Abstract
Flavocytochrome b558, the catalytic core of the phagocytic NADPH oxidase, mediates the transfer of electrons from NADPH to molecular oxygen to generate superoxide for host defense. Flavocytochrome b is a membrane heterodimer consisting of a large subunit gp91phox (NOX2) and a smaller subunit, p22phox. Although in neutrophils flavocytochrome b has been shown to localize to the plasma membrane and specific granules, little is known about its distribution in macrophages. Using immunofluorescent staining and live cell imaging of fluorescently tagged gp91phox and p22phox, we demonstrate in a Chinese hamster ovary cell model system and in RAW 264.7 and primary murine bone marrow-derived macrophages that flavocytochrome b is found in the Rab11-positive recycling endocytic compartment, as well as in Rab5-positive early endosomes and plasma membrane. Additionally, we show that unassembled p22phox and gp91phox subunits localize to the endoplasmic reticulum, which redistribute to the cell surface and endosomal compartments following heterodimer formation. These studies show for the first time that flavocytochrome b localizes to intracellular compartments in macrophages that recycle to the plasma membrane, which may act as a reservoir to deliver flavocytochrome b to the cell surface and phagosome membranes.
The generation of superoxide by the phagocytic NADPH oxidase provides antimicrobial defense essential to innate immunity. The NADPH oxidase is a multicomponent enzyme, comprised of a membrane-bound heterodimer, flavocytochrome b558, which consists of a large subunit, gp91phox (NOX2), and a small subunit, p22phox, and cytosolic regulatory subunits p67phox, p47phox, p40phox, and Rac (1). Genetic deficiencies in the NADPH oxidase impair superoxide production and result in chronic granulomatous disease, which is characterized by a marked inability to kill certain microorganisms, resulting in frequent and life-threatening fungal and bacterial infections (2). Proper targeting of the NADPH oxidase complex is also required for microbe killing. Thus, while several pathogens prevent NADPH oxidase assembly at the phagosome in macrophages and/or neutrophils as a means to evade killing by toxic oxidants (3–7), others such as Helicobacter plyori (8) disrupt enzyme targeting such that active NADPH oxidase complexes accumulate at the cell surface, and superoxide is generated in the extracellular space instead of the phagosome lumen.
The subcellular distribution of flavocytochrome b has been well characterized in neutrophils. gp91phox and p22phox are synthesized as separate polypeptides in the endoplasmic reticulum (ER).3 Human gp91phox is generated from a core protein of 58 kDa (9) that is subsequently glycosylated to gp65, a high-mannose 65-kDa form in the ER that binds to heme, allowing for heterodimer formation with p22phox (10–13). The heterodimer then traffics to the Golgi where gp91phox is further glycosylated to the mature, 91-kDa form (13). In resting neutrophils, the mature flavocytochrome is found primarily in the membrane of secondary granules, but it is also present in secretory vesicles, a type of endosome, and in the plasma membrane (14, 15). Upon neutrophil activation by soluble or particulate stimuli, these internal compartments are mobilized to the cell surface or phagosomal membrane (16).
Although the subcellular localization of flavocytochrome b is well established in neutrophils, its distribution in monocytes and, particularly, macrophages is incompletely defined, and the membrane compartments that contribute to delivery of flavocytochrome b to the phagosome are unknown. Studies using immunoelectron microscopy showed that relatively little flavocytochrome b localized to the plasma membrane in resting human monocytes, and it was mainly in small peroxidase-negative granules and vesicles, which, as in neutrophils, appear to be a pool that is rapidly mobilized to the plasma membrane upon cellular activation (16–18). Further characterization of these intracellular flavocytochrome b compartments in monocytes has not been reported. Studies in macrophages are even more limited. In human monocyte-derived macrophages, p22phox and gp91phox have been detected in the plasma membrane as well as in reticulo-vesicular intracellular structures, which were not further characterized (6, 19), and in the plasma membrane of murine peritoneal macrophages (20). A better understanding of the subcellular distribution of flavocytochrome b in macrophages may illuminate potential mechanism(s) that regulate localization of flavocytochrome b under homeostatic conditions and in response to phagocytic stimuli, and such understanding may also provide insight into how some pathogens subvert oxidase targeting to evade oxidative killing.
Thus, the goal of our studies was to more fully characterize the distribution and trafficking of flavocytochrome b in macrophages. We generated fluorescently tagged p22phox and gp91phox probes and rigorously tested their functionality in Chinese hamster ovary cells (CHO-K1). Initial studies in CHO cells also investigated the role of the individual subunits in directing trafficking of the heterodimer. Localization of flavocytochrome b was then examined in both RAW 264.7 murine macrophages and primary murine bone marrow-derived macrophages (BMDM). Our results show that endogenous and transfected flavocytochrome b localizes to similar intracellular compartments in these three cell types, and we demonstrate for the first time that macrophage flavocytochrome b is present in intracellular compartments that recycle to the plasma membrane.
Materials and Methods
Abs and reagents
Anti-gp91phox mAbs 54.1, and CL5 (21, 22), as well as anti-p22phox mAbs NS2 and 44.1 (23), were kindly provided by A. J. Jesaitis (Montana State University). 7D5 mAb (anti-gp91phox), collected from hybridoma cells kindly provided by M. Nakamura (Nagasaki University) (24), and a rabbit polyclonal anti-p22phox (25) were described previously. The following Abs were purchased: anti-calnexin (Stressgen), anti-Rab11 (Zymed Laboratories), anti-β-actin (Sigma-Aldrich), anti-GFP (Santa Cruz Biotechnology, catalog no. sc-8334), secondary HRP-conjugated Abs (Promega), and R-PE-conjugated rat anti-mouse IgG1 and anti-mouse CD16/CD32 (BD Pharmingen). Alexa Fluor-labeled secondary Abs and Alexa Fluor 647-labeled dextran (10,000 m.w., catalog no. D22914) were from Molecular Probes/Invitrogen. Mouse transferrin (Sigma-Aldrich) was iron loaded and tagged to Alexa Fluor 647 (Molecular Probes) to generate mTfn-AF647 as previously described (26). PBS (pH 7.2), penicillin/streptomycin, neomycin, trypsin/EDTA, DMEM with low glucose, α-MEM, Ham’s F12K medium, cell dissociation buffer, and Lipofectamine 2000 were purchased from Invitrogen. FCS and bovine growth serum were purchased from Hy-Clone Laboratories. All other reagents were from Sigma-Aldrich.
