Abstract
The objective of this study was to determine whether green tea (GT) inhibits the expression of genes regulating hepatic lipogenesis and intestinal lipid transport in fructose-fed ovariectomized (OX) rats. OX rats were assigned to: 1) a control group (S) fed the AIN-93G diet with corn starch as the major carbohydrate source; 2) another control group (F) fed the same diet but containing fructose at 60% as the major carbohydrate source; 3) a group fed the F diet but containing 0.5% GT; and 4) a group fed the F diet containing 1% GT. At 6 wk, plasma and liver triglyceride (TG) and cholesterol and expression of liver sterol regulatory element-binding protein-1c (SREBP-1c) and selected genes involved in lipogenesis and lipid transport were measured. Fructose elevated plasma TG and cholesterol compared with the S group. GT at 0.5 and 1.0% markedly lowered plasma and liver TG. Fructose increased the expression of SREBP-1c, fatty acid synthase, and stearoyl-CoA desaturase 1 mRNA in the liver, whereas GT decreased the expression of these lipogenic genes. Similarly, fructose increased the abundance of hepatic 3-hydroxy-3-methyl-glutaryl-CoA reductase mRNA, whereas GT significantly decreased its expression. GT did not alter the expression of scavenger receptor class B, type 1, microsomal TG transfer protein, and apobec 1 in the liver and intestine. The results suggest that the lipid-lowering effect of GT is mediated partly by its inhibition of hepatic lipogenesis involving SREBP-1c and its responsive genes without affecting lipoprotein assembly.
Introduction
Green tea (GT)6 is a widely consumed popular beverage derived from the tea plant, Camellia sinensis. Evidence suggests that GT and its catechins possess antioxidant, antiatherogenic, antiinflammatory, and anticarcinogenic properties, as suggested by numerous in vitro cell culture and animal studies (1–3). Among the potential health benefits of GT, its lipid-lowering effect has been well documented in animal models of hyperlipidemia and atherosclerosis (4,5), although the evidence still remains inconclusive for definitive relationships between GT intake and the risk of cardiovascular disease in humans (6,7). At present, the mechanisms underlying the lipid-lowering effect of GT are not well understood. Previously, using rats with mesenteric lymph cannulas, we presented evidence that GT inhibits the intestinal absorption of dietary lipids, including triglyceride (TG), cholesterol, and other lipophilic compounds such as α-tocopherol (8–11). GT and its catechins, particularly (-)-epigallocatechin gallate (EGCG), interfere with the emulsification, digestion, and micellar solubilization of lipids, critical steps involved in the intestinal absorption of dietary lipids. Whether the intestinal uptake and packaging of lipids and/or chylomicron assembly is affected by GT is currently unknown (11,12).
Evidence also suggests that GT and its principal catechin, EGCG, may inhibit lipogenesis by suppressing the expression of lipogenic genes (13–15). For example, EGCG has been shown to downregulate such genes as fatty acid synthase (FAS) and stearoly-CoA desaturase 1 (SCD1) in H4IIE rat hepatoma cells in vitro (16). An in vivo study (17) using C57BL/6J mice fed a high-fat diet containing EGCG at 1.0% demonstrated that EGCG lowers plasma TG and downregulates the adipose expression of acetyl-CoA carboxylase, the rate-limiting enzyme for fatty acid synthesis, as well as FAS and SCD1.
The above-cited observations suggest that the hypolipidemic effect of GT and its catechins may be mediated primarily via the inhibition of intestinal lipid absorption and suppression of hepatic and adipose lipogenesis. However, it remains to be determined whether GT, rather than pure EGCG, at levels commonly consumed, affects the expression of genes involved in the regulation of hepatic lipogenesis and intestinal lipid absorption. In the present study, we examined whether dietary GT, as supplemented at 0.5 and 1.0%, affects the expression of genes regulating hepatic lipogenesis and intestinal uptake and absorption of lipids. The present study used ovariectomized (OX) rats fed a fructose-enriched diet to stimulate hepatic de novo lipogenesis (18) and also to mimic the postmenopausal state in which plasma levels of lipids are elevated with increased risk of coronary heart disease (19).
Materials and Methods
Rats and diet.
