Abstract
Introduction:
The behavioral mechanisms by which bupropion reduces smoking have been explored in laboratory behavioral studies, with some inconsistent results. Intention to quit smoking has been found to moderate some effects of nicotine replacement, and the degree to which that characteristic may affect responses to other smoking pharmacotherapies is unknown.
Methods:
This laboratory study examined the effects of 300 mg/day bupropion, compared with placebo, on baseline and smoking cue–elicited urge to smoke and other measures in smokers who stated that they did (n = 8) or did not (n = 17) intend to quit smoking within 6 months.
Results:
Significant interactions indicated that bupropion reduced the effects of acute abstinence on smoking urges in the presence of neutral cues, only in those who intended to quit. Bupropion and intention to quit did not reduce the effects of acute abstinence on urges in the presence of smoking cues and did not reduce nicotine withdrawal symptoms or smoking behavior between sessions.
Discussion:
This study is one of the first placebo-controlled examinations of the effects of bupropion on cue reactivity and provides support for the idea that laboratory smoking studies may be more likely to detect effects of pharmacological treatments for smoking when they enroll smokers who intend to quit.
Introduction
Human behavioral laboratory studies offer an efficient method of screening potential pharmacological treatments for smoking and of identifying behavioral mechanisms by which such treatments work, but they have been criticized on the grounds that they often fail to detect effects of medications that are known to be clinically effective (Perkins, Stitzer, & Lerman, 2006). This insensitivity may be due, at least in part, to the fact that participants in these studies typically are not trying to quit smoking permanently (Perkins et al., 2006). Perkins et al. (2008) found that transdermal nicotine replacement increased abstinence and reduced cigarette craving in smokers who intended to quit smoking within 30 days, but not in those who did not intend to quit within 6 months. The degree to which intention to quit may moderate the effects of other pharmacological treatments for smoking is unknown.
Bupropion approximately doubles smoking cessation rates in randomized clinical trials (Hughes, Stead, & Lancaster, 2007). Although some studies have found that bupropion reduces abstinence-induced craving and negative affect (Shiffman et al., 2000; Teneggi et al., 2005), these effects have not been detected consistently (reviewed in Mooney & Sofuoglu, 2006; Perkins et al., 2006). To our knowledge, only one study has been published on the effects of bupropion on smoking cue reactivity (Brody et al., 2004). Results of that study indicated that participants taking bupropion for 4 weeks had lower baseline and cue-elicited craving levels than did untreated smokers (Brody et al., 2004). However, because that study used an untreated control group rather than a placebo control group, the extent to which its results were due to a pharmacological effect versus an expectancy effect are unclear.
The present study was designed initially to examine the effects of bupropion compared with placebo on baseline and cue-elicited smoking urges in adult smokers. In view of the suggestion that treatment-seeking status may moderate the effects of medications for smoking cessation (Perkins et al., 2008), we also examined whether intention to quit moderated the effects of bupropion. Given the clinical efficacy of bupropion in treatment-seeking smokers and its ability to reduce craving and withdrawal symptoms in some human laboratory studies (Mooney & Sofuoglu, 2006), we hypothesized that bupropion would reduce the effects of abstinence on these measures, particularly in participants who intended to quit smoking.
Methods
Participants
Participants were required to be 18 years or older, to have smoked at least 20 cigarettes/day for at least the past year, and to have Fagerström Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker & Fagerström, 1991) scores of 6 or higher. The Structured Clinical Interview for Diagnostic and statistical manual of mental disorders, fourth edition (First, Spitzer, Gibbon, & Williams, 1994) was used to rule out those with current Axis I disorders. Other exclusionary criteria included positive urine drug or pregnancy tests, positive breath alcohol levels at any visit, use of any psychoactive medication, and the presence of any medical condition that might interact with bupropion. Procedures were approved by the institutional review board involved. Of the 27 participants enrolled, 25 completed the study.
