Abstract
Glutamine (Gln) is important for intestinal barrier function and regulation of tight junction (TJ) proteins, but the intracellular mechanisms of action remain undefined. The purpose of this study was to test the hypothesis that Gln regulates intercellular junction integrity and TJ proteins through the phosphatidylinositol 3-kinase (PI3K)/Akt pathway in Caco-2 cells. Deprivation of exogenous and endogenous glutamine decreased transepithelial electrical resistance (TER) (P < 0.01) and increased permeability (P < 0.01). Both wortmannin and LY294002, PI3K inhibitors, prevented the TER decrease and the permeability increase induced by Gln deprivation (P < 0.001). Gln deprivation also caused decreased TJ protein claudin-1 (P < 0.001). Both wortmannin and LY294002 treatment prevented this effect (P < 0.001). Deprivation of Gln increased phosphor-Akt protein. Gln supplementation reversed this effect. Decreased TER and increased permeability associated with Gln deprivation were not observed in small interfering RNA for p85 transfected Caco-2 cells. In conclusion, Gln regulates intercellular junction integrity and TJ proteins through the PI3-Kinase/Akt pathway.
Introduction
Only a single layer of epithelial cells separates the luminal contents from effector immune cells in the lamina propria and the internal milieu of the body. Breaching this single layer of epithelium can lead to pathological exposure of the highly immunoreactive subepithelium to the vast number of microbes and antigens in the lumen. Breakdown of the barrier is implicated in bacterial translocation, leading to sepsis, and in the pathogenesis of acute illnesses such as multiple organ system failure (1). Increased permeability early in life has been implicated in the pathogenesis of several diseases that manifest in later life, including atopy, food allergies, celiac enteropathy, type 1 diabetes, inflammatory bowel disease (2,3), and autism (4). Studies in rodents show that stresses such as physical restraint can lead to increased ileal permeability (5), and early separation of the mother from the infant is a stress that is associated with intestinal permeability disorders later in life (6).
Tight junctions (TJ)3 forming the paracellular apical junctions of intestinal epithelia are composed of specific proteins such as occludin (7), claudins (8), and zonula occludens (ZO)-1, ZO-2, and ZO-3, which interact with the actin cytoskeleton to stabilize the TJ apical end of the cell (9). Very little is known about specific nutrients and how they affect intestinal epithelial junctions. Previous studies from our laboratory demonstrated that deprivation of glutamine (Gln) from cell culture medium and inhibition of Gln synthetase (GS) using methionine sulfoximine (MS) led to significant decreases in transepithelial resistance of Caco-2 cell monolayers and increased permeability (10,11). In the absence of added Gln, sufficient Gln may be provided de novo by GS. Similar results have been found in a cell culture stress model (12,13). Although these studies demonstrate a relationship between TJ proteins and paracellular biophysical, physiological properties, and intestinal epithelial Gln status, the mechanisms of these effects remain unclear.
There currently are several candidate pathways that might be related to nutrition and stress-related signaling mechanisms of TJ assembly (14). Several lines of evidence have implicated phosphatidylinositol 3-kinase (PI3K) in regulating TJ assembly. Previous investigators have found that prostaglandins stimulate recovery of paracellular resistance via a mechanism involving transepithelial osmotic gradients and PI3K-dependent restoration of TJ protein distribution (15). Rao et al. (16) have shown that oxidative stress activated PI3K in intestinal cell monolayers, causing increased permeability. Inhibition of PI3K prevented the increase in permeability. In the current study, the PI3K pathway was inhibited by PI3K inhibitors, wortmannin or LY294002, or blocked by small interfering RNA for p85 (siRNA p85). We tested the hypothesis that PI3K-dependent signaling processes play an important role in glutamine-mediated modulation of the junction integrity.
Materials and Methods
Reagents.