Fluorescently tagged p22phox and gp91phox expression constructs
Fluorescently tagged probes utilized in our studies are listed in Table I. For generation of p22phox full-length (wild-type) and p22phox C-terminal deletion fragments, primers specific to p22phox (available on request) were developed to amplify the desired region of p22phox and to add HindIII and EcoR1 restriction sites at the 5′ and 3′ regions of the amplified human p22phox cDNA. Amplified fragments were cloned into the HindIII and EcoR1 sites of the mammalian expression vector enhanced yellow fluorescent protein (eYFP)-N1 (Clontech) to generate p22phox (1–195)/eYFP-N1 (referred to as 22YFP), p22phox (1–171)/eYFP-N1 (referred to as 172YFP), p22phox (1–148)/eYFP-N1 (referred to as 149YFP), and p22phox (1–130)/eYFP-N1 (referred to as 131YFP) constructs. Full-length p22phox (1–195) was also subcloned from 22YFP into the mDsRED-N1 (Clontech) and the monomeric cyan fluorescent protein (mCFP)-N1 (provided by J. Swanson, University of Michigan) vectors using the HindIII and EcoR1 restriction sites to generate 22RED and 22CFP.
Table I.
Fluorescently tagged proteins used in this studya
| Fluorescently Tagged Proteins | Abbreviation |
|---|---|
| p22phox (1–195)/eYFP | 22YFP |
| p22phox (1–171)/eYFP | 172YFP |
| p22phox (1–148)/eYFP | 149YFP |
| p22phox (1–130)/eYFP | 131YFP |
| p22phox/mCFP | 22CFP |
| p22phox/mDsRED | 22RED |
| eCFP-C1/gp91phox | CFP91 |
| mCIT-C1/gp91phox | YFP91 |
| Rab5eGFP | Rab5GFP |
| Rab7eGFP | Rab7GFP |
| Rab11eCFP* | CFPRab11 |
| eGFP/Rab11a* | GFPRab11 |
The fluorescently tagged proteins used in this study are shown along with the abbreviated nomenclature. All transgenes were of human origin except for those marked with an asterisk, which are of rabbit origin. For constructs expressing YFP-tagged p22phox and C-terminal-deleted derivatives, the amino acids present in full-length (amino acids 1–195) and truncated forms are indicated.
The human gp91phox cDNA was excised from gp91phox/Bluescript KS+ (27) using BamH1, and ligated into the BamH1 site in pcDNA3.1+ (Invitrogen) to generate gp91phox/pcDNA3.1+. The gp91phox cDNA was then subcloned into the HindIII and SalI sites of eCFP-C1 (Clontech) by digesting gp91phox/pCDNA3.1+ with HindIII and XhoI to generate eCFP-C1/gp91phox (referred to as CFP91, sequence available upon request). This construct expressed gp91phox tagged at its N terminus with CFP, with an intervening 31 amino acids, where 22 were derived from polylinker sequence carried over from the insert in gp91phox/Bluescript KS+ and nine were from the multiple cloning site within eCFP-C1. gp91phox was further subcloned from eCFP-C1/gp91phox into the monomeric citrine fluorescent protein (mCIT)-C1 (provided by J. Swanson, University of Michigan) to generate mCIT-C1/gp91phox using the HindIII and XhoI multiple cloning sites to maintain the same linker between the fluorescent tag and gp91phox. Citrine is a derivative of YFP with a single amino acid change that reduces potential changes in fluorescence due to low pH, but otherwise functions essentially the same as YFP (28); for simplicity, mCIT-C1/gp91phox will be referred to as YFP91 (see Table I).
pEF- and pRK5-based vectors for expression of p47phox and p67phox were described previously (29). Rab11eCFP (30) and Rab11eGFP (31) were described previously. The following expression vectors were generous gifts: Rab5eGFP (G. Li, Washington University), Rab7eGFP (A. Wandinger-Ness, University of New Mexico), and FcγRIIa-GFP and PH-(PLCγ)-GFP (S. Grinstein, Hospital for Sick Children, Toronto).
Retroviral vectors
A YFP-expressing retroviral vector, MSCV-eYFPN1, was generated by subcloning the eYFP cDNA and the multiple cloning sites up to and including BglII at the 5′ end of eYFP from peYFP-N1 (Clontech) into the MSCV-pac vector backbone (Clontech) after removing the puromycin expression cassette. Human p22phox was then subcloned from p22eYFP-N1 (described above) using the HindIII and EcoR1 sites and inserted into pMSCV-eYFP-N1 to generate pMSCV-p22eYFP-N1 (referred to as MSCV-22YFP). Retroviruses were packaged using a pantropic retroviral expression system (Clontech) and stored at −80°C until use, according to the manufacturer’s instructions.
Transgene expression in CHO-K1 and RAW 264.7 cell lines
CHO-K1 and RAW 264.7 cell lines utilized in these studies are listed in Table II. Parental CHO cells or derivatives expressing NADPH oxidase transgenes were transfected using Lipofectamine 2000 according to manufacturer’s instructions. CHO-22 and CHO-91-67-47, which will be referred to as CHO-22 and CHO-91 (32), respectively, were previously generated for stable expression of untagged p22phox and gp91phox. Similarly, CHO-K1 cells were transfected with YFP91 or cotransfected with p47phox and p67phox to generate CHO-YFP91 and CHO-47-67 cell lines. 22YFP was transfected in the CHO-91 cell line to generate CHO-91-22YFP cells. CHO-91-22YFP cells express p47phox and p67phox, but for the purposes of these experiments, the absence or presence of p47phox and p67phox has no significant effect on the localization of p22phox, which is illustrated by the similar distributions of 22CFP, expressed in CHO-YFP91 cells that lack p47phox and p67phox, and 22YFP, expressed in CHO-91-22YFP cells that express p47phox and p67phox (see Figs. 2B and 3C). For stable expression of YFP-tagged proteins in CHO-YFP91 and CHO-91-22YFP cell lines, cells were selected in 1.6 mg/ml neomycin for at least 2 wk, followed by flow cytometric cell sorting (FACSAria, BD Biosciences) to collect strongly fluorescent cells and to discard cells with little to no fluorescence. Cell sorting increased the percentage of positive 22YFP cells from 18% to 75% in the CHO-91-22YFP cell line and 22% to 70% in the CHO-YFP91 cell line. After sorting, CHO-YFP91 cells were maintained in 0.8 mg/ml neomycin, and CHO-91-22YFP cells were maintained in 0.8 mg/ml neomycin, 1 μg/ml puromycin, and 0.2 mg/ml hygromycin. CHO cells were cotransfected with expression vectors for p47phox and p67phox, and clones were obtained by limiting dilution in medium containing puromycin and hygromycin as described previously (29). CHO parental and derivative cell lines were maintained in Ham’s F12 medium, 10% bovine growth serum, 0.15% sodium bicarbonate, 10 U/ml penicillin, and 100 μg/ml streptomycin as previously described (33).
Table II.