Study design and medication
Participants first completed questionnaires on demographics and other individual difference measures. Intention to quit smoking was measured using the stages-of-change algorithm (DiClemente et al., 1991). Participants then underwent three laboratory sessions, spaced 7 days apart. In Session 1, participants were not abstinent and not taking any medication. In Sessions 2–3, participants were tested under 5-hr abstinent conditions after 1 week of either 300 mg/day sustained-release bupropion (GlaxoSmithKline, Research Triangle Park, NC) or matching placebo (ProClinical Inc., Phoenixville, PA). The bupropion dose was 150 mg/day for the first 3 days and was increased on day 4 to 300 mg/day (150 mg twice daily), according to the manufacturer's instructions. Medications were administered under double-blind conditions, in divided organizers, with dose order counterbalanced across participants. Medication compliance was checked weekly by pill count.
Laboratory procedures
Upon arrival, participants provided breath samples for the assessment of carbon monoxide (CO) levels (Smokerlyzer; Bedfont Scientific Ltd., Kent, United Kingdom). Daily smoking rate over the past week was gathered using the Timeline Followback interview for smoking (Brown et al., 1998). Participants then either smoked freely or were not permitted to smoke for the next 5 hr. Participants were under continuous observation, with CO monitoring before and after lunch or bathroom breaks to assure compliance with abstinence. At the end of these 5-hr periods, CO levels were measured and participants completed the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1986, 1998), with the insomnia item omitted because participants did not undergo overnight abstinence. Responses were reported on a 100-mm Visual Analog Scale (VAS) with the anchors 0 = “not present” and 100 = “severe.” The total score provided is the mean of ratings on the included items.
Participants then underwent a smoking cue reactivity assessment as described previously (Tidey, Rohsenow, Kaplan, & Swift, 2005). After a 10-min relaxation period, participants viewed and handled neutral cues (a pencil, 25 × 65 mm eraser, and small pad of paper) for 4 min and then rated their urge to smoke using the Questionnaire on Smoking Urges—Brief (QSU-Brief; Cox, Tiffany, & Christen, 2001) and the item “How much is your urge to smoke right now?” recorded on a 100-mm VAS, with the anchors 0 = “no urge at all” and 100 = “strongest urge you’ve ever had.” Next, participants viewed and handled the smoking cues (a cigarette, lighter, and ashtray) for 4 min and then completed the same measures. Neutral cues were always presented first due to known effects of order on cue presentation (Rickard-Figueroa & Zeichner, 1985).
Data analyses
Dependent variables were examined for distributional assumptions and collinearity. The Huynh–Feldt correction was used for violations of sphericity. The QSU-Brief and urge VAS scores were highly collinear during neutral and smoking cue exposure (Cronbach's α’s ≥ .90), so only the single-item urge score was retained in analyses. Group comparisons on demographic and smoking history measures were conducted using independent-samples t tests for continuous variables and chi-square tests for categorical variables. Mixed-factor 3 × 2 analyses of variance (ANOVAs) were used to examine the effects of condition (nonabstinent, abstinent + placebo, abstinent + bupropion) and intention to quit (intention negative, intention positive) on arrival CO levels, daily smoking rate in the week before the session, and precue CO levels and MNWS scores. Mixed-factor 3 × 2 × 2 ANOVAs were used to examine the effects of condition, intention, and cue (neutral, smoking) on smoking urge. Significant interactions were followed by simple effects tests. Differences were considered significant for p values of .05 or smaller. Effect sizes (η2) are provided for marginal effects, with η2 ≤ .05 for small, η2 = .06 – .13 for medium, and η2 ≥ .14 for large effect sizes (Cohen, 1988). Analyses were conducted using SPSS version 14.0 for Windows.
Results
Individual difference measures
The 17 participants who indicated that they were not interested in quitting smoking within the next 6 months were classified as intention negative. The eight participants who indicated that they wanted to quit within 6 months, including four who intended to quit within 30 days, were classified as intention positive. We found no significant differences between the groups on any demographic or smoking history measure (Table 1). No participant missed more than one pill in the week prior to each session.
Table 1.