Vendor prepared solutions of Trypsin/EDTA, minimum essential medium, fetal bovine serum, and antibiotic antimycotic solution were from GIBCO BRL. Biocoat Cell Culture Inserts (Fibrillar Collagen, type I rat tail) and MITO+ Serum Extender were from Collaborative Biomedical Products. MS [l-S-(3-amino-3-carboxypropyl)-S-methylsulfoximine], l-glutamine, wortmannin, or LY294002 and all other chemical reagents were from Sigma Chemical. Antibodies [anti-claudin-1, p85, Akt, and phosphorylated Akt (pAkt)] were obtained from Zymed Laboratory and Santa Cruz. siRNA p85 and Lipofectmine 2000 were purchased from Invitrogen.
Cell culture.
Caco-2 cells were purchased from ATCC and grown in a humidified incubator at 37oC with 5% CO2 and 95% air. Cells between passage 20 and 30 were used for all experiments. For each experiment, cells were collected by dissociation of a confluent stock culture with 0.05% trypsin and 0.53 mmol/L EDTA, counted using a hemacytometer. Cells were seeded into 24-well Biocoat Cell Culture Inserts (Collaborative Biomedical Products) at 200,000 cells per well for transepithelial electrical resistance (TER) measurement, Nunc flasks (Nalge Nunc International) for immunoblotting, and 12-well plates for transfection. Culture media consisted of 8:2 Gln-free minimum essential medium and fetal bovine serum. Cells were cultured with 4 mmol/L Gln for 2 wk before treatment. Then cells were fed with Gln-free DMEM with MITO+ Serum Extender (Collaborative Biomedical Products), a substitute of serum, including hormones, growth factors, and defined metabolites but not Gln. Media was changed every other day. Media also contained penicillin, streptomycin, and amphotericin B at concentrations of 200 kU/L, 200 mg/L, and 50 μg/L, respectively. Gln availability was controlled by adding 4 mmol/L to the medium and treatment with MS (4 mmol/L) to inhibit GS as a source of endogenous Gln production. Wortmannin or LY294002 were used as specific inhibitors of PI3K.
TER measurement.
TER across the monolayer was measured by placing one of the electrodes in the upper well and the other in the lower well using a Millicell Electrical Resistance System with a dual electrode (Millipore) according to the manufacturer's instructions. Two readings were taken per well and averaged. The resistance from each well was subtracted by its appropriate blank, the inserts without cells, to arrive at the resistance of the monolayer, which was multiplied by the effective growing area to obtain TER (Ω·cm2).
Transepithelial mannitol flux studies.
Caco-2 cells grown on collagen-coated membranes were treated with MS, in the presence of absence of Gln. d-[14C-mannitol] (specific activity, 2.15 GBq/mmol; molecular weight, 184) was added at 7.4 MBq/L to the apical compartments. Cells were then incubated at 37°C. Basal medium samples were taken after 2 h, 20 μL for liquid scintillation counting. Apparent permeability coefficients (Papps) were calculated by the formula given below:
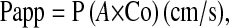 |
where P is the permeability rate (mol/s), Co is the initial apical concentration of test substance (mol/ml), and A is the surface area of the monolayer.
Protein preparation and western blotting.
Caco-2 cells were washed with ice-cold PBS and immediately incubated in Nonidet P-40 extraction buffer (25 mmol/L HEPES, pH 7.4, 150 mmol/L NaCl, 4 mmol/L EDTA, 1% Nonidet P-40, 25 mmol/L NaF, 1 mmol/L Na3VO4, 10 mmol/L sodium pyrophosphate, and protease inhibitors) on ice for 30 min and centrifuged at 10,000 × g; 15 min at 4°C. Supernatants were then collected. Protein concentrations were measured using a Bio-Rad Dc Protein Assay kit (Bio-Rad Laboratories) according to the instructions. Equal amounts of proteins (20 μg) for each sample were separated on SDS-PAGE (12.5% polyacrylamide gel) and transferred to Immobilon Transfer polyvinylidene fluoride membranes (Millpore). Blots were blocked with 5% nonfat milk and incubated sequentially with primary antibody (1:2000 for claudin-1, 1:1000 for ZO-1 and occludin) and horseradish peroxidase-conjugated secondary antibody (1:2000), and detected with ECL plus (Amersham Pharmacia Biotech). Protein bands were quantified by densitometry using Adobe Photoshop software.