OX Sprague-Dawley rats were purchased from Harlan Sprague Dawley and were individually housed in a temperature- and humidity-controlled room with a 12-h-light/12-h-dark cycle (light from 0700 to 1900). All rats were acclimated for 5 d while being fed a rodent nonpurified diet. Rats were assigned to the following 4 groups: 1) a negative control group (S) fed a modified AIN-93G diet (20,21) with corn starch as the major carbohydrate source (Dyets) (Table 1); 2) a positive control group (F) fed the same diet but containing fructose in place of corn starch at 60% to stimulate lipogenesis (18) (Table 1); 3) a group (FT0.5%) fed the F diet but containing 0.5% GT; and 4) another group (FT1.0%) fed the F diet containing 1% GT. GT (lyophilized powder) was a kind gift from Unilever Bestfoods North America. The GT contained 29.2 mg total catechins/100 mg (wt:wt) consisting of 48% EGCG, 31% epigallocatechin, 13% epicatechin gallate, and 8% epicatechin, as determined by HPLC. The dietary levels of GT at 0.5 and 1.0% were equivalent to human intakes of 4 and 8 cups (2.0 g GT/cup) per day, respectively, as estimated based on an energy intake of 8360 kJ/d). Rats were fed the diets 3 times/wk and were weighed weekly. At the end of 6 wk of feeding, rats were anesthetized under isoflurane vapor after overnight food deprivation. Blood samples were collected via the retro-orbital sinus into tubes containing 4 mg Na2-EDTA. Plasma was collected after centrifuging at 2000 × g; 20 min at 4°C and stored at −80°C in aliquots in a preservation cocktail (0.5 mL aprotinin, 0.1 mL sodium azide, and 0.1 mL phenylmethane sulfonyl fluoride/100 mL). After blood collection, rats were killed with isoflurane overdosing followed by cervical dislocation. From the central lobe of the liver, ∼0.5 g tissue was excised from each rat and placed in a plastic tube containing RNALater solution (Ambion). The remainder of the liver was weighed and snap-frozen in liquid nitrogen. The mid-segment of the intestine (jejunum) was washed 3 times with 10 mL ice-cold saline containing 16.5 mmol/L sodium taurocholate (Sigma-Aldrich) and stored in RNALater solution. The liver and intestinal tissues in RNALater were kept at 4°C for 24 h and then stored at −80°C. The protocol for the care and use of animals was approved by the Institutional Animal Care and Use Committee at the University of Connecticut.
TABLE 1.
Composition of diets containing starch and fructose as the major source of carbohydrate1
| Ingredient | S | F | |
|---|---|---|---|
| g/kg | |||
| Egg whites | 200 | 200 | |
| Corn starch | 530.7 | 30.7 | |
| Fructose | 100 | 600 | |
| Cellulose | 50 | 50 | |
| Soybean oil2 | 70 | 70 | |
| Mineral mix (AIN-93G-EGG-MX) | 35 | 35 | |
| Vitamin mix (AIN-93-VX) | 10 | 10 | |
| Biotin premix (1 mg/g sucrose) | 1.8 | 1.8 | |
| Choline bitartrate | 2.5 | 2.5 | |
Plasma and liver lipids.
Plasma total cholesterol (TC) was determined as described previously (22). The HDL cholesterol (HDL-C) concentration in the supernatant was measured by an enzymatic method after selective precipitation of apolipoprotein (apo) B-containing lipoproteins by magnesium chloride and dextran sulfate (23). Liver cholesterol and TG concentrations were determined as previously described following extraction of hepatic lipids with chloroform-methanol 2:1 (24). Esterified cholesterol (EC) concentrations were calculated by subtracting free cholesterol (FC) from TC.
Plasma glucose.
Plasma glucose was determined enzymatically (25) using a kit from Wako Chemicals.
RNA isolation and quantitative real-time PCR.
Total RNA was isolated from liver and intestine samples using TRIzol reagent (Invitrogen) following the manufacturer's protocol. One microgram of total RNA was treated with DNase I to remove any genomic DNA contamination, after which it was reverse transcribed by Moloney murine leukemia virus RT (Promega). Quantitative real-time PCR analysis was performed using the Sybr Green procedure and an ABI 7300 instrument (Applied Biosystems). Primers were used, as designed according to GenBank database using the Primer Express software (Applied Biosystems). Primer sequences are given in Supplemental Table 1. Expression of mRNA values was normalized relative to glyceraldehyde-3-phosphate dehydrogenase as an internal control and data were analyzed, as described previously (26).
Statistical analysis.
All statistical analyses were performed using PC SAS (27) (SAS Institute). One-way ANOVA and Tukey's post hoc test with least significant difference were used to compare group means. Homogeneity of variances was tested by Levene's test and Welch's ANOVA was used to compare group means when the group variances were unequal. Differences were considered significant at P < 0.05. Values in tables and figures are expressed as means ± SD.
Results
General observations.
