Abstract
Purpose
We hypothesized that corneal epithelium plays a role in the innate immune response by sensing the presence of pathogens and providing signals that activate the corneal defense system. We sought to determine the mechanisms involved in the activation of the signaling pathways and subsequent production of proinflammatory cytokines in human corneal epithelial cells (HCECs) in response to Pseudomonas aeruginosa infection.
Methods
Epithelial monolayers of a telomerase-immortalized HCEC line, HUCL, and primary cultures of HCECs were exposed to P. aeruginosa (PA01 strain) with or without the presence of the NF-κB inhibitor kamebakaurin, the p38 inhibitor SB203580, or the JNK inhibitor SP600125. IκB-α phosphorylation and degradation and p38 and JNK phosphorylation were assessed at different time points by Western blot analysis. Interleukin (IL)-6, IL-8, and TNF-αlevels were determined by reverse transcription-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA).
Results
Exposure of HUCL cells and primary HCECs to P. aeruginosa resulted in rapid activation of NF-κB as indicated by an increase in IκB-α phosphorylation observed within 15 min and by IκB-αdegradation, which peaked in 1 hr. Two stress-activated mitogen-activated protein kinases, p38 and JNK, were also activated as their phosphorylation was induced by P. aeruginosa infection. Concomitant with the activation of these Toll-like receptor–mediated signaling pathways, transcriptional expression and subsequent secretion of IL-6 and IL-8 in HUCL cells were also induced by P. aeruginosa. Presence of the NF-κB inhibitor kamebakaurin in culture medium blocked P. aeruginosa–induced NF-κB activation and inhibited IL-6, IL-8, and TNF-αexpression and secretion. Inhibition of p38 or JNK also resulted in a decrease in bacteria-induced expression and secretion of these cytokines.
Conclusions: P. aeruginosa
triggers an innate immune response in HCECs, and NF-κB and, to a lesser extent, the p38/JNK signal pathways are responsible for P. aeruginosa–induced proinflammatory cytokine production in these cells.
Keywords: bacterial keratitis, corneal epithelium, proinflammatory cytokines, Pseudomonas aeruginosa, Toll-like receptors
INTRODUCTION
Pseudomonas aeruginosa is an opportunistic bacterial pathogen that can cause bacterial keratitis in patients who use extended-wear contact lenses.1 Human corneal epithelial cells (HCECs), like other mucosal epithelial linings in the body,2,3 constitute the first line of defense against microbial pathogens and are believed to possess the ability to sense the presence of pathogenic bacteria such as P. aeruginosa and Staphylococcus aureus.4–7 Recent studies have shown that the ability of epithelial cells to recognize pathogens is largely due to the expression of Toll-like receptors (TLRs), an evolutionarily conserved family of receptors that function in innate immunity via recognition of pathogen-associated molecular patterns.8–10 Pattern recognition by TLRs then leads to activation of NF-κB and the mitogen-activated protein kinases (MAPKs)—e.g., p38, c-Jun N-terminal kinase (JNK)—through MyD88-dependent signaling pathways and production of proinflammatory cytokines.2,11–14 Early studies have shown that exposure of cultured HCECs to P. aeruginosa,15,16 its derived lipopolysaccharide,6 and its flagellin5 resulted in the induction of the proinflammatory cytokine IL-6 and the chemokine IL-8. The release of these cytokines in resident corneal cells can augment or prolong the inflammatory response, a consequence required to contain the infection.4,17 The host inflammatory response, however, also contributes to corneal destruction.4,18,19
In this study, we investigated the activation of signaling pathways that are known to be effectors of TLR downstream signaling, including NF-κB, p38, and JNK, and their role in induction of proinflammatory cytokine production in response to P. aeruginosa infection. Our results showed that P. aeruginosa–induced inflammatory responses in human cornea epithelium are through NF-κB and the p38 and JNK signaling pathways. Based on this and our previous studies,5 we suggest that one or more TLRs, such as TLR5, might be involved in recognition of P. aeruginosa in HCECs.
MATERIALS AND METHODS
Human Corneal Epithelial Cell Culture and P. aeruginosa Challenge
The human telomerase-immortalized HCEC line, HUCL, kindly provided by Dr. Rheinwald and Dr. Gipson,20,21 was maintained in defined keratinocyte-SFM (serum-free medium; Invitrogen Life Technologies, Carlsbad, CA, USA) in a humidified 5% CO2 incubator at 37°C. Before treatment, cells were split into culture dishes precoated with FNC (Athena Environmental Service, Inc., Baltimore, MD, USA) and cultured in antibiotic-free defined keratinocyte-SFM. After cells were attached, the medium was replaced with Keratinocyte Basic Medium (KBM, BioWhittaker, Walkersville, MD, USA), and the cells were further cultured overnight (growth factor starvation).
Primary HCECs isolated from human donor corneas obtained from the Georgia Eye Bank were also used for verifying the results obtained from HUCL cells. The epithelial sheet was separated from the underlying stroma after overnight dispase (2.5 U/ml, Sigma-Aldrich, St. Louis, MO, USA) treatment at 4°C. The dissected epithelial sheet was trypsinized and cells collected by centrifugation (500 × g, 5 min). HCECs were cultured in T25 flasks coated with fibronectin-collagen (FNC, Athena Environmental Service, Inc.) and used at passage 3.
