactose (PDB 1UXA) (Burmeister, et al. 2004).
Penton base-integrin associations
Association of the adenovirus penton base protein with a separate set of cell receptors known as αv integrins, promotes virus infection by stimulating internalization into clathrin-coated vesicles rather than enhancing virus attachment (Bai et al., 1994; Wickham et al., 1993). Integrins are heterodimeric cell membrane proteins that participate in many important cell biological processes including cell adhesion, cell migration, differentiation and proliferation (Arnaout et al., 2005; Luo et al., 2007). These host cell molecules have been usurped by many different viral pathogens for cell invasion (Stewart and Nemerow, 2007). Structural insights into integrin—HAdV interaction originated from initial cryoEM image reconstructions of HAdV type 2 alone (Stewart et al., 1991) or Ad2 in complex with Fab fragments of a monoclonal antibody that recognizes the integrin-binding RGD motif present on the penton base protein (Stewart et al., 1997). These earlier studies showed that highly flexible loops located on the top of the penton base serve as the site of antibody binding and thus are the likely site of integrin attachment. Interestingly, structural modeling as well as functional experiments indicated that due to the close spacing (∼60 Å) of each of the five RGD loops on the penton base and the close apposition of the fiber protein, Fab fragments but not the intact anti-penton base MAb were capable of binding to and neutralizing the virus. Thus the precise arrangement of the RGD loops on adenovirus restricts the binding of a potentially neutralizing antibody while allowing integrin association. In order to confirm the location and functional consequences of HAdV association with integrins, subsequent cryoEM structural and kinetic binding analyses were performed with soluble recombinant integrin (Chiu et al., 1999). These studies used adenovirus type 12 since sequence alignment indicated that the penton base protein of this HAdV type contains a short RGD loop that should restrain the mobility of bound integrins, which is favorable for structural studies. A kinetic study indicated that at close to saturation, 4.2 integrins are bound to each penton base. The cryoEM structure with imposed icosahedral symmetry revealed that integrin molecules form a compact ring above the penton base. The reconstructed integrin molecules showed two distinct domains, a globular region in contact with the penton base and a flexible region extending away from the capsid (Fig. 8). As these cryoEM images of the Ad—integrin complex were obtained at ∼20 Å, precise knowledge of the integrin interaction with the penton base RGD loop could not be obtained and thus further high resolution structural studies are needed. Nonetheless, these early structural studies of HAdV complexed with integrin suggested that the particular spatial arrangement of five RGD loops on the penton base may promote integrin clustering and the subsequent signaling responses involved in virus internalization. In keeping with this possibility, integrin ligation on live cells by either intact Ad particles or the isolated penton base was shown to trigger specific cell signaling events (Li et al., 1998a, b). Thus, Ad binding to integrins activates phosphoinositide-3-OH kinase (PI3K), p130CAS, and the Rho family GTPases. This signaling pathway serves to facilitate actin polymerization, a process needed for efficient virus internalization (Li et al., 1998a). Not surprisingly, a growing number of enveloped and nonenveloped viruses have been shown to use integrins for either cell attachment and/or cell entry, perhaps due to the unique signaling properties of integrins (Stewart and Nemerow, 2007).
Figure 8.

CryoEM structure of Ad12 complexed with a soluble recombinant form of integrin αvβ5. The structure is shown artistically as if one virion were interacting with a host cell in the foreground. A second Ad virion with modeled full length fibers is shown in the distance. The cryoEM structure revealed a compact ring of integrin density over each penton base with the RGD spacing of the penton base promoting interaction between integrin heterodimers (red) (bottom). Segmented density regions corresponding roughly to individual integrin molecules are shown in solution on the lower left. Copyright American Society for Microbiology, Journal of Virology, Volume 73, Number 8, p. 6759-6768, cover image, 1999.
