Abstract
Branched-chain amino acids (BCAA), Leu, and the signaling pathways they regulate have been reported to either improve or worsen adiposity and insulin sensitivity. Therefore, it is unclear whether dietary supplementation of Leu would be beneficial. To help address this question, we examined the effect of adding Leu (150 mmol/L; Expt. 1 and Expt. 2) or BCAA (109 mmol/L of each; Expt. 3) to the drinking water on diet-induced obesity (induced with a 60-kJ% fat diet) in singly housed C57BL6/J male mice for at least 14 wk. Liquid and solid food intakes were evaluated weekly along with body weight. During the last few weeks, several blood samples were taken at different times for plasma glucose, total cholesterol, or Leu measurements. Metabolic rate by indirect calorimetry, locomotor activity by light beam breaking, body composition by H1-NMR, and insulin tolerance were also determined. Compared with control, supplementation did not affect body weight, food intake, oxygen consumption, locomotor activity, body composition, insulin tolerance, or total cholesterol. In fed mice, this method of Leu supplementation only increased plasma Leu by 76% when the supplemented group was compared with control. On the other hand, after overnight food deprivation, the plasma Leu did not differ between these 2 groups, even though the mice in the supplemented group had continuous access to Leu-containing water during the solid food deprivation. Taken together, the results do not provide evidence that either Leu or BCAA supplementation of drinking water ameliorates diet-induced obesity in mice, although it may improve glycemia.
Introduction
The use of high-protein diets or dietary supplementation with branched-chain amino acids (BCAA)4 or Leu has been explored for therapeutic intervention of obesity and body weight control (1,2). Similarly, Leu has been shown to decrease food intake through activation of mammalian target of rapamycin signaling in the hypothalamus (3) and increases the prandial rise in the satiety hormone, leptin (4).
Supplementation of dietary protein, BCAA, or Leu has also been reported to affect energy expenditure. Diet-induced thermogenesis in response to protein ingestion does not appear to be solely due to heat released from digestion; even BCAA or Leu alone increase thermogenesis when administered i.v. or orally (5,6). Leu and BCAA improve body weight control when coupled with exercise or energy restriction (7–9). In light of these findings, it would seem reasonable to expect that Leu supplementation would alleviate obesity.
However, a review of the supplementation literature concluded that the data supporting this efficacy were promising, although not entirely consistent (1). A potential caveat is that persistently overactivating mammalian target of rapamycin nutrient signaling through overnutrition, as might occur with chronic dietary supplementation of amino acids or Leu, has been implicated in Ser phosphorylation of IRS1 leading to insulin resistance and worsening of diabetes (10,11). Another paradox is that BCAA are already elevated in obesity and their concentrations decline in response to a weight loss intervention [for review, see (12)]. Thus, further studies are needed to understand the therapeutic potential of BCAA supplementation for obesity.
To begin to address this question, we examined the effects of supplementation by adding near-saturating concentrations of Leu or BCAA to the drinking water of C57BL/6 mice provided a 60% high-fat diet. We measured longitudinal changes in body weight, food intake, insulin sensitivity, fasting plasma glucose, metabolic rate, body composition, and total plasma cholesterol during the development of diet-induced obesity.
Methods
Animal and treatment protocol.
The Penn State College of Medicine Institutional Animal Care and Use Committee approved the animal protocols in this study. Three experiments were conducted with different cohorts of 6- to 7–wk-old C57BL/6J mice. This strain was used because they develop obesity and insulin resistance in response to a high-fat diet (13). Mice were purchased from Jackson Laboratories and maintained at our facility for a minimum of 1 wk before the start of treatments of Harlan Teklad 2018 Global 18% Protein Rodent diet (14). The light cycle for the mice began at 0700 and the dark cycle began at 1900. Mice were caged separately with free access to food and water or other liquid as indicated below.
