Abstract
Livers and hearts from mice deficient in glycerol-3-phosphate acyltransferase 1 (GPAT1; Gpat1−/−) have a decreased content of glycerolipid intermediates and triacylglycerol, an altered composition of liver phospholipids, and elevated markers of oxidative stress. Compared with control C57BL/6 mice, infection of Gpat1−/− mice with coxsackievirus B3 (CVB3) resulted in higher mortality, an ∼50% increase in heart pathology, a significant increase in liver viral titers, and a 100-fold increase in heart viral titers. Moreover, heart mRNA levels for proinflammatory cytokines tumor necrosis factor-α, interleukin (IL)-6, and IL-1B were increased in the Gpat1−/− mice. Loss of Gpat1 also resulted in dysregulation of specific immune cells. Splenic dendritic cells from Gpat1−/− mice were fully capable of stimulating T cells from control mice; however, splenic T cells from Gpat1−/− mice were defective in their response to CVB3 antigen. Our data indicate that a lack of GPAT1 activity affects both innate and adaptive immune mechanisms. Innate mechanisms may be affected by altered membrane composition or host redox status, whereas the adaptive response may require GPAT1 activity itself.
Introduction
Effective control and viral clearance during infection requires a number of highly synchronized immune cells that can each, in turn, be affected by individual metabolic processes such as glycolysis and lipogenesis. Glycerol-3-phosphate acyltransferase 1 (GPAT1)4 is located on the mitochondrial outer membrane and catalyzes the first and rate-limiting step in the synthesis of glycerophospholipids and triacylglycerol (TAG). GPAT1 is 1 of 4 GPAT isoforms that initiate de novo membrane glycerophospholipid biosynthesis and it esterifies primarily SFA at the sn-1 position of glycerol-3-phosphate (1–3). In most tissues, GPAT1 comprises ∼10% of the total GPAT activity; however, in liver, it comprises ∼30–50% of total GPAT activity. GPAT1 may play a critical role in disorders such as obesity, diabetes, and atherosclerosis, because the expression of GPAT1 mRNA has been shown to be nutritionally and hormonally regulated when TAG synthesis is increased (4,5).
Although it is clear that GPAT1 plays a pivotal role in TAG synthesis, recent evidence suggests an important role for the enzyme in immune function. GPAT1 influences biological membrane composition (2), which can have a profound effect on immune cell interactions and signaling pathways. Absence of GPAT1 results in decreases in the second messengers diacylglycerol and lysophosphatidic acid and increases in the amount of arachidonate in membrane phospholipids in the liver and heart (6–9). In addition, a decrease of GPAT1 activity in aging T-lymphocytes may contribute to age-dependent immune depression. Compared with T-cells from young controls, T cells isolated from aged mice show decreased GPAT1 activity and proliferative capacity when stimulated with mitogen. T cells isolated from GPAT1 knockout mice (Gpat1−/−) undergo similar changes (10–12).
Although GPAT1 activity has the potential to affect immune status of the host in vitro, the importance of GPAT1 activity for the immune response and the subsequent control and clearance of a viral infection has not yet been elucidated. We hypothesized that the absence of GPAT1 activity might alter the immune response to a viral challenge. To study the effect of GPAT1 during infection, we used coxsackievirus B3 (CVB3), a major cause of viral myocarditis in humans. Acute CVB3 myocarditis is a principal cause of heart failure in young adults and can progress to chronic myocarditis, dilated cardiomyopathy, and congestive heart failure (13–15). Viral infectivity, viral persistence, and host immune response may all contribute to the severity of CVB3 pathogenesis (16). Mice with CVB3-induced myocarditis have been used a model for the human disease for over 50 y (17). After the initial infection in the intestinal tract by intraperitoneal injection, the virus infects the liver and pancreas by d 2 or 3 postinfection (p.i.) and can be detected in the heart between d 4 and 5 p.i. Acute myocarditis usually occurs at d 7–14 p.i (18,19). The pathogenesis of the virus in the mouse is identical to the progression observed in the human disease.