Additional insights into the three dimensional structure of the penton base at 3.3 Å resolution were recently obtained using X-ray diffraction (Zubieta et al., 2005). These studies revealed that the somewhat elongated penton base is comprised of a jellyroll domain that faces the virus capsid. Unfortunately, the RGD loop on top of the penton monomer was disordered and therefore was not seen in the X-ray model. However, the residues involved in multimerization of the pentameric protein were identified. Moreover, a co-crystal structure of the penton base and a peptide corresponding to the highly conserved N-terminus of the fiber protein, FNPVYPY, revealed the location of fiber binding. The fiber peptide lies in a depression on top of the penton base formed between two adjacent monomers and binding of this peptide results in a modest conformational change in an exposed helix α7 on the penton base. Steric considerations suggest that three non-consecutive sites on the penton base are likely utilized to achieve a highly stable noncovalent interaction. Thus, the crystallographic studies provided a plausible explanation for how a trimeric fiber protein engages the pentameric penton base protein (i.e. symmetry mismatch).
Soluble blood proteins influence HAdV tropism in vivo
As is frequently the case with various microbial pathogens, the cell entry pathways that have been discovered using in vitro assays may not be relevant for certain in vivo situations. This turns out to be the case upon intravascular administration of adenoviral vectors in rodent animal models which results in substantial sequestration of the virus in liver hepatocytes and Kupffer cells (Huard et al., 1995; Tao et al., 2001). Modified HAdV vectors (i.e. containing mutations in the fiber and penton base) that lack the ability to use CAR or integrins on cultured cell lines, retain the ability to efficiently transduce multiple cell types in the liver (Smith et al., 2003) suggesting that other entry pathways exist for in vivo gene delivery. Subsequent in vivo studies revealed that certain soluble blood proteins including several complement and clotting factors appear to associate with systemically delivered Ad5-based vectors and thereby direct these agents into the liver (Shayakhmetov et al., 2005).
Recently, several investigators demonstrated that the vitamin K-dependent coagulation factor X (FX) directly binds to Ad5 and mediates liver hepatocyte transduction (Kalyuzhniy et al., 2008; Waddington et al., 2008). FX binds in a central depression at the top of the hexon trimer of species C HAdV types via its Gla domain as revealed by molecular studies combined with cryoEM structural analyses. Interestingly, HAdV types belonging to species B and D exhibit either weak or no binding to FX suggesting that the non-conserved sequences in the hypervariable loops at the top of the hexon contribute to FX association. Ad infection/transduction of liver hepatocytes is mediated by the heparin-binding epitope in the serine protease domain of FX. These studies provide a valuable molecular description of how Ad5-based vectors circumvent the normal receptor interactions to transduce liver cells in vivo. They also provide potential new avenues to avoid this situation for future clinical applications. However, it remains to be determined whether these small animal models of liver transduction are relevant for non-human primates and man.
Immune factors impact HAdV cell entry
Association of soluble host proteins does not always lead to increased Ad transduction and in fact can have just the opposite effect. This was recently observed with a neutralizing monoclonal antibody directed against the Ad hexon protein (Varghese et al., 2004). CryoEM structure analyses showed that 240 copies (i.e. one per hexon trimer) of the 9C12 Mab were bound to each virion. The antibody mediated bivalent binding interactions and bridged adjacent exposed loops of neighboring hexon trimers. These studies suggest that the antibody might be capable of cross-linking in such a way as to prevent virus uncoating. Interestingly however subsequent studies demonstrated that the 9C12 Mab did not prevent uncoating or escape from early endosomes. Instead, the actual mode of neutralization involved interruption of Ad particle trafficking along microtubules mediated by dynein motors thus arresting nuclear trafficking (Smith et al., 2008). The precise mechanism of 9C12 inhibition of adenovirus association with microtubule motors remains to be elucidated however. These studies illustrate the diverse manner in which specific components of the immune system can alter Ad cell entry and infection.
The HAdV cell entry pathway can also be altered by associations with several members of the family of naturally occurring antimicrobial peptides known as alpha defensins (Smith and Nemerow, 2008). These relatively small (18–45 amino acid residues) cationic peptides comprise part of the innate immune system and are conserved throughout evolution (Ganz, 2003). Defensins are released from certain cell types (i.e. Paneth cells of the small intestine) and can achieve relatively high concentrations (1–10 mM) on or near mucosal surfaces. These peptides have been primarily associated with antibacterial activity, likely causing disruption of the pathogen cell membrane. However, recent studies have shown that certain defensins can also restrict infection by both enveloped (Hazrati et al., 2006) and nonenveloped viruses (Buck et al., 2006), although the mechanism of inactivation of this latter group of viral pathogens is less clear. Studies with HAdV have provided new knowledge of how alpha defensins can neutralize a nonenveloped virus (Smith and Nemerow, 2008). Alpha defensins HD5 and HNP1, at concentrations of 5–10 mM, bind to HAdV particles and prevent infection of cells in vitro. These molecules do not block binding or internalization but potently inhibit virus disassembly and release of the membrane lytic protein pVI, thereby preventing endosome disruption (endosomalysis). While HD5/HNP1-mediated virus neutralization represents a novel mechanism of inactivation, their precise sites of interaction with the virus remain to be defined. Such knowledge could aid in the development of antiviral compounds as well as provide insights for the mechanism of adenovirus disassembly during endosome penetration.