In each of the 3 experiments, body weight, food intake, liquid intake, and Leu intake were measured weekly for 14–15 wk. The starting body weights ranged from 17.1 to 22.1 g in Expt. 1, from 21.1 to 25.0 g in Expt. 2, and from 16.5 to 23.7 in Expt. 3. Food and fluid consumption were measured every week for 2-d periods. Food remaining in cages and crumbs were weighed and accounted for. The water or liquid supplements were provided by means of graduated cylinders topped with a 1-hole rubber stopper holding a metal drinking nipple. To account for fluid spillage (generally 1–2 mL/2 d), a graduated cylinder with a drinking nipple was hung on an empty cage. Leu intake was calculated from the fluid and food concentration (2.3 g Leu/100 g of the D12492 high-fat diet). BCAA provided as crystals or flakes were ground to a fine powder with a ceramic mortar and pestle to improve solubility.
In Expt. 1, mice were allocated to 1 of 2 experimental groups: HF (control group; n = 12 mice) or HF+Leu (experimental group; n = 12). HF mice were provided free access to a high-fat diet containing 60 kJ% fat [D12492, Research Diets; previously described in (14)] and drank water ad libitum throughout the study. The HF+Leu group was treated the same prior to t = 0 wk, when their water switched to Leu containing water at a concentration of 150 mmol/L. During the last few weeks, various endpoints were measured in the days following weekly body weight and intake measurements. These included calorimetry, locomotor activity assessment, and insulin tolerance tests. On 1 occasion, blood was taken for plasma amino acid analysis from fed mice. On several other occasions, blood was taken after a period of food deprivation (5 h or overnight as indicated). These were timed to have the least effect on weekly food intake and body weight measurements.
Expt. 2 was designed similarly to Expt. 1 except that there were 15 mice in each group. During the last few weeks, calorimetry, locomotor activity assessment, body composition measurement, and an insulin tolerance test were measured. At 2 times during this period, blood was taken after the 5-h period of food deprivation. However, a difference from Expt. 1 is that the Leu was removed from the water during the food deprivation period.
The methods for Expt. 3 are found in the Supplemental material.
Blood glucose and insulin sensitivity.
Blood glucose concentration was measured from the tail vein with Glucometer ONETOUCH Ultra (Life Scan, Johnson & Johnson). Insulin tolerance tests were performed in 5-h food-deprived mice. We measured blood glucose just before and 30, 60, 90, and 120 min after t = zero, i.e. the time when the mice received an intraperitoneal injection of insulin at a dose of 5.2 pmol/g body weight (human insulin, Eli Lilly, 5.2 pmol = 0.75 mU).
Energy expenditure and activity.
Energy expenditure was assessed using indirect calorimetry for 24 h following a 2-h acclimatization period as previously described (15). Constant airflow (0.6 L/min) was drawn through the chamber and monitored by a mass-sensitive flow meter. The concentrations of oxygen and carbon dioxide were monitored at the inlet and outlet of the sealed chambers to calculate oxygen consumption and RQ. Each chamber was measured for 1 min at 15-min intervals. Locomotor activity was measured using infrared technology (OPT-M3, Columbus Instruments). The counts of 3-dimensional beam breaking (X total, X ambulatory, and Z) were calculated using movement detection software provided by Columbus Instruments.
Body composition.
Body composition was determined using an LF90 Minispec Time Domain Nuclear Magnetic Resonance Spectrometer (Bruker Optics).
Analytical procedures.
Plasma concentrations of triglyceride and cholesterol were measured using a Vitros Chemistry Analyzer (Ortho-Clinical Diagnosis). Plasma amino acid concentrations were measured using fluorometric HPLC methods (15).
Statistical analysis.
Data are reported as means ± SEM. In all statistical tests, differences were considered significant at P < 0.05. The ANOVA repeated-measures test (Prism, GraphPad Software) was used to determine differences between the control (HF) and experimental (HF+Leu) groups when endpoints were measured repeatedly over time. These endpoints included body weight, food intake, Leu intake, and time points in the insulin tolerance test. Differences between the control (e.g. HF) and experimental (e.g. HF+Leu) groups in body composition, O2 consumption, RQ, locomotor activity, and plasma analyte concentrations were assessed using a 2-tailed t test (InStat3, GraphPad Software). Calorimetry and locomotor activity data were analyzed for differences over the whole day and during only the light cycle or dark cycle.
Post hoc power analysis of the body weight data for wk 14 for an α = 0.05 (2-tail) was performed using the Statmate computer program (Graphpad software).