CHO-K1 and RAW 264.7 cell lines used in this studya
| Cell Line | Parental Cells | Transgene(s) |
|---|---|---|
| CHO-WT | CHO-K1 | |
| CHO-22* | CHO-K1 | p22phox/PGKneo |
| CHO-YFP91 | CHO-K1 | mCIT/gp91phox |
| CHO-91 | CHO-K1 | MFG-S/gp91phox 1 p47phox/PGKhygro 1 p67phox/PGKpuro |
| CHO-91-22YFP | CHO-91 | p22phox (1–195)/eYFP |
| CHO-47-67* | CHO-K1 | p47phox/PGKhygro 1 p67phox/PGKpuro |
| RAW-WT | RAW 264.7 | |
| RAW-22YFP* | RAW 264.7 | p22phox (1–195)/eYFP |
| RAW-YFP91 | RAW 264.7 | mCIT-C1/gp91phox |
The nomenclature for CHO-K1 and RAW264.7 cell lines used in this study are shown, along with the parental cell line and transgenes. An asterisk denotes a clonal cell line.
FIGURE 2.
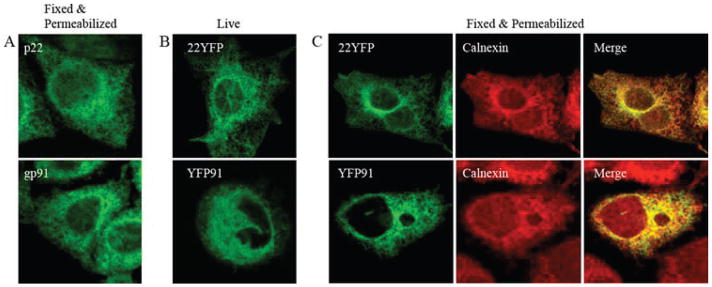
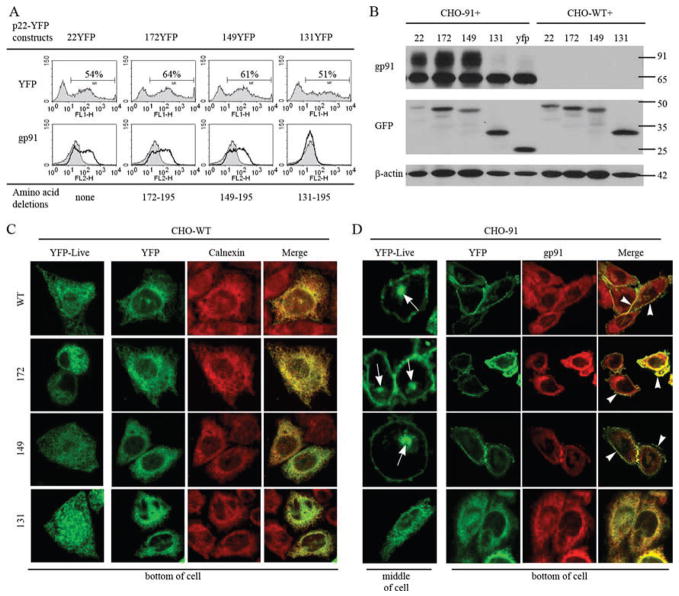
The subunits of flavocytochrome b, gp91phox, and p22phox localize to the ER when expressed individually in CHO cells. A, CHO cells stably expressing untagged p22phox (top panel) or gp91phox (bottom panel) were fixed, permeabilized, and stained for p22phox (mAb 44.1) or gp91phox (mAb 54.1). 22YFP or YFP91 transiently expressed in CHO-WT cells was imaged 48 h posttransfection in (B) living cells and (C) cells fixed, permeabilized, and costained for calnexin. Merged images show 22YFP and calnexin (top panel) and YFP91 and calnexin (bottom panel), and they are single plane z-stack slices acquired from the bottom of the cell.
FIGURE 3.
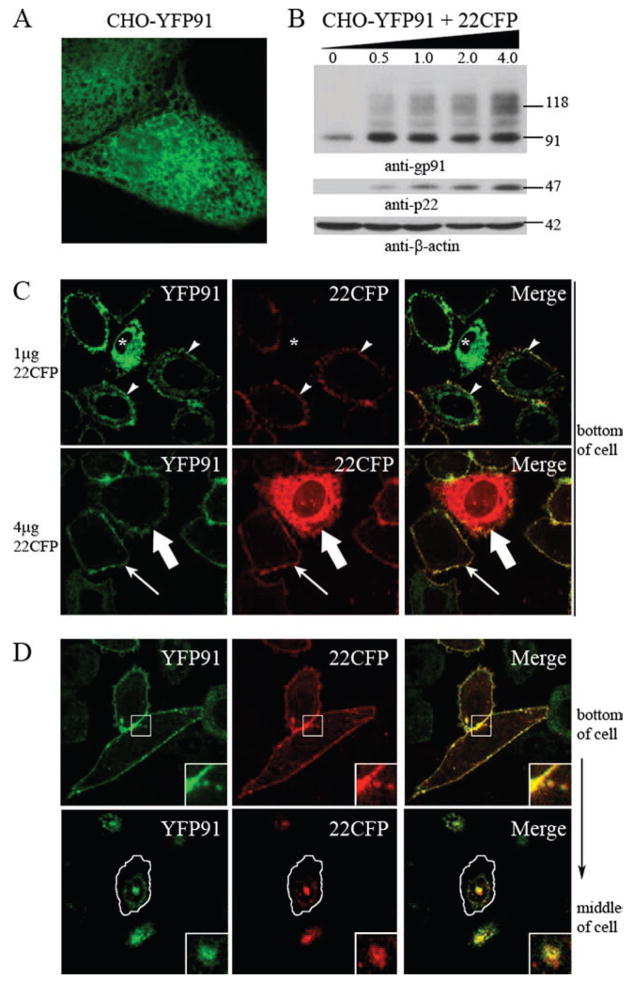
Heterodimers of p22phox and gp91phox traffic to the plasma membrane, while the subunit in “excess” localizes to the ER. CHO-YFP91 cells were transiently transfected with 0.5, 1.0, 2.0, or 4.0 μg of the 22CFP vector. A, YFP91 stably expressed in CHO-YFP91 cells was imaged in living cells. A single-plane z-stack slice acquired from the bottom of the cell is shown. B, Cell lysates were analyzed for gp91phox, p22phox, and β-actin protein expression 48 h posttransfection (n = 2 experiments in which protein expression was evaluated). C, Confocal microscopy of YFP91 (left column), 22CFP (middle column), and merged images (right column) following transient expression of 1.0 μg of 22CFP (top panels) or 4.0 μg of 22CFP (bottom panels) 72 h posttransfection of CHO-YFP91 cells. YFP91 localized to the ER in cells that lacked expression of 22CFP (asterisks), to the ER and plasma membrane in some cells expressing 22CFP (arrowheads), and to the plasma membrane but not the ER in other CHO-YFP91 cells that coexpressed 22CFP (arrows). 22CFP localized to the plasma membrane in most CHO-YFP91 cells (arrows), although it was present in both the plasma membrane and ER in some cells overexpressing 22CFP (wide arrows). Single-plane z-stack slices acquired from the bottom of the cell are shown. D, YFP91 and 22CFP colocalize in CHO-YFP91 cells transiently transfected with 0.5 μg of 22CFP. Single-plane z-stack slices acquired from the bottom and middle of the cell are shown.