Summary and future perspectives
Modern molecular and cell biological techniques have been utilized to identify and characterize the primary and secondary receptors that mediate adenovirus attachment and internalization into host cells. However, these biological approaches alone have failed to provide a complete understanding of the entry process. Fortunately, the steady improvement in cryoEM techniques combined with well-established X-ray crystallographic methods have accelerated knowledge of HAdV entry mechanisms. These structural approaches have provided a more precise molecular description of the regions of the virus involved in receptor association as well as the binding sites for immune and coagulation factors. Atomic resolution crystallographic structures of HAdV fiber with domains of CAR and CD46 and with sialyl lactose have provided detailed knowledge of the molecular interactions necessary for viral attachment to the cell. We envision that future higher resolution structural studies will provide even greater insight into HAdV structure, its interactions with the numerous host cell factors, and the conformational changes in the virion that must occur during productive cell entry. While this endeavor will certainly require a major effort, it is worth noting that during this decade, near atomic resolution structures have been obtained for other large viruses by X-ray diffraction (Abrescia et al., 2004; Reinisch et al., 2000) or by cryoEM (Jiang et al., 2008; Yu et al., 2008; Zhang et al., 2008).
Acknowledgments
Sources of funding for this work include: NIH EY011431 (GRN); NIH HL054352 (GRN); NIH AI42929 (PLS). This is TSRI Manuscript #19715.
References
- Abrescia NG, Cockburn JJ, Grimes JM, Sutton GC, Diprose JM, Butcher SJ, Fuller SD, Martin C. San, Burnett RM, Stuart DI, Bamford DH, Bamford JK. Insights into assembly from structural analysis of bacteriophage PRD1. Nature. 2004;432:68–74. doi: 10.1038/nature03056. [DOI] [PubMed] [Google Scholar]
- Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell. Dev. Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- Arnberg N, Edlund K, Kidd AH, Wadell G. Adenovirus type 37 uses sialic acid as a cellular receptor. J. Virol. 2000;74:42–48. [PMC free article] [PubMed] [Google Scholar]
- Bai M, Campisi L, Freimuth P. Vitronectin receptor antibodies inhibit infection of HeLa and A549 cells by adenovirus type 12 but not by adenovirus type 2. J. Virol. 1994;68:5925–5932. doi: 10.1128/jvi.68.9.5925-5932.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton ES, Forrest JC, Connolly JL, Chappell JD, Liu Y, Schnell FJ, Nusrat A, Parkos CA, Dermody S. Junction adhesion molecule is a receptor for reovirus. Cell. 2001;104:441–451. doi: 10.1016/s0092-8674(01)00231-8. [DOI] [PubMed] [Google Scholar]
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Berget SM, Moore C, Sharp PA. Spliced segments at the 5′ terminus of adenovirus 2 late mRNA. Proc. Natl. Acad. Sci. U. S. A. 1977;74:3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley MC, Springer K, Zhang YB. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science. 1999;286:1579–1583. doi: 10.1126/science.286.5444.1579. [DOI] [PubMed] [Google Scholar]
- Bordigoni P, Carret AS, Venard V, Witz F, LeFaou A. Treatment of adenovirus infections in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis. 2001;32:1290–1297. doi: 10.1086/319984. [DOI] [PubMed] [Google Scholar]
