Abstract
The dietary essential PUFA docosahexaenoic acid [DHA; 22:6(n-3)] is a critical contributor to cell structure and function in the nervous system, and deficits in DHA abundance are associated with cognitive decline during aging and in neurodegenerative disease. Recent studies underscore the importance of DHA-derived neuroprotectin D1 (NPD1) in the homeostatic regulation of brain cell survival and repair involving neurotrophic, antiapoptotic and antiinflammatory signaling. Emerging evidence suggests that NPD1 synthesis is activated by growth factors and neurotrophins. Evolving research indicates that NPD1 has important determinant and regulatory interactions with the molecular-genetic mechanisms affecting β-amyloid precursor protein (βAPP) and amyloid beta (Aβ) peptide neurobiology. Deficits in DHA or its peroxidation appear to contribute to inflammatory signaling, apoptosis, and neuronal dysfunction in Alzheimer disease (AD), a common and progressive age-related neurological disorder unique to structures and processes of the human brain. This article briefly reviews our current understanding of the interactions of DHA and NPD1 on βAPP processing and Aβ peptide signaling and how this contributes to oxidative and pathogenic processes characteristic of aging and AD pathology.
Introduction
Docosahexaenoic acid [DHA;4 22:6(n-3); cervonic acid; MW 327] is a dietary essential (n-3) PUFA highly enriched in fish oils and concentrated up the food chain from photosynthetic and heterotrophic microalgae. In addition to these essential marine sources, DHA is also synthesized via an elongation and desaturation of the 20-carbon eicosapentanoic acid [EPA; 20:5(n-3)], or elongation of the 18-carbon (n-3) fatty acid, α-linolenic acid [ALA; 18:3(n-3)] enriched in flax (Linaceae), walnut (Juglandaceae), chia (Salvia hispanica), and other photosynthesizing terrestrial plants (1–3). In the brain, glia and endothelial cells of the microvasculature, but not neurons, have some capacity to synthesize DHA from ALA and other (n-3) precursor fatty acids, but whether or not this contributes significantly to total brain DHA is not clear. The high concentration of DHA in the capillary endothelium suggests that DHA is taken up from the diet via blood plasma DHA transporters including specific fatty-acid-binding lipoprotein carriers (3–5). DHA is an absolute requirement for the development of the human central nervous system (CNS), and the continuous maintenance of brain cell function, illustrating the strong mechanistic link between an adequate supply of essential PUFA in the diet and the sustenance of cognitive health. During postnatal development, rapid accretion of DHA in brain and retina takes place (2–4). DHA attains its highest concentration in CNS synapses and in retinal photoreceptors; in fact, up to 60% of all fatty acids esterified in neuronal plasma membrane phospholipid consist of DHA. By use of the postnatal development of mice as a model, it has been determined that dietary linolenic acid is first taken up by the liver, where elongation and desaturation to DHA occurs, followed by its supply through the bloodstream to brain and retina, coinciding with photoreceptor development and synaptogenesis (2–5). Brain and retinal cells therefore have a convenient and readily accessible supply of DHA that, through highly regulated, phospholipase-mediated exoprotease activities, liberates membrane-bound DHA to serve in neuroprotective and cell fate-signaling roles (6–12). The beneficial neurophysiological actions of DHA occur in part through its direct maintenance of neuronal plasma membrane fluidity and functional integrity, and in part through the generation of docosanoids. The first identified DHA-derived mediator, neuroprotectin D1 (NPD1; MW 359), is formed through tandem phospholipase A2 (PLA2)-lipoxygenase (LOX) action on free DHA, via a 16,17S-DHA epoxide intermediate (6,10–14).