RAW 264.7 macrophages were transfected using a cell line Nucleofector solution kit V (Amaxa), according to the manufacturer’s instructions. RAW 264.7 cells were transfected with the 22YFP vector to generate a RAW-22YFP stable cell line. After at least 2 wk in selection in 1.6 mg/ml neomycin, RAW-22YFP clones were selected by limiting dilution in 96-well plates followed by screening using flow cytometry for YFP. RAW 264.7 cells were also transfected with YFP91, FcγRIIa-GFP, or PH-(PLCγ)-GFP and selected in 0.8 mg/ml neomycin to generate RAW-mCIT-C1/gp91phox (referred to as RAW-YFP91), RAW-FcγRIIa-GFP (referred to as RAW-FcR-GFP), and RAW-PH-(PLCγ)-GFP (referred to as RAW-PH-GFP) cell lines. RAW 264.7 cells were maintained with or without selection antibiotics in low-glucose DMEM, 10% heat-inactivated FCS, 10 U/ml penicillin, and 100 μg/ml streptomycin.
For passaging of cells in culture or collecting cells for analysis, CHO and RAW 264.7 cells were harvested using trypsin (0.05% in EDTA) as described previously for CHO cells (33).
Mice
Homozygous nmf333 mice (A.B6-Tyr+/J genetic background), which harbor a T to C point mutation in the Cyba gene encoding p22phox and lack expression of p22phox, were kindly provided by Botond Banfi (University of Iowa, Iowa City, IA) (34). C57BL/6J mice were obtained from The Jackson Laboratory.
Retroviral transduction of murine bone marrow (BM) and macrophage differentiation
Retroviral transduction of murine BM cells with MSCV-22YFP was performed as previously described (35). Briefly, 3 days following i.p. injection of 150 mg/kg 5-fluorouracil, BM cells were harvested from femurs and tibias by flushing with α-MEM. The day of BM harvest was considered day 0. BM cells were pelleted and resuspended in prestimulation media (α-MEM with 20% heat-inactivated FCS, 100 ng/ml SCF, 100 U/ml IL-6, 10 U/ml penicillin, and 100 μg/ml streptomycin) for 48 h. On days 2–4, cells were transduced every 24 h with retrovirus supernatant. On day 4, after the last 4 h of transduction, the combined suspended and adherent cells were collected using cell dissociation buffer and differentiated into BMDM as described previously, with some minor changes (36). Transduced BM cells (5 × 105) were suspended in 3 ml of α-MEM media containing 20% heat-inactivated FCS, 25 ng/ml murine M-CSF (PeproTech), 10 U/ml penicillin, and 100 μg/ml streptomycin instead of DME-10F media containing 20% heat-inactivated FCS and 30% L cell-conditioned medium. Additionally, we initially plated BM cells in one well of a 6-well tissue culture dish for 3 days in differentiation media and then transferred the nonadherent cells to a 150-mm petri dish (non-tissue culture plastic) for an additional 3–4 days in differentiation media, which replaced the previous method of differentiation in petri dishes for 6–7 days.
SDS-PAGE and Western blotting
Ten micrograms of total protein from Triton X-100 cell extracts was added to each well of a 9% or 12% SDS-polyacrylamide gel, electrophoresed, transferred to nitrocellulose membranes, and probed for protein expression by immunoblotting as described (29, 33). Primary Abs were used at the following dilutions in Tris-buffered saline with 0.1% Tween 20 (TBST): rabbit anti-GFP, which also recognizes YFP (1/1,000), mouse anti-gp91phox (1/1,000 –5,000 for either 54.1 or CL5), rabbit anti-p22phox (1/5,000 –10,000), mouse anti-p22phox (1/500 –1,000), mouse β-actin (1/10,000), and secondary HRP-conjugated Abs (1/10,000).
Flow cytometry
gp91phox cell surface expression and the transfection efficiency of fluorescently tagged proteins were evaluated by flow cytometry. CHO cells were suspended at 0.5–1.0 × 106 cells/ml in FACS buffer (0.1% BSA in PBS) with 10% normal goat serum for 30 min and kept at 4°C throughout the staining procedure. 7D5 supernatant and PE-conjugated secondary Ab (1/200) were diluted in FACS buffer and incubated with cells for 30 min. Fluorescence was measured by FACScan (BD Biosciences). In some experiments, stained cells were fixed with 1% paraformaldehyde at 4°C overnight and subsequently analyzed 24–48 h later, with results the same as those obtained with freshly stained, unfixed cells.
NADPH oxidase activity
Superoxide dismutase-inhibitable NADPH oxidase activity in CHO cells was measured by isoluminol-ECL following stimulation with arachidonic acid as described previously (33). The number of cells per assay was increased from 1 × 105 to 2 × 105 cells/well for CHO-22 cells transfected with p47phox, p67phox, and CFP91 or CFP (Fig. 1D) due to lower transfection efficiency when introducing three plasmids.
FIGURE 1.
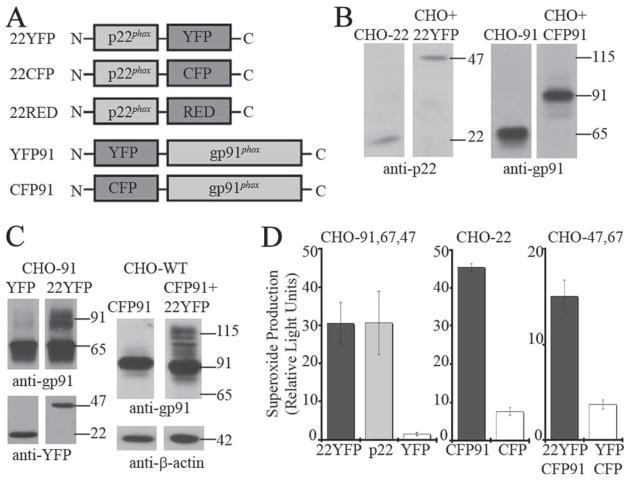
Fluorescently tagged p22phox and gp91phox form functional heterodimers. A, Schematic of constructs encoding fluorescently tagged p22phox and gp91phox. B, 22YFP or CFP91 were transiently expressed in CHO-WT cell lines. Cell lysates prepared 48 h posttransfection were analyzed by Western blotting using polyclonal anti-p22phox and monoclonal anti-gp91phox (CL5) Abs. Cell lysates from CHO-22 and CHO-91 cells were used as controls. C, Cell lysates from CHO-91 cells transiently expressing YFP or 22YFP, as well as CHO-WT cells expressing CFP91 or coexpressing CFP91 and 22YFP, were analyzed by Western blotting at 48 h posttransfection for maturation of untagged and CFP-tagged gp91phox (mAb CL5). YFP and 22YFP protein expression were validated with an Ab to YFP. Data shown are representative of at least three independent experiments. D, NADPH oxidase activity was measured using isoluminol chemiluminescence in CHO cells transiently and/or stably expressing p47phox and p67phox with untagged or fluorescently tagged flavocytochrome subunits as indicated. Integrated relative light units are shown. From left to right: CHO-91-67-47 cells transfected with 22YFP (n = 5), untagged p22phox (n = 3), or YFP (n = 2); CHO-22 cells transfected with p47phox, p67phox, CFP91 (n = 2), or CFP (n = 1); CHO-47-67 cells transfected with 22YFP and CFP91 (n = 5) or YFP and CFP (n = 2). Mean ± SD of data for the number of experiments listed above.