Fructose feeding for 6 wk did not affect body weight or food intake of the rats. Dietary GT both at 0.5 and 1.0% resulted in moderate increases in body weight and food intake compared with the F-fed group. A moderate increase in body weight was consistently noted in OX rats when GT was fed in combination with fructose, whereas no such change occurred when they were fed with starch under similar experimental conditions (S. Shrestha and S. I. Koo, unpublished data). Fructose feeding resulted in a decrease in retroperitoneal adipose weight. GT did not significantly affect the adipose weight in fructose-fed rats (Table 2). Fructose significantly increased liver weight compared with the S group. GT did not affect liver weight in the F-fed rats (Table 3).
TABLE 2.
Body weight, food intake, plasma lipids, and plasma glucose in OX rats fed diets containing S or F diet or the F diet with 0.5% or 1.0% GT for 6 wk1
| Variables | S | F | FT 0.5% | FT 1.0% |
|---|---|---|---|---|
| Body weight, g | 303 ± 11bc | 290 ± 13c | 319 ± 13a | 310 ± 16b |
| Retroperitoneal adipose, g | 1.8 ± 0.6a | 1.3 ± 0.3bc | 1.6 ± 0.4ab | 1.1 ± 0.1c |
| Food intake, g/d | 19 ± 2.0b | 19 ± 3.0b | 22 ± 2.0a | 24 ± 2.0a |
| TG, mmol/L | 0.3 ± 0.1b | 0.4 ± 0.1a | 0.3 ± 0.1b | 0.3 ± 0.1b |
| TC, mmol/L | 2.3 ± 0.2c | 2.7 ± 0.4b | 2.9 ± 0.2ab | 3.2 ± 0.3a |
| HDL-C, mmol/L | 1.5 ± 0.3c | 1.8 ± 0.1b | 2.0 ± 0.2ab | 2.2 ± 0.3a |
| Non-HDL-C, mmol/L | 0.8 ± 0.3 | 0.9 ± 0.4 | 0.9 ± 0.2 | 1.0 ± 0.3 |
| Glucose, mmol/L | 9.0 ± 1.5b | 11.7 ± 2.0a | 10.5 ± 1.4ab | 9.2 ± 2.4b |
Data are means ± SD, n = 7. Means in a row with superscripts without a common letter differ, P < 0.05.
TABLE 3.
Liver weight and lipids in OX rats fed diets containing S or F diet or the F diet with 0.5% or 1.0% GT for 6 wk1
| Variables | S | F | FT 0.5% | FT 1.0% |
|---|---|---|---|---|
| Liver weight, g | 7.0 ± 0.7b | 8.5 ± 0.7a | 8.8 ± 0.6a | 8.8 ± 1.0a |
| TG, μmol/g | 7.9 ± 4.3a | 8.7 ± 2.3a | 2.4 ± 1.1b | 2.4 ± 0.6b |
| TC, μmol/g | 11.1 ± 2.8a | 8.5 ± 2.1b | 7.5 ± 0.8b | 7.8 ± 1.8b |
| FC, μmol/g | 4.1 ± 2.6 | 3.6 ± 0.8 | 3.4 ± 1.0 | 2.9 ± 1.3 |
| EC, μmol/g | 7.0 ± 3.6a | 4.9 ± 1.0ab | 4.1 ± 1.8b | 4.9 ± 2.1ab |
Data are means ± SD, n = 7. Means in a row with superscripts without a common letter differ, P < 0.05.
Plasma lipids and glucose.
Fructose feeding significantly elevated plasma TG, whereas GT lowered plasma TG to the control level. Fructose feeding also elevated plasma TC compared with the controls. GT at 0.5% did not affect plasma TC, whereas GT at 1.0% significantly increased TC due to increased plasma HDL-C, with no change in non-HDL-C. Dietary fructose significantly increased plasma glucose and GT lowered it at 1.0%, with no significant effect at 0.5% (Table 2).
Liver lipids.
Fructose feeding for 6 wk did not alter the concentration of liver TG. GT at both 0.5 and 1.0% had a profound effect on liver TG, reducing it to 27–30% of the controls fed the F and S diets (Table 3). In contrast, GT did not affect liver TC concentrations in the F-fed rats, whereas dietary fructose lowered liver TC concentrations compared with starch (Table 3).
Expression of genes involved in lipid transport and metabolism.