P. aeruginosa (PAO1 strain from Pseudomonas Genetic Stock Center at East Carolina University) were shaken in tryptic soy broth (Sigma-Aldrich) at 37°C until absorbance at 600 nm reached O.D. 0.3–0.4. The bacterial culture was centrifuged at 6000 × g for 10 min. Bacteria were resuspended in KBM and then used to challenge the growth factor–starved HCECs at a ratio of 50:1 (bacteria to cell). Heat-killed bacteria were prepared in the same manner, but after suspension in KBM, they were heated to 80°C for 10 min and were assessed for non-viability by plating on TSB agar plates. Three different ratios (50:1, 100:1, and 200:1) of heat-killed bacteria to cells were used to test the effects of heat-killed bacteria on HCEC cells. Resuspended bacteria were added to HUCL culture dishes, and the dishes were then centrifuged at 150 × g for 5 min to allow the bacteria to readily contact the cells. The cells were further cultured in bacteria-containing medium for 2 hr at 37°C in a humidified 5% CO2 chamber, the medium with bacteria was then removed, and the cells were washed three times with PBS. Fresh medium containing 100 μg/ml gentamicin was added to the culture to kill the bacteria outside cells, and the cells were further cultured in gentamicin-containing media for the indicated times. Cells were then processed for RNA preparation or immunoblotting and conditioned media collected for cytokine determination.
To determine the role of signaling pathways in cytokine production, HCECs were pretreated for 30 min at 37°C with the NF-κB inhibitor kamebakaurin (Isodon japonicus, 25 μM), the p38 inhibitor SB203580 (10 μM), or the JNK inhibitor SP600125 (0.5 μM) (Calbiochem, La Jolla, CA, USA), prior to P. aeruginosa exposure. Inhibitors were then continually present in culture media of infected HCECs.
Western Blot Analysis
P. aeruginosa–treated HCECs were lysed with RIPA buffer (150 mM NaCl, 100 mM Tris-HCl, pH 7.5, 1% deoxycholate, 0.1% sodium dodecyl sulfate, 1% Triton X-100, 50 mM NaF, 100 mM sodium pyrophosphate, 3.5 mM sodium orthovanadate, proteinase inhibitor cocktails, and 0.1 mM PMSF) and centrifuged at 12,000 rpm at 4°C for 30 min. Protein concentration was determined with Micro BCA kit (Pierce Biotechnology, Rockford, IL, USA). Cell lysate was immunoblotted and developed with Supersignal reagents from Pierce Biotechnology. IκB-α phosphorylation and degradation were detected with rabbit anti-IκB-αand anti-phospho-IκB-α; p38 and JNK activation were detected with rabbit anti-phospho-p38 and mouse anti-phospho-JNK antibody; and p38 and JNK were detected with rabbit anti-p38 and anti-JNK as controls. The antibodies were purchased from Cell Signaling Technologies (Beverly, MA, USA).
RNA Extraction and Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
RNA was isolated using Trizol (Invitrogen), and 2 μg of total RNA was reverse-transcribed with SuperScript first-strand synthesis system for RT-PCR (Invitrogen). The primers for amplifying IL-6 and IL-8 transcripts by PCR are listed in Table 1 with GAPDH as internal control. IL-6 was amplified 22 cycles with annealing temperature 60°C; IL-8 was amplified 30 cycles with annealing temperature 58°C; and TNF-αwas amplified 25 cycles with annealing temperature 60°C. The PCR products (5 μl) were subjected to electrophoresis on 2% agarose gels containing ethidium bromide. Staining was captured using the Kodak EDAS 290 system.
TABLE 1.
Sequences and Product Size of PCR Primers
| Gene | Primer | Sequence | Product size |
|---|---|---|---|
| IL-6 | Forward | CTCCTTCTCCACAAGCGCCTTC | 583 bp |
| Reverse | GCGCAGAATGAGATGAGTTGTC | ||
| IL-8 | Forward | GCAGTTTTGCCAAGGAGTGCT | 347 bp |
| Reverse | GCATCTGGCAACCCTACAACA | ||
| TNF-α | Forward | GAAAGCATGATCCGGGACGTG | 510 bp |
| Reverse | GATGGCAGAGAGGAGGTTGAC | ||
| GAPDH | Forward | CACCACCAACTGCTTAGCAC | 515 bp |
| Reverse | CCCTGTTGCTGTAGCCAAAT |
Determination of IL-6 and IL-8 Secretion
Secretion of IL-6, IL-8, and TNF-αwas determined with ELISAs. HCECs were plated at 4 × 105 cells/well in 12-well plates. After growth factor starvation, cells were treated with P. aeruginosa and/or inhibitors for the indicated times, and the conditioned media were centrifuged at 20,000 × g at 4°C for 20 min to remove bacteria and cell debris. Supernatants were harvested for measurement of IL-6, IL-8, and TNF-α by ELISAs according to the manufacturer’s instructions (R& D Systems, Minneapolis, MN, USA). The amount of these cytokines in culture media was normalized with the total amount of cellular protein lysed with RIPA buffer. Results were expressed as means of pg of cytokine per mg cell lysate ±SE (n = 3). Each figure shows the results of experiments repeated at least three times. Statistical analysis was performed using ANOVA with a p value <0.05 considered statistically significant.