Labeling of cells with fluorescently tagged ligands
Cells plated on coverslips were washed once in their corresponding media (37°C, no serum) and then incubated with mTfn-AF647 or Alexa Fluor 647-labeled dextran (Dex-AF647) diluted in the same media for the times indicated. For live cell imaging, cells plated on MatTek coverslip dishes were washed once in PBS (37°C) and then incubated with fluorescently tagged ligands diluted in 0.01 M HEPES buffer in PBSG. mTfn-AF647 (20 μg/ml) was used for labeling recycling endosomes in CHO cells and 5 μg/ml for labeling recycling endosomes in macrophages. Dex-AF647 (10 μg/ml) was used for labeling lysosomes in CHO cells. In some experiments, cells were chased with media (37°C) as described.
Serum opsonization of zymosan
Human serum was collected from six donors as described previously (37) using a protocol approved by the Institutional Review Board of Indiana University, and all donors provided informed consent. Zymosan A (from Saccharomyces cerevisiae; Sigma-Aldrich) was suspended at 20 mg/ml in PBS, sonicated at maximum speed on ice for 10-s intervals (repeated five times), washed in PBS, and resuspended to 20 mg/ml. Sonicated zymosan was opsonized with human serum as described previously (37).
Synchronized phagocytosis assay
RAW 264.7 cells and BMDM were plated on coverslips at ~50–70% confluency 24 h before phagocytosis assay. Serum-opsonized zymosan (SOZ) was diluted 1/100–1/500 to result in approximately three to five zymosan particles per cell. After adding SOZ, cells were centrifuged at room temperature for 2 min at 800 × g and then fixed immediately or incubated for an additional 13 min at 37°C for a total of 2 or 15 min to allow for internalization. Cells were then washed twice with PBS (room temperature) and then fixed, permeabilized, and stained as described below.
Indirect immunofluorescence microscopy
CHO and RAW 264.7 cells were plated on glass coverslips coated with 0.01% gelatin, while BMDM were plated on uncoated, acid-washed coverslips. Following 24–48 h culture, when cells were 50–80% confluent, monolayers were washed once in PBS, fixed in 4% paraformaldehyde for 10 min at room temperature, permeabilized in methanol/acetone for 5 min at 4°C as described (38), washed in PAB (2% BSA, 0.05 g/L NaN3 in PBS), and then blocked in 10% goat serum in PAB for 30 min at room temperature or overnight at 4°C. Primary Abs were diluted in 5% goat serum in PAB at the following concentrations: anti-calnexin (1/400), anti-Rab11 (1/400), 54.1 mAb anti-gp91phox (1/1000), 44.1 mAb anti-p22phox (1/2), and anti-CD16/CD32-PE (1/50). After 1 h of incubation, coverslips were washed and incubated with Alexa Fluor secondary Abs (1/600) in 10% goat serum in PAB for 45–60 min at room temperature. Coverslips were washed and mounted with Dako fluorescent mounting media. The specificity and optimal concentration of mAb 54.1 for immunofluorescence microscopy was determined by comparing gp91phox in CHO parental to CHO-91-67-47 cells (not shown), and by comparing wild-type BMDM to BMDM obtained from gp91phox-null chronic granulomatous disease mice (39).
Live cell imaging
Cells were plated onto MatTek coverslip dishes 24–72 h before imaging to result in ~50–70% confluency at the time of analysis. Cells were washed with PBSG (29) and then imaged in 0.01 M HEPES buffer (Sigma-Aldrich) in PBSG at 37°C.
Confocal microscopy
Images from cells grown on coverslips or MatTek coverslip dishes were acquired on one of two confocal systems: a Zeiss LSM510 or a PerkinElmer-Cetus Ultraview mounted on a Nikon TE 2000U inverted microscope equipped with an Andor CCD camera. On both systems, a vertical series of images was collected using a Nikon 100×, NA 1.4× oil immersion planapochromatic objective. For most images, the optimal single plane for each subcellular compartment is shown as follows: for the ER or the plasma membrane, the substrate-adherent surface is shown, and for the endocytic recycling compartment, the middle of the cell is shown.
Image analysis using ImageJ
Images acquired from confocal systems were opened in ImageJ and single plane images (0.16–0.5 μm Z sections) were saved as individual TIFF files after only cropping or adjusting the brightness and/or contrast; no other modifications were performed. For some images acquired from BMDM, a vertical stack was merged using the z-stack projection tool of ImageJ (40). A total of at least 50 cells from at least three independent experiments were analyzed unless otherwise stated in the figure legend.
Results
Fluorescently tagged flavocytochrome b subunits form functional heterodimers
In neutrophils, the stability of the individual flavocytochrome b subunits (gp91phox and p22phox) is dependent on heterodimer formation, and free monomers are rapidly degraded by the proteasome. However, in CHO cells, which lack detectable endogenous gp91phox or p22phox, the individual subunits are stable and can be highly expressed (32, 33). In the absence of p22phox, gp91phox is expressed in CHO cells predominantly as gp65, with little detected on the cell surface. Coexpressed gp91phox and p22phox form heterodimers and coimmunoprecipitate in CHO cells, which correlates with carbohydrate maturation of gp91phox, resulting in an increase in size to 91 kDa and a marked increase in gp91phox cell surface expression (33). These results suggest that either p22phox or the heterodimer harbor localization signals that direct trafficking of flavocytochrome b to the cell surface, although the intracellular compartments where each subunit was localized in the absence or presence of its binding partner were not well defined in these studies.