NPD1 biological actions
Adequate dietary intake of PUFA and, in particular, life-long DHA bioavailability have been shown to provide visual, neurovascular, cardiovascular, and neurological health benefits (1–6,10–14). The positive regulatory actions of DHA and NPD1 occur via several interdependent mechanisms that include the following: 1) membrane functional integrity including lipid bilayer fluidity and membrane rafts; 2) the recruitment and up-regulation of antiapoptotic members of the Bcl-2 gene family such as Bcl-xl and Bfl-1 (A1); 3) the repression of the activation of inflammatory signaling mediators such as the prostaglandin-synthesizing arachidonic acid cascade enzyme cyclooxygenase-2 (COX-2); 4) the modulation of kinase-mediated Bcl-2 gene family phosphorylation, such as directed inhibition of the SAPK/JNK survival signaling cascade; and 5) the repression of the expression of proapoptotic signaling (Fig. 1) (1–6,10–16).
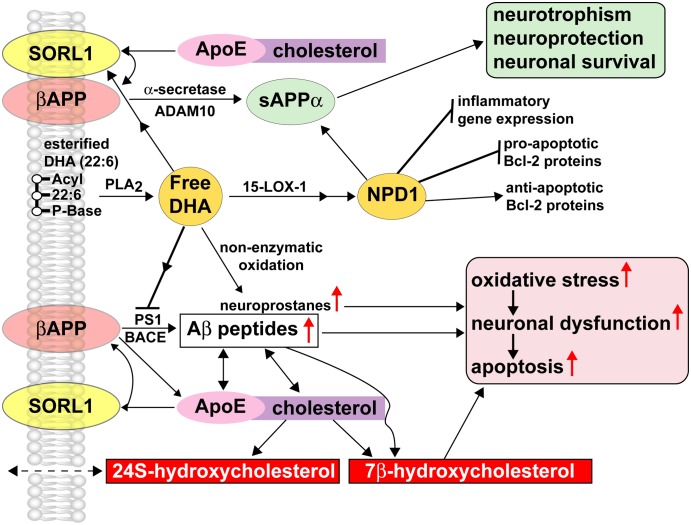
FIGURE 1 .
DHA, NPD1, and βAPP-derived Aβ peptide signaling circuits in homeostatic aging and in AD. DHA and NPD1 act as PLA2- and 15-LOX-mediated neuroprotectants in the βAPP-sAPPα-Aβ peptide signaling pathway. Free DHA is liberated from membrane-bound stores via the action of a highly regulated membrane-associated PLA2 that may be subsequently converted into a potent neurotrophic NPD1 through an enzyme-mediated lipoxygenation via 15-LOX-1 or 15-LOX-like activities. NPD1 has been shown to convey multiple neuroprotective effects including induction of antiapoptotic Bcl-2 proteins, inhibition in the expression of proapoptotic Bcl-2 proteins, and suppression of inflammatory gene expression. Various ROS are more abundant in AD than in control brain, suggesting a possible role for oxidation-related decrease in protein function in processes such as depletion of the cellular redox balance, loss of specific protein function, interference with the cell cycle, and abnormal clearance of proteins and neurodegeneration leading ultimately to neuronal death. Nonenzymatic oxidation of free DHA results in the formation of neuroprostanes, a class of peroxidized lipids that further support oxidative stress, neuronal dysfunction, and apoptosis. Nonenzymatic reactions may be quenched by specific antioxidants and free radical scavengers, indicating that the redox state of brain cells has bearing on neurotrophic or oxidative-neurotoxic pathways for DHA. Enriched within neuronal plasma and endoplasmic membranes, the integral βAPP gives rise to sAPPα via an α-secretase/ADAM (a disintegrin and metalloprotease) 10-mediated pathway that is nonamyloidogenic and neurotrophic and whose synthesis is supported by free DHA and NPD1 (upper pathways). The βAPP membrane-integral sorting receptor sortilin-1 (SORL1), when proximal to βAPP, has direct effects on βAPP trafficking, and decreased abundance of SORL1, or βAPP-SORL1 dissociation, is coupled to activation of the amyloidogenic pathway from βAPP and the increased generation and secretion of Aβ peptides (lower pathways) (46–49). SORL-1 further interacts with the type E apolipoprotein (ApoE), a major biolipid and cholesterol transporter in the brain, and the interaction of βAPP and ApoE within cholesterol-enriched lipid raft membrane domains, especially in the absence of SORL-1, gives rise to an increased generation of Aβ peptides via stimulation of β-amyloid cleavage enzyme (BACE) and presenilin 1 (PS1). The tandem actions of BACE and PS1 are sometimes referred to as the β-γ-secretase signaling pathway, an integral component of the amyloid cascade hypothesis, and known to contribute to Aβ peptide accumulation, neuropathology, and neurodegeneration. Aβ peptides bind directly to ApoE and cholesterol, and both Aβ peptides and βAPP oxidize ApoE-cholesterol to form the proapoptotic neurotoxic oxysterol 7β-hydroxycholesterol (7β-HC) or 24S hydroxycholesterol (24S-HC) via the action of CYP46A1 (48–50). 