A Pearson correlation analysis (Prism, GraphPad Software) was performed to correlate the individual body weight and plasma cholesterol values from Expt. 2. The analysis provided the following for each group: the slope ± SEM, r2, and an the results of an F-test, for which a P-value is provided, to assess the difference in the slope from zero. A t test was then used to determine differences in the HF and HF+Leu slopes from each other.
Results
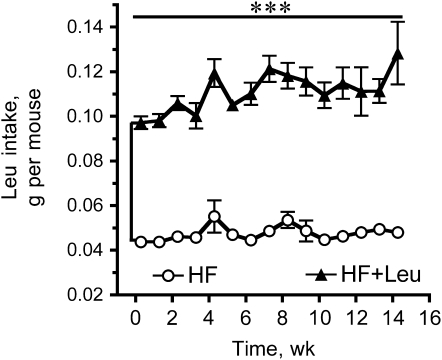
Body weights were not significantly different at any time between the HF and HF+Leu groups in either Expt. 1 or Expt. 2 (Supplemental Fig. 1). The amount of food consumed also did not differ (Supplemental Fig. 2). Our protocol for Leu supplementation more than doubled the daily Leu intake in the HF+Leu group compared with the HF group (Fig. 1 and data not shown for Expt. 1); however, it did not affect fluid intake per se (data not shown). Post hoc power analysis of the 2 Leu experiments indicated they had 80% power to detect an ∼11–12% difference in body weight at wk 14 between the HF and HF+Leu groups.
FIGURE 1 .
Weekly Leu intake of mice fed HF and HF+Leu diets (Expt. 2). Values are means ± SE, n = 15. ***Groups differ, P < 0.001.
VO2, respiratory quotient, locomotor activity, and body composition.
O2 consumption, respiratory quotient (RQ), and activity were measured in the last few weeks of Expt. 1 (data not shown) and Expt. 2 (Supplemental Fig. 3). O2 consumption, RQ, and locomotor activity did not differ between the HF and HF+Leu groups. Light to dark cycle changes in activity, RQ, or VO2 also did not differ between these 2 groups. Body composition was assessed at wk 8 and 16. Chronic Leu supplementation did not change the proportion of fat or lean tissue when the HF and HF+Leu groups were compared (data not shown).
Total cholesterol and triglyceride.
Leu supplementation did not cause a significant difference in the plasma cholesterol or triglyceride concentration between the HF and HF+Leu groups. Plasma total cholesterol was 3.98 ± 0.21 mmol/L in HF compared with 3.80 ± 0.16 mmol/L in HF+Leu group at wk 13 after the overnight food deprivation. In Expt. 2, plasma total cholesterol was 4.95 ± 0.14 mmol/L in the HF group compared with 4.63 ± 0.16 mmol/L in the HF+Leu group on wk 15 after the 5 h of food deprivation. The plasma triglyceride concentration in Expt. 1 was 0.67 ± 0.031 mmol/L in the HF group compared with 0.70 ± 0.049 mmol/L in the HF+Leu group at wk 13 after the overnight food deprivation. In Expt. 2, plasma triglyceride was 0.88 ± 0.057 mmol/L in the HF group compared with 0.82 ± 0.035 mmol/L in the HF+Leu group on wk 15 after the 5 h of food deprivation.
Plasma glucose and insulin sensitivity.
Plasma glucose was measured weekly during the last 2 or 3 wk of Expt. 1 and 2 after either overnight or 5 h of food deprivation (Table 1). The plasma glucose concentration was 9–12% lower in the HF+Leu group compared with the HF group in Expt. 1, but the groups did not differ in Expt. 2 (Table 1).
TABLE 1.
Effect of Leu supplementation on plasma glucose concentration in mice deprived of food overnight or for 5 h1
| Expt. | Blood sample2 | Group | Glucose, mmol/L | P-value |
|---|---|---|---|---|
| 13 | 1 | HF | 13.8 ± 0.25 | 0.049 |
| HF+Leu | 12.7 ± 0.49 | |||
| 13 | 2 | HF | 15.6 ± 0.49 | 0.026 |
| HF+Leu | 13.7 ± 0.66 | |||
| 14 | 3 | HF | 11.0 ± 0.41 | 0.03 |
| HF+Leu | 9.6 ± 0.43 | |||
| 23 | 1 | HF | 12.0 ± 0.60 | 0.11 |
| HF+Leu | 10.8 ± 0.47 | |||
| 23 | 2 | HF | 11.7 ± 0.48 | 0.2 |
| HF+Leu | 12.7 ± 0.54 |
Values are means ± SE, n = 12 (Expt. 1) or 15 (Expt. 2).
