Abstract
Introduction:
Our goals were to determine whether acute exposure to nicotine alters nitric oxide synthase (NOS)–dependent responses of the basilar artery and to identify a potential role for activation of NAD(P)H oxidase in nicotine-induced impairment in NOS-dependent responses of the basilar artery.
Methods:
We measured in vivo diameter of the basilar artery in response to NOS-dependent (acetylcholine) and NOS-independent (nitroglycerin) agonists before and during an acute infusion of nicotine (2 μg/kg/min intravenously for 30 min followed by a maintenance dose of 0.35 μg/kg/min). In addition, we measured superoxide anion production (lucigenin chemiluminescence) by the basilar artery in response to nicotine in the absence or presence of apocynin.
Results:
We found that NOS-dependent, but not NOS-independent, vasodilation was impaired during infusion of nicotine. In addition, treatment of the basilar artery with apocynin (100 μM, 30 min prior to infusion of nicotine) prevented nicotine-induced impairment in NOS-dependent vasodilation. Further, the production of superoxide anion was increased in the basilar artery by nicotine, and this increase could be inhibited by apocynin.
Discussion:
Our findings suggest that acute exposure to nicotine impairs NOS-dependent dilation of the basilar artery by a mechanism that appears to be related to the release of superoxide anion. A possible source of superoxide may be via the activation of NAD(P)H oxidase.
Introduction
Cigarette smoking and the use of smokeless tobacco products increase the risk for ischemic and hemorrhagic stroke (Asplund, Nasic, Janlert, & Stegmayr, 2003; Burns, 2003; Hawkins, Brown, & Davis, 2002; Higa & Davanipour, 1991). Although the precise components of cigarette smoke and smokeless tobacco that contribute to the pathogenesis of vascular damage remains uncertain, several lines of evidence suggest that nicotine may play an important role. Investigators have shown that nicotine produces toxic effects on the endothelium (Hladovec, 1978; Lakier, 1992) and exposure (acute and chronic) to nicotine impairs nitric oxide synthase (NOS)–dependent dilation of large (Pellaton, Kubli, Feihl, & Waeber, 2002; Puranik & Celermajer, 2003) and small (Ijzerman, Serne, van Weissenbruch, de Jongh, & Stehouwer, 2003; Mayhan & Sharpe, 1999) peripheral blood vessels. In addition, we have shown that acute and chronic exposure to nicotine impairs NOS-dependent reactivity of pial arterioles located on the parietal cortex of rats (Fang, Sun, Arrick, & Mayhan, 2006; Fang, Sun, & Mayhan, 2003, 2004; Mayhan & Patel, 1997). Further, we have shown that impaired NOS-dependent responses of peripheral (Mayhan & Sharpe, 1998b) and cerebral (pial; Fang et al., 2003, 2006) arterioles during exposure to nicotine are related to the production of superoxide anion. Thus, oxidative stress appears to be an important contributor to nicotine-induced alterations in endothelial/vascular function.
Researchers have found important regional differences in responses of cerebral blood vessels to agonists that stimulate the synthesis/release of nitric oxide and regional differences regarding mechanisms that contribute to impaired responses of cerebral vessels during disease states. For example, mechanisms that contribute to impaired responses of cerebral arterioles during chronic hypertension appear to differ from those contributing to impaired responses of the basilar artery during chronic hypertension (Mayhan, 1990; Mayhan, Faraci, & Heistad, 1988). We are not aware of any studies that have examined the regional effects of nicotine on the cerebral microcirculation. Thus, the first goal of this study was to examine the acute influence of nicotine on reactivity of the basilar artery. Given that oxidative stress is an important contributor to vascular dysfunction in a variety of disease states, including during exposure to nicotine (Fang et al., 2003, 2006), our second goal was to examine whether oxidative stress also contributes to impaired NOS-dependent reactivity of the basilar artery during acute exposure to nicotine.
Methods
Preparation of animals
Adult male Sprague–Dawley rats (280–350 g) were used in these studies. All rats were housed in an animal care facility at the University of Nebraska Medical Center that is approved by the American Association for the Accreditation of Laboratory Animal Care, and all protocols were reviewed and approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee. Rats were anesthetized with thiobutabarbital sodium (100 mg/kg body weight, intraperitoneally). A tracheotomy was performed, and the animals were mechanically ventilated with room air and supplemental oxygen. A catheter was placed in a femoral vein for infusion of supplemental anesthetic (10–20 mg/kg, as necessary), for infusion of nicotine (2 μg/kg/min for 30 min followed by a maintenance dose of 0.35 μg/kg/min for the duration of the experiment), and for infusion of saline. This protocol, which we have used previously (Mayhan & Patel, 1997; Mayhan & Sharpe, 1998a), produces plasma levels of nicotine similar to that seen in chronic smokers (10–40 ng/ml; Benowitz, 1997; Benowitz, Zevin, & Jacob, 1997; Pomerleau, 1992; Russell, Jarvis, Iyer, & Feyerabend, 1980). A femoral artery was cannulated to measure arterial blood pressure.