24S-HC, highly enriched in the human CNS, is membrane permeable and is associated with amyloidogenesis and AD pathology (49). The actions of DHA or NPD1 on CYP46A1 and oxidation of cholesterol to 7β-HC or 24S-HC are not well understood. Current and emerging pharmaceutical strategies aim at the modulation of secretase activities through the actions of SALA to favor the more neurotrophic βAPP-cleavage signaling pathways (upper pathways) over the neurotoxic, amyloidogenic BACE-PS1 β-γ-secretase pathways (lower pathways; 50). The therapeutic use of statins, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, that lower serum cholesterol has also been shown to reduce Aβ peptide abundance in in vitro models of AD using human brain cell primary cultures and in some clinical trials, and large phase III studies are currently in progress (48–50). The interactions of DHA and NPD1 with SALA drugs or statins are not well understood; however, early clinical trials using DHA and antioxidants together as enhancers of cognition in aged patients showed synergistic beneficial effects (23,49,50).
Enzyme-mediated oxygenation of DHA to NPD1
The bioavailability of free unesterified DHA is a highly regulated event, and free unesterified DHA, normally undetectable under basal conditions, increases during brain injury, cerebral ischemia, seizures, and other pathological conditions. The arachidonic acid cascade is also activated under these conditions (12,15–19). Up-regulation of PLA2 activity is observed in the neocortex and hippocampus of AD, during hypoxia, and in Aβ peptide- or IL-1β-stressed human neural cells in primary culture (18–20). Unesterified DHA may be enzymatically oxygenated to generate NPD1, which in turn elicits potent bioactivity against excessive oxidative stress and neuroprotective functions in immediate proximity to the site of DHA liberation (16–22). Changes in the redox balance of brain cells, modulated in part by bioavailable antioxidants, may further affect the kinetics of these DHA-processing systems (19–23). For example, the overall bioavailabilty of DHA may be in part dependent on the redox state of brain cells, and the supplementation of DHA with antioxidants such as antioxidant carotenoids has been shown to significantly improve cognitive abilities in elderly populations (23).
Nonenzymatic oxidation of DHA
DHA is also a primary lipid peroxidation target in oxidative retinal and brain cell injury, and reduced free DHA levels are associated with retinal and neurological dysfunction and visual and cognitive decline. During periods of excessive oxidative stress, DHA may be oxidized nonenzymatically into F4-, D4-, E4-, A4-, and J4-neuroprostanes. These prostaglandin-like compounds formed independently of cyclooxygenase trigger reactive oxygen species (ROS) evolution and oxidative stress at the cytoplasmic or extracellular interface of the plasma membrane (24–27). Synthesis of F4-neuroprostane-containing aminophospholipids may further adversely affect neuronal function as a result of alterations they induce in the biophysical properties of neuronal plasma membranes (27,28). The abundance and speciation of F4-neuroprostanes and hydroxynonenal, which reflect the general state of lipid peroxidation and abundance of ROS in stressed brain cells, may be useful biomarkers for the extent of brain cell oxidation, degeneration, and neuronal dysfunction as well as for the therapeutic efficacy of antioxidative drugs and their neuroprotective actions (27–30). The nature of the switch from neuroprotective to membrane disruptive and oxidative roles for DHA, such as the generation of NPD1 vs. neuroprostanes, is under intense research study, as the signaling axis along PLA2-15-LOX, and related enzyme pathways, may be profitably exploited to modulate NPD1 generation and their brain cell survival bioactivity.