Blood samples were obtained during the last 2–3 wk of each experiment and are presented in chronological order.
5-h food deprivation.
Overnight food deprivation.
An insulin tolerance test was performed in the last few weeks to assess insulin sensitivity between the HF and HF+Leu groups in Expt. 1 (data not shown) and Expt. 2 (Supplemental Fig. 4). Percent changes in the plasma glucose concentration after insulin injection did not differ between the groups in either experiment.
Plasma amino acid concentrations.
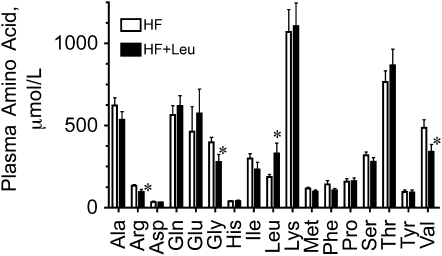
The concentrations of amino acids in plasma were determined in the fed state near the end of Expt. 1 (Fig. 2). Fed plasma Leu concentration was 76% higher in the HF+Leu group compared with the HF group, whereas the valine, glycine, and arginine concentrations were significantly lower in the HF+Leu group compared with the HF group. Plasma Leu concentrations were also measured after overnight food deprivation. In this case, plasma Leu did not significantly differ between the HF and HF+Leu groups (data not shown).
FIGURE 2 .
Plasma amino acid concentrations in mice fed HF and HF+Leu diets for wk 13 (Expt. 1). Values are means ± SE, n = 8. *Different from HF, P < 0.05.
Variability of body weight and correlation of body weight with plasma total cholesterol.
In both experiments, there was large variability in the body weight response to high-fat feeding (Supplemental Fig. 5). Thus, body weight increases ranged from 16 to 20 g in the HF and HF+Leu groups of both experiments, even though the mice started with a narrow weight range of a few grams. There was no significant correlation between the starting and wk 15 body weights in either experiment (data not shown). Thus, the variability in the end body weights appears to represent variability in the response (i.e. change in body weight) to high-fat feeding.
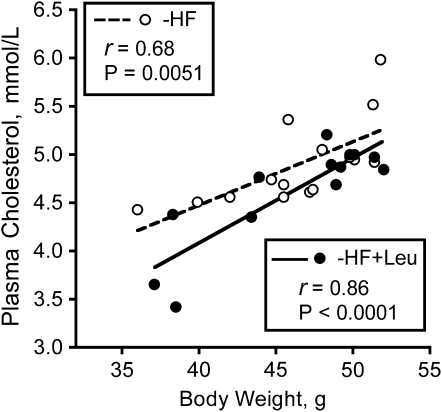
Individual plasma total cholesterol concentrations and wk 15 body weights, from either the HF group or HF+Leu group, were significantly correlated (Fig. 3). However, the best fit slopes for the HF group (0.066 ± 0.020) and for the HF+Leu group (0.088 ± 0.015) did not differ.
FIGURE 3 .
Correlations between body weights and plasma total cholesterol concentrations in mice fed HF and HF+Leu diets for 15 wk (Expt. 2). The symbols represent individual mice.
Discussion
We examined the effect of supplementing drinking water with saturating concentrations of Leu or BCAA on diet-induced obesity in C57BL6 mice. Neither Leu nor BCAA supplementation affected body weight or body composition. Not surprisingly, other obesity-related metabolic endpoints were similarly unaffected by these interventions. Plasma glucose was significantly lower when measured at several times during the last few weeks of Expt. 1, but not in Exp 2 or 3. However, in the latter experiments, the Leu or BCAA was removed from the water during the food deprivation period. Because plasma glucose has been shown to decline in response to BCAA or Leu in studies from several laboratories and in different species, the glucose-lowering effect would seem to warrant further mechanistic studies (16) [for review, see (2)].