Transfection of Caco-2 Cells with SiRNA.
Caco-2 cells (75,000 cells/well) were plated in a 12-well plate using media conditions as described above and incubated for 24 h. Cells were transfected with chemically synthesized siRNA p85 (0.5 μg/well) using Lipofectamine 2000 and serum-free medium according to the manufacturer's recommendations. All transfections were done in triplicate and cells were untreated or treated with Gln or MS.
Statistical analysis.
Sigmastat statistical software (SPSS) was used to analyze densitometry results of TER, permeability assays, and protein relative expression levels for Western blots. All data were reported as means ± SD from 3 independent experiments. A 1-way or 2-way ANOVA was used to determine whether a significant difference was present among all treatment groups. Additionally, Bonferroni t tests were performed for comparisons when the ANOVA was significant at P < 0.05.
Results
Effects of signaling inhibitors on TER and 14C-mannitol permeability.
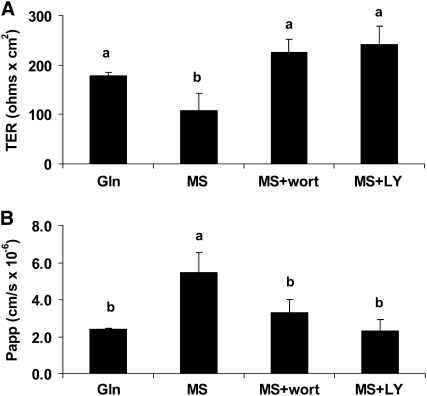
To evaluate the role of PI3K pathway in the TJ regulation process by Gln, Caco-2 cells were cultured and analyzed. Gln deprivation for 5 d (exogenous and endogenous) decreased TER by 39% compared with Gln-treated cells (P < 0.01; Fig. 1A). Both wortmannin (10 nmol/L) and LY294002 (10 μmol/L) reversed this effect (Fig. 1A). Similar results were seen in the 14C-mannitol permeability assay on the same cells (Fig. 1B). The deprivation of Gln resulted in an increased monolayer permeability for 14C-mannitol by 129% compared with Gln-treated cells (P < 0.01). Both wortmannin and LY294002 replacement reversed this effect (Fig. 1B). Inhibition of PI3K by wortmannin or LY294002 did not change the TER and permeability in Gln-supplemented cells (data not shown). These results suggest a role of the PI3K pathway in Gln-mediated changes in TER and permeability in Caco-2 cells.
FIGURE 1 .
Effects of signaling inhibitors wortmannin (wort) and LY294002 (LY) on TER (A) and 14C-mannitol permeability (B) in spontaneously differentiated Caco-2 cells that were or were not treated with 4 mmol/L Gln, in the presence or absence of 4 mmol/L MS treated or not with wortmannin or LY294002 for 5 d. Data are means ± SD, n = 3. Means without a common letter differ, P < 0.05.
Effects of signaling inhibitors on intercellular junction protein claudin-1.
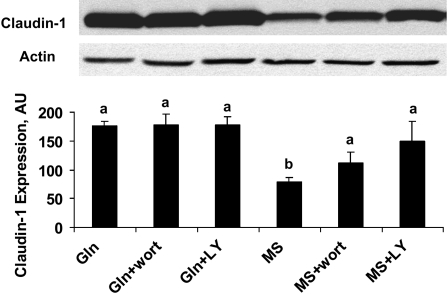
To further investigate the effect of the PI3K pathway on Gln regulation of the epithelia TJ, Caco-2 monolayers were treated as above. Immunoblotting was done to evaluate the specific TJ protein claudin-1 expression. Gln deprivation markedly decreased claudin-1 expression (P < 0.01); this effect was also reversed with both wortmannin and LY294002, the inhibitors of PI3K. However, inhibition of PI3K by wortmannin or LY294002 did not alter the expression level of claudin-1 in Gln-supplemented cells (Fig. 2). These results suggested that the PI3K pathway plays a role in Gln mediation of TJ protein expression.