RESULTS
P. aeruginosa Induces NF-κB, p38, and JNK Activation in HUCL Cells
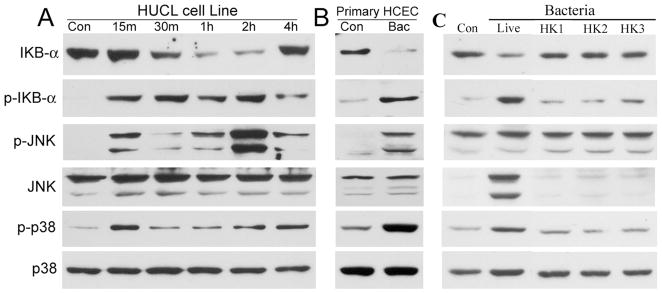
Activation of NF-κB in HCECs in response to P. aeruginosa infection was assessed by immunodetection of IκB-α phosphorylation and degradation (Fig. 1). HUCL cells challenged with P. aeruginosa at a bacteria to cell ratio of 50:1 resulted in IκB-αphosphorylation and degradation in a time-dependent manner (panel A, Fig. 1). An elevated level of phospho-IκB-α was detected at 15 min and remained fairly consistent through 2 hr postinfection (p.i.), and then declined, but was still detectable at 4 hr. The increase in IκB-α phosphorylation preceded IκB-α degradation, which was detectable 30 min p.i. By 1 hr, the level of IκB-α was barely detectable; however, it reappeared 4 hr poststimulation.
FIGURE 1.
P. aeruginosa stimulates IκB-α degradation/phosphorylation in HUCL or primary HCE cells. HUCL (A) or primary HCE cells (B) were grown to ~90% confluence on 6-well plates and were serum-starved overnight. Cells were then infected with live P. aeruginosa at a cell-to-bacterium ratio of 1:50 (A and B) over a 4-hr time course. HUCL cells were also challenged with heat-killed P. aeruginosa (C) for 2 hr at different cell-to-bacterium ratio 1:50 (HK1), 1:100 (HK2), or 1:200 (HK3); Live, positive control with live P. aeruginosa (ratio 1:50). Cell lysates were prepared at the designated time points p.i. and 20 μg of protein was subjected to SDS-PAGE and immunoblotting using phospho-IκB-α (p-IκB-α), IκB-α, phospho-p38 (p-p38), and phospho-JNK (p-JNK), with anti-p38 and JNK as loading control. These results are representative for three independent experiments.
We next assessed the activation of the MAP kinases p38 and JNK in response to P. aeruginosa. Activation of both p38 and JNK was detectable by 15 min p.i. Interestingly, there appeared to be a second peak of activation observed 2 hr p.i. for JNK phosphorylation and 4 hr p.i. for p38 phosphorylation. There were no significant changes observed for the total protein levels of either MAPK in HCECs during the time course.
To verify the results obtained from HUCL cells, primary HCECs were also infected with P. aeruginosa (panel B, Fig. 1). As in HUCL cells, exposure of primary HCECs to P. aeruginosa for 1 hr resulted in a great decrease in the level of IκB-α, corresponding to a significant rise in the level of phospho-IκB-α. Similarly, phospho-p38 and phospho-JNK were also increased in primary HCECs in response to P. aeruginosa infection.
Heat-killed P. aeruginosa at bacteria to cell ratio 50:1 or 100:1 were unable to stimulate the activation (phosphorylation) of these signal pathways (Fig. 1C). At 200:1, the heat-killed bacteria stimulated detectable but much lower phosphorylation of IκB when compared to live P. aeruginosa (ratio 50:1).
Taken together, we conclude that HCECs respond to P. aeruginosa infection by activating NF-κB, p38, and JNK signal pathways, and bacterial-HCEC interaction is required for activation of these signaling pathways.
P. aeruginosa Stimulates IL-6 and IL-8 Expression and Secretion in HCECs
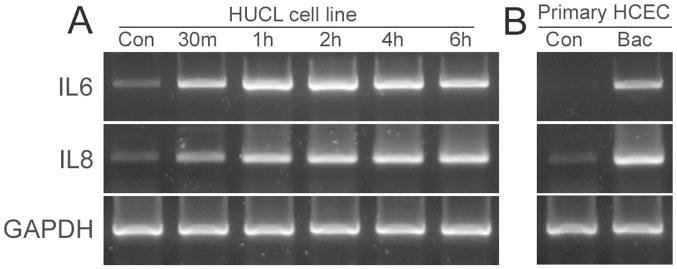
The effect of P. aeruginosa on IL-6 and IL-8 mRNA expression was determined by RT-PCR (Fig. 2). A trace amount of IL-6 mRNA was detected in control noninfected HUCL cells (panel A, Fig. 2). The band intensity increased at 30 min, reached a peak at 1 hr, and remained at the elevated level 6 hr p.i. IL-8 transcripts (347 bp) were also observed in control HUCL cells, but the level increased in P. aeruginosa–treated cells, starting at 30 min and peaking at 1 hr. The levels of GAPDH (controls for RT-PCR) remained unchanged in control and in P. aeruginosa–infected cells. Similarly, P. aeruginosa infection also induced IL-6 and IL-8 expression in primary HCECs (panel B, Fig. 2).