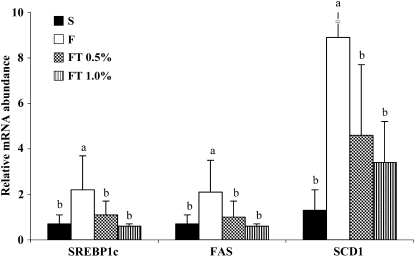
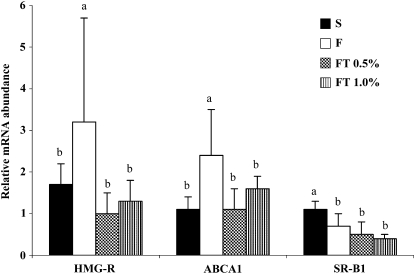
To explore the molecular mechanism underlying the hypolipidemic effect of GT, we examined the expression of hepatic and intestinal genes that regulate lipogenesis, lipid uptake, and lipoprotein assembly. Dietary fructose, compared with starch, markedly increased the expression of hepatic sterol regulatory element-binding protein-1c (SREBP-1c) and also the expression of its target lipogenic genes, FAS and SCD1. GT at both 0.5 and 1.0% prevented the fructose-induced increases in SREBP-1c, FAS, and SCD1 and decreased their expression to the level expressed in the S-fed rats (Fig. 1). Fructose feeding also increased the expression of hepatic 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMG-R) and ATP-binding cassette transporter A1 (ABCA1) mRNAs, whereas GT at 0.5 and 1.0% lowered their expression to the S control level (Fig. 2). Dietary fructose significantly decreased the expression of liver scavenger receptor class B type 1 (SR-B1). Similarly, fructose markedly decreased the expression of intestinal SR-B1 mRNA but did not affect ABCA1 mRNA. GT did not affect hepatic and intestinal expression of SR-B1, whereas GT at 0.5% moderately increased intestinal Niemann-Pick C1-like 1 (NPC1L1) mRNA involved in intestinal cholesterol uptake (Table 4).
FIGURE 1 .
The expression of liver SREBP-1c, FAS, and SCD1 in OX rats fed diets containing S or F diet or the F diet with 0.5% or 1.0% GT for 6 wk. Bars are means ± SD, n = 7. Means without a common letter differ, P < 0.05.
FIGURE 2 .
The expression of liver HMG-R, ABCA1, and SR-B1 in OX rats fed diets containing S or F diet or the F diet with 0.5% or 1.0% GT for 6 wk. Bars are means ± SD, n = 7. Means without a common letter differ, P < 0.05.
TABLE 4.
Expression of selected genes in the liver and intestine of OX rats fed diets containing S or F diet or the F diet with 0.5% or 1.0% GT for 6 wk1
| Relative mRNA abundance | S | F | FT 0.5% | FT 1.0% |
|---|---|---|---|---|
| Liver | ||||
| ACAT1 | 1.6 ± 0.5 | 1.5 ± 1.0 | 1.3 ± 0.4 | 1.4 ± 0.2 |
| ACAT2 | 1.0 ± 0.5a | 0.5 ± 0.3b | 0.3 ± 0.2b | 0.6 ± 0.3b |
| Apobec-1 | 1.4 ± 0.3b | 1.4 ± 0.7b | 2.0 ± 0.5ab | 2.3 ± 0.7a |
| MTP | 2.2 ± 0.6 | 2.2 ± 2.2 | 2.0 ± 0.4 | 2.7 ± 0.4 |
| Intestine | ||||
| ACAT1 | 1.1 ± 0.6 | 0.9 ± 0.6 | 0.8 ± 0.2 | 0.8 ± 0.1 |
| ACAT2 | 1.3 ± 0.4a | 0.7 ± 0.1b | 0.7 ± 0.3b | 0.6 ± 0.3b |
| Apobec1 | 1.2 ± 0.4 | 1.1 ± 0.7 | 1.7 ± 0.9 | 1.8 ± 0.5 |
| MTP | 1.5 ± 0.4 | 1.0 ± 0.6 | 1.9 ± 0.8 | 1.9 ± 1.2 |
| NPC1L1 | 0.7 ± 0.4b | 0.9 ± 0.2b | 1.2 ± 0.3a | 1.0 ± 0.2ab |
| SR-BI | 0.8 ± 0.3a | 0.3 ± 0.2b | 0.2 ± 0.1b | 0.2 ± 0.1b |
| ABCA1 | 0.7 ± 0.4 | 0.8 ± 0.5 | 1.1 ± 0.4 | 0.7 ± 0.1 |
Data are means ± SD, n = 6–8. Means in a row with superscripts without a common letter differ, P < 0.05.
Fructose, compared with starch, significantly decreased acyl-CoA:cholesterol acyltransferase 2 (ACAT2) mRNA in both the liver and intestine without affecting ACAT1 mRNA, whereas GT did not affect ACAT1 or ACAT2 in either tissue (Table 4). Fructose had no effect on apobec1 and microsomal TG transfer protein (MTP), genes regulating apoB mRNA editing and lipoprotein assembly, respectively, in the liver and intestine. GT only at 1.0% significantly increased liver apobec1 mRNA but did not affect its expression in the intestine (Table 4).