FIGURE 2 .
Effects of signaling inhibitors wortmannin (wort) and LY294002 (LY) on intercellular junction protein claudin-1 in spontaneously differentiated Caco-2 cells that were or were not treated with 4 mmol/L Gln, in the presence or absence of 4 mmol/L MS treated or not with wortmannin or LY294002 for 5 d. Data are means ± SD, n = 3. Means without a common letter differ, P < 0.05.
Effects of Gln on the PI3kinase pathway.
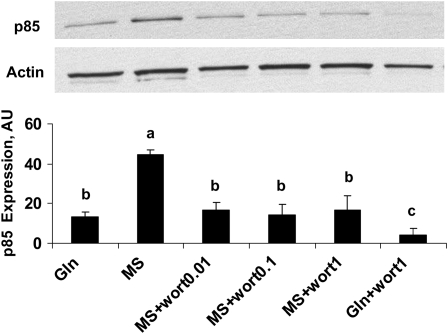
To demonstrate if Gln could act as a signaling molecule to regulate PI3K, Caco-2 cells were cultured as described before. Spontaneously differentiated cells were or were not treated with 4 mmol/L Gln, in the presence or absence of 4 mmol/L MS and various doses of wortmannin for 4 d. The expression of p85, one of the subunits of PI3K, was evaluated using Western blotting. The absence of Gln increased the p85 component of PI3K expression (P < 0.001) and this effect was reversed with wortmannin administration from 1 nmol/L to 1 μmol/L, whereas this downregulatory effect in Gln-free cells was less than in Gln-supplemented cells (P < 0.01; Fig. 3).
FIGURE 3 .
Effects of Gln on p85 expression in Western blots in spontaneously differentiated Caco-2 cells that were or were not treated with 4 mmol/L Gln, in the presence and absence of 4 mmol/L MS with various doses of wortmannin for 4 d. Data are means ± SD, n = 3. Means without a common letter differ, P < 0.05.
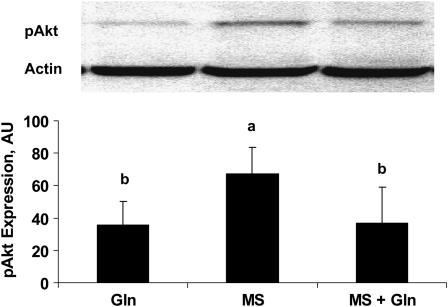
To study the regulatory effect of Gln on the PI3K pathway, a downstream protein of PI3K, Akt was also evaluated. Caco-2 cells were or were not treated with 4 mmol/L Gln, in the presence or absence of 4 mmol/L MS for 3 d. Western blots were done to evaluate the expression level of Akt and pAkt. Gln deprivation increased the expression of pAkt (P < 0.05; Fig. 4) but not Akt (data not shown). Gln supplementation reversed this effect (P < 0.05; Fig. 4).
FIGURE 4 .
Effects of Gln on Akt expression in Western blots in spontaneously Caco-2 cells that were treated or not with 4 mmol/L Gln, in the presence or absence of 4 mmol/L MS for 3 d. Data are means ± SD, n = 3. Means without a common letter differ, P < 0.05.
These results demonstrate that Gln deprivation upregulated the PI3K/Akt pathway. Glutamine supplementation reversed this effect.
Effect of Gln on TER and C14-mannitol permeability in siRNA p85-Caco-2 cells.