Demographic and smoking characteristics of study participants
| Intention negative (n = 17) | Intention positive (n = 8) | |
| Age (years) | 43.9 (10.8) | 48.5 (7.5) |
| Gender (male) | 71% | 63% |
| Race | 59% W, 35% B, 6% M | 75% W, 25% B |
| Employed full- or part-time | 29% | 25% |
| Annual income ≥US$10,000 | 65% | 63% |
| ≥12 years of education | 82% | 100% |
| Cigarettes/day | 26.5 (10.6) | 26.9 (9.2) |
| Nicotine dependence (FTND score) | 7.0 (1.8) | 8.0 (1.5) |
| Years of daily smoking | 25.8 (12.6) | 30.6 (10.4) |
| CO level at enrollment (ppm) | 23.4 (11.1) | 19.3 (5.9) |
| CO level at session arrival (ppm) | 20.4 (13.2) | 18.0 (8.6) |
| At least one quit attempt in past year | 35% | 50% |
Note. W = White; B = Black; M = mixed race; FTND = Fagerström Test for Nicotine Dependence; CO = carbon monoxide; ppm = parts per million. Values are means with SDs or percentages.
Smoking behavior between sessions
There were no main effects of intention or condition, and no intention × condition interaction, on CO level measured upon arrival for sessions (see Table 1). We found a significant effect of condition on average daily smoking rate in the week prior to each session, F(2, 46) = 12.47, p < .001, with post-hoc tests indicating that smoking rates were higher in the week prior to Session 1 than in subsequent weeks (p < .01). We found no indication that bupropion reduced daily smoking rate and no main effect of intention or intention × condition interaction on daily smoking rate. The intention-negative group smoked 22.5 cigarettes/day (SD = 10.9), 19.0 cigarettes/day (SD = 11.5), and 18.7 cigarettes/day (SD = 11.3) in the weeks before the nonabstinent, abstinent plus placebo, and abstinent plus bupropion sessions, respectively. The corresponding rates for the intention-positive group were 24.3 (SD = 11.5), 19.4 (SD = 14.4), and 19.6 (SD = 12.0).
Nicotine withdrawal symptoms and smoking urge levels
We found a main effect of condition on precue breath CO level, F(2, 46) = 27.87, p < .001, with post-hoc tests indicating that abstinence significantly reduced CO levels (p < .001). There was no main effect of intention or intention × condition interaction on precue CO level. For the intention-negative group, precue breath CO levels in the nonabstinent, abstinent plus placebo, and abstinent plus bupropion conditions were 20.8 ppm (SD = 7.2), 10.5 ppm (SD = 6.1), and 10.9 ppm (SD = 5.7), respectively. The corresponding values for the intention-positive group were 19.5 ppm (SD = 10.1), 10.3 ppm (SD = 3.8), and 10.5 ppm (SD = 5.6). We found a marginal effect of condition on precue MNWS score, F(2, 46) = 2.95, p = .06; η2 = .11. Means indicated that abstinence tended to increase MNWS score, but bupropion did not reduce this effect. We found no main effect of intention or intention × condition interaction on MNWS score (η2 ≤ .05). For the intention-negative group, MNWS scores in the nonabstinent, abstinent plus placebo, and abstinent plus bupropion conditions were 12.5 (SD = 18.8), 19.9 (SD = 22.9), and 21.4 (SD = 24.8), respectively. The corresponding MNWS scores for the intention-positive group were 7.8 (SD = 8.6), 10.6 (SD = 9.3), and 11.6 (SD = 10.9).
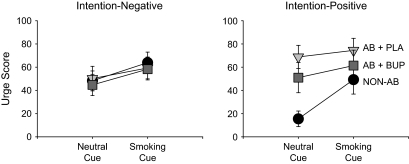
We found significant main effects of cue and condition on urge score, F(1, 23) = 25.98, p < .001, and F(2, 46) = 4.67, p < .05, respectively, and a significant cue × condition interaction on urge score, F(2, 46) = 10.60, p < .001. Moreover, we found significant intention × condition and intention × condition × cue interactions on urge score, F(2, 46) = 5.65, p < .05, and F(2, 46) = 5.11, p < .05, respectively. Separate condition × cue ANOVAs were performed within each group to clarify the interactions. In the intention-negative group, smoking cues significantly increased urge scores, F(1, 16) = 15.53, p < .01, but the main effect of condition and the condition × cue interaction were not significant (Figure 1, left). In the intention-positive group, we found significant main effects of condition and cue and a significant condition × cue interaction on urge levels, F(2, 14) = 6.75, p = .01, F(1, 7) = 12.06, p < .05, F(2, 14) = 6.93, p < .05, respectively. Simple effects tests indicated that abstinence increased (p < .01) and bupropion decreased (p < .05) urge levels during neutral cue exposure (Figure 1, right). During smoking cue exposure, the effect of condition was not significant, although the effect size was large, F(2, 14) = 1.92, p = .20; η2 = .22.