Discussion
Using an OX rat model fed a high-fructose diet, the present study provides the following new evidence that GT: 1) lowered plasma and liver TG concentrations; 2) downregulated the expression of SREBP-1c mRNA and its target lipogenic genes, FAS and SCD1, and genes regulating hepatic cholesterol synthesis and transport (HMG-R, ACAT2, and ABCA1), with no effect on SR-B1; 3) did not affect the expression of apobec1 and MTP involved in intestinal apoB mRNA editing and chylomicron assembly; and 4) did not affect the intestinal expression of SR-B1 and ABCA1 that mediate cholesterol uptake and transport.
Dietary fructose is a strong inducer of SREBP-1c, FAS, SCD1, and other enzymes regulating hepatic lipogenesis (18,28–30) and produces a pronounced elevation of hepatic TG concentrations with an increase in plasma TG leading to the development of insulin resistance and impaired glucose tolerance (18). Evidence shows that the induction of hepatic SREBP-1c by dietary sugars is insulin independent in refed states and that SREBP-1c and its target genes such as FAS, SCD1, and acetyl-CoA carboxylase are highly inducible by fructose and glucose under insulin-depleted conditions (30). However, fructose is more potent than glucose at inducing these lipogenic genes (29). The induction of SREBP-1c by fructose occurs more gradually with an increased expression of FAS for an extended period in refed mice (30). This phenomenon may partly explain the fructose-induced hypertriglyceridemia and development of the metabolic syndrome with chronic fructose feeding. The present findings demonstrate that GT is effective in lowering liver and plasma TG in an OX rat model of hypertriglyceridemia induced by a high-fructose diet. The findings are consistent with our recent observation of pronounced decreases in liver and serum TG concentrations by GT in ob/ob mice, a genetic model of spontaneous hypertriglyceridemia (31). Previously, female rats with intact ovaries were shown to be protected against the fructose-induced hypertriglyceridemia (32) and metabolic syndrome (33). The present study demonstrates that fructose feeding in OX rats increases plasma TG and glucose concentrations and that GT prevents the fructose-induced increases in plasma TG and glucose concentrations in this rat model. This finding suggests a potential benefit of GT for postmenopausal women, considering the increased prevalence of the metabolic syndrome and insulin resistance after menopause (34).
The upregulation of hepatic HMG-R mRNA by fructose is consistent with the increased plasma cholesterol concentration here and as reported by others in fructose-fed male rats (35). Based on information available, the hypercholesterolemic effect of fructose is likely mediated via interactions of multiple factors regulating its synthesis, packaging, and release via VLDL, and plasma clearance (18,28). It is also possible that the increase in the plasma cholesterol concentration by fructose may be due partly to increased cholesterol efflux from liver to plasma by upregulation of ABCA1, as suggested by our data. This may also explain the lower levels of liver cholesterol observed in fructose-fed rats. The decrease in liver ACAT2 expression by fructose may result in a transient increase in FC, while its efflux from the liver is facilitated via the upregulation of ABCA1 that transfers cellular cholesterol to HDL apolipoproteins, including A1 (36). The fructose-induced decrease in SR-B1 expression in the liver, as shown here, may further contribute to the elevation of plasma HDL-C, because liver SR-B1 is largely responsible for selective uptake/transfer of cholesterol esters from HDL to the liver. Our data show that GT suppresses HMG-R expression in the liver. GT, however, was ineffective in preventing the fructose-induced increase in plasma cholesterol concentrations when fed at 0.5%, but rather increased the cholesterol concentrations when fed at a higher level (1.0%). This finding is in direct contrast with the cholesterol-lowering effect of GT in normal male animals fed high-fat or high-sucrose diets (4,5). Previously, the cholesterol-raising effect of GT was reported by another study (37) in male mice susceptible to diet-induced obesity and insulin resistance. Similar to our observations here, GT (94% EGCG) at 0.5% did not affect the plasma cholesterol concentration, but at 1.0%, it raised the cholesterol concentration (37). In the present study, the increase in the plasma cholesterol concentration by GT at 1.0% was due to a significant rise in the HDL fraction, with no change in non-HDL-C. This increase appears unrelated to changes in hepatic expression of SR-B1 or ABCA1, because GT did not further decrease the expression of SR-B1 while decreasing ABCA1 expression. The decrease in ABCA1 expression by GT may reflect the inhibition of hepatic cholesterol synthesis, as suggested by a decrease in HMG-R expression. Evidence shows that ABCA1 is downregulated by HMG-R inhibition but is inducible by cholesterol loading (36).