To determine the effect of a lack of GPAT1 activity on both the immune response and on viral replication kinetics, we infected Gpat1−/− and wild-type C57BL/6 mice with CVB3 and measured the pathological outcome, the amount of viral replication, and the host immune response. We found that a deficiency in GPAT1 clearly altered the ability of the host to respond effectively to CVB3 infection.
Materials and Methods
Gpat1−/− mice and CVB3 infection.
Gpat1−/− mice were generated previously in the Coleman laboratory on a C57BL/6 background (1) and knockout and wild-type mice were bred in-house. Male C57BL/6J and Gpat1−/− mice were housed in the University of North Carolina Animal Facility, which is fully accredited by the American Association for Accreditation of Laboratory Animal Care. Mice were maintained under protocols approved by the Institutional Animal Use and Care Committee. All mice were housed under a 12–h-light/-dark schedule with free access to food and water. Male C57BL/6J and Gpat1−/− mice at 2 to 3 mo of age were inoculated intraperitoneally with 105 median tissue culture infective dose (TCID50) CVB3/59 in 0.1-mL sterile minimum essential media. CVB3/59 has been shown to produce myocardial pathology in BL/6 mice (19,20).
Pathology.
Mice were killed at d 10 p.i. by cervical dislocation and their hearts were removed and transversely cut in half. One half of each heart was embedded in Optimal Cutting Temperature Compound (Tissue-Tek) and frozen on dry ice. Frozen sections (6 μm) were stained with hematoxylin and eosin. The extent of heart pathology was graded in a semiquantitative manner according to the relative degree (from heart to heart) of mononuclear cell infiltrate and the extent of necrosis.
Quantitation of viral titers.
HeLa cells were plated in 96-well flat-bottom plates. Tissue samples were weighed, ground in minimum essential media, frozen and thawed 5 times, and centrifuged at 9300 × g; 10 min at 4°C. The supernate was collected and filtered. Serial 10-fold dilutions of supernate were added in replicates of 6 to the HeLa cell plates. Following 2–3 d of incubation, viral cytopathic effect was scored for each well and TCID50 was determined by the method of Reed and Muench (21).
Quantitation of heart mRNA cytokine levels.
Heart samples were collected on d 5 p.i. and total RNA was isolated using the TRIzol method (Invitrogen). RT was carried out with a Superscript II First Strand Synthesis kit (Invitrogen) using oligo (dT) primers. Following previously described methods (22), mRNA levels for murine interferon (IFN) α, IFNβ, IFNγ, interleukin (IL)-1β, IL-6, IL-10, tumor necrosis factor-α (TNFα), inducible nitric oxide synthase (iNOS), and glyceraldehyde-3-phosphate dehydrogenase (G3PDH) were determined using quantitative real-time PCR.
Determination of natural killer cell cytotoxicity.
Following previously published methods (22), isolated Gpat1−/− and wild-type spleen cells were analyzed in a natural killer (NK) cell cytotoxicity assay (23) using 51Cr-labeled YAC-1 tumor cells (American Type Culture Collection) as targets.
Antigen presentation by dendritic cells.
Following previously published methods (24), spleens from uninfected Gpat1−/− and wild-type mice were collected and dendritic cells (DC) were isolated using a DC enrichment kit (Dynal). T cells were isolated from the spleens of wild-type C57BL/6 mice that had been infected with CVB3/59 10 d before. DC were incubated at a multiplicity of infection of 10 with UV-inactivated CVB3/59 for 2 h followed by extensive washing to remove excess virus. Serial dilutions of 0.1 mL DC starting at 1 × 106 cells/mL were plated with 1 × 105 T cells in a 96-well plate, resulting in DC:T ratios of 1:1, 1:2, and 1:4. All samples were prepared in triplicate and incubated for 2 h at 37°C followed by the addition of Golgi Plug (BD Biosciences) and incubation for an additional 4 h. Cells were then stained with fluorescein isothiocyanate-anti-CD3, allophycocyanin-anti-CD4, and peridinin-chlorophyll-protein complex anti-CD8 (BD Biosciences) followed by fixation and permeabilization for subsequent intracellular staining with PE-anti-IFNγ. PE-goat-anti-IgG (Sigma) was used as a staining control. Cells were then analyzed on a flow cytometer (FACSCalibur).