After placement of all catheters, the animal was placed in a head holder in a supine position. The larynx and esophagus were retracted rostrally and laterally, and the musculature covering the basioccipital bone was removed. Then, a craniotomy was made in the bone at the base of the skull. The dura was incised to expose the basilar artery. We (Mayhan, 1990, 1991) and others (Faraci, 1990) have used this method to expose the basilar artery. The cranial window was suffused with artificial cerebral spinal fluid (37 ± 1 °C) and bubbled continuously to maintain gases within normal limits. Blood gases were monitored and maintained within normal limits throughout the experiment. The inner diameter of the basilar artery was measured online using a video image-shearing device (Model 908, Instrumentation for Physiology and Medicine, San Diego, CA). Diameter of the basilar artery was measured prior to, at 1-min intervals for 5 min during application of agonists, and after application of agonists was completed. We used the baseline diameter measured immediately before application of the agonist to determine the response of the basilar artery to that given agonist (percentage change in diameter). Application of vehicle did not alter baseline diameter, and diameter of the basilar artery returned to control levels (prior to application of agonists) within 2–3 min after application of agonists was stopped. Agonists were applied to the basilar artery in a cumulative manner (low concentration followed by high concentration). Application of agonists was separated by a period of at least 10 min.
Experimental protocol
The cranial window was suffused for 30 min before testing responses to the agonists. In the first group of rats (n = 5), we examined the effects of acute treatment with nicotine on reactivity of the basilar artery to an NOS-dependent agonist (acetylcholine, 1.0 and 10 μM) and to an NOS-independent agonist (nitroglycerin, 0.1 and 1.0 μM). Thus, in this series of studies, we first examined responses of the basilar artery to the agonists. Then we started an intravenous infusion of nicotine (2 μg/kg/min for 30 min followed by a maintenance dose of 0.35 μg/kg/min for the duration of the experiment). We again measured responses of the basilar artery to the agonists 30 min after starting the infusion of nicotine. In a second group of rats (n = 5), we examined whether topical treatment with apocynin (100 μM) could influence reactivity of the basilar artery during acute infusion of nicotine. In these studies, we first examined responses of the basilar artery to the agonists. Then we started a continuous suffusion of apocynin over the cranial window. We began the acute infusion of nicotine 30 min after starting the suffusion with apocynin. We again examined responses of the basilar artery to the agonists 30 min after starting the infusion of nicotine.
Superoxide anion measurement
In another group of rats (n = 8), we measured superoxide anion production using lucigenin-enhanced chemiluminescence (Arrick & Mayhan, 2007; Mayhan, Arrick, Sharpe, Patel, & Sun, 2006). After the rat was exsanguinated, the basilar artery was removed and immersed in a modified Krebs–N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES) buffer containing (in mmol/L): 118 NaCl, 4.7 KCl, 1.3 CaCl2, 1.2 MgCl2, 1.2 KH2PO4, 25 NaHCO3, 10 HEPES, and 5 glucose (pH 7.4). Samples of the basilar artery were placed in polypropylene tubes containing 5 μmol/L lucigenin and then read in a Fentomaster FB12 (Zytox) luminometer, which reports relative light units emitted integrated over 30-s intervals for 5 min. Data were corrected for background activity and normalized to tissue protein content. In these studies, we measured superoxide anion production under basal conditions, during exposure to nicotine (20 ng/ml) for 30 min, and during exposure to nicotine in the presence of apocynin (100 μM).
Data analyses
Paired Student t tests were used to compare functional responses of the basilar artery before and during treatment with nicotine and before and during treatment with nicotine in the presence of apocynin. Analysis of variance with Student–Newman–Keuls test for significance was used to compare the dose–response relationship to the agonists (low vs. high dose under baseline conditions) and superoxide anion production during treatment with nicotine in the absence and presence of apocynin. A p value of .05 or less was considered to be significant.
Results
Influence of nicotine on reactivity of the basilar artery
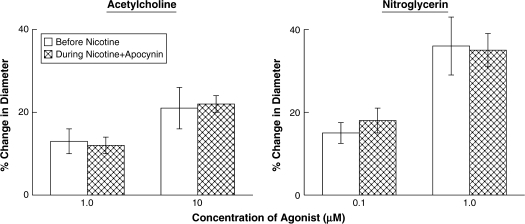
Baseline diameter of the basilar artery prior to the application of agonists was 253 ± 21 microns. Application of acetylcholine and nitroglycerin produced dose-related dilation of the basilar artery (Figure 1). Under control conditions, the response of the basilar artery to the low dose of the agonist (acetylcholine or nitroglycerin) was significantly less than that observed to the high dose of the agonist (p < .05). Acute infusion of nicotine did not alter baseline diameter of cerebral arterioles (248 ± 22 microns before infusion of nicotine vs. 238 ± 21 microns during infusion of nicotine; p > .05). However, acute infusion of nicotine impaired reactivity of the basilar artery to acetylcholine but not to nitroglycerin (see Figure 1).