FIGURE 2.
P. aeruginosa induces IL-6 and IL-8 mRNA expression in human corneal epithelial cells. HUCL (panel A) or primary HCE (panel B) cells were grown to ~90% confluence on 6-well plates and were serum-starved overnight. Cells were then infected with P. aeruginosa at a cell-to-bacterium ratio of 1:50 over a 4-hr time course. At the indicated times, cells were lysed, and total RNA was extracted, reverse transcribed, and amplified using IL-6 and IL-8 primers (Table 1) with GAPDH as control. PCR products were separated and stained as described in “Materials and Methods.” Results are representative of three independent experiments.
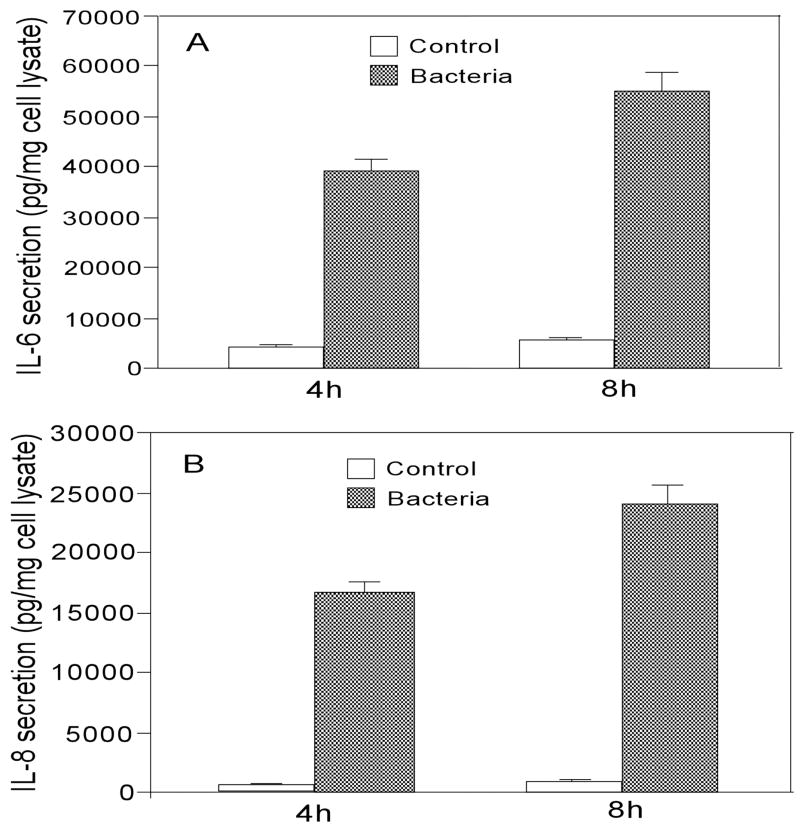
The effects of P. aeruginosa on IL-6 and IL-8 secretion in HUCL cells were assessed through ELISAs (Fig. 3). The amount of IL-6 and IL-8 accumulated in 4 hr cultures of P. aeruginosa–infected HUCL cells increased 9.5-fold and 35-fold, respectively, when compared to the control noninfected cells for the same period. The amount of secreted IL-6 and IL-8 continued to increase in P. aeruginosa–infected cells (8 hr p.i. measured), consistent with elevated levels of mRNA for these cytokines in HUCL cells 6 hr p.i. (Fig. 2).
FIGURE 3.
P. aeruginosa induces IL-6 and IL-8 secretion in HUCL cells. HUCL cells grown overnight in KBM were infected with P. aeruginosa and culture media collected 4 hr or 8 hr p.i. The accumulation of IL-6 (panel A) and IL-8 (panel B) was measured in cell culture supernatants by ELISA. Data shown are representative of triplicate experiments. All values are expressed as mean ± SEM. Statistically significant differences in secreted IL-6 and IL-8 in P. aeruginosa–infected HUCL cells were determined by the ANOVA (p < 0.001).
Taken together, these data suggest that P. aeruginosa stimulates IL-6 and IL-8 expression and secretion in HCECs.