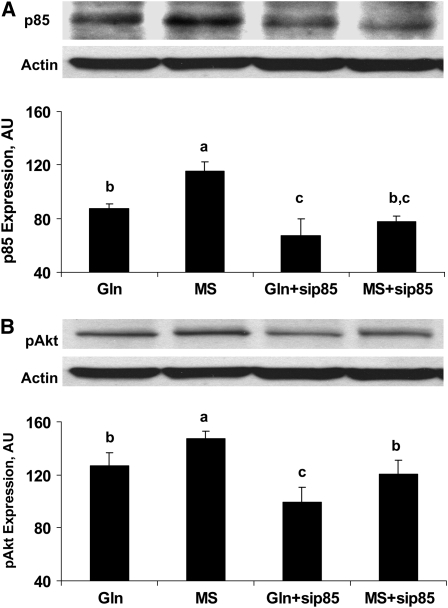
To further demonstrate the direct effects of PI3K on the TJ regulation process by Gln, siRNA p85 was transfected to knockdown the PI3K pathway with or without 4 mmol/L Gln, in the presence or absence of 4 mmol/L MS. At d 3, p85 and pAkt expression were evaluated by Western blot to verify the effect of siRNA (Fig. 5). In a separate experiment 3 d after transfection, Caco-2 cells were or were not treated with 4 mmol/L Gln, in the presence or absence of 4 mmol/L MS for 5 d. We measured TER (Fig. 6A) and evaluated permeability using C14-mannitol fluxes (Fig. 6B).
FIGURE 5 .
Downregulation of p85 (A) and pAkt (B) expression in Western blots in siRNA p85-Caco-2 Cells. SiRNA p85 was transfected in Caco-2 cells treated or not with 4 mmol/L Gln, in the presence or absence of 4 mmol/L MS for 3 d. Data are means ± SD, n = 3. Means without a common letter differ, P < 0.05.
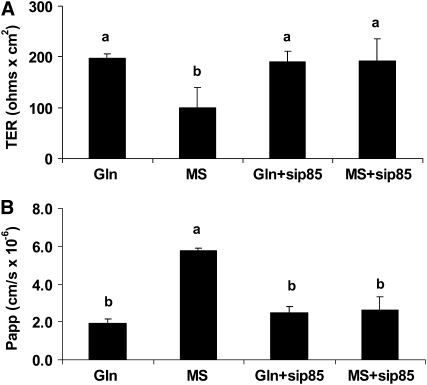
FIGURE 6 .
Effect of Gln on TER (A) and C14-mannitol permeability (B) in siRNA p85-Caco-2 Cells. siRNA p85 was transfected to knockdown the PI3k pathway. Three days after transfection, Caco-2 cells were treated or not with 4 mmol/L Gln, in the presence or absence of 4 mmol/L MS for 5 d. Data are means ± SD, n = 3. Means without a common letter differ, P < 0.05.
Gln deprivation increased p85 expression (P < 0.05). SiRNA p85 downregulated p85 in the presence or absence of MS (P < 0.01; Fig. 5A). Additionally, Gln deprivation increased pAkt expression (P < 0.05). SiRNA p85 downregulated pAkt in the presence or absence of MS (P < 0.01; Fig. 5B). These results suggest that the PI3K pathway was blocked successfully by siRNA.
The downregulatory effect of Gln deprivation on TER in nontransfected Caco-2 cells (P < 0.01) was not observed in PI3K silenced cells. The siRNA p85 itself did not alter the TER (Fig. 6A). Similarly, the increased permeability due to Gln deprivation in nontransfected Caco-2 cells (P < 0.01) was not observed in PI3K silenced cells. The siRNA p85 itself did not alter the permeability (Fig. 6B).
These findings are consistent with the results using PI3K inhibitors, supporting an important role of PI3K as an intermediate in the pathway for the TJ regulation with Gln.
Discussion
These studies were designed to identify the mechanism by which Gln transduces signals to maintain intercellular junction resistance and permeability. To test the hypothesis that Gln regulates intercellular barrier function and TJ proteins through the PI3K/Akt pathway, as suggested by previous studies (15), the extracellular Gln concentration was manipulated in Caco-2 cells while also inhibiting the capability of the cells to synthesize Gln via the GS reaction using the inhibitor MS.