Evidence shows that GT also inhibits the intestinal absorption of lipids, including TG and other lipophilic compounds (8–11). The inhibitory effect of GT on lipid absorption is largely due to its interference with the intraluminal emulsification, hydrolysis, and micellar solubilization of lipids (11,12). Our findings here provide evidence that GT does not significantly affect the expression of genes involved in the uptake and subsequent packaging of lipids into chylomicrons in the enterocyte, such as intestinal SR-B1, ABCA1, apobec1, and MTP. SR-B1, present in the brush-border and basolateral membranes of the enterocyte, mediates the uptake and efflux of cholesterol (38,39), whereas ABCA1 in the basolateral membrane is thought to promote cholesterol efflux from the enterocyte (40). GT, when fed at 0.5%, caused only a slight increase in gene expression of NPC1L1, a brush-border protein involved in cholesterol uptake. GT did not affect intestinal ACAT2 mRNA expression, whereas fructose significantly downregulated intestinal ACAT2, as in the liver. Moreover, GT did not alter the intestinal expression of apobec1, the apoB mRNA-editing enzyme that produces ApoB48, and of MTP, which catalyzes the transfer of lipids to apoB48 during chylomicron assembly (41). Based on our findings here and earlier observations (8–11), GT or catechins may not appreciably alter the intestinal processes of lipid uptake, packaging, and chylomicron assembly but mainly interfere with the intraluminal events critical to luminal lipolysis and transfer of lipolytic products to the enterocyte.
At present, the mechanism underlying the inhibitory effect of GT on the expression of lipogenic genes remains unclear. Because our data here indicate that GT inhibits the hepatic expression of SREBP-1c and ABCA1, which are liver X receptor (LXR)-target genes, the possibility exists that the inhibition of lipogenic gene expression by GT may be mediated in part via its modulation of LXR. In fact, a study using human mononuclear cells (42) showed that GT polyphenols dose dependently decreased the expression of LXRα and PPARγ that upregulate the expression of lipogenic genes, including SREBP-1c. Whether the inhibition of liver HMG-R expression by GT is mediated via its regulation of SREBP-2 and other transcription factors (e.g. LXR) is unknown at present. A study with HepG2 cells in vitro (43) showed that EGCG increased the active form of SREBP-2 by inhibiting its degradation by the ubiquitin-proteasome pathway. Another study with HepG2 cells (44) suggests that the regulation of HMG-R by GT catechins may be concentration dependent, because cholesterol synthesis was significantly inhibited at a low concentration of EGCG (50 μmol/L) but increased at a higher concentration. The dual effects of GT on cholesterol synthesis remain to be further examined under in vivo conditions with catechin levels attainable by dietary means.
In summary, this study, using OX rats fed fructose, an animal model of diet-induced hypertriglyceridemia, provides new evidence that GT significantly downregulates the hepatic expression of SREBP-1c and its target genes such as FAS and SCD1, and the genes that regulate hepatic cholesterol synthesis (HMG-R) and efflux (ABCA1). Data here suggest that GT may not alter the expression of genes involved in intestinal lipid uptake and chylomicron assembly. Thus, based on the information available, the TG-lowering effect of GT in plasma and liver may be mediated partly via the suppression of lipogenesis and inhibition of luminal hydrolysis and micellar transfer of lipids to the enterocyte. Further studies are warranted to determine the expression of lipogenic gene products and to elucidate the mechanisms whereby GT suppresses the expression of these genes, particularly in relation to postprandial dyslipidemia and insulin resistance.
Supplementary Material
Supported by grant from the NIH (NCCAM. R21AT001363-01 to S. I. Koo) and the USDA (Hatch CONS00761 to S.I. Koo).
Author disclosures: S. Shrestha, S. J. Ehlers, J.-Y. Lee, M.-L. Fernandez, and S. I. Koo, no conflicts of interest.
Supplemental Table 1 is available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: ABCA1, ATP-binding cassette transporter A1; ACAT, acyl CoA: cholesterol acyltransferase; apo, apolipoprotein; EC, esterified cholesterol; EGCG, (-)-epigallocatechin gallate; F, fructose (fructose-fed); FAS, fatty acid synthase; FC, free cholesterol; FT0.5%, fructose-fed with 0.5% green tea; FT1.0%, fructose-fed with 1.0% green tea; GT, green tea (extract); HDL-C, HDL-cholesterol; HMG-R, 3-hydroxy-3-methyl-glutaryl-CoA reductase; LXR, liver X receptor; MTP, microsomal triglyceride transfer protein; NPC1L1, Niemann-Pick C1 Like 1; OX, ovariectomized; S, starch (starch-fed); SCD1, stearoyl-CoA desaturase 1; SR-B1, scavenger receptor class B type 1; SREBP, sterol regulatory element-binding protein; TC, total cholesterol; TG, triglyceride.