As mentioned earlier, several previous studies have observed improved body weight control after Leu or BCAA supplementation (2,7–9,16). Thus, we were surprised by the lack of effects of Leu and BCAA supplementation on body weight in our study. This may relate to the mode of supplementation we employed. Because the solubility of the BCAA in drinking water is relatively limited (e.g. compared with other amino acids), the average Leu concentration in plasma from the supplemented (HF+Leu) mice was only 76% higher than that of the HF group. Although this was significant in mice with free access to food, it was not significantly different when the mice were food deprived. Thus, we cannot be sure that plasma Leu is always elevated with this type of supplementation. In comparison, prandial Leu concentrations increased by 2–300% in meal-trained rats (4). In mitochondrial branched-chain aminotransferase isozyme (BCATm) knockout mice, which had strong effects on insulin sensitivity and body weight, Leu concentrations were typically elevated by ∼400–3100% depending on the diet provided (15). Therefore, the concentration of Leu we achieved in this study may not be great enough to observe the changes in body mass observed in other studies.
Another difference between this and some of the previous studies is that, in some cases, Leu supplementation was coupled to some other intervention such as dietary restriction or exercise. Alternatively, supplementation was provided in the solid portion of the diet by providing proteins with extra BCAA (7,9).
On the other hand, the design of our study is similar, although not in all respects, to a recent study by Zhang et al. (16). In their study, Leu supplementation in drinking water was associated with an ∼8–10% difference in body weight following high-fat feeding. We used an identical high-fat diet and even slightly more Leu in the water than that study, so the results of each of these studies are inconsistent. One difference between the 2 studies is that the housing for the mice was somewhat different. Ours were singly housed during the entire study, whereas Zhang et al. (16) only housed singly for 14 d near the end of the study to measure food intake. Single housing is known to cause stress in mice and might have influenced our results and thereby explain these differences. Another difference is that the number in the study by Zhang et al. (16) was only 5 per group. This could be a potential factor because several groups have reported large inter-mouse variability in the responses of C57BL6 mice to high-fat diet feeding (17–20). For example, Koza et al. (18) reported considerable variability in the response of 107 mice to high-fat feeding. The magnitude of that variability was similar to what we observed here. In both our present study and previous studies by others (17–20), cohorts of mice could be selected that significantly differed in their response to high-fat feeding compared with other mice fed the same diet. This variability in the response to high-fat feeding appears to arise from epigenetic factors (18). The random chance of a type 1 error (e.g. having a sample mean and SD that is different from the population) is increased as the variability of the response increases and the sample size decreases. If such an error occurred, body weight related responses such as adiposity, VO2, insulin sensitivity, and plasma cholesterol (shown here and elsewhere to correlate with body weight) would be expected to follow.
In the present study, rates of oxygen consumption were typically within the range of 2000–3000 mL·kg−1·h−1, consistent with our previous study (15), and typical for mice of this strain, the diet and daily food intake (e.g. 21,22). These were not affected by Leu supplementation in contrast to the significant effects observed by Zhang et al. (16). However, their VO2 values were ∼10,000 mL·kg−1·h−1 (∼209.2 kJ·mouse−1·d−1). This is far greater than their reported energy intakes, suggesting a systematic calorimetry problem.
In conclusion, our study suggests that neither BCAA nor Leu supplementation of water leads to a significant reduction in body weight or plasma cholesterol in response to high-fat feeding. If Leu is able to stimulate diet-induced thermogenesis, a higher plasma Leu concentration may be needed throughout the day to see a significant effect on body weight, because plasma Leu elevation achieved by supplementation of the drinking water was not as high as that observed in BCATm knockout mice. To achieve such concentrations, an inhibitor of BCATm might be needed in addition to supplementation.
Supplementary Material
Acknowledgments
We thank Vance Albaugh, Jamie Spicer, and Stephanie Goshorn for technical assistance.
Supported by NIH RO1 grants (DK053843, DK062880, GM39277, and AA12814).
Author disclosures: A. Nairizi, P. She, T. C. Vary, and C. J. Lynch, no conflicts of interest.