Figure 1.
Response of the basilar artery to acetylcholine and nitroglycerin before (open bars) and during infusion of nicotine (closed bars). Values are means with SEs. *p < .05 versus response before infusion of nicotine.
Influence of apocynin
To determine whether infusion of nicotine impaired responses of the basilar artery via an increase in oxidative stress, presumably via activation of NAD(P)H oxidase, we examined the influence of apocynin on reactivity of the basilar artery during infusion of nicotine. Baseline diameter of the basilar artery prior to application of agonists was 224 ± 28 microns. Prior to treatment with apocynin and infusion of nicotine, acetylcholine, and nitroglycerin produced dose-related dilation of the basilar artery (Figure 2). Topical application of apocynin to the basilar artery did not affect baseline diameter (227 ± 29 microns before application of apocynin vs. 218 ± 30 microns during application of apocynin; p > .05). In addition, infusion of nicotine in the presence of apocynin did not influence baseline diameter of cerebral arterioles (218 ± 30 microns before infusion of nicotine vs. 217 ± 28 microns during infusion of nicotine in the presence of apocynin; p > .05). In contrast to our findings during infusion of nicotine in the absence of apocynin (Figure 1), treatment with apocynin prior to infusion of nicotine could prevent nicotine-induced impairment in reactivity of the basilar artery to acetylcholine (Figure 2). However, treatment with apocynin did not influence reactivity of the basilar artery to nitroglycerin.
Figure 2.
Response of the basilar artery to acetylcholine and nitroglycerin before (open bars) and during infusion of nicotine in the presence of apocynin (cross-hatched bars). Values are means with SEs.
Superoxide anion production
Basal production of superoxide anion increased dramatically when the basilar artery was exposed to nicotine for 30 min (Figure 3). In addition, treatment of the basilar artery with apocynin significantly decreased nicotine-induced superoxide anion production.
Figure 3.
Superoxide anion production (relative light units) from the basilar artery under basal conditions (open bars), during treatment with nicotine (20 ng/ml for 30 min; closed bars), and during treatment with nicotine in the presence of apocynin (100 μM; cross-hatched bars). Values are means with SEs. *p < .05 versus response under basal conditions; **p < .05 versus during treatment with nicotine.
Discussion
The present study resulted in three new findings. First, acute treatment with nicotine specifically impaired NOS-dependent dilation of the basilar artery. This finding cannot be explained by a nonspecific impairment in vasodilation since nitroglycerin produced similar dose-related dilation of the basilar artery before and during treatment with nicotine. Second, exposure of the basilar artery to nicotine produced a significant increase in superoxide anion production. Third, treatment with apocynin could prevent nicotine-induced impairment in NOS-dependent dilation of the basilar artery and nicotine-induced increases in superoxide anion production. Based on these findings, we suggest that nicotine impairs NOS-dependent dilation of the basilar artery via a mechanism that involves an increase in the production of superoxide anion. In addition, we speculate that activation of NAD(P)H oxidase by nicotine may contribute to the production of superoxide anion.
Consideration of methods
We used acetylcholine to examine the influence of nicotine on NOS-dependent dilation of the basilar artery. Many studies using in vitro methodologies have shown that acetylcholine produces marked relaxation of the basilar artery in rats (Benyo, Lacza, Hortobagyi, Gorlach, & Wahl, 2000; Lai et al., 1989; Soltis & Bohr, 1987), rabbits (Fujiwara, Kassell, Sasaki, Nakagomi, & Lehman, 1986; Nakagomi et al., 1988; Wellman & Bevan, 1995), and humans (Kanamaru, Waga, Fujimoto, Itoh, & Kubo, 1989; Whalley, Amure, & Lye, 1987). In addition, we (Mayhan, 1990, 1992; Mayhan, Sun, Mayhan, & Patel, 2004) and others (Benyo et al., 2000; Faraci, 1990, 1991) have shown that acetylcholine-induced relaxation/dilation of the basilar artery in rats could be inhibited by enzymatic inhibitors of NOS. In contrast, vasodilation in response to nitrovasodilators was not altered by inhibition of NOS (Faraci, 1990, 1991; Mayhan, 1990). It is difficult to determine the precise role of the various isoforms of NOS on dilation of the basilar artery. Most studies that have examined a role for NOS in dilation of the basilar artery in response to acetylcholine have used nonspecific inhibitors of NOS (Faraci, 1990, 1991; Mayhan, 1990, 1992; Mayhan et al., 2004). However, Benyo et al. (2000) found that inhibition of neuronal nitric oxide synthase (nNOS) using 7-nitroindazole could significantly inhibit relaxation of the basilar artery in response to acetylcholine. In addition, relaxation of the basilar artery in response to acetylcholine could be completely abolished by removal of the endothelium (Benyo et al., 2000). Thus, Benyo et al. concluded that nNOS must be present in the endothelium of the basilar artery and that activation of nNOS contributes to relaxation of the basilar artery in response to acetylcholine. In the present study, we found that nicotine could impair acetylcholine-induced dilation of the basilar artery via an increase in oxidative stress. Although we did not specifically examine which isoform of NOS accounts for dilation of the basilar artery in response to acetylcholine, the increase in oxidative stress produced by nicotine may influence the contribution of both nNOS and endothelial nitric oxide synthase (eNOS) in dilation of the basilar artery in response to acetylcholine.