References
- 1.Wolfram S. Effects of green tea and EGCG on cardiovascular and metabolic health. J Am Coll Nutr. 2007;26:S373–88. [DOI] [PubMed] [Google Scholar]
- 2.Crespy V, Williamson G. A review of the health effects of green tea catechins in in vivo animal models. J Nutr. 2004;134:S3431–40. [DOI] [PubMed] [Google Scholar]
- 3.Henning SM, Fajardo-Lira C, Lee HW, Youssefian AA, Go VL, Heber D. Catechin content of 18 teas and a green tea extract supplement correlates with the antioxidant capacity. Nutr Cancer. 2003;45:226–35. [DOI] [PubMed] [Google Scholar]
- 4.Chan PT, Fong WP, Cheung YL, Huang Y, Ho WKK, Chen ZY. Jasmine green tea epicatechins are hypolipidemic in hamsters (Mesocricetus auratus) fed a high fat diet. J Nutr. 1999;129:1094–101. [DOI] [PubMed] [Google Scholar]
- 5.Yang M, Wang C, Chen HL. Green, oolong and black tea extracts modulate lipid metabolism in hyperlipidemia rats fed high-sucrose diet. J Nutr Biochem. 2001;12:14–20. [DOI] [PubMed] [Google Scholar]
- 6.Erba D, Riso P, Bordoni A, Foti P, Biagi PL, Testolin G. Effectiveness of moderate green tea consumption on antioxidative status and plasma lipid profile in humans. J Nutr Biochem. 2005;16:144–9. [DOI] [PubMed] [Google Scholar]
- 7.Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81:S317–25. [DOI] [PubMed] [Google Scholar]
- 8.Löest HB, Noh SK, Koo SI. Green tea extract lowers the lymphatic absorption of cholesterol and α-tocopherol in ovariectomized rats. J Nutr. 2002;132:1282–8. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Noh SK, Koo S. I. Epigallocatechin gallate and caffeine differentially inhibit the intestinal absorption of cholesterol and fat in ovariectomized rats. J Nutr. 2006;136:2791–6. [DOI] [PubMed] [Google Scholar]
- 10.Wang S, Noh SK, Koo SI. Green tea catechins inhibit pancreatic phospholipase A2 and intestinal absorption of lipids in ovariectomized rats. J Nutr Biochem. 2006;17:492–8. [DOI] [PubMed] [Google Scholar]
- 11.Koo SI, Noh SK. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. J Nutr Biochem. 2007;18:179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shishikura Y, Khokhar S, Murray BS. Effect of tea polyphenols on emulsification of olive oil in a small intestine model system. J Agric Food Chem. 2006;54:1906–13. [DOI] [PubMed] [Google Scholar]
- 13.Moon HS, Lee HG, Choi YJ, Kim TG, Cho CS. Proposed mechanisms of (-)- epigallocatechin-3-gallate for anti-obesity. Chem Biol Interact. 2007;167:85–98. [DOI] [PubMed] [Google Scholar]
- 14.Lin JK, Lin-Shiau SY. Mechanisms of hypolipidemic and anti-obesity effects of tea and tea polyphenols. Mol Nutr Food Res. 2006;50:211–7. [DOI] [PubMed] [Google Scholar]
- 15.Kao YH, Chang HH, Lee MJ, Chen CL. Tea, obesity, and diabetes. Mol Nutr Food Res. 2006;50:188–210. [DOI] [PubMed] [Google Scholar]
- 16.Wolfram S, Raederstorff D, Preller M, Wang Y, Teixeira SR, Riegger C, Weber P. Epigallocatechin gallate supplementation alleviates diabetes in rodents. J Nutr. 2006;136:2512–8. [DOI] [PubMed] [Google Scholar]
- 17.Wolfram S, Raederstorff D, Wang Y, Teixeira SR, Elste V, Weber P. TEAVIGO (epigallocatechin gallate) supplementation prevents obesity in rodents by reducing adipose tissue mass. Ann Nutr Metab. 2005;49:54–63. [DOI] [PubMed] [Google Scholar]
- 18.Basciano H, Federico L, Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab. 2005;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dallongeville J, Marecaux N, Isorez D, Zylbergberg G, Fruchart J-C, Amouyel P. Multiple coronary heart disease risk factors are associated with menopause and influenced by substitutive hormonal therapy in a cohort of French women. Atherosclerosis. 1995;118:123–33. [DOI] [PubMed] [Google Scholar]
- 20.Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition Ad Hoc Writing Committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 21.Reeves PG. AIN-93 purified diets for the study of trace element metabolism in rodents. In: Watson RR, editor. Trace elements in laboratory rodents. Boca Raton (FL): CRC Press; 1996. p. 3–37.