- Buck CB, Day PM, Thompson CD, Lubkowski J, Lu W, Lowy DR, Schiller JT. Human alpha-defensins block papillomavirus infection. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1516–1521. doi: 10.1073/pnas.0508033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister WP, Guilligay D, Cusack S, Wadell G, Arnberg N. Crystal structure of species D adenovirus fiber knobs and their sialic acid binding sites. J. Virol. 2004;78:7727–7736. doi: 10.1128/JVI.78.14.7727-7736.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R. Four viruses, two bacteria, and one receptor: membrane cofactor protein (CD46) as pathogens’ magnet. J. Virol. 2004;78:4385–4388. doi: 10.1128/JVI.78.9.4385-4388.2004. [comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell JD, Prota AE, Dermody TS, Stehle T. Crystal structure of reovirus attachment protein σ1 reveals evolutionary relationship to adenovirus fiber. EMBO J. 2002;21:1–11. doi: 10.1093/emboj/21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnadurai G. Adenovirus 2 Ip+ locus codes for a 19 kd tumor antigen that plays an essential role in cell transformation. Cell. 1983;33:759–766. doi: 10.1016/0092-8674(83)90018-1. [DOI] [PubMed] [Google Scholar]
- Chiu CY, Mathias P, Nemerow GR, Stewart PL. Structure of adenovirus complexed with its internalization receptor, αv β5 integrin. J. Virol. 1999;73:6759–6768. doi: 10.1128/jvi.73.8.6759-6768.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CY, Wu E, Brown SL, Von Seggern DJ, Nemerow GR, Stewart PL. Structural analysis of a fiber-pseudotyped adenovirus with ocular tropism suggests differential modes of cell receptor interactions. J. Virol. 2001;75:5375–5380. doi: 10.1128/JVI.75.11.5375-5380.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow LT, Gelinas RE, Broker TR, Roberts RJ. An amazing sequence arrangement at the 5′ ends of adenovirus 2 messenger RNA. Cell. 1977;12:1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Chroboczek J, Ruigrok RW, Cusack S. Adenovirus fiber. Curr. Top Microbiol. Immunol. 1995;199(Pt 1):163–200. doi: 10.1007/978-3-642-79496-4_10. [DOI] [PubMed] [Google Scholar]
- Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. U. S. A. 2001;98:15191–15196. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124:119–131. doi: 10.1016/j.cell.2005.10.035. [see comment] [DOI] [PubMed] [Google Scholar]
- Fabry CM, Rosa-Calatrava M, Conway JF, Zubieta C, Cusack S, Ruigrok RW, Schoehn G. A quasi-atomic model of human adenovirus type 5 capsid. EMBO J. 2005;24:1645–1654. doi: 10.1038/sj.emboj.7600653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischli C, Verhaagh S, Havenga M, Sirena D, Schaffner W, Cattaneo R, Greber UF, Hemmi S. The distal short consensus repeats 1 and 2 of the membrane cofactor protein CD46 and their distance from the cell membrane determine productive entry of species B adenovirus serotype 35. J. Virol. 2005;79:10013–10022. doi: 10.1128/JVI.79.15.10013-10022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- Gaggar A, Shayakhmetov DM, Liszewski MK, Atkinson JP, Lieber A. Localization of regions in CD46 that interact with adenovirus. J. Virol. 2005;79:7503–7513. doi: 10.1128/JVI.79.12.7503-7513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev., Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Greber UF, Singh I, Helenius A. Mechanisms of virus uncoating. Trends Microbiol. 1994;2:52–56. doi: 10.1016/0966-842x(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Gustafsson DJ, Segerman A, Lindman K, Mei Y-F, Wadell G. The Arg279Gln [corrected] substitution in the adenovirus type 11p (Ad11p) fiber knob abolishes EDTA-resistant binding to A549 and CHO-CD46 cells, converting the phenotype to that of Ad7p. J. Virol. 2006;80:1897–1905. doi: 10.1128/JVI.80.4.1897-1905.2006. [erratum appears in J Virol. 2006 May;80(10):5101] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey B-G, Maroni J, O’Donoghue KA, Chu KW, Muscat JC, Pippo AL, Wright CE, Hollmann C, Wisnivesky JP, Kessler PD, Rasmussen HS, Rosengart TK, Crystal RG. Safety of local delivery of low-and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of morbid conditions. Hum. Gene Ther. 2002;13:15–63. doi: 10.1089/10430340152712638. [DOI] [PubMed] [Google Scholar]
- Hazrati E, Galen B, Lu W, Wang W, Ouyang Y, Keller MJ, Lehrer RI, Herold BC. Human alpha-and beta-defensins block multiple steps in herpes simplex virus infection. J. Immunol. 2006;177:8658–8666. doi: 10.4049/jimmunol.177.12.8658. [DOI] [PubMed] [Google Scholar]