T cell response to presented antigen.
DC were isolated from uninfected wild-type mice and incubated with UV-inactivated virus as described above. Splenic T cells from Gpat1−/− and wild-type mice infected 10 d before were isolated as above. Wild-type DC and T cell groups were incubated together and analyzed as above.
Subcellular fractionation and GPAT enzyme assays.
Livers from wild-type and Gpat1−/− were collected at d 0 (uninfected), 2, 5, and 10 p.i. Livers were homogenized with 10 up-and-down strokes in a Teflon-glass motorized homogenizer in 10 mmol/L Tris, pH 7.4, 250 mmol/L sucrose, 1 mmol/L dithiothreitol, and 1 mmol/L EDTA. The total membrane fraction was isolated by centrifugation at 100,000 × g; 1 h. The protein concentration was measured by the bicinchoninic acid method (Pierce) with bovine serum albumin as the standard. GPAT activity was assayed for 10 min at 25°C with 800 μmol/L [3H]glycerol-3-phosphate and 100 μmol/L palmitoyl-CoA in the presence or absence of 2 mmol/L N-ethylmaleimide (NEM), which inhibits the microsomal isoforms GPAT3 and 4 (25). The NEM-sensitive mitochondrial GPAT2 is present at very low levels in liver (26). [3H]glycerol-3-phosphate was synthesized enzymatically (27).
Statistical analysis.
Statistical analyses were performed using JMP Statistical software (SAS Institute). Nonparametric data were analyzed using the Kruskal Wallis test (α = 0.05). Differences were considered significant at P < 0.05. T cell response data were analyzed by 2-way ANOVA with mouse type and effector:target ratio as main effects. A Tukey test was used for post hoc analysis. Relationships between liver viral titer and cytokine mRNA expression were analyzed by the nonparametric Spearman's rank-order correlation analysis (Spearman's ρ).
Results
Following a coxsackievirus challenge, Gpat1−/− mice exhibited increased mortality and significantly increased heart pathology. At age 3 mo, mice were infected with CVB3. Although no wild-type mice died, Gpat1−/− mice had an 8% mortality rate (data not shown). In addition, cardiac pathology, characterized by heart inflammatory infiltrate, was significantly greater in the Gpat1−/− mice at d 10 p.i. compared with wild-type controls (data not shown).
Increased inflammation was associated with increased CVB3 viral load in Gpat1−/− mice.
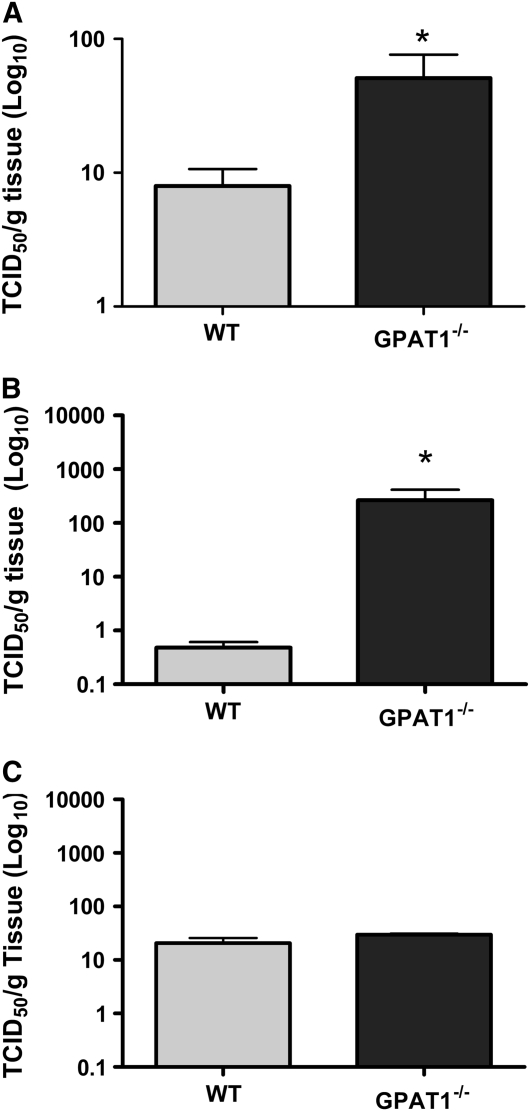
The virus titer was significantly greater in the liver of Gpat1−/− mice at d 2 p.i (Fig. 1A) and in the heart at d 5 p.i. (Fig. 1B) compared with wild-type mice. Although viral titers did not differ between the groups at d 10 p.i. (Fig. 1C), Gpat1−/− mice still had severe cardiac inflammation compared with wild-type mice (Fig. 1).