NAD(P)H oxidase is a multicomponent enzyme complex that includes two membrane-associated subunits (p22phox and gp91phox) and at least three cytosolic subunits (p47phox, p67phox, and p40phox; Brandes & Kreuzer, 2005; Chabrashvili et al., 2002; Droge, 2001). Although NAD(P)H oxidase has been studied widely in phagocytic cells, it is also present in vascular smooth muscle cells (Griendling, Minieri, Ollerenshaw, & Alexander, 1994; Lassegue et al., 2001; Suh et al., 1999) and endothelial cells (De Keulenaer et al., 1998; Mohazzab, Kaminski, & Wolin, 1994), including endothelial cells of cerebral vessels (Ago et al., 2005). Thus, NAD(P)H could be a major source of superoxide anion production during disease states. In the present study, we applied apocynin to the cranial window to determine a potential role for activation of NAD(P)H oxidase and subsequent formation of superoxide anion by nicotine in impaired NOS-dependent reactivity of the basilar artery. Our assumption was that apocynin is a specific inhibitor of NAD(P)H oxidase because it inhibits the translocation of p47phox. This assumption is supported by a number of studies that have used apocynin (acute and chronic treatment) to examine the effects of oxidative stress, via activation of NAD(P)H oxidase, on vascular dysfunction during a variety of disease states (Beswick, Dorrance, Leite, & Webb, 2001; Hamilton, Brosnan, Al-Benna, Berg, & Dominiczak, 2002; Hamilton, Brosnan, McIntyre, Graham, & Dominiczak, 2002; Rey, Li, Carretero, Garvin, & Pagano, 2002; Sun et al., 2006; Ungvari et al., 2003). However, whether apocynin is specific for NAD(P)H oxidase or is merely acting as an antioxidant is the subject of debate (Heumuller et al., 2008; Touyz, 2008). In any event, we found that apocynin alleviated impaired NOS-dependent reactivity of the basilar artery during infusion of nicotine and inhibited superoxide anion formation by the basilar artery during exposure to nicotine. The influence of apocynin on vascular function appears to be specific for NOS-dependent agonists because apocynin did not alter responses to nitroglycerin. We cannot precisely determine whether apocynin is acting as an antioxidant or as a specific inhibitor of NAD(P)H oxidase, but our findings suggest an important role for superoxide anion in impaired responses of the basilar artery during exposure to nicotine.
Consideration of previous studies
Active and passive exposure to cigarette smoke impairs NOS-dependent reactivity of large and small peripheral vessels in animals (Mays et al., 1999; Murohara, Kugiyama, Ohgushi, Sugiyama, & Yasue, 1994; Rubinstein, Yong, Rennard, & Mayhan, 1991) and humans (Celermajer et al., 1996; Sumida et al., 1998). The cellular mechanism that contributes to impaired vascular function during exposure to cigarette smoke appears to involve oxygen-derived free radicals (Mays et al., 1999; Murohara et al., 1994; Ota et al., 1997). Although cigarette smoke contains many toxic substances, we (Mayhan & Sharpe, 1998b, 1999) and others (Chalon, Moreno, Benowitz, Hoffman, & Blaschke, 2000; Miller et al., 2000; Sabha et al., 2000) have suggested that nicotine may be an important candidate contributing to vascular dysfunction. Exposure of humans (Chalon et al., 2000; Sabha et al., 2000) and animals (Mayhan & Sharpe, 1998b, 1999; Miller et al., 2000) to nicotine impairs NOS-dependent reactivity of large and small peripheral vessels. In addition, acute and chronic treatment of rats with nicotine, at concentrations found in smokers and users of tobacco products (Benowitz et al., 1997; Sarabi & Lind, 2000), selectively impairs NOS-dependent (eNOS and nNOS) responses of cerebral (pial) arterioles via an increase in superoxide anion production (Arrick & Mayhan, 2007; Fang et al., 2003, 2006). The findings of the present study agree with those of our previous studies (Arrick & Mayhan, 2007; Fang et al., 2003, 2006). The present study also extends our previous findings by examining important regional influences of nicotine on cerebrovascular function and by examining the mechanism for the effects of nicotine on the basilar artery. We found that acute nicotine infusion impairs NOS-dependent reactivity of the basilar artery and that this impairment could be reversed by acute treatment with apocynin. In addition, we found that exposure of the basilar artery to nicotine increased superoxide anion production that could be inhibited by apocynin.