P. aeruginosa–Induced Expression and Secretion of IL-6, IL-8, and TNF-α was NF-κB–dependent and p38- and JNK-enhanced
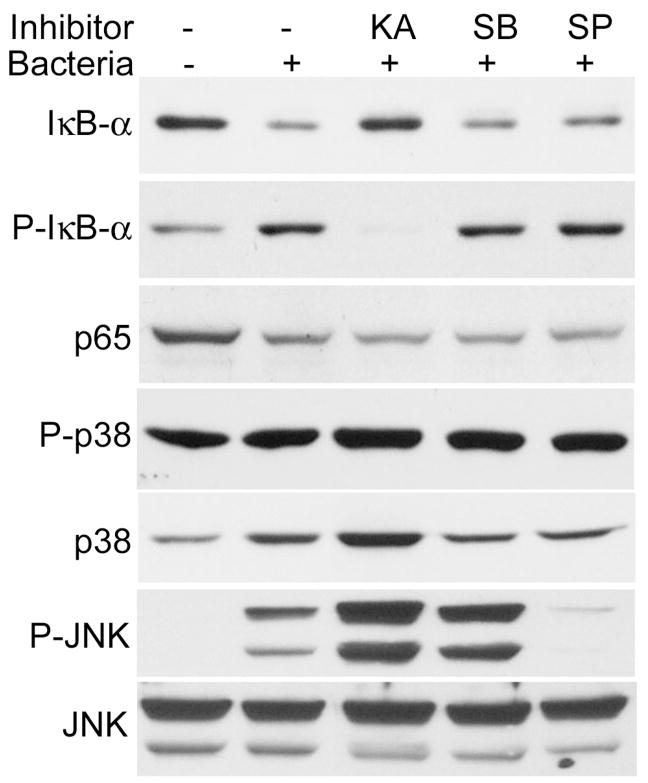
To determine the role of the NF-κB, p38, and JNK signaling pathways in innate responses of HCECs, the specific inhibitors for these pathways were used (Fig. 4). Exposure of HUCL cells to P. aeruginosa for 1 hr induced NF-kB activation, but treatment of cells with the NF-κB inhibitor kamebakaurin inhibited the P. aeruginosa–induced IκB-α phosphorylation and degradation. The p38 inhibitor SB203580 attenuated the P. aeruginosa–induced p38 activation but did not totally block p38 phosphorylation. The JNK inhibitor SP600125 also inhibited the P. aeruginosa–induced JNK activation. Whereas treatment of both SB203580 and SP600125 resulted in a decrease in total IκB-αB, both inhibitors exhibited no apparent effects on IκB-α phosphorylation.
FIGURE 4.
Effects of inhibitors on P. aeruginosa–induced signal activation. HUCL cells were preincubated with 25 μM kamebakaurin, 10 μM SB203580, or 0.5 μM SP600125 for 30 min and infected with P. aeruginosa at a cell-to-bacterium ratio of 1:50 for 2 hr. Cells were then lysed and subjected to Western blotting analysis with anti-phospho-IκB-α (p-IκB-α), IκB-α, p65 of NF-κB (p65), phospho-p38 (P-p38), and phospho-JNK (p-JNK), with anti-p38 and JNK as loading controls. Results are representative of two independent experiments.
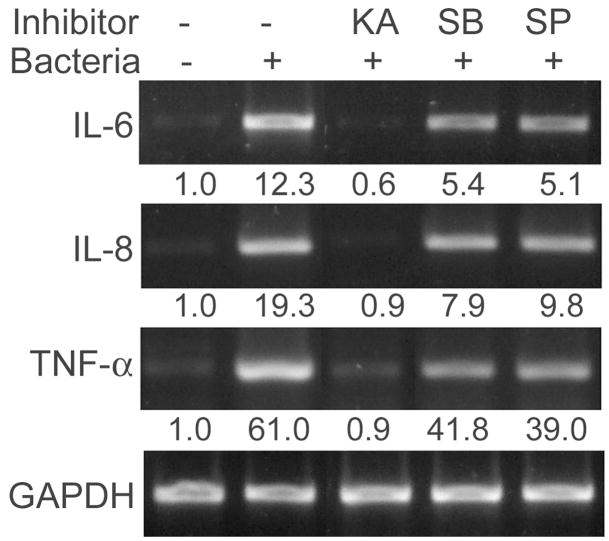
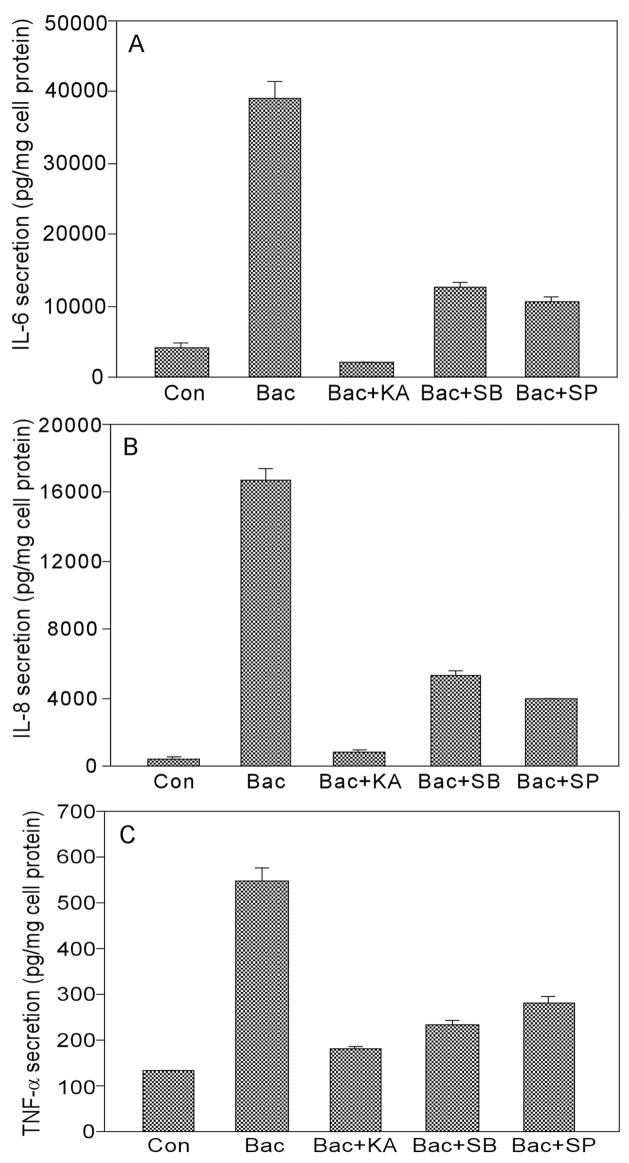
Figure 5 shows that treatment of HUCL cells with kamebakaurin totally blocked P. aeruginosa–induced IL-6, IL-8, and TNF-α expression assessed by RT-PCR. B203580 and SP600125, on the other hand, showed partial inhibition, as noted by quantitation of band intensity shown below the panel. Similarly, kamebakaurin retarded P. aeruginosa–induced IL-6, IL-8, and TNF-α accumulation, whereas p38 and JNK inhibitors significantly reduced P. aeruginosa–induced IL-6 (67% by SB203580 and 73% by SP600125, p < 0.01), IL-8 secretion (68% by SB203580 and 77% by SP600125, 0 p < 0.01), and TNF-α secretion (77.8% by SB203580 and 63.8% by SP600125 after background was subtracted, p < 0.01) (Fig. 6). Compared to kamebakaurin, these inhibitors were significantly less effective in inhibiting secretion of IL-6 and IL-8 (p < 0.05) but not TNF-α. These data indicated that the NF-κB, p38, and JNK signaling pathways are required for maximal production of IL-6 and IL-8 in HCECs.