- He Y, Chipman PR, Howitt J, Bator CM, Whitt MA, Baker TS, Kuhn RJ, Anderson CW, Freimuth P, Rossmann MG. Interaction of coxsackievirus B3 with the full length coxsackievirus—adenovirus receptor. Nat. Struct. Biol. 2001;8:874–878. doi: 10.1038/nsb1001-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry LJ, Xia D, Wilke ME, Deisenhofer J, Gerard RD. Characterization of the knob domain of the adenovirus type 5 fiber protein expressed in Escherichia coli. J. Virol. 1994;68:5239–5246. doi: 10.1128/jvi.68.8.5239-5246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry TD, Grines CL, Watkins MW, Dib N, Barbeau G, Moreadith R, Andrasfay T, Engler RL. Effects of Ad5FGF-4 in patients with angina: an analysis of pooled data from the AGENT-3 and AGENT-4 trials. J. Am. Coll. Cardiol. 2007;50:1038–1046. doi: 10.1016/j.jacc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Hilleman MR, Werner JH. Recovery of new agent from patients with acute respiratory illness. Proc. Soc. Exp. Biol. Med. 1954;85:183–188. doi: 10.3181/00379727-85-20825. [DOI] [PubMed] [Google Scholar]
- Hoffman JA. Adenoviral disease in pediatric solid organ transplant recipients. Pediatr. Transplant. 2006;10:17–25. doi: 10.1111/j.1399-3046.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- Huard J, Lochmuller H, Acsadi G, Jani A, Massie B, Karpati G. The route of administration is a major determinant of the transduction efficiency of rat tissues by adenoviral recombinants. Gene Ther. 1995;2:107–115. [PubMed] [Google Scholar]
- Jiang W, Baker ML, Jakana J, Weigele PR, King J, Chiu W. Backbone structure of the infectious epsilon15 virus capsid revealed by electron cryomicroscopy. Nature. 2008;451:1130–1134. doi: 10.1038/nature06665. [DOI] [PubMed] [Google Scholar]
- Kajon AE, Moseley JM, Metzgar D, Huong H-S, Wadleigh A, Ryan MAK, Russell KL. Molecular epidemiology of adenovirus type 4 infections in US military recruits in the postvaccination era (1997–2003) J. Infect. Dis. 2007;196:67–75. doi: 10.1086/518442. [DOI] [PubMed] [Google Scholar]
- Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL, Shayakhmetov DM. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law LK, Davidson BL. What does it take to bind CAR? Mol. Ther. 2005;12:599–609. doi: 10.1016/j.ymthe.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Li E, Stupack D, Bokoch G, Nemerow GR. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J. Virol. 1998a;72:8806–8812. doi: 10.1128/jvi.72.11.8806-8812.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E, Stupack D, Klemke R, Cheresh DA, Nemerow GR. Adenovirus endocytosis via alpha(v) integrins requires phosphoinositide-3-OH kinase. J. Virol. 1998b;72:2055–2061. doi: 10.1128/jvi.72.3.2055-2061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QG, Lindman K, Wadell G. Hydropathic characteristics of adenovirus hexons. Arch. Virol. 1997;142:1307–1322. doi: 10.1007/s007050050162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lortat-Jacob H, Chouin E, Cusack S, van Raaij MJ. Kinetic analysis of adenovirus fiber binding to its receptor reveals an avidity mechanism for trimeric receptor—ligand interactions. J. Biol. Chem. 2001;276:9009–9015. doi: 10.1074/jbc.M009304200. [DOI] [PubMed] [Google Scholar]
- Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Ann. Rev. Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey JK, Rigden PM, Green M. Do highly oncogenic group A human adenoviruses cause human cancer? Analysis of human tumors for adenovirus 12 transforming DNA sequences. Proc. Natl. Acad. Sci. U. S. A. 1976;73:4657–4661. doi: 10.1073/pnas.73.12.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier GT, Campbell JA, Chappell JD, Stehle T, Dermody TS, Barry MA. A chimeric adenovirus vector encoding reovirus attachment protein sigma1 targets cells expressing junctional adhesion molecule 1. Proc. Natl. Acad. Sci. U. S. A. 2004;101:6188–6193. doi: 10.1073/pnas.0400542101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepomuceno RR, Pache L, Nemerow GR. Enhancement of gene transfer to human myeloid cells by adenovirus—fiber complexes. Mol. Ther. 2007;15:571–578. doi: 10.1038/sj.mt.6300048. [DOI] [PubMed] [Google Scholar]