If we speculate that activation of NAD(P)H oxidase is important based on our findings using apocynin, then the precise cellular pathway by which nicotine may increase NAD(P)H oxidase activity remains uncertain. One possibility is that angiotensin II plays a critical role. Acute and chronic cigarette smoking have been shown to increase the conversion of angiotensin I to angiotensin II in rats (Yu, Jin, & Wang, 1992). Angiotensin II has been shown to increase the activity of NAD(P)H oxidase, vascular p47phox expression, and production of oxygen radicals by vascular cells (Fukui et al., 1997; Griendling et al., 1994; Griendling, Sorescu, & Ushio-Fukai, 2000; Landmesser et al., 2002; Rajagopalan et al., 1996). In addition, treatment of chronic smokers with an angiotensin-converting enzyme inhibitor (lisinopril) can improve impaired endothelial function (Butler, Morris, & Struthers, 2001). Thus, it is conceivable that an increased generation of angiotensin II during infusion of nicotine may contribute to cerebrovascular dysfunction. Future studies will be required to precisely determine the role of NAD(P)H oxidase in impaired NOS-dependent responses of the basilar artery during infusion of nicotine.
In summary, we examined the acute effects of nicotine on NOS-dependent reactivity of the basilar artery. We found that acute infusion of nicotine in rats significantly impaired NOS-dependent, but not NOS-independent, reactivity of the basilar artery. In addition, we found that inhibition of superoxide anion formation by treatment with apocynin could prevent impaired NOS-dependent reactivity of the basilar artery during infusion of nicotine. Further, exposure of the basilar artery to nicotine dramatically increased superoxide anion formation, and this increase in superoxide anion formation could be inhibited by apocynin. We suggest that impaired NOS-dependent responses of the basilar artery during infusion of nicotine are related to the production of superoxide anion. We speculate that activation of NAD(P)H oxidase could presumably be involved in the formation of superoxide anion during exposure to nicotine. Our findings may have important implications for the pathogenesis of cerebrovascular abnormalities, including ischemic stroke, observed in smokers and users of tobacco products.
Funding
National Institutes of Health (DA-14258, HL-79587, and AA-11288); a scientist development grant from the American Heart Association (0635052N); University of Nebraska Medical Center.
Declaration of Interests
None declared.
Supplementary Material
References
- Ago T, Kitazono T, Kuroda J, Kumai Y, Kamouchi M, Ooboshi H, et al. NAD(P)H oxidases in rat basilar arterial endothelial cells. Stroke. 2005;36:1040–1046. doi: 10.1161/01.STR.0000163111.05825.0b. [DOI] [PubMed] [Google Scholar]
- Arrick DM, Mayhan WG. Acute infusion of nicotine impairs nNOS-dependent reactivity of cerebral arterioles via an increase in oxidative stress. Journal of Applied Physiology. 2007;103:2062–2067. doi: 10.1152/japplphysiol.00411.2007. [DOI] [PubMed] [Google Scholar]
- Asplund K, Nasic S, Janlert U, Stegmayr B. Smokeless tobacco as a possible risk factor for stroke in men: A nested case-control study. Stroke. 2003;34:1754–1759. doi: 10.1161/01.STR.0000076011.02935.A1. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. The role of nicotine in smoking-related cardiovascular disease. Preventive Medicine. 1997;26:412–417. doi: 10.1006/pmed.1997.0175. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Zevin S, Jacob P. Sources of variability in nicotine and cotinine levels with use of nicotine nasal spray, transdermal nicotine and cigarette smoking. British Journal of Clinical Pharmacology. 1997;43:259–267. doi: 10.1111/j.1365-2125.1997.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyo Z, Lacza Z, Hortobagyi T, Gorlach C, Wahl M. Functional importance of neuronal nitric oxide synthase in the endothelium of rat basilar arteries. Brain Research. 2000;877:79–84. doi: 10.1016/s0006-8993(00)02611-1. [DOI] [PubMed] [Google Scholar]
- Beswick RA, Dorrance AM, Leite R, Webb RC. NADH-NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension. 2001;38:1107–1111. doi: 10.1161/hy1101.093423. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Kreuzer J. Vascular NADPH oxidases: Molecular mechanisms of activation. Cardiovascular Research. 2005;65:16–27. doi: 10.1016/j.cardiores.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Burns DM. Epidemiology of smoking-induced cardiovascular disease. Progress in Cardiovascular Disease. 2003;46:11–29. doi: 10.1016/s0033-0620(03)00079-3. [DOI] [PubMed] [Google Scholar]