FIGURE 1 .
Liver virus titers at d 2 (A) and heart virus titers at d 5 (B) and d 10 p.i. with coxsackievirus in wild-type and Gpat1−/− mice. Values are mean + SEM on a Log10 scale, n = 6–8. *Different from wild type, P < 0.05.
Gpat1−/− hearts expressed increased cytokine mRNA levels after CVB3 challenge.
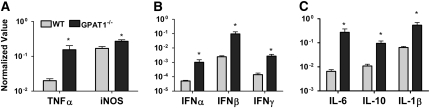
Cytokines help to orchestrate immune responses both by controlling inflammatory responses and by attracting immune cells to the site of infection. Levels of mRNA for TNFα and iNOS (Fig. 2A) as well as IL-1β, IL-6 (Fig. 2B), and IFNα, IFNβ, and IFNγ (Fig. 2C) were significantly elevated by 1- to 2-fold in the hearts of Gpat1−/− mice at 5 d p.i. compared with wild-type mice. Additionally, mRNA levels of the antiinflammatory cytokine IL-10 were also significantly increased (Fig. 3B). Increased cytokine mRNA levels were positively correlated with virus titer on an individual mouse basis [P ≤ 0.05; ρ = 0.7692 (IFNβ), 0.5385 (IFNγ), 0.5692 (TNFα), 0.7473 (IL-6), 0.5209 (IL-10)]. Liver cytokine concentrations did not differ between the groups at d 2 p.i. (data not shown).
FIGURE 2 .
Heart mRNA levels for murine cytokines, chemokines, and G3PDH at d 5 p.i. mRNA levels of TNFα, iNOS (A), IL-6, -10, and -1β (B), as well as IFNα, -β, and -γ (C) were tested. Values are mean ± SEM by normalization to G3PDHs, n = 6–8. *Different from wild type, P < 0.05.
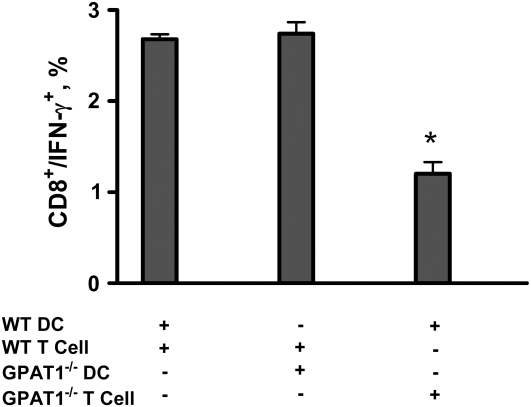
FIGURE 3 .
Gpat1−/− DC present antigen to T cells normally, but Gpat1−/− T cells have a reduced ability to respond to antigen. Gpat1−/− and wild-type DC were incubated with UV-inactivated CVB3 (multiplicity of infection = 10), and then incubated with T cells isolated from the spleens of wild-type and Gpat1−/− mice at d 10 p.i. at a DC:T cells ratio of 1:1. Numbers of T cells that responded to presentation were measured by flow cytometry. Values are percent of CD8+ cells expressing IFNγ ± SEM, n = 3, and are representative of 2 separate experiments. *Different from wild type, P < 0.01.