Alzheimer disease, β-amyloid precursor protein, and DHA
Abundant clinical, epidemiological, molecular, and neuropathological evidence supports the idea that Alzheimer disease (AD) evolves from a complex interplay of environmental and molecular-genetic factors that develops over decades, over and above the more subtle neurochemical changes that accompany healthy brain aging. Pathogenic processes that typify AD neuropathology exhibit 3 key features: 1) they are highly specific to the unique structures and functions of human brain cells; 2) they act in a chronic, cooperative, and accumulative fashion over a lengthy time course involving decades of aging; and 3) once initiated, their deleterious effects exhibit positive feedback, often perpetuating until brain cell defenses are exhausted, leading to progressive and irreversible neuronal cell damage and death. Mitochondrial dysfunction and focused oxidative damage, including primary peroxidation of membrane lipids and PUFA by ROS, appear to be among the earliest events in pathological aging, as exemplified in the initiation and progression of AD (17–25). Emerging epidemiological and molecular-genetic evidence further suggests that dietary lipids such as DHA and high-fat cholesterol (HF-C) diets are active modulators of the oxidative processes that either prevent or support brain cell neurodegeneration, respectively (28–31). Free radical oxidative damage to brain plasma membrane integral proteins and lipids, the latter of which contain a high proportion of DHA, therefore appears to be one of the early critical and determining events involved in initiating brain cell membrane instability and neural cell dysfunction.
The membrane-embedded, ∼110-kDa integral type-1 transmembrane glycoprotein βAPP holoprotein central to the “amyloid cascade hypothesis” of AD, comprising the substrate of the γ-secretase complex that consists of βAPP, presenilin 1 and/or 2 (PS1/PS2; essential components of γ-secretase) and nicastrin, gives rise to neurotoxic Aβ peptides 37 to 43 amino acids in length (Aβ37–Aβ43). Of these, Aβ40 is associated with neurovascular deposition and vascular pathology, whereas a self-aggregating, highly amyloidogenic Aβ42 dimer is thought to be particularly detrimental to neuronal activity, in part through its promotion of oxidative stress and synaptotoxic effects (6–11,31). Alternatively, βAPP can be processed via a membrane-associated disintegrin metalloprotein α-secretase into a soluble form of APP (sAPPα), which is neuritogenic, neurotrophic, promotes neuronal survival, and further precludes the generation of toxic Aβ peptides. Both DHA and NPD1 promote the generation of sAPPα via stimulation of α-secretase activities, but whether this is a membrane biophysical-lipid microenvironment effect or a result of direct α-secretase-DHA or NPD1 effect remains unclear (21,31).
The βAPP-containing γ-secretase complex thereby contains a family of both peripheral and transmembrane polytopic proteins intimately associated with lipid raft domains of neuronal, lysosomal, Golgi, endoplasmic reticular, and plasma membranes (9,19,29,30). One fundamental feature of the amyloid cascade hypothesis of AD is the progressive evolution of Aβ peptides derived from the tandem β-γ secretase pathway that processes βAPP into the more pathogenic forms of βAPP-derived Aβ42 peptide fragments. The unusual γ-secretase cleavage site within the hydrophobic transmembrane domain of βAPP suggests that pathological events that alter or disorganize lipid bilayer structure or fluidity contribute to Aβ40 and Aβ42 peptide generation. The progressive accumulation, condensation, and aggregation of fibrillar Aβ peptides into neuritic plaques further support ROS generation, oxidative stress, proinflammatory, and proapoptotic gene expression and signaling, resulting in neuronal dysfunction and irreversible loss of brain cell homeostasis (31–35). Aβ peptide speciation, solubility, aggregation, and downstream consequences of Aβ peptide accumulation, such as microglia activation, are clearly prooxidative, proinflammatory, proapoptotic, and toxic to adjacent neurons. Mechanisms responsible for generating Aβ peptides and their neurotoxic consequences such as driving brain stress increase with age and may potentially predispose aging human brain cells to progressive neurological dysfunction (36,37). The chronic nature of AD suggests that brain survival factors are progressively diminished or exhausted over decades of life as self-perpetuating neuropathology slowly takes over and spreads throughout the neocortex (38,39). Part of this pathology promotes an up-regulation in the expression of apoptotic factors coupled to decreases in the