As in our previous studies (13), the absence of Gln (exogenous and endogenous) resulted in the lowest TER and highest permeability and decreased the expression of the intercellular TJ protein claudin-1. Sheth et al. (16) reported that oxidative stress likewise activated p85 in intestinal cell monolayers, resulting in an association between p85 and occludin, phosphorylation of occludin, and increased permeability. LY294002 prevented the increase in permeability. Basuroy et al. (17) also showed that Gln protects from oxidative stress. In this study, both wortmannin and LY294002, inhibitors of PI3K, reversed these effects. Similar reversing effects were seen in PI3K-silenced Caco-2 cells by using siRNA p85. Concurrent to the alterations in permeability and TER, Gln deprivation also markedly decreases claudin-1 expression, an effect that was also reversed with both wortmannin and LY294002, the inhibitors of PI3K. However, inhibition of PI3K by wortmannin or LY294002 did not alter the TER, permeability, or expression levels of claudin-1 in Gln-supplemented cells. These results suggest the PI3K pathway plays a role in Gln mediation of TJ protein expression. A recent study has shown that Gln deprivation increased apoptosis by causing phosphorylation of Akt. Akt phosphorylation in response to Gln deprivation appeared to represent a protective mechanism (18).
Our previous studies and this current study demonstrated the beneficial effect of Gln supplementation on TJ and intestinal barrier function in vitro. The effect of Gln supplementation on intestinal permeability in vivo is still controversial. A recent study examined the effect of a diet supplemented with alanyl-glutamine on intestinal barrier function on 107 children aged 7.9–82.2 mo. The percentage of lactulose urinary excretion significantly improved (decreased), suggesting a beneficial effect on the barrier function paracellular pathway (19). However, a randomized trial of Gln-enriched enteral nutrition on intestinal permeability in very low birth weight infants showed that Gln-enriched enteral nutrition did not enhance the postnatal decrease in intestinal permeability in very low birth weight infants (20). Therefore, the effect of Gln supplementation on intestinal barrier function in vivo requires further evaluation.
Determination of the mechanisms of how Gln signals affect the TJ relates to how Gln helps maintain intestinal mucosal integrity, especially during stresses, such as radiation therapy (21), chemotherapy (22), and total parenteral nutrition (23), and blunts increased gut permeability associated with experimental sepsis (24). Several stressors have been found to have profound effects on intestinal TJ. These include bacterial toxins (25) and allergens, including gliadin (26).
In summary, to our knowledge, the current studies are the first to establish a relationship between intestinal cell Gln status and intercellular junction proteins and the P13K pathway and provide an improved understanding of the overall mechanisms of how deprivation of this amino acid may relate to various diseases and pathologic conditions.
Supported by NIH grant RO1HD38954.
Author disclosures: N. Li and J. Neu, no conflicts of interest.
Abbreviations used: GS, glutamine synthetase; MS, methionine sulfoximine; pAkt, phosphorylated Akt; PI3K, phosphatidylinositol 3-kinase; siRNA p85, small interfering RNA for p85; TER, transepithelial electrical resistance; TJ, tight junction; ZO, zonula occludens.