Splenic NK cell activity was normal in Gpat1−/− mice.
NK cells act to eliminate virally infected cells, reducing viral spread until adaptive mechanisms can be marshaled. Because viral titers were higher in Gpat1−/− mice, NK cell cytotoxicity was tested in the Gpat1−/− mice. Cytotoxicity did not differ between cells isolated from wild-type and Gpat1−/− mice (data not shown). The percent of NK cells (%CD3−/DX5+) of total cells isolated from the spleen as determined by flow cytometry also did not differ (data not shown).
DC from Gpat1−/− mice were able to present antigen normally.
Effective viral clearance depends on the generation of a functional adaptive immune response. The generation of this virus-specific response stems from the ability of DC to take up and present antigen to T cells. DC from both groups activated equivalent numbers of wild-type CD8+ T cells to produce IFNγ, indicating that Gpat1−/− DC were able to take up and present antigen normally (Fig. 3). In addition to the ability of DC to present antigen, it is equally important for T cells to be able to respond to the presented antigen. CD8+ T cells from Gpat1−/− mice had lower IFNγ production compared with those from wild-type mice, indicating that GPAT1 activity is required for T cells to properly respond to antigen presentation (Fig. 3). This decreased T cell response in Gpat1−/− mice may have contributed to the increases in viral loads and pathology. The total numbers and populations of T cells did not differ at any time in the hearts of these mice (data not shown).
GPAT1 activity does not change in the liver following CVB3 infection.
Because lack of Gpat1 resulted in an altered immune response, we measured GPAT-specific activity in the livers of CVB3-infected mice over the course of the infection. As expected, GPAT1-associated activity (NEM-resistant) was virtually absent in the livers of Gpat1−/− mice. NEM-sensitive GPAT activity did not change in either group during the course of the infection (data not shown).
Discussion
Response to a viral infection requires a coordinated response of immune cells, cytokine expression, and antigen processing and presentation. GPAT1, a mitochondrial membrane-associated enzyme that initiates glycerolipid synthesis, has been previously shown to alter potential immunoregulatory factors, including T cell function, in a noninfectious model. To determine whether GPAT1 activity could influence the response to a viral infection, we infected Gpat1−/− mice with CVB3. Similar to infection in humans, CVB3 replicates in murine liver and heart tissue and ultimately induces myocarditis in the infected host. Thus, the resulting heart pathology is caused by inflammation associated with the immune response directed against virus-infected cardiomyocytes. Although the immune response contributes to the pathology, it is also required for recovery and viral clearance. The balance is delicate between the effectiveness of the immune response and the potential for harmful cardiac inflammation.
Compared with wild-type C57BL/6 mice, Gpat1−/− mice had greater mortality and cardiac pathology after CVB3 challenge. On d 5 p.i., viral titers and cytokine mRNA levels were greater in the hearts of the Gpat1−/− mice. These data suggest that the infectivity and virulence of coxsackievirus is influenced by the absence of GPAT1 activity. The increased viral titers likely induced the increased inflammatory response; however, the reason for the increased viral titers in the Gpat1−/− mice remains unclear. Loss of GPAT1 may alter the liver microenvironment and allow greater replication or spread of CVB3. For example, liver oxidative stress is increased in Gpat1−/− mice (6). Earlier work in our laboratory and others has demonstrated increased CVB3 viral replication under conditions of increasing oxidative stress (28–30). Similarly, heart tissue from Gpat1−/− mice may also exhibit increased oxidative stress, leading to increased viral replication.