expression of antiapoptotic members of the Bcl-2 gene family (6,39–43). Unlike cholesterol and HF-C dietary intake, DHA and DHA-derived NPD1 decrease the rate of Aβ peptide generation and shedding from brain cells and diminish Aβ peptide-mediated proinflammatory, proapoptotic, and pathogenic consequences (6,16,19,43). NPD1 also influences apoptosis-induced brain cell damage in part by shifting the balance from the expression of pro-apoptotic factors toward the expression of antiapoptotic, survival-promoting members of the Bcl-2 gene family (6,39–45). Neurotrophins, including pigment epithelium-derived factor and brain-derived neurotrophic factor, stimulate NPD1 synthesis, which in turn modifies the expression of Bcl-2 family members by activating antiapoptotic proteins, by decreasing proapoptotic proteins, and by attenuating caspase-3 downstream during oxidative stress (11–16). BACE (β-secretase) and/or PS1/PS2 (γ-secretase) activities, which down-regulate neurotoxic Aβ peptide production, and subsequent ROS generation appear to be affected by the lipid raft composition and microenvironment of these membrane integral and peripheral enzyme systems (29–37).
Oxidative stress and additional lipid membrane-associated factors
Neurological diseases that exhibit excessive markers for oxidative stress also display reduced NPD1 abundance and are associated with the progressive neuronal decline and neurodegeneration that characterize AD-affected brains (6,43). Transcription and translation of βAPP, secretase, and membrane-associated factors linked to Aβ peptide speciation and trafficking in AD and in experimental models of AD are influenced by the availability of DHA and by the composition of the lipid raft domains (21,26–31). Additional membrane-associated factors such as the presence of the transmembrane sorting receptor sortilin 1 (SORL1; LR11) and the serum lipid carrier apolipoprotein E (apoE) that modulate βAPP processing and signaling, and secretase-mediated Aβ peptide synthesis, are also impacted by the presence of DHA or NPD1 (Fig. 1; 46,47). DHA suppresses age-related Aβ42 peptide shedding from human neural cells (6,11), represses Aβ peptide-related pathology in Tg2576 transgenic mouse models of AD (19,21), and stimulates nonamyloidogenic βAPP processing, which reduces both intracellular and extracellular levels of Aβ peptide in aged SH-SY5Y cells (47). DHA interactions with and recruitment of neural membrane-associated factors modulating βAPP catabolism therefore appear to be highly complex and interactive in the maintenance of normal membrane signaling and synaptic, intercellular, and extracellular secretory functions (6,39,43–47).
Prospects
In conclusion, important insights into the neurobiology of DHA and NPD1 in the aging brain and in AD have to date been obtained; however, several important areas of research involving the functional importance of these PUFA and their derivatives in the maintenance of cognitive mechanisms and brain health remain to be investigated. The roles of NPD1 as a potential modulator of apoE-mediated transport, biosynthesis, and trafficking and their influencing βAPP processing, sAPPα or Aβ peptide speciation, generation, and secretion during aging, and in cytokine-, hypoxia-, and oxidation-stressed human brain cell models of AD are incompletely understood. It has been reported that DHA itself exerts actions on some of these events (6,10–16,18,19,21). It remains to be established if, under those conditions, DHA is converted into NPD1 or if there are alternative processing mechanisms. In this connection, the addition of 50 nmol/L DHA was found to markedly inhibit cytokine-mediated production and secretion of Aβ40 and Aβ42 peptides from aging human neural cells in culture (6,11). Remarkably, in that and related studies, NPD1 was rapidly biosynthesized (6,11,16). The effects of NPD1, if any, on the biophysics and kinetics of the membrane-embedded secretase-mediated cleavage mechanisms of βAPP remain to be explored (6,19,21,48–50). Knowledge of how NPD1 impacts specific secretase activities is essential to the future design of more effective and selective Aβ peptide-lowering agents (SALA drugs; 49,50). The bioactivity of NPD1 in development and in aging human brain, its role in the onset and progression of AD neuropathology, and further mechanistic studies in vitro and in vivo on how NPD1 promotes neuroprotection via multiple and interactive mechanisms should further define how this endogenously derived lipid mediator protects against oxidative stress, apoptosis, and inflammation-triggered neuronal decline while promoting brain cell survival and maximizing cognitive function throughout the human lifespan.