FIGURE 5.
Requirement of NF-κB, p38, and JNK signaling for P. aeruginosa–induced IL-6 and IL8 expression. HUCL cells were preincubated with 25 μM kamebakaurin, 10 μM SB203580, or 0.5 μM SP600125 for 30 min and infected with P. aeruginosa at a cell-to-bacterium ratio of 1:50 for 2 hr. Cells were then lysed, total RNA was extracted, reverse transcribed, and amplified using IL-6, IL-8, and TNF-α primers with GAPDH as control. PCR products were separated and stained as described in “Materials and Methods.” The underlining numbers represent relative band intensity of cytokine PCR products normalized with band intensity of GAPDH. Results are representative of three independent experiments.
FIGURE 6.
Requirement of NF-κB, p38, and JNK signaling for maximal IL-6, IL-8, and TNF-α secretion. HUCL cells were pre-treated as described in Figure 5. HUCL cell culture media were collected 4 hr post PA-infection, and released IL-6 (A), IL-8 (B), and TNF-α were measured by ELISA. The data shown are representative of triplicate experiments. All values are expressed as mean ± SD. Statistical analysis was performed using ANOVA, and each pair showed significant differences in IL-6 and IL-8 secretion in inhibitor-treated PA-infected HUCL cells (p < 0.001).
DISCUSSION
In this study, we demonstrated that corneal epithelial cells in culture possess the ability to recognize the presence of P. aeruginosa and respond by activation of multiple signaling pathways, including the NF-κB, p38, and JNK pathways. Exposure of epithelial cells to P. aeruginosa also resulted in a significant increase in the expression and secretion of the proinflammatory cytokines IL-6 and IL-8. These results showed, for the first time, that the expression and secretion of the proinflammatory cytokines in HCECs in response to P. aeruginosa infection is NF-κB–dependent. p38 and JNK may also contribute to the regulation of cytokines expression directly or by influencing NF-κB activation. The P. aeruginosa–induced signaling pathway activation and proinflammatory cytokine production were also confirmed in primary human corneal epithelial cells. Thus, our data indicate that corneal epithelial cells can recognize and respond to P. aeruginosa infection and initiate innate immune responses in the cornea that may clear the pathogen. However, an excessive inflammatory response of the epithelium may also contribute to corneal scarring and the loss of vision associated with bacterial keratitis.