To combat viral infection, macrophages, neutrophils, and DC are recruited to the infected area where they secrete proinflammatory cytokines. These mediators then act both to reduce viral replication and to attract and activate adaptive immune cells capable of clearing the remaining viral infection (31). The acute myocarditis that occurs in the heart around d 5 p.i. is comprised of inflammatory infiltrates of macrophages, neutrophils, DC, NK cells, and CD8+ T cells (19,32). Altered functionality of these cells could result in the increased pathology in the Gpat1−/− mice. DC isolated from both Gpat1−/− and wild-type mice were able to induce similar numbers of CD8+ T cells to secrete IFNγ. Additionally, NK cell cytotoxicity was not reduced in Gpat1−/− mice. Thus, GPAT1 activity is not necessary for DC or NK function and, taken together, these results suggest that these cells of the innate immune response are not directly affected by a lack of GPAT1 activity. However, an early increase in viral titers at a time during which viral replication is controlled by the innate immune response as well as an increase in innate proinflammatory cytokines suggests that GPAT1 does influence the innate immune response.
Because DC from Gpat1−/− mice presented antigen normally, we tested whether Gpat1−/− T cells were able to respond to antigen presentation. The finding of elevated viral titers and enhanced heart inflammation in the Gpat1−/− mice suggested that T cells were not adequately regulating the viral infection. T lymphocytes play a varied role in coxsackievirus B3-induced myocarditis (33). The severity of myocardial damage and the associated mortality depend on the predominant T cell type that is available to respond to CVB3 infection (20,34). The response of T cells from Gpat1−/− mice was greatly reduced when presented with antigen displayed on wild-type DC. Thus, T cell function was impaired in these mice. These results indicate that viral clearance by T cells is inadequate in Gpat1−/− mice and may contribute to the increased myocarditis. In previous studies, knockout of CD8+ T cells resulted in greater disease severity compared with a knockout of CD4+ cells; however, both types of knockout mice displayed greater mortality than wild-type controls (35). The exact mechanism by which GPAT1 activity affects T cells is unknown. Because lipid 2nd messengers such as phospholipids and diacylglycerol are extremely important in T cell activation (36–38), future studies will focus on the contribution of GPAT1 to the generation of these lipid 2nd messengers as well as the effects of these lipids on T cell-specific activation proteins such as protein kinase C θ, phospholipase C, and diacylglycerol kinase.
Taken together, these data obtained from Gpat1−/− mice indicate that the adaptive arm of the immune response requires GPAT1 activity. Increased viral infectivity and replication as well as reduced viral clearance results in increased pathology and increased mortality in the Gpat1−/− mice. Thus, glycerolipid synthesis may play a more important role in the immune response than previously suspected.
Acknowledgments
The authors thank Dr. Alexia Smith and Dr. Patricia Sheridan for their excellent technical assistance.
Supported by grants from the NIH (DK56598) and from grants from the NIH to the Clinical Nutrition Research Unit (DK56350).
Author disclosures: E. A. Karlsson, S. Wang, Q. Shi, R. A. Coleman, and M. A. Beck, no conflicts of interest.
Abbreviations used: CVB3, coxsackievirus B3; DC, dendritic cell; GPAT1, glycerol-3-phosphate acyltransferase 1; Gpat1−/−, glycerol-3-phosphate acyltransferase 1 knockout; G3PDH, glyceraldehyde-3-phosphate dehydrogenase; IFN, interferon; IL, interleukin; iNOS, inducible nitric oxide synthase; NEM, N-ethylmaleimide; NK, natural killer cells; p.i., post infection; TAG, triacylglycerol; TCID50, median tissue culture infectious dose; TNFα, tumor necrosis factor-α.