- Butler R, Morris AD, Struthers AD. Lisinopril improves endothelial function in chronic cigarette smokers. Clinical Science. 2001;101:53–58. [PubMed] [Google Scholar]
- Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, et al. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. New England Journal of Medicine. 1996;334:150–154. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- Chabrashvili T, Tojo A, Onosato ML, Kitiyakara C, Quinn MT, Fujita T, et al. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension. 2002;39:269–274. doi: 10.1161/hy0202.103264. [DOI] [PubMed] [Google Scholar]
- Chalon S, Moreno H, Benowitz NL, Hoffman BB, Blaschke TF. Nicotine impairs endothelium-dependent dilatation in human veins in vivo. Clinical Pharmacology and Therapeutics. 2000;67:391–397. doi: 10.1067/mcp.2000.105153. [DOI] [PubMed] [Google Scholar]
- De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: Role of a superoxide producing NADH oxidase. Circulation Research. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiological Reviews. 2001;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Fang Q, Sun H, Arrick DM, Mayhan WG. Inhibition of NADPH oxidase improves impaired reactivity of pial arterioles during chronic exposure to nicotine. Journal of Applied Physiology. 2006;100:631–636. doi: 10.1152/japplphysiol.00975.2005. [DOI] [PubMed] [Google Scholar]
- Fang Q, Sun H, Mayhan WG. Impairment of nitric oxide synthase-dependent dilatation of cerebral arterioles during infusion of nicotine. American Journal of Physiology. 2003;284:H528–H534. doi: 10.1152/ajpheart.00752.2002. [DOI] [PubMed] [Google Scholar]
- Fang Q, Sun H, Mayhan WG. L-arginine prevents impaired endothelium-dependent cerebral arteriolar dilatation during acute infusion of nicotine. Nicotine and Tobacco Research. 2004;6:1009–1014. doi: 10.1080/14622200412331324949. [DOI] [PubMed] [Google Scholar]
- Faraci FM. Role of nitric oxide in regulation of basilar artery tone in vivo. American Journal of Physiology. 1990;259:H1216–H1221. doi: 10.1152/ajpheart.1990.259.4.H1216. [DOI] [PubMed] [Google Scholar]
- Faraci FM. Role of endothelium-derived relaxing factor in cerebral circulation: Large arteries vs. microcirculation. American Journal of Physiology. 1991;261:H1038–H1042. doi: 10.1152/ajpheart.1991.261.4.H1038. [DOI] [PubMed] [Google Scholar]
- Fujiwara S, Kassell NF, Sasaki T, Nakagomi T, Lehman RM. Selective hemoglobin inhibition of endothelium dependent vasodilation of rabbit basilar artery. Journal of Neurosurgery. 1986;64:445–452. doi: 10.3171/jns.1986.64.3.0445. [DOI] [PubMed] [Google Scholar]
- Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Q, Taylor WR, et al. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circulation Research. 1997;80:45–51. doi: 10.1161/01.res.80.1.45. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circulation Research. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase. Role in cardiovascular biology and disease. Circulation Research. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, Al-Benna S, Berg G, Dominiczak AF. NAD(P)H oxidase inhibition improves endothelial function in rat and human blood vessels. Hypertension. 2002;40:755–762. doi: 10.1161/01.hyp.0000037063.90643.0b. [DOI] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging. A common cause of endothelial dysfunction. Hypertension. 2002;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Brown RC, Davis TP. Smoking and ischemic stroke: A role for nicotine. Trends in Pharmacological Sciences. 2002;23:78–82. doi: 10.1016/s0165-6147(02)01893-x. [DOI] [PubMed] [Google Scholar]
- Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, et al. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- Higa M, Davanipour Z. Smoking and stroke. Neuroepidemiology. 1991;10:211–222. doi: 10.1159/000110272. [DOI] [PubMed] [Google Scholar]
- Hladovec J. Endothelial injury by nicotine and its prevention. Experientia. 1978;34:1585–1586. doi: 10.1007/BF02034689. [DOI] [PubMed] [Google Scholar]
- Ijzerman RG, Serne EH, van Weissenbruch MM, de Jongh RT, Stehouwer CD. Cigarette smoking is associated with an acute impairment of microvascular function in humans. Clinical Science (London) 2003;104:247–252. doi: 10.1042/CS20020318. [DOI] [PubMed] [Google Scholar]