- Pache L, Venkataraman S, Reddy VS, Nemerow GR. Structural variations in species B adenovirus fibers impact CD46 association. J. Virol. 2008;82:7923–7931. doi: 10.1128/JVI.00754-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson BD, Reiter DM, Marttila M, Mei Y-F, Casasnovas JM, Arnberg N, Stehle T. Adenovirus type 11 binding alters the conformation of its receptor CD46. Nat. Struct. Mol. Biol. 2007;14:164–166. doi: 10.1038/nsmb1190. [DOI] [PubMed] [Google Scholar]
- Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao G-P, Wilson JM, Batshaw ML. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Molec. Genet. Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Reinisch KM, Nibert ML, Harrison SC. Structure of the reovirus core at 3.6 Å resolution. Nature. 2000;404:960–967. doi: 10.1038/35010041. [DOI] [PubMed] [Google Scholar]
- Roelvink PW, Lee GM, Einfeld DA, Kovesdi I, Wickham TJ. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science. 1999;286:1568–1571. doi: 10.1126/science.286.5444.1568. [DOI] [PubMed] [Google Scholar]
- Rowe WP, Huebner RJ, Hartley JW, Ward TG, Parrott RH. Studies of the adenoidal—pharyngeal—conjunctival (Apc) group of viruses. Am. J. Hyg. 1955;61:197–218. [Google Scholar]
- Ruigrok RW, Barge A, Albiges-Rizo C, Dayan S. Structure of adenovirus fibre. II. Morphology of single fibres. J. Mol. Biol. 1990;215:589–596. doi: 10.1016/S0022-2836(05)80170-6. [DOI] [PubMed] [Google Scholar]
- Saban SD, Silvestry M, Nemerow GR, Stewart PL. Visualization of alpha-helices in a 6-angstrom resolution cryoelectron microscopy structure of adenovirus allows refinement of capsid protein assignments. J. Virol. 2006;80:12049–12059. doi: 10.1128/JVI.01652-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter NK, Bednarski MD, Wurzburg BA, Hanson JE, Whitesides GM, Skehel JJ, Wiley DC. Hemagglutinins from two influenza virus variants bind to sialic acid derivatives with millimolar dissociation constants: a 500-MHz proton nuclear magnetic resonance study. Biochemistry. 1989;28:8388–8396. doi: 10.1021/bi00447a018. [DOI] [PubMed] [Google Scholar]
- Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayakhmetov DM, Lieber A. Dependence of adenovirus infectivity on length of the fiber shaft domain. J. Virol. 2000;74:10274–10286. doi: 10.1128/jvi.74.22.10274-10286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JG, Cassany A, Gerace L, Ralston R, Nemerow GR. Neutralizing antibody blocks adenovirus infection by arresting microtubule-dependent cytoplasmic transport. J. Virol. 2008;82:6492–6500. doi: 10.1128/JVI.00557-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JG, Nemerow GR. Mechanism of adenovirus neutralization by human alpha-defensins. Cell Host Microbe. 2008;3:11–19. doi: 10.1016/j.chom.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Smith TA, Idamakanti N, Marshall-Neff J, Rollence ML, Wright P, Kaloss M, King L, Mech C, Dinges L, Iverson WO, Sherer AD, Markovits JE, Lyons RM, Kaleko M, Stevenson SC. Receptor interactions involved in adenoviral-mediated gene delivery after systemic administration in non-human primates. Hum. Gene Ther. 2003;14:1595–1604. doi: 10.1089/104303403322542248. [DOI] [PubMed] [Google Scholar]
- Stewart PL, Burnett RM, Cyrklaff M, Fuller SD. Image reconstruction reveals the complex molecular organization of adenovirus. Cell. 1991;67:145–154. doi: 10.1016/0092-8674(91)90578-m. [DOI] [PubMed] [Google Scholar]