References
- 1.Hammond LE, Gallagher PA, Wang S, Hiller S, Kluckman KD, Posey-Marcos EL, Maeda N, Coleman RA. Mitochondrial glycerol-3-phosphate acyltransferase-deficient mice have reduced weight and liver triacylglycerol content and altered glycerolipid fatty acid composition. Mol Cell Biol. 2002;22:8204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haldar D, Tso WW, Pullman ME. The acylation of sn-glycerol 3-phosphate in mammalian organs and Ehrlich ascites tumor cells. J Biol Chem. 1979;254:4502–9. [PubMed] [Google Scholar]
- 3.Gonzalez-Baro MR, Lewin TM, Coleman RA. Regulation of triglyceride metabolism II. Function of mitochondrial GPAT1 in the regulation of triacylglycerol biosynthesis and insulin action. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res. 2004;43:134–76. [DOI] [PubMed] [Google Scholar]
- 5.Coleman RA, Lewin TM, Muoio DM. Physiological and nutritional regulation of enzymes of triacylglycerol synthesis. Annu Rev Nutr. 2000;20:77–103. [DOI] [PubMed] [Google Scholar]
- 6.Hammond LE, Albright CD, He L, Rusyn I, Watkins SM, Doughman SD, Lemasters JJ, Coleman RA. Increased oxidative stress is associated with balanced increases in hepatocyte apoptosis and proliferation in glycerol-3-phosphate acyltransferase-1 deficient mice. Exp Mol Pathol. 2007;82:210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaqoob P. Lipids and the immune response: from molecular mechanisms to clinical applications. Curr Opin Clin Nutr Metab Care. 2003;6:133–50. [DOI] [PubMed] [Google Scholar]
- 8.Calder PC, Yaqoob P, Thies F, Wallace FA, Miles EA. Fatty acids and lymphocyte function. Br J Nutr. 2002;87 Suppl 1:S31–48. [DOI] [PubMed] [Google Scholar]
- 9.Lewin TM, de Jong H, Schwerbrock NJM, Hammond LE, Watkins SM, Combs TP, Coleman RA. Mice deficient in mitochondrial glycerol-3-phosphate acyltransferase-1 have diminished myocardial triacylglycerol accumulation during lipogenic diet and altered phospholipid fatty acid composition. Biochim Biophys Acta. 2008;1781:352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collison LW, Jolly CA. Phosphorylation regulates mitochondrial glycerol-3-phosphate-1 acyltransferase activity in T-lymphocytes. Biochim Biophys Acta. 2006;1761:129–39. [DOI] [PubMed] [Google Scholar]
- 11.Collison LW, Kannan L, Onorato TM, Knudsen J, Haldar D, Jolly CA. Aging reduces glycerol-3-phosphate acyltransferase activity in activated rat splenic T-lymphocytes. Biochim Biophys Acta. 2005;1687:164–72. [DOI] [PubMed] [Google Scholar]
- 12.Kannan L, Knudsen J, Jolly CA. Aging and acyl-CoA binding protein alter mitochondrial glycerol-3-phosphate acyltransferase activity. Biochim Biophys Acta. 2003;1631:12–6. [DOI] [PubMed] [Google Scholar]
- 13.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases. Part I. General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–53. [DOI] [PubMed] [Google Scholar]
- 14.Woodruff JF. Viral myocarditis. A review. Am J Pathol. 1980;101:425–84. [PMC free article] [PubMed] [Google Scholar]
- 15.Reyes MP, Lerner AM. Coxsackievirus myocarditis–with special reference to acute and chronic effects. Prog Cardiovasc Dis. 1985;27:373–94. [DOI] [PubMed] [Google Scholar]
- 16.Tam PE. Coxsackievirus myocarditis: interplay between virus and host in the pathogenesis of heart disease. Viral Immunol. 2006;19:133–46. [DOI] [PubMed] [Google Scholar]
- 17.Fairweather D, Kaya Z, Shellam GR, Lawson CM, Rose NR. From infection to autoimmunity. J Autoimmun. 2001;16:175–86. [DOI] [PubMed] [Google Scholar]
- 18.Pallansch M, Roos R. Enteroviruses: polioviruses, coxsackieviruses, echoviruses and newer enteroviruses. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007.