To examine the subcellular distribution of individual subunits and the flavocytochrome b heterodimer, we developed constructs to express fluorescently tagged derivatives of p22phox and gp91phox to visualize their location by confocal microscopy. YFP-, CFP-, and RED-tagged constructs were generated (Fig. 1A) to allow analysis of subcellular targeting and the extent of subunit colocalization. Tags were placed on the C terminus of p22phox and the N terminus of gp91phox, as depicted in Fig. 1A. We hypothesized that tags at these positions would less likely impair protein function based on previous studies that found that the N terminus of p22phox and the C terminus of gp91phox were sensitive to mutagenesis and/or placement of an epitope tag (33, 41). Fluorescently tagged subunits were each expressed in parental CHO cells following transient transfection, and Western blotting of cell lysates confirmed that the chimeras were expressed at the predicted molecular masses (Fig. 1B and not shown). For example, with the addition of the YFP or CFP tag (~25 kDa), 22YFP was expressed at 47 kDa (Fig. 1B) and CFP91 was expressed predominantly as a 91-kDa protein, corresponding to gp65 detected in CHO-91 cells (Fig. 1B).
It was essential that the tagged subunits were capable of heterodimer formation. As mentioned earlier, this correlates with increased molecular mass of gp91phox as a result of carbohydrate maturation, and increased gp91phox cell surface expression. In CHO-91 cells, transient expression of 22YFP but not YFP induces formation of the 91-kDa form of gp91phox, consistent with heterodimer formation between 22YFP and gp91phox (Fig. 1C, left panel). Conversely, transient expression of CFP91 and 22YFP resulted in increased maturation of CFP91 to the 115-kDa form of CFP91, showing that CFP91 is capable of heterodimer formation with fluorescently tagged p22phox (Fig. 1C, right panel). Additionally, untagged and fluorescently tagged gp91phox cell surface expression increased when coexpressed with tagged or untagged p22phox (supplemental Fig. S1).4 Thus, fluorescent tags on p22phox or gp91phox do not interfere with heterodimer formation.
Next, we analyzed whether flavocytochrome b containing the fluorescent-tagged subunits was functional. Exogenous expression of p22phox, gp91phox, p47phox, and p67phox in CHO cells reconstitutes NADPH oxidase activity (32, 33). Transient expression of 22YFP but not YFP in CHO cells stably expressing gp91phox, p47phox, and p67phox supported superoxide production similar to transient expression of untagged p22phox (Fig. 1D, left panel). CFP-tagged gp91phox but not CFP alone was also functional when transiently coexpressed with p47phox and p67phox in CHO-22 cells (Fig. 1D, middle panel). Transient coexpression of CFP91 and 22YFP in CHO cells with stable expression of p47phox and p67phox also supported NADPH oxidase activity (Fig. 1D, right panel). These results establish that fluorescently tagged gp91phox and p22phox form functional heterodimers with each other and with the untagged partners.
The individual subunits p22phox and gp91phox localize to the ER in CHO cells
The intracellular compartments in CHO cells where p22phox and gp91phox reside in the absence or presence of each other were not well characterized in previous studies (32, 33). In promyelocytic PLB-985 cells, which are an undifferentiated neutrophil precursor cell line, unassembled gp91phox and p22phox reside in the ER membrane before heterodimer formation (13). To determine whether the distribution of unassembled subunits in CHO cells is similar to neutrophil precursors and to examine whether placement of the fluorescent tag altered this distribution, the subcellular location of untagged and fluorescently tagged p22phox and gp91phox was evaluated by confocal microscopy. Both untagged p22phox and gp91phox in CHO-22 and CHO-91 cell lines, respectively, had an intracellular reticular distribution as visualized by immunofluorescence microscopy (Fig. 2A). A similar distribution for 22YFP and for YFP91 when each was transiently expressed in CHO-WT cells was detected in living cells (Fig. 2B) and in cells fixed and permeabilized (Fig. 2C, left panel). These results show that neither subunit localized predominantly to the plasma membrane and that neither the fluorescent tag nor cell fixation and permeabilization altered the apparent location of p22phox or gp91phox.
The intracellular reticular distribution of p22phox and gp91phox shown in Fig. 2, A and B, is characteristic of the ER. Moreover, 22YFP and the ER marker calnexin strongly colocalized as shown in Fig. 2C (top right panel). Similar data were obtained for YFP91 and calnexin (Fig. 2C, bottom panels). Thus, these data indicate that p22phox and gp91phox localize primarily to the ER when each is expressed individually in CHO-WT cells, consistent with studies performed using immature myeloid cells (13).
Heterodimers of p22phox and gp91phox traffic to the plasma membrane
Formation of heterodimers upon coexpression of p22phox and gp91phox in CHO cells correlates with a marked increase in gp91phox cell surface expression as detected by flow cytometry (33). Based on these studies and our present results localizing the individual subunits to the ER, we hypothesized that heterodimer formation may direct trafficking of flavocytochrome b to the plasma membrane. To test this, we transiently expressed increasing amounts of a plasmid for 22CFP into CHO cells with stable expression of YFP91 (CHO-YFP91 cells). Western blot analysis showed that increasing expression of 22CFP resulted in increasing amounts of the mature ~115-kDa form of YFP91 (Fig. 3A), consistent with increasing formation of the flavocytochrome b heterodimer.
We next evaluated the subcellular distribution of YFP91 and 22CFP following transient expression of increasing amounts of 22CFP in CHO-YFP91 cells. As previously shown for transiently expressed YFP91, stably expressed YFP91 was present in a reticular pattern in the absence of 22CFP (Fig. 3A), consistent with localization to the ER (Fig. 2, A and B). The reticular distribution of YFP91 disappeared in parallel with increasing expression of 22CFP and was replaced by enrichment of YFP91 in the plasma membrane (Fig. 3C, arrowheads and arrows). The change in YFP91 distribution was dependent on the amount of 22CFP. In transfections with only 1.0 μg of 22CFP plasmid DNA, cells showed localization of YFP91 to either the ER (asterisks), to the ER and plasma membrane (arrowheads), or to the plasma membrane (arrows) (Fig. 3C). In contrast, for cells transfected with 4.0 μg of 22CFP plasmid DNA, YFP91 is almost always detected in the plasma membrane.
Parallel effects were seen for the distribution of p22phox. Unlike the ER localization of p22phox when expressed in the absence of gp91phox (Fig. 2, A and B), 22CFP expressed in the presence of YFP91 localized to the plasma membrane (Fig. 3C). In some cells overexpressing 22CFP, 22CFP was present both in the plasma membrane and a reticular distribution (Fig. 3C, wide arrows), but only the plasma membrane protein colocalized with YFP91, suggesting that the reticular pattern corresponded to unassembled 22CFP subunits expressed in excess of YFP91. Thus, the partner in excess stays in the ER while the assembled heterodimer localizes to the plasma membrane. Taken together, these results clearly illustrate a change in subunit location with their coexpression, and they show that heterodimer formation is accompanied by trafficking to the plasma membrane. These results also support a requirement for heterodimer formation, rather than determinants from one of the individual subunits, to efficiently traffic flavocytochrome b to the cell surface.