- Stewart PL, Chiu CY, Huang S, Muir T, Zhao Y, Chait B, Mathias P, Nemerow GR. Cryo-EM visualization of an exposed RGD eptitope on adenovirus that escapes antibody neutralization. EMBO J. 1997;16:1189–1198. doi: 10.1093/emboj/16.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PL, Nemerow GR. Cell integrins: commonly used receptors for diverse viral pathogens. Trends Microbiol. 2007;15:500–507. doi: 10.1016/j.tim.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Suomalainen M, Nakano MY, Keller S, Boucke K, Stidwill RP, Greber UF. Microtuble-dependent plus-and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J. Cell Biol. 1999;144:657–672. doi: 10.1083/jcb.144.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao N, Gao GP, Parr M, Johnston J, Baradet T, Wilson JM, Barsoum J, Fawell SE. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol. Ther. 2001;3:28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- Trentin JJ, Yabe Y, Taylor G. The quest for human cancer viruses. Science. 1962;137:835–841. doi: 10.1126/science.137.3533.835. [DOI] [PubMed] [Google Scholar]
- van Raaij MJ, Mitraki A, Lavigne G, Cusack S. A triple [beta]-spiral in the adenovirus fibre shaft reveals a new structural motif for a fibrous protein. Nature. 1999;401:935–938. doi: 10.1038/44880. [DOI] [PubMed] [Google Scholar]
- Varghese R, Mikyas Y, Stewart PL, Ralston R. Postentry neutralization of adenovirus type 5 by an antihexon antibody. J. Virol. 2004;78:12320–12332. doi: 10.1128/JVI.78.22.12320-12332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Seggern DJ, Chiu CY, Fleck SK, Stewart PL, Nemerow GR. A helper-independent adenovirus vector with E1, E3, and fiber deleted: Structure and infectivity of fiberless particles. J. Virol. 1999;73:1601–1608. doi: 10.1128/jvi.73.2.1601-1608.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SMK, Greig JA, Denby L, Custers J, Morita T, Francischetti IMB, Monteiro RQ, Barouch DH, van Rooijen N, Napoli C, Havenga MJE, Nicklin SA, Baker AH. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Walters RW, Freimuth P, Moninger TO, Ganske I, Zabner J, Welsh MJ. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell. 2002;110:789–799. doi: 10.1016/s0092-8674(02)00912-1. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins αv β3 and αv β5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Wiethoff CM, Wodrich H, Gerace L, Nemerow GR. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J. Virol. 2005;79:1992–2000. doi: 10.1128/JVI.79.4.1992-2000.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu E, Pache L, VonSeggern DJ, Mullen TM, Mikyas Y, Stewart PL, Nemerow GR. Flexibility of the adenovirus fiber is required for efficient receptor (CAR) interaction. J. Virol. 2003;77:7225–7235. doi: 10.1128/JVI.77.13.7225-7235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu E, Trauger SA, Pache L, Mullen T-M, von Seggern DJ, Siuzdak G, Nemerow GR. Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J. Virol. 2004;78:3897–3905. doi: 10.1128/JVI.78.8.3897-3905.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia D, Henry LJ, Gerard RD, Deisenhofer J. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 Å resolution. Structure. 1994;2:1259–1270. doi: 10.1016/s0969-2126(94)00126-x. [DOI] [PubMed] [Google Scholar]
- Yang XJ, Ogryzko VV, Nishikawa J, Howard BH, Nakatani Y. A p300/CBPassociated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- Yu X, Jin L, Zhou ZH. 3.88 A structure of cytoplasmic polyhedrosis virus by cryo-electron microscopy. Nature. 2008;453:415–419. doi: 10.1038/nature06893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Settembre E, Xu C, Dormitzer PR, Bellamy R, Harrison SC, Grigorieff N. Near-atomic resolution using electron cryomicroscopy and single-particle reconstruction. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1867–1872. doi: 10.1073/pnas.0711623105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta C, Schoehn G, Chroboczek J, Cusack S. The structure of the human adenovirus 2 penton. Mol. Cell. 2005;17:121–135. doi: 10.1016/j.molcel.2004.11.041. [DOI] [PubMed] [Google Scholar]