- 22.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 23.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantification of high-density lipoprotein cholesteol. Clin Chem. 1982;28:1379–88. [PubMed] [Google Scholar]
- 24.Carr TP, Andresen CJ, Rudel LL. Enzymatic determination of triglycerides, free cholesterol, and total cholesterol in tissue lipid extracts. Clin Biochem. 1993;26:39–42. [DOI] [PubMed] [Google Scholar]
- 25.Reljic R, Ries M, Anić N, Ries B. New chromogen for assay of glucose in serum. Clin Chem. 1992;38:522–5. [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 27.SAS Institute, Inc. SAS user's guide: statistics, version 5. Cary (NC): SAS Institute; 1985. p. 433–506.
- 28.Rutledge AC, Adeli K. Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms. Nutr Rev. 2007;65:S13–23. [DOI] [PubMed] [Google Scholar]
- 29.Miyazaki M, Dobrzyn A, Man WC, Chu K, Sampath H, Kim HJ, Ntambi JM. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J Biol Chem. 2004;279:25164–71. [DOI] [PubMed] [Google Scholar]
- 30.Matsuzaka T, Shimano H, Yahagi N, Amemiya-Kudo M, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, Tomita S, et al. Insulin-independent induction of sterol regulatory element-binding protein-1c expression in the livers of streptozotocin-treated mice. Diabetes. 2004;53:560–9. [DOI] [PubMed] [Google Scholar]
- 31.Bruno RS, Dugan CE, Smyth JA, DiNatale DA, Koo SI. Green tea extract protects leptin-deficient spontaneously obese mice from hepatic steatosis and injury. J Nutr. 2008;138:323–31. [DOI] [PubMed] [Google Scholar]
- 32.Busserolles J, Mazur A, Gueux E, Rock E, Rayssiguier Y. Metabolic syndrome in the rat: females are protected against the pro-oxidant effect of a high sucrose diet. Exp Biol Med (Maywood). 2002;227:837–42. [DOI] [PubMed] [Google Scholar]
- 33.Galipeau D, Verma S, McNeill JH. Female rats are protected against fructose-induced changes in metabolism and blood pressure. Am J Physiol Heart Circ Physiol. 2002;283:H2478–84. [DOI] [PubMed] [Google Scholar]
- 34.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–11. [DOI] [PubMed] [Google Scholar]
- 35.Huang BW, Chiang MT, Yao HT, Chiang W. The effect of high-fat and high-fructose diets on glucose tolerance and plasma lipid and leptin levels in rats. Diabetes Obes Metab. 2004;6:120–6. [DOI] [PubMed] [Google Scholar]
- 36.Oram JF, Vaughan AM. ATP-binding cassette cholesterol transporters and cardiovascular disease. Circ Res. 2006;99:1031–43. [DOI] [PubMed] [Google Scholar]
- 37.Klaus S, Pültz S, Thöne-Reineke C, Wolfram S. Epigallocatechin gallate attenuates diet- induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int J Obes (Lond). 2005;29:615–23. [DOI] [PubMed] [Google Scholar]
- 38.Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26:1702–11. [DOI] [PubMed] [Google Scholar]
- 39.Levy E, Spahis S, Sinnett D, Peretti N, Maupas-Schwalm F, Delvin E, Lambert M, Lavoie MA. Intestinal cholesterol transport proteins: an update and beyond. Curr Opin Lipidol. 2007;18:310–8. [DOI] [PubMed] [Google Scholar]
- 40.Temel RE, Lee RG, Kelley KL, Davis MA, Shah R, Sawyer JK, Wilson MD, Rudel LL. Intestinal cholesterol absorption is substantially reduced in mice deficient in both ABCA1 and ACAT2. J Lipid Res. 2005;46:2423–31. [DOI] [PubMed] [Google Scholar]
- 41.Davidson NO, Shelness GS. Apolipoprotein B: mRNA editing, lipoprotein assembly, and presecretory degradation. Annu Rev Nutr. 2000;20:169–93. [DOI] [PubMed] [Google Scholar]
- 42.Kaul D, Sikand K, Shukla AR. Effect of green tea polyphenols on the genes with atherosclerotic potential. Phytother Res. 2004;18:177–9. [DOI] [PubMed] [Google Scholar]
- 43.Kuhn DJ, Burns AC, Kazi A, Dou QP. Direct inhibition of the ubiquitin-proteasome pathway by ester bond-containing green tea polyphenols is associated with increased expression of sterol regulatory element-binding protein 2 and LDL receptor. Biochim Biophys Acta. 2004;1682:1–10. [DOI] [PubMed] [Google Scholar]
- 44.Bursill CA, Roach PD. Modulation of cholesterol metabolism by the green tea polyphenol (-)-epigallocatechin gallate in cultured human liver (HepG2) cells. J Agric Food Chem. 2006;54:1621–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.