Of interest, we also detected flavocytochrome b in CHO-YFP91 cells coexpressing 22CFP in intracellular vesicles near the plasma membrane (Fig. 3D, top panel) and in a perinuclear location (Fig. 3D, bottom panel). These findings suggested that a portion of flavocytochrome b in CHO cells resides in endocytic compartments that may recycle to the plasma membrane. The perinuclear vesicles were best seen in images collected from the middle of the cell (Fig. 3D, bottom panel), consistent with the perinuclear accumulation of the endocytic recycling compartment characteristic for CHO cells (42).
To exclude the alternative possibility that coexpression of gp91phox and p22phox and not heterodimer formation per se changed the subcellular distribution of the individual subunits, CHO-WT cells were transiently cotransfected with 22YFP and a H115L mutant of gp91phox (43). This residue is thought to contribute to heme binding, and replacement with a leucine residue prevents heme incorporation and heterodimer formation with p22phox (43). Western blot analysis showed that gp91phox H115L was expressed in CHO cells predominantly as the 58-kDa and 65-kDa forms with no apparent gp91phox maturation (supplemental Fig. S2), as seen previously in PLB-985 promyelocyte and COS7 cell lines (43). Confocal analysis of 22YFP and gp91phox H115L showed both proteins localized to a reticular compartment that resembled the ER (supplemental Fig. S2). These results support the notion that heterodimer formation is required to efficiently traffic p22phox and gp91phox to the plasma membrane, while the unassembled subunits remain in the ER.
Characterization of a CHO-91-22YFP cell line for evaluating trafficking of flavocytochrome b in CHO cells
To characterize the intracellular compartments to which flavocytochrome b is trafficked in CHO cells (Fig. 3), we developed a CHO-91 cell line that stably expressed 22YFP (CHO-91-22YFP cells) to allow simultaneous localization of flavocytochrome b and tagged markers of different membrane compartments. Western blot analysis and flow cytometry showed increased gp91phox maturation to the 91-kDa form and increased gp91phox cell surface expression in CHO-91-22YFP cells compared with CHO-91 cells (Fig. 4, A and B). However, some gp65 was still present (Fig. 4A), indicating that not all the gp91phox was incorporated into flavocytochrome b. Confocal microscopy of CHO-91-22YFP cells showed that 22YFP localized to the plasma membrane and intracellular vesicles near the plasma membrane, as well as a perinuclear compartment (Fig. 4C, left panels), similar to CHO-91 cells that transiently expressed 22YFP (Fig. 3). 22YFP did not colocalize with the ER marker calnexin in CHO-91-22YFP cells (Fig. 4C, middle panels), as shown in images collected from the bottom of the cell. In contrast, gp91phox was present in both a reticular pattern, which colocalized with calnexin (Fig. 4E, arrowheads), and at the plasma membrane, where it colocalized with 22YFP (Fig. 4D, arrowheads). Taken together, these data indicate that gp91phox was expressed in excess of 22YFP in the CHO-91-22YFP cell line, resulting in unassembled gp91phox accumulation in the ER, while 22YFP and gp91phox colocalized in the plasma membrane and intracellular compartments distinct from the ER. Thus, we used 22YFP as an indicator of flavocytochrome b targeting in this cell line.
FIGURE 4.
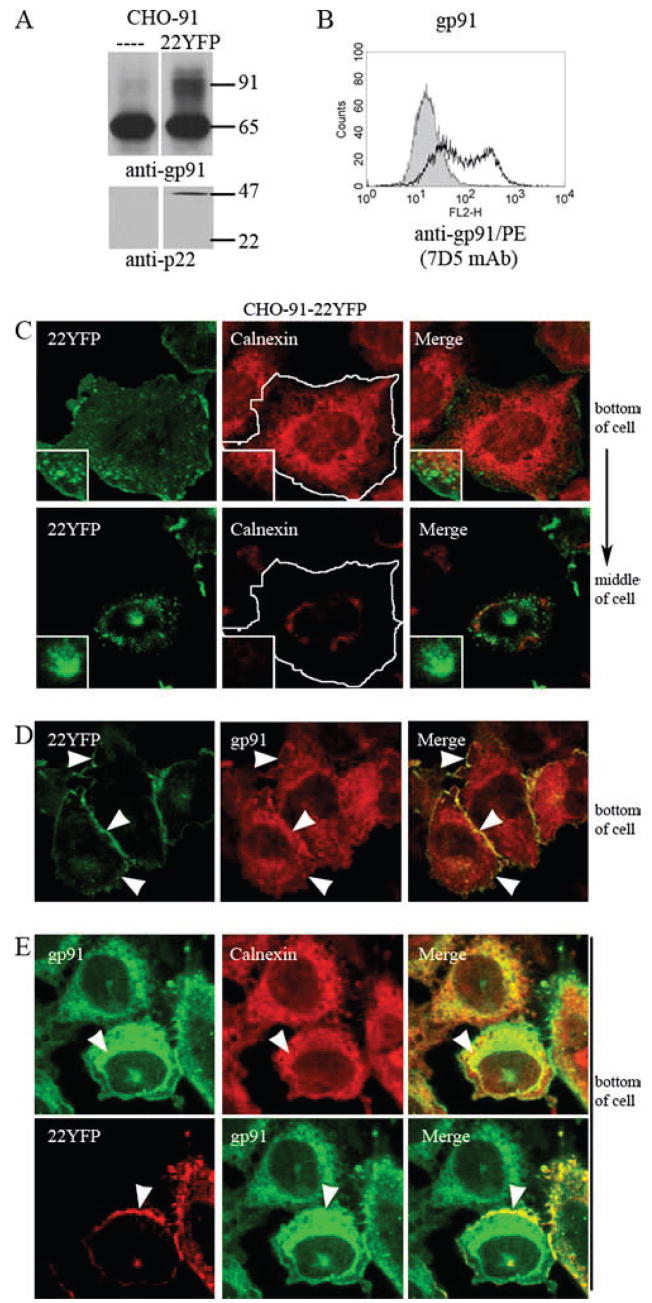
Characterization of model cell line, CHO-91-22YFP, to investigate trafficking of flavocytochrome b. A, Immunoblots of cell lysates from CHO-91 and CHO-91-22YFP cells probed with mAb 54.1 and mAb NS2 to detect gp91phox maturation and 22YFP protein expression, respectively. B, Cell surface gp91phox detected by 7D5 (mAb) using flow cytometry in CHO-91 cells (gray) and CHO-91-22YFP cells (black line). CHO-91-22YFP cells were fixed, permeabilized, and stained to detect calnexin (C) gp91phox (D), or gp91phox and calnexin (E). Arrowheads denote areas of colocalization. Single-plane z-stack slices acquired from the bottom and/or middle of the cell are shown.