Figure 1.
Effects of neutral and smoking cues under nonabstinent (circles), abstinent plus placebo (inverted triangle), and abstinent plus 300-mg sustained-release bupropion (squares) on smoking urge levels in smokers who reported intention (intention positive) or no intention (intention negative) to quit smoking within the next 6 months. Data points represent means with SEM.
Discussion
The results of this study, while preliminary, support the idea that intention to quit may moderate the effects of medications for smoking cessation. We would like to comment here on several points. First, while viewing neutral cues immediately after a 5-hr ad libitum smoking period, the intention-positive group reported very low urge levels, as expected, but urge levels in the intention-negative group were considerably higher. Because of the cross-sectional nature of this study, the direction of causality, if any, in the relationship between these variables cannot be determined, and the small sample sizes may make these effects unreliable. Longitudinal studies could clarify whether progression toward smoking cessation leads to lower urge levels under nonabstinent, neutral conditions, or whether having high urge levels under such conditions impedes progression toward cessation. The two groups had very similar baseline and cue-elicited urge levels after 5-hr abstinence, which is consistent with previous findings (McDermut & Haaga, 1998).
Second, smokers who intended to quit smoking within 6 months were more sensitive to the effects of abstinence and bupropion on smoking urge levels during neutral cue exposure than were those who did not intend to quit. This is consistent with the finding that treatment-seeking participants tended to be more sensitive than non–treatment seekers to the effects of nicotine replacement on smoking urge levels (Perkins et al., 2008). Bupropion did not significantly reduce urges in the presence of smoking cues, but this may be due to the small sample size given that the effect size for condition was large in the intention-positive group. Why a medication for smoking would be more effective in those who intend to quit is unknown. Intention to quit might heighten the salience of the medication's effects (Perkins et al., 2008). Alternatively, an unknown variable, such as a genetic polymorphism, might underlie the relationship between intention to quit and response to bupropion. Although Shiffman et al. (2000) found that bupropion reduced the effects of 72-hr abstinence on depression, irritability, and difficulty concentrating, bupropion did not reduce the effects of abstinence on withdrawal symptoms in the present study. The shorter abstinence period in the present study may have reduced our ability to detect effects of bupropion on withdrawal symptoms. Because participants were not encouraged to quit smoking during the study, no effect of bupropion on intersession smoking rate was expected, and none was observed.
Strengths of the study include its within-subject design and the 1-week treatment with bupropion or matched placebo prior to the sessions. The study also has at least two design limitations. Because it was not designed initially to test the moderating effects of intention to quit, the intention-positive sample was small and it was a broader category than that studied by Perkins et al. (2008). Another limitation is that the nonabstinent session was conducted first in all participants. Hence, the finding of lower urge levels during the nonabstinent condition may be confounded if smokers generally report lower urge levels during their first study session compared with subsequent sessions. Other smoking cue reactivity studies have found this not to be the case (LaRowe, Saladin, Carpenter, & Upadhyaya, 2007; Miranda, Rohsenow, Monti, Tidey, & Ray, 2008). Furthermore, this limitation does not extend to the abstinence plus placebo and abstinence plus bupropion conditions, the order of which was counterbalanced across participants. However, in view of these limitations, particularly the small sample sizes, the results of the present study should be considered preliminary and the study should be repeated using a larger sample and a fully counterbalanced design.
A final point is that the results of the study suggest that bupropion may not help to reduce the effects of acute abstinence on urge levels in smokers who are ambivalent about quitting. This possibility is disconcerting given that the majority of current smokers fall into this category (Etter, Perneger, & Ronchi, 1997; Wewers, Stillman, Hartman, & Shopland, 2003). However, this concern is mitigated by the finding that intention to quit can be increased through brief motivational interventions (Steinberg, Ziedonis, Krejci, & Brandon, 2004).
Funding
This research was supported by National Institute on Drug Abuse grant DA14002 to JWT and a Research Career Scientist Award from the Department of Veterans Affairs to DJR.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
The authors thank Amy Adolfo, Elizabeth Cathers, and Laura Dionne for their technical assistance and the individuals who participated in this study for their contributions.