Our studies reveal that corneal epithelial cells respond to the presence of P. aeruginosa and initiate a rapid innate immune response by the activation of NF-κB and two stress-related MAPKs, leading to the production of proinflammatory cytokines and chemokines such as IL-6 and IL–8.22–24 It is generally believed that many types of epithelial cells, including corneal epithelial cells, have the intrinsic ability to sense the presence of microbial organisms and respond specifically through the identification of conserved microbial components termed pathogen-associated molecular patterns (PAMPs).25 Several Gram-negative components, including lipopolysaccharide (LPS), flagellin, and bacterial DNA (rich in unmethylated CpG-containing DNA), are shown to be PAMPs that are recognized by TLR-4,26,27, −5,28 and −9,29 respectively. We recently reported flagellin isolated from P. aeruginosa stimulate rapid, TLR5-dependent NF-κB activation and IL-6 and IL-8 production with a time course similar to that observed in this study by P. aeruginosa exposure.5 LPS was also reported to induce a rapid intracellular calcium response as well as the secretion of multiple proinflammatory cytokines and chemokines in cultured HCECs in a CD14-dependent manner.6 However, the secretion of the cytokines was not observed until 24 hr post-LPS treatment. The temporal responses of epithelial cells to LPS,6 flagellin,5 and P. aeruginosa suggest that TLR5, which recognizes flagellin, but not TLR4, which recognizes LPS, is a Gram-negative bacterial sensor in the cornea. However, comparing the time courses of NF-κB activation and IL-6 and -8 mRNA expression induced by P. aeruginosa infection presented here and also by Xue et al.15 with that induced by flagellin treatment5 revealed that the latter is more transient: NF-κB activation was detected in HCECs 2 hr post–P. aeruginosa infection but not post–flagellin exposure; elevated IL-6 and IL-8 expression lasted for 2 hr in flagellin-exposed cells and more than 6 hr in P. aeruginosa–infected HCECs. Thus, it is likely that another bacterial pattern recognizing receptor such as intracellularly located TLR-9, which recognizes bacterial DNA,30,31 might be involved. Indeed, our preliminary studies showed the expression of TLR-9 in HCECs (data not shown). The role of TLR-9 in detecting the presence of bacteria in HCECs is under investigation in the laboratory. Nevertheless, the available data suggest that blocking TLR5 activation in epithelial cells should be part of a therapeutic strategy for preventing some of the destructive consequences of ocular Gram-negative infections.
NF-κB, p38, and JNK are three signaling pathways known to be activated by TLRs when they interact with their prospective PAMPs.2,11–14 Demonstration of rapid activation of all three pathways by exposure of P. aeruginosa in epithelial cells suggests the involvement of TLRs. We showed that presence of the inhibitors of NF-κB, a master regulator of inflammation,32 totally blocks IL-6, IL-8, and TNF-α production. However, both transcription and secretion of these cytokines are only partially sensitive to JNK and p38 MAPK inhibition. Thus, p38 and JNK activities are required for maximal P. aeruginosa–stimulated IL-8/IL-6 and TNF-α expression and secretion. However, whether MAPK regulates proinflammatory cytokine expression through effects on NF-κB expression and/or activation or in an NF-κB–independent manner remains to be determined.
In summary, our studies suggest that HCECs can recognize Gram-negative bacteria and initiate innate immune responses, including the release of proinflammatory cytokines. Understanding the molecular events of bacterial-epithelial interactions and the inflammatory consequences of cytokine production may permit the development of novel, specific therapies that can promote innate defense and prevent some of the destructive consequences of ocular Gram-negative infections.
Acknowledgments
This work was supported in part by National Institutes of Health research grants RO1 EY14080 and RO1EY10869, and the work in X.Y.W.’s lab was supported by a grant from the National Natural Science Foundation of China (30328026). The authors thank Dr. Rhea-Beth Markowitz Medical College of Georgia for critical reading of the manuscript.
Contributor Information
Jing Zhang, Department of Cellular Biology and Anatomy, Medical College of Georgia, Augusta, Georgia, USA.
Xin-Yi Wu, Department of Ophthalmology, Qilu Hospital, Shandong University, Jinan, Shandong, People’s Republic of China.