Supplemental Procedures and Results, Supplemental Tables 1 and 2, and Supplemental Figures 1–8 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: BCAA, branched-chain amino acid; BCATm, mitochondrial branched-chain aminotransferase isozyme (gene name: BCAT2); HF, high-fat control group; HF+Leu, experimental group receiving high-fat diet and water supplemented with Leu; HF+BCAA, high-fat diet and water supplemented with branched-chain amino acids group; RQ, respiratory quotient (also known as RER).
References
- 1.Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr. 2004;23:373–85. [DOI] [PubMed] [Google Scholar]
- 2.Layman DK. The role of leucine in weight loss diets and glucose homeostasis. J Nutr. 2003;133:S261–7. [DOI] [PubMed] [Google Scholar]
- 3.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–30. [DOI] [PubMed] [Google Scholar]
- 4.Lynch CJ, Gern B, Lloyd C, Hutson SM, Eicher R, Vary TC. Leucine in food mediates some of the postprandial rise in plasma leptin concentrations. Am J Physiol Endocrinol Metab. 2006;291:E621–30. [DOI] [PubMed] [Google Scholar]
- 5.Tappy L, Jequier E, Acheson K. Thermic effect of infused amino acids in healthy humans and in subjects with insulin resistance. Am J Clin Nutr. 1993;57:912–6. [DOI] [PubMed] [Google Scholar]
- 6.Sellden E, Brundin T, Wahren J. Augmented thermic effect of amino acids under general anaesthesia: a mechanism useful for prevention of anaesthesia-induced hypothermia. Clin Sci (Lond). 1994;86:611–8. [DOI] [PubMed] [Google Scholar]
- 7.Donato J Jr, Pedrosa RG, Cruzat VF, Pires IS, Tirapegui J. Effects of leucine supplementation on the body composition and protein status of rats submitted to food restriction. Nutrition. 2006;22:520–7. [DOI] [PubMed] [Google Scholar]
- 8.Layman DK, Walker DA. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr. 2006;136:S319–23. [DOI] [PubMed] [Google Scholar]
- 9.Mourier A, Bigard AX, de Kerviler E, Roger B, Legrand H, Guezennec CY. Combined effects of caloric restriction and branched-chain amino acid supplementation on body composition and exercise performance in elite wrestlers. Int J Sports Med. 1997;18:47–55. [DOI] [PubMed] [Google Scholar]
- 10.Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–81. [DOI] [PubMed] [Google Scholar]
- 11.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age-and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–5. [DOI] [PubMed] [Google Scholar]
- 12.She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab. 2007;293:E1552–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller RS, Becker KG, Prabhu V, Cooke DW. Adipocyte gene expression is altered in formerly obese mice and as a function of diet composition. J Nutr. 2008;138:1033–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pruznak AM, Kazi AA, Frost RA, Vary TC, Lang CH. Activation of AMP-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-beta-D-ribonucleoside prevents leucine-stimulated protein synthesis in rat skeletal muscle. J Nutr. 2008;138:1887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007;6:181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via ltimechanisms. Diabetes. 2007;56:1647–54. [DOI] [PubMed] [Google Scholar]
- 17.Ahren B, Pacini G. Insufficient islet compensation to insulin resistance vs. reduced glucose effectiveness in glucose-intolerant mice. Am J Physiol Endocrinol Metab. 2002;283:E738–44. [DOI] [PubMed] [Google Scholar]
- 18.Koza RA, Nikonova L, Hogan J, Rim JS, Mendoza T, Faulk C, Skaf J, Kozak LP. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet. 2006;2:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burcelin R, Crivelli V, Dacosta A, Roy-Tirelli A, Thorens B. Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am J Physiol Endocrinol Metab. 2002;282:E834–42. [DOI] [PubMed] [Google Scholar]
- 20.Rossmeisl M, Rim JS, Koza RA, Kozak LP. Variation in type 2 diabetes-related traits in mouse strains susceptible to diet-induced obesity. Diabetes. 2003;52:1958–66. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Rossmeisl M, McClaine J, Riachi M, Harper ME, Kozak LP. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest. 2003;111:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams TD, Chambers JB, Roberts LM, Henderson RP, Overton JM. Diet-induced obesity and cardiovascular regulation in C57BL/6J mice. Clin Exp Pharmacol Physiol. 2003;30:769–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.