Other articles in this symposium include references (51) and (52).
Published as a supplement to The Journal of Nutrition. Presented as part of the symposium “Dietary PUFA and the Aging Brain: Food for Thought” given at the 2008 Experimental Biology meeting on April 7, 2008, in San Diego, CA. The symposium was sponsored by the American Society for Nutrition and supported by an educational grant from Martek BioSciences Corporation. The symposium was chaired by Jay Whelan.
Supported by grants AG18031, NS23002, NS046741, and EY005121 from the NIH.
Author disclosures: W. Lukiw and N. Bazan, no conflicts of interest. Part of this work was presented at the International Congress of Alzheimer's disease (ICAD) 2008 11th biannual meeting held in Chicago IL, August 25–31, 2008.
Abbreviations used: Aβ42, amyloid-β 42-amino-acid peptide; ADAM, a disintegrin and metalloprotease; AD, Alzheimer disease; ALA, α-linolenic acid; ApoE, apolipoprotein E; BACE, β-amyloid cleavage enzyme; βAPP, β-amyloid precursor protein; CNS, central nervous system; COX-2, inducible cyclooxygenase-2; DHA, docosahexaenoic acid; EPA, eicosapentanoic acid; HF-C, high-fat cholesterol; LOX, lipoxygenase; NPD1, neuroprotectin D1; PLA2, phospholipase A2; PS1, presenilin 1; sAPPα, soluble amyloid precursor protein α fragment; ROS, reactive oxygen species; SALA, selective Aβ42-lowering agents; SORL1, sortilin 1.
References
- 1.Marszalek JR, Lodish HF. Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function. Annu Rev Cell Dev Biol. 2005;21:633–57. [DOI] [PubMed] [Google Scholar]
- 2.Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot Essent Fatty Acids. 2007;77:251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Innis SM. Dietary (n-3) fatty acids and brain development. J Nutr. 2007;137:855–9. [DOI] [PubMed] [Google Scholar]
- 4.Spector AA. Plasma free fatty acid and lipoproteins as sources of polyunsaturated fatty acid for the brain. J Mol Neurosci. 2001;16:159–65. [DOI] [PubMed] [Google Scholar]
- 5.Scott BL, Bazan NG. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci USA. 1989;86:2903–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lukiw WJ, Cui JG, Marcheselli VL, Boedker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mancuso M, Coppede F, Migliore L, Siciliano G, Murri L. Mitochondrial dysfunction, oxidative stress and neurodegeneration. J Alzheimers Dis. 2006;10:59–73. [DOI] [PubMed] [Google Scholar]
- 8.Kazantsev AG. Cellular pathways leading to neuronal dysfunction and degeneration. Drug News Perspect. 2007;20:501–9. [DOI] [PubMed] [Google Scholar]
- 9.Smith DG, Cappai R, Barnham KJ. The redox chemistry of the Alzheimer's disease amyloid beta peptide. Biochim Biophys Acta. 2007;1768:1976–1990. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee PK, Chawla A, Loayza MS, Bazan NG. Docosanoids are multifunctional regulators of neural cell integrity and fate: significance in aging and disease. Prostaglandins Leukot Essent Fatty Acids. 2007;77:233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukiw WJ, Bazan NG. Survival signaling in Alzheimer's disease. Biochem Soc Trans. 2006;34:1277–82. [DOI] [PubMed] [Google Scholar]
- 12.Bazan NG. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J Lipid Res. 2003;44:2221–33. [DOI] [PubMed] [Google Scholar]
- 13.Brand A, Schonfeld E, Isharel I, Yavin E. Docosahexaenoic acid-dependent iron accumulation in oligodendroglia cells protects from hydrogen peroxide-induced damage. J Neurochem. 2008;105:1325–35. [DOI] [PubMed] [Google Scholar]