Fu-Shin X. Yu, Department of Cellular Biology and Anatomy, Medical College of Georgia, Augusta, Georgia, USA
References
- 1.Cohen E, Laibson P, Arentsen J, Clemons C. Corneal ulcers associated with cosmetic extended wear soft contact lenses. Ophthalmology. 1987;94:109–114. doi: 10.1016/s0161-6420(87)33491-8. [DOI] [PubMed] [Google Scholar]
- 2.Gewirtz AT. Intestinal epithelial toll-like receptors: to protect. And serve? Curr Pharm Des. 2003;9:1–5. doi: 10.2174/1381612033392422. [DOI] [PubMed] [Google Scholar]
- 3.Fisette PL, Ram S, Andersen JM, Guo W, Ingalls RR. The lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-{kappa}B activation in epithelial cells in a Toll-like receptor 2-dependent manner. J Biol Chem. 2003;278:46252–46260. doi: 10.1074/jbc.M306587200. [DOI] [PubMed] [Google Scholar]
- 4.Kurpakus-Wheater M, Kernacki KA, Hazlett LD. Maintaining corneal integrity how the “window” stays clear. Prog Histochem Cytochem. 2001;36:185–259. [PubMed] [Google Scholar]
- 5.Zhang J, Xu K, Ambati B, Yu FS. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Invest Ophthalmol Vis Sci. 2003;44:4247–4254. doi: 10.1167/iovs.03-0219. [DOI] [PubMed] [Google Scholar]
- 6.Song PI, Abraham TA, Park Y, et al. The expression of functional LPS receptor proteins CD14 and toll-like receptor 4 in human corneal cells. Invest Ophthalmol Vis Sci. 2001;42:2867–2877. [PubMed] [Google Scholar]
- 7.You L, Kruse FE, Bacher S, Schmitz ML. Lipoteichoic acid selectively induces the ERK signaling pathway in the cornea. Invest Ophthalmol Vis Sci. 2002;43:2272–2277. [PubMed] [Google Scholar]
- 8.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 10.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity [see comments] Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 11.Philpott DJ, Girardin SE, Sansonetti PJ. Innate immune responses of epithelial cells following infection with bacterial pathogens. Curr Opin Immunol. 2001;13:410–416. doi: 10.1016/s0952-7915(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 12.Li JD. Exploitation of host epithelial signaling networks by respiratory bacterial pathogens. J Pharmacol Sci. 2003;91:1–7. doi: 10.1254/jphs.91.1. [DOI] [PubMed] [Google Scholar]
- 13.Keates S, Keates AC, Warny M, Peek RM, Jr, Murray PG, Kelly CP. Differential activation of mitogen-activated protein kinases in AGS gastric epithelial cells by cag+ and cag- Helicobacter pylori. J Immunol. 1999;163:5552–5559. [PubMed] [Google Scholar]
- 14.Guillot L, Medjane S, Le-Barillec K, et al. Response of human pulmonary epithelial cells to lipopolysaccharide involves Toll-like receptor 4 (TLR4)-dependent signaling pathways: evidence for an Intracellular Compartmentalization of TLR4. J Biol Chem. 2004;279:2712–2718. doi: 10.1074/jbc.M305790200. [DOI] [PubMed] [Google Scholar]
- 15.Xue ML, Thakur A, Lutze-Mann L, Willcox MD. Pro-inflammatory cytokine/chemokine gene expression in human corneal epithelial cells colonized by Pseudomonas aeruginosa. Clin Experiment Ophthalmol. 2000;28:197–200. doi: 10.1046/j.1442-9071.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- 16.Xue ML, Willcox MD, Lloyd A, Wakefield D, Thakur A. Regulatory role of IL-1beta in the expression of IL-6 and IL-8 in human corneal epithelial cells during Pseudomonas aeruginosa colonization. Clin Experiment Ophthalmol. 2001;29:171–174. doi: 10.1046/j.1442-9071.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- 17.Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2:1051–1060. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- 18.Steadman R, Irwin MH, St JPL, Blackburn WD, Heck LW, Abrahamson DR. Laminin cleavage by activated human neutrophils yields proteolytic fragments with selective migratory properties. J Cell Biol. 1993;121:923–929. doi: 10.1002/jlb.53.4.354. [DOI] [PubMed] [Google Scholar]
- 19.Steuhl KP, Doring G, Henni A, Thiel HJ, Botzenhart K. Relevance of host-derived and bacterial factors in Pseudomonas aeruginosa corneal infections. Invest Ophthalmol Vis Sci. 1987;28:1559–1568. [PubMed] [Google Scholar]
- 20.Rheinwald JG, Hahn WC, Ramsey MR, et al. A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol Cell Biol. 2002;22:5157–5172. doi: 10.1128/MCB.22.14.5157-5172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- 22.Cole N, Krockenberger M, Bao S, Beagley KW, Husband AJ, Willcox M. Effects of exogenous interleukin-6 during Pseudomonas aeruginosa corneal infection. Infect Immun. 2001;69:4116–4119. doi: 10.1128/IAI.69.6.4116-4119.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobden JA, Masinick SA, Barrett RP, Hazlett LD. Proinflammatory cytokine deficiency and pathogenesis of Pseudomonas aeruginosa keratitis in aged mice. Infect Immun. 1997;65:2754–2758. doi: 10.1128/iai.65.7.2754-2758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbaum J, Planck S, Huang X, Rich L, Ansel J. Detection of mRNA for the cytokines, interleukin-1 alpha and interleukin-8, in corneas from patients with pseudophakic bullous keratopathy. Invest Ophthalmol Vis Sci. 1995;36:2151–2155. [PubMed] [Google Scholar]
- 25.Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–18. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 26.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki K, Akashi S, Shimazu R, Yoshida T, Miyake K, Nishijima M. Mouse toll-like receptor 4.MD-2 complex mediates lipopolysaccharide- mimetic signal transduction by Taxol. J Biol Chem. 2000;275:2251–2254. doi: 10.1074/jbc.275.4.2251. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 29.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad-Nejad P, Hacker H, Rutz M, Bauer S, Vabulas RM, Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol. 2002;32:1958–1968. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 31.Akhtar M, Watson JL, Nazli A, McKay DM. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependent, NF-kappaB-independent pathway. FASEB J. 2003;17:1319–1321. doi: 10.1096/fj.03-0950fje. [DOI] [PubMed] [Google Scholar]
- 32.Barnes P, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]