- Kanamaru K, Waga S, Fujimoto K, Itoh H, Kubo Y. Endothelium-dependent relaxation of human basilar arteries. Stroke. 1989;20:1208–1211. doi: 10.1161/01.str.20.9.1208. [DOI] [PubMed] [Google Scholar]
- Lai FM, Cobuzzi A, Shepherd C, Tanikella T, Hoffman A, Cervoni P. Endothelium-dependent basilar and aortic vascular responses in normotensive and coarctation hypertensive rats. Life Sciences. 1989;45:607–614. doi: 10.1016/0024-3205(89)90046-5. [DOI] [PubMed] [Google Scholar]
- Lakier JB. Smoking and cardiovascular disease. American Journal of Medicine. 1992;93(Suppl. 1A):8S–12S. doi: 10.1016/0002-9343(92)90620-q. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, et al. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, et al. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circulation Research. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- Mayhan WG. Impairment of endothelium-dependent dilatation of basilar artery during chronic hypertension. American Journal of Physiology. 1990;259:H1455–H1462. doi: 10.1152/ajpheart.1990.259.5.H1455. [DOI] [PubMed] [Google Scholar]
- Mayhan WG. Responses of the basilar artery to products released by platelets during chronic hypertension. Brain Research. 1991;545:97–102. doi: 10.1016/0006-8993(91)91274-5. [DOI] [PubMed] [Google Scholar]
- Mayhan WG. Impairment of endothelium-dependent dilatation of the basilar artery during diabetes mellitus. Brain Research. 1992;580:297–302. doi: 10.1016/0006-8993(92)90957-b. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Arrick DM, Sharpe GM, Patel KP, Sun H. Inhibition of NAD(P)H oxidase alleviates impaired NOS-dependent responses of pial arterioles in type 1 diabetes mellitus. Microcirculation. 2006;13:567–575. doi: 10.1080/10739680600885194. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Faraci FM, Heistad DD. Responses of cerebral arterioles to adenosine 5′-diphosphate, serotonin, and the thromboxane analogue U-46619 during chronic hypertension. Hypertension. 1988;12:556–561. doi: 10.1161/01.hyp.12.6.556. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Patel KP. Effect of nicotine on endothelium-dependent arteriolar dilatation in vivo. American Journal of Physiology. 1997;272:H2337–H2342. doi: 10.1152/ajpheart.1997.272.5.H2337. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Sharpe GM. Nicotine impairs histamine induced increases in macromolecular efflux: Role of oxygen radicals. Journal of Applied Physiology. 1998a;84:1589–1595. doi: 10.1152/jappl.1998.84.5.1589. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Sharpe GM. Superoxide dismutase restores endothelium-dependent arteriolar dilatation during acute infusion of nicotine. Journal of Applied Physiology. 1998b;85:1292–1298. doi: 10.1152/jappl.1998.85.4.1292. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Sharpe GM. Chronic exposure to nicotine alters endothelium-dependent arteriolar dilatation: Effect of superoxide dismutase. Journal of Applied Physiology. 1999;86:1126–1134. doi: 10.1152/jappl.1999.86.4.1126. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Sun H, Mayhan JF, Patel KP. Influence of exercise on dilatation of the basilar artery during diabetes mellitus. Journal of Applied Physiology. 2004;96:1730–1737. doi: 10.1152/japplphysiol.01185.2003. [DOI] [PubMed] [Google Scholar]
- Mays BW, Freischlag JA, Eginton MT, Cambria RA, Seabrook GR, Towne JB. Ascorbic acid prevents cigarette smoke injury to endothelium-dependent arterial relaxation. Journal of Surgical Research. 1999;84:35–39. doi: 10.1006/jsre.1999.5601. [DOI] [PubMed] [Google Scholar]
- Miller VM, Clouse WD, Tonnessen BH, Boston US, Severson SR, Bonde S, et al. Time and dose effect of transdermal nicotine on endothelial function. American Journal of Physiology. 2000;279:H1913–H1921. doi: 10.1152/ajpheart.2000.279.4.H1913. [DOI] [PubMed] [Google Scholar]
- Mohazzab KM, Kaminski PM, Wolin MS. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. American Journal of Physiology. 1994;266:H2568–H2572. doi: 10.1152/ajpheart.1994.266.6.H2568. [DOI] [PubMed] [Google Scholar]
- Murohara T, Kugiyama K, Ohgushi M, Sugiyama S, Yasue H. Cigarette smoke extract contracts isolated porcine coronary arteries by superoxide anion-mediated degradation of EDRF. American Journal of Physiology. 1994;266:H874–H880. doi: 10.1152/ajpheart.1994.266.3.H874. [DOI] [PubMed] [Google Scholar]