- 14.Uauy R, Dangour AD. Nutrition in brain development and aging: role of essential fatty acids. Nutr Rev. 2006;64:S24–33. [DOI] [PubMed] [Google Scholar]
- 15.Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photo-receptors. Trends Neurosci. 2006;29:263–71. [DOI] [PubMed] [Google Scholar]
- 16.Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care. 2007;10:136–41. [DOI] [PubMed] [Google Scholar]
- 17.Sun GY, Xu J, Jensen MD, Simonyi A. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J Lipid Res. 2004;45:205–13. [DOI] [PubMed] [Google Scholar]
- 18.Kim H-Y, Akbar M, Lau A, Edsall L. Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6 n-3). J Biol Chem. 2000;275:35215–23. [DOI] [PubMed] [Google Scholar]
- 19.Cole GM, Frautschy SA. Docosahexaenoic acid protects from amyloid and dendritic pathology in an Alzheimer's disease mouse model. Nutr Health. 2006;18:249–59. [DOI] [PubMed] [Google Scholar]
- 20.Kim YJ, Chung HY. Antioxidative and anti-inflammatory actions of docosahexaenoic acid and eicosapentaenoic acid in renal epithelial cells and macrophages. J Med Food. 2007;10:225–31. [DOI] [PubMed] [Google Scholar]
- 21.Calon F, Cole G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: evidence from animal studies. Prostaglandins Leukot Essent Fatty Acids. 2007;77:287–93. [DOI] [PubMed] [Google Scholar]
- 22.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–17. [DOI] [PubMed] [Google Scholar]
- 23.Johnson EJ, McDonald K, Caldarella SM, Chung HY, Troen AM, Snodderly DM. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr Neurosci. 2008;11:75–83. [DOI] [PubMed] [Google Scholar]
- 24.Leuner K, Hauptmann S, Abdel-Kader R, Scherping I, Keil U, Strosznajder JB, Eckert A, Muller WE. Mitochondrial dysfunction: the first domino in brain aging and Alzheimer's disease? Antioxid Redox Signal. 2007;9:1659–75. [DOI] [PubMed] [Google Scholar]
- 25.Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci USA. 2004;101:2070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montine KS, Quinn JF, Zhang J, Fessel JP, Roberts LJ 2nd, Morrow JD, Montine TJ. Isoprostanes and related products of lipid peroxidation in neurodegenerative diseases. Chem Phys Lipids. 2004;128:117–24. [DOI] [PubMed] [Google Scholar]
- 27.Hoozemans JJ, Veerhuis R, Rozemuller AJ, Eikelenboom P. The pathological cascade of Alzheimer's disease: the role of inflammation and its therapeutic implications. Drugs Today (Barc). 2002;38:429–43. [DOI] [PubMed] [Google Scholar]
- 28.Roberts LJ 2nd, Fessel JP, Davies SS. The biochemistry of the isoprostane, neuroprostane, and isofuran pathways of lipid peroxidation. Brain Pathol. 2005;15:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poon HF, Calabrese V, Scapagnini G, Butterfield DA. Free radicals: key to brain aging and heme oxygenase as a cellular response to oxidative stress. J. Gerontol A Biol Sci Med Sci. 2004;59:478–93. [DOI] [PubMed] [Google Scholar]
- 30.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–14. [DOI] [PubMed] [Google Scholar]
- 31.Osenkowski P, Ye W, Wang R, Wolfe MS, Selkoe DJ. Direct and potent regulation of gamma-secretase by its lipid microenvironment. J Biol Chem. 2008. Jun 6. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 32.Colangelo V, Schurr J, Ball MJ, Pelaez RP, Bazan NG, Lukiw WJ. Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J Neurosci Res. 2002;70:462–73. [DOI] [PubMed] [Google Scholar]