- 19.Whitton JL. Immunopathology during coxsackievirus infection. Springer Semin Immunopathol. 2002;24:201–13. [DOI] [PubMed] [Google Scholar]
- 20.Kishimoto C, Ochiai H, Sasayama S. Immunological mechanisms in experimental coxsackievirus B3 myocarditis in mice. Jpn Circ J. 1991;55:1144–8. [DOI] [PubMed] [Google Scholar]
- 21.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–7. [Google Scholar]
- 22.Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137:1236–43. [DOI] [PubMed] [Google Scholar]
- 23.Leung KN, Ada GL. Induction of natural killer cells during murine influenza virus infection. Immunobiology. 1981;160:352–66. [DOI] [PubMed] [Google Scholar]
- 24.Smith AG, Sheridan PA, Tseng RJ, Sheridan JF, Beck MA. Selective impairment in dendritic cell function and altered antigen-specific CD8+ T-cell responses in diet-induced obese mice infected with influenza virus. Immunology. 2009;126: 268–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coleman RA, Haynes EB. Selective changes in microsomal enzymes of triacylglycerol and phosphatidylcholine synthesis in fetal and postnatal rat liver. Induction of microsomal sn-glycerol 3-phosphate and dihydroxyacetonephosphate acyltransferase activities. J Biol Chem. 1983;258:450–6. [PubMed] [Google Scholar]
- 26.Wang S, Lee DP, Gong N, Schwerbrock NMJ, Mashek DG, Gonzalez-Baró MR, Stapleton C, Li LO, Lewin TM, et al. Cloning and functional characterization of a novel mitochondrial N-ethylmaleimide-sensitive glycerol-3-phosphate acyltransferase (GPAT2). Arch Biochem Biophys. 2007;465:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang Y-Y, Kennedy EP. Biosynthesis of phosphatidyl glycerophosphate in Escherichia coli. J Lipid Res. 1967;8:447–55. [PubMed] [Google Scholar]
- 28.Beck MA, Handy J, Levander OA. The role of oxidative stress in viral infections. Ann N Y Acad Sci. 2000;917:906–12. [DOI] [PubMed] [Google Scholar]
- 29.Xie B, Zhou JF, Lu Q, Li CJ, Chen P. Oxidative stress in patients with acute coxsackie virus myocarditis. Biomed Environ Sci. 2002;15:48–57. [PubMed] [Google Scholar]
- 30.Beck MA. Nutritionally induced oxidative stress: effect on viral disease. Am J Clin Nutr. 2000;71:S1676–81. [DOI] [PubMed] [Google Scholar]
- 31.Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology: the immune system in health and disease. 6th ed. New York: Garland Science; 2005.
- 32.Godeny EK, Gauntt CJ. In situ immune autoradiographic identification of cells in heart tissues of mice with coxsackievirus B3-induced myocarditis. Am J Pathol. 1987;129:267–76. [PMC free article] [PubMed] [Google Scholar]
- 33.Henke A, Huber S, Stelzner A, Whitton J. The role of CD8+ T lymphocytes in coxsackievirus B3-induced myocarditis. J Virol. 1995;69:6720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kishimoto C, Kuribayashi K, Masuda T, Tomioka N, Kawai C. Immunologic behavior of lymphocytes in experimental viral myocarditis: significance of T lymphocytes in the severity of myocarditis and silent myocarditis in BALB/c-nu/nu mice. Circulation. 1985;71:1247–54. [DOI] [PubMed] [Google Scholar]
- 35.Opavsky MA, Penninger J, Aitken K, Wen W-H, Dawood F, Mak T, Liu P. Susceptibility to myocarditis is dependent on the response of {alpha}{beta} T lymphocytes to coxsackieviral infection. Circ Res. 1999;85:551–8. [DOI] [PubMed] [Google Scholar]
- 36.Altman A, Villalba M. Protein kinase C{theta} (PKC{theta}): a key enzyme in T cell life and death. J Biochem. 2002;132:841–6. [DOI] [PubMed] [Google Scholar]
- 37.Merida I, Avila-flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J. 2008;409:1–18. [DOI] [PubMed] [Google Scholar]
- 38.Carrasco S, Merida I. Diacylglycerol, when simplicity becomes complex. Trends Biochem Sci. 2007;32:27–36. [DOI] [PubMed] [Google Scholar]