- Nakagomi T, Hongo K, Kassell NF, Sasaki T, Lehman RM, Ogawa H, et al. Pharmacological comparison of endothelium-dependent relaxation in isolated cerebral and extracerebral arteries. Journal of Neurosurgery. 1988;69:580–587. doi: 10.3171/jns.1988.69.4.0580. [DOI] [PubMed] [Google Scholar]
- Ota Y, Kugiyama K, Sugiyama S, Ohgushi M, Matsumura T, Doi H, et al. Impairment of endothelium-dependent relaxation of rabbit aortas by cigarette smoke extract-role of free radicals and attenuation by captopril. Atherosclerosis. 1997;131:195–202. doi: 10.1016/s0021-9150(97)06106-6. [DOI] [PubMed] [Google Scholar]
- Pellaton C, Kubli S, Feihl F, Waeber B. Blunted vasodilatory responses in the cutaneous microcirculation of cigarette smokers. American Heart Journal. 2002;144:269–274. doi: 10.1067/mhj.2002.123842. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF. Nicotine and the central nervous system: Biobehavioral effects of cigarette smoking. American Journal of Medicine. 1992;93:2S–7S. doi: 10.1016/0002-9343(92)90619-m. [DOI] [PubMed] [Google Scholar]
- Puranik R, Celermajer DS. Smoking and endothelial function. Progress in Cardiovascular Disease. 2003;45:443–458. doi: 10.1053/pcad.2003.YPCAD13. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation: Contribution to alterations in vascular tone. Journal of Clinical Investigation. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey FE, Li XC, Carretero OA, Garvin JL, Pagano PJ. Perivascular superoxide anion contributes to impairment of endothelium-dependent relaxation: Role of gp91(phox) Circulation. 2002;106:2497–2502. doi: 10.1161/01.cir.0000038108.71560.70. [DOI] [PubMed] [Google Scholar]
- Rubinstein I, Yong T, Rennard SI, Mayhan WG. Cigarette smoke extract attenuates endothelium-dependent arteriolar dilatation in vivo. American Journal of Physiology. 1991;261:H1913–H1918. doi: 10.1152/ajpheart.1991.261.6.H1913. [DOI] [PubMed] [Google Scholar]
- Russell MA, Jarvis M, Iyer R, Feyerabend C. Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers. British Medical Journal. 1980;3:972–976. doi: 10.1136/bmj.280.6219.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabha M, Tanus-Santos JE, Toledo JCY, Cittadino M, Rocha JC, Moreno H. Transdermal nicotine mimics the smoking-induced endothelial dysfunction. Clinical Pharmacology and Therapeutics. 2000;68:167–174. doi: 10.1067/mcp.2000.108851. [DOI] [PubMed] [Google Scholar]
- Sarabi M, Lind L. Short-term effects of smoking and nicotine chewing gum on endothelium-dependent vasodilation in young healthy habitual smokers. Journal of Cardiovascular Pharmacology. 2000;35:451–456. doi: 10.1097/00005344-200003000-00016. [DOI] [PubMed] [Google Scholar]
- Soltis EE, Bohr DF. Cerebral vascular responsiveness in DOCA hypertensive rats. American Journal of Physiology. 1987;252:H198–H203. doi: 10.1152/ajpheart.1987.252.1.H198. [DOI] [PubMed] [Google Scholar]
- Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- Sumida H, Watanabe H, Kugiyama K, Ohgushi M, Matsumura T, Yasue H. Does passive smoking impair endothelium-dependent coronary artery dilation in women? Journal of the American College of Cardiology. 1998;31:811–815. doi: 10.1016/s0735-1097(98)00010-2. [DOI] [PubMed] [Google Scholar]
- Sun H, Molacek E, Zheng H, Fang Q, Patel KP, Mayhan WG. Alcohol-induced impairment of neuronal nitric oxide synthase (nNOS)-dependent dilation of cerebral arterioles: Role of NAD(P)H oxidase. Journal of Molecular and Cellular Cardiology. 2006;40:321–328. doi: 10.1016/j.yjmcc.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Apocynin, NADPH oxidase, and vascular cells: A complex matter. Hypertension. 2008;51:172–174. doi: 10.1161/HYPERTENSIONAHA.107.103200. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation. 2003;108:1253–1258. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- Wellman GC, Bevan JA. Barium inhibits the endothelium-dependent component of flow but not acetylcholine-induced relaxation in isolated rabbit cerebral arteries. Journal of Pharmacology and Experimental Therapuetics. 1995;274:47–53. [PubMed] [Google Scholar]
- Whalley ET, Amure YO, Lye RH. Analysis of the mechanism of action of bradykinin on human basilar artery in vitro. Archives of Pharmacology. 1987;335:433–437. doi: 10.1007/BF00165559. [DOI] [PubMed] [Google Scholar]
- Yu XL, Jin XR, Wang DX. Effects of cigarette smoking on the function of metabolizing arachidonic acid and angiotensin I in the isolated perfused rat lungs. Journal of Tongji Medical University. 1992;12:201–204. doi: 10.1007/BF02887849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.