- 33.Lukiw WJ. Gene expression profiling in fetal, aged and Alzheimer hippocampus—a continuum of stress-related signaling. Neurochem Res. 2004;29:1287–97. [DOI] [PubMed] [Google Scholar]
- 34.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153:S200–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaya J, Schipper HM. Oxysterols, cholesterol homeostasis, and Alzheimer disease. J Neurochem. 2007;102:1727–37. [DOI] [PubMed] [Google Scholar]
- 36.Fukumoto H, Rosene DL, Moss MB, Raju S, Hyman BT, Irizarry MC. Beta-secretase activity increases with aging in human, monkey, and mouse brain. Am J Pathol. 2004;164:719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci USA. 2007;104:13152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bazan NG, Marcheselli VL, Cole-Edwards K. Brain response to injury and neurodegeneration: endogenous neuroprotective signaling. Ann N Y Acad Sci. 2005;1053:137–47. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Y, Cui JG, Lukiw WJ. Natural secretory products of human neural and microvessel endothelial cells: Implications in pathogenic “spreading” and Alzheimer's disease. Mol Neurobiol. 2006;34:181–92. [DOI] [PubMed] [Google Scholar]
- 40.Shibata N, Kobayashi M. The role for oxidative stress in neurodegenerative diseases. Brain Nerve. 2008;60:157–70. [PubMed] [Google Scholar]
- 41.Akhtar RS, Ness JM, Roth KA. Bcl-2 family regulation of neuronal development and neuro-degeneration. Biochim Biophys Acta. 2004;1644:189–203. [DOI] [PubMed] [Google Scholar]
- 42.Bazan NG. Homeostatic regulation of photoreceptor cell integrity: significance of the potent mediator neuroprotectin D1 biosynthesized from docosahexaenoic acid: the Proctor Lecture. Invest Ophthalmol Vis Sci. 2007;48:4866–81. [DOI] [PubMed] [Google Scholar]
- 43.Yavin E, Brand A, Green P. Docosahexaenoic acid abundance in the brain: a biodevice to combat oxidative stress. Nutr Neurosci. 2002;5:149–57. [DOI] [PubMed] [Google Scholar]
- 44.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Culmsee C, Landshamer S. Molecular insights into mechanisms of the cell death program: role in the progression of neurodegenerative disorders. Curr Alzheimer Res. 2006;3:269–83. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y, Cui JG, Lukiw WJ. Reduction of sortilin-1 in Alzheimer hippocampus and in cytokine-stressed human brain cells. Neuroreport. 2007;18:1187–91. [DOI] [PubMed] [Google Scholar]
- 47.Sahlin C, Pettersson FE, Nilsson LN, Lannfelt L, Johansson AS. Docosahexaenoic acid stimulates non-amyloidogenic APP processing resulting in reduced Aβ levels in cellular models of Alzheimer's disease. Eur J Neurosci. 2007;26:882–9. [DOI] [PubMed] [Google Scholar]
- 48.Nelson TJ, Alkon DL. Protection against beta-amyloid-induced apoptosis by peptides interacting with beta-amyloid. J Biol Chem. 2007;282:31238–49. [DOI] [PubMed] [Google Scholar]
- 49.Lukiw WJ. Cholesterol and 24S-hydroxycholesterol trafficking in Alzheimer's disease. Expert Rev Neurother. 2006;6:683–93. [DOI] [PubMed] [Google Scholar]
- 50.Lukiw WJ. Emerging amyloid beta (Aβ) peptide modulators for the treatment of Alzheimer's disease (AD). Expert Opin Emerg Drugs. 2008;13:255–71. [DOI] [PubMed] [Google Scholar]
- 51.Rapoport SI. Arachidonic acid and the brain. J Nutr. 2008;138:2515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whelan J. (n-6) and (n-3) polyunsaturated fatty acids and the aging brain: food for thought. J Nutr. 2008;138:2521–2. [DOI] [PubMed] [Google Scholar]