References
- 1.Soeters PB, Luyer MD, Greve JW, Buurman WA. The significance of bowel permeability. Curr Opin Clin Nutr Metab Care. 2007;10:632–8. [DOI] [PubMed] [Google Scholar]
- 2.Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003;52:439–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol. 2003;18:479–97. [DOI] [PubMed] [Google Scholar]
- 4.White JF. Intestinal pathophysiology in autism. Exp Biol Med (Maywood). 2003;228:639–49. [DOI] [PubMed] [Google Scholar]
- 5.Mazzon E, Sturniolo GC, Puzzolo D, Frisina N, Fries W. Effect of stress on the paracellular barrier in the rat ileum. Gut. 2002;51:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soderholm JD, Yates DA, Gareau MG, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1257–63. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol Gastrointest Liver Physiol. 1995;269:G467–75. [DOI] [PubMed] [Google Scholar]
- 8.Tsukita S, Furuse M. Occludin and claudins in tight junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–73. [DOI] [PubMed] [Google Scholar]
- 9.Wittchen ES, Haskins J, Stevenson BR. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J Biol Chem. 1999;274:35179–85. [DOI] [PubMed] [Google Scholar]
- 10.DeMarco VG, Li N, Thomas J, West CM, Neu J. Glutamine and barrier function in cultured caco-2 epithelial cell monolayers. J Nutr. 2003;133:2176–9. [DOI] [PubMed] [Google Scholar]
- 11.Weiss MD, DeMarco V, Strauss DM, Samuelson DA, Lane ME, Neu J. Glutamine synthetase: a key enzyme for intestinal epithelial differentiation? JPEN J Parenter Enteral Nutr. 1999;23:140–6. [DOI] [PubMed] [Google Scholar]
- 12.Li N, DeMarco VG, West CM, Neu J. Glutamine supports recovery from loss of transepithelial resistance and increase of permeability induced by media change in Caco-2 cells. J Nutr Biochem. 2003;14:401–8. [DOI] [PubMed] [Google Scholar]
- 13.Li N, Lewis P, Samuelson D, Liboni K, Neu J. Glutamine regulates Caco-2 cell tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2004;287:G726–33. [DOI] [PubMed] [Google Scholar]
- 14.Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4:225–36. [DOI] [PubMed] [Google Scholar]
- 15.Little D, Dean RA, Young KM, McKane SA, Martin LD, Jones SL, Blikslager, AT. PI3K signaling is required for prostaglandin-induced mucosal recovery in ischemia-injured porcine ileum. Am J Physiol Gastrointest Liver Physiol. 2003;284:G46–56. [DOI] [PubMed] [Google Scholar]
- 16.Sheth P, Basuroy S, Li C, Naren AP, Rao RK. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem. 2003;278:49239–45. [DOI] [PubMed] [Google Scholar]
- 17.Basuroy S, Sheth P, Mansbach CM, Rao RK. Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and L-glutamine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G367–75. [DOI] [PubMed] [Google Scholar]
- 18.Larson SD, Li J, Chung DH, Evers BM. Molecular mechanisms contributing to glutamine-mediated intestinal cell survival. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima NL, Soares AM, Mota RM, Monteiro HS, Guerrant RL, Lima AA. Wasting and intestinal barrier function in children taking alanyl-glutamine-supplemented enteral formula. J Pediatr Gastroenterol Nutr. 2007;44:365–74. [DOI] [PubMed] [Google Scholar]
- 20.van den Berg A, Fetter WP, Westerbeek EA, van der Vegt IM, van der Molen HR, van Elburg RM. The effect of glutamine-enriched enteral nutrition on intestinal permeability in very-low-birth-weight infants: a randomized controlled trial. JPEN J Parenter Enteral Nutr. 2006;30:408–14. [DOI] [PubMed] [Google Scholar]
- 21.Chun H, Sasaki M, Fujiyama Y, Bamba T. Effect of enteral glutamine on intestinal permeability and bacterial translocation after abdominal radiation injury in rats. J Gastroenterol. 1997;32:189–95. [DOI] [PubMed] [Google Scholar]
- 22.Bai M, Jiang Z, Liu Y. Glutamine dipeptide attenuate mucosal atrophic changes and preservation of gut barrier function following 5-FU intervention. Zhonghua Wai Ke Za Zhi. 1996;34:370–3. [PubMed] [Google Scholar]
- 23.Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, Langkamp-Henken B. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39:44–52. [DOI] [PubMed] [Google Scholar]
- 24.Dugan ME, McBurney MI. Luminal glutamine perfusion alters endotoxin-related changes in ileal permeability of the piglet. JPEN J Parenter Enteral Nutr. 1995;19:83–7. [DOI] [PubMed] [Google Scholar]
- 25.Nusrat A, von Eichel-Streiber C, Turner JR, Verkade P, Madara JL, Parkos CA. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun. 2001;69:1329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clemente MG, De Virgiliis S, Kang JS, Macatagney R, Musu MP, Di Pierro MR, Drago S, Congia M, Fansano A. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. 2003;52:218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]