References
- Brody AL, Mandelkern MA, Lee G, Smith E, Sadeghi M, Saxena S, et al. Attenuation of cue-induced cigarette craving and anterior cingulated cortex activation in bupropion-treated smokers: A preliminary study. Psychiatry Research: Neuroimaging. 2004;130:269–281. doi: 10.1016/j.pscychresns.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors. 1998;12:101–111. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the Brief Questionnaire of Smoking Urges (QSU-Brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- DiClemente CC, Prochaska JO, Fairhurst SK, Velicer WF, Velasquez MM, Rossi JS. The process of smoking cessation: An analysis of precontemplation, contemplation and preparation stages of change. Journal of Consulting and Clinical Psychology. 1991;59:295–304. doi: 10.1037//0022-006x.59.2.295. [DOI] [PubMed] [Google Scholar]
- Etter JF, Perneger TV, Ronchi A. Distributions of smokers by stage: International comparison and association with smoking prevalence. Preventive Medicine. 1997;26:580–585. doi: 10.1006/pmed.1997.0179. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV axis-I disorders—patient edition (SCID-I/P, version 2.0) New York: Biometric Research Department; 1994. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Errors in using tobacco withdrawal scale. Tobacco Control. 1998;7:92–93. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews. 2007 doi: 10.1002/14651858.CD000031.pub3. CD000031. [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Saladin ME, Carpenter MJ, Upadhyaya HP. Reactivity to nicotine cues over repeated cue reactivity sessions. Addictive Behaviors. 2007;32:2888–2899. doi: 10.1016/j.addbeh.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermut W, Haaga DAF. Effect of stage of change on cue reactivity in continuing smokers. Experimental and Clinical Psychopharmacology. 1998;6:316–324. doi: 10.1037//1064-1297.6.3.316. [DOI] [PubMed] [Google Scholar]
- Miranda R, Rohsenow DJ, Monti PM, Tidey J, Ray L. Effects of repeated days of smoking cue exposure on urge to smoke and physiological reactivity. Addictive Behaviors. 2008;33:347–353. doi: 10.1016/j.addbeh.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Sofuoglu M. Bupropion for the treatment of nicotine withdrawal and craving. Expert Review of Neurotherapeutics. 2006;6:965–981. doi: 10.1586/14737175.6.7.965. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Stitzer ML, Fonte CA, Briski JL, Scott JA, et al. Development of procedures for early screening of smoking cessation medications in humans. Clinical Pharmacology & Therapeutics. 2008;84:216–221. doi: 10.1038/clpt.2008.30. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: A proposal for new methodologies. Psychopharmacology. 2006;184:628–636. doi: 10.1007/s00213-005-0105-5. [DOI] [PubMed] [Google Scholar]
- Rickard-Figueroa K, Zeichner A. Assessment of smoking urge and its concomitants under an environmental smoking cue manipulation. Addictive Behaviors. 1985;10:249–256. doi: 10.1016/0306-4603(85)90005-x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty J, et al. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology. 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Steinberg ML, Ziedonis DM, Krejci JA, Brandon TH. Motivational interviewing with personalized feedback: A brief intervention for motivating smokers with schizophrenia to seek treatment for tobacco dependence. Journal of Consulting and Clinical Psychology. 2004;72:723–728. doi: 10.1037/0022-006X.72.4.723. [DOI] [PubMed] [Google Scholar]
- Teneggi V, Tiffany ST, Squassante L, Milleri S, Ziviani L, Bye A. Effect of sustained-release (SR) bupropion on craving and withdrawal in smokers deprived of cigarettes for 72 h. Psychopharmacology. 2005;183:1–12. doi: 10.1007/s00213-005-0145-x. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Subjective and physiological responses to smoking cues in smokers with schizophrenia. Nicotine & Tobacco Research. 2005;7:421–429. doi: 10.1080/14622200500125724. [DOI] [PubMed] [Google Scholar]
- Wewers ME, Stillman FA, Hartman AM, Shopland DR. Distribution of daily smokers by stage of change: Current population survey results. Preventive Medicine. 2003;36:710–20. doi: 10.1016/s0091-7435(03)00044-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.