Flavocytochrome b localizes to the endocytic recycling compartment in CHO cells
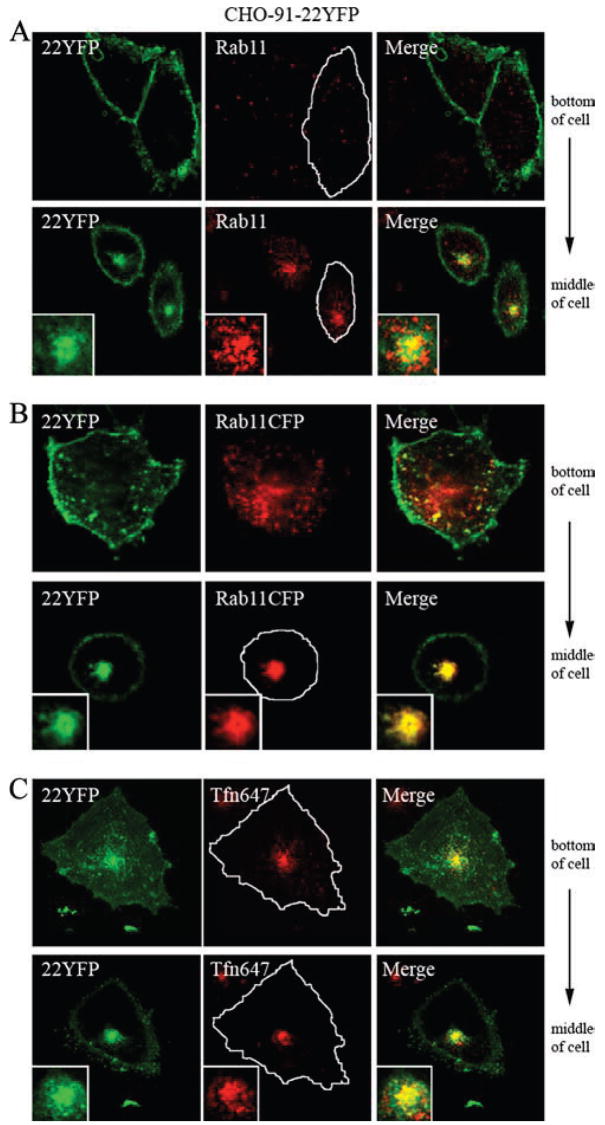
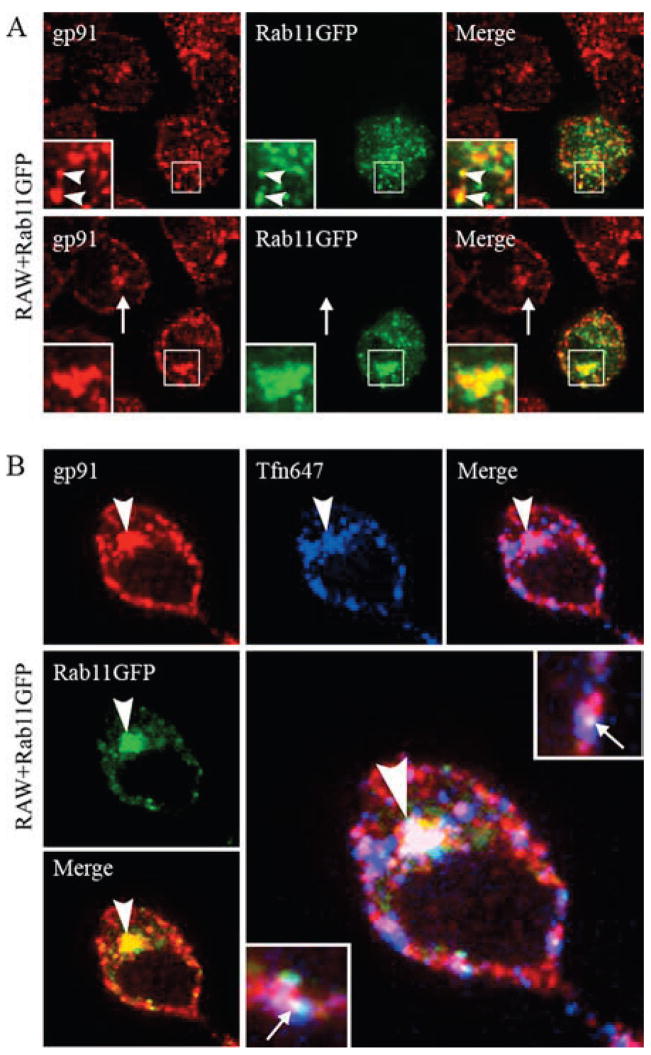
The CHO-91-22YFP cell line described above (Fig. 4) was used to investigate trafficking of flavocytochrome b via detection of the YFP tag on p22phox. The perinuclear location of flavocytochrome b-containing vesicles (Figs. 3D and 4C) is characteristic of the endocytic recycling compartment in some cell types, including CHO cells (44, 45), which is defined by the GTPase Rab11 (42). As shown in Fig. 5A, 22YFP strongly colocalized with Rab11 near the nucleus of CHO-91-22YFP cells. Transiently expressed Rab11CFP and 22YFP also colocalized in CHO-91-22YFP cells (Fig. 5B). In contrast, 22YFP expressed in CHO cells in the absence of gp91phox did not colocalize with Rab11 (not shown). These results suggest that a portion of flavocytochrome b in CHO cells resides in the endocytic recycling compartment.
FIGURE 5.
Flavocytochrome b localizes to the Rab-11-positive endocytic recycling compartment in CHO cells. A, CHO-91-22YFP cells were fixed, permeabilized, and stained for Rab11. Insets within the merged image (bottom panel) show colocalization of 22YFP and Rab11 in a perinuclear compartment. B, CHO-91-22YFP cells transiently expressing Rab11CFP were imaged in living cells 24 h posttransfection. C, CHO-91-22YFP cells were incubated with Alexa Fluor 647-conjugated transferrin (TfnAF647, 20 μg/ml) for 5 min at 37°C followed by a 25-min chase at 37°C to label the endocytic recycling compartment. Living cells were imaged, and colocalization of 22YFP and Tfn was evaluated in the merged image (n = 2 experiments where 22YFP and Tfn colocalization was evaluated). Single-plane z-stack slices taken from the bottom and middle of the cell are shown.
Although widely used as a marker for recycling endosomes (42, 46), Rab11 may also traffic between the trans-Golgi network and the cell surface (47). Thus, to better define the Rab11-positive perinuclear compartment in our studies, we also analyzed the extent to which flavocytochrome b colocalized with internalized transferrin, a marker for recycling endosomes that does not traffic to the Golgi. Transferrin is endocytosed upon binding to the transferrin receptor, is subsequently delivered to the recycling compartment, and then traffics back to the plasma membrane (26, 48). Recycling endosomes in CHO-91-22YFP cells were loaded with tagged transferrin, Tfn-AF647, using a 5-min pulse followed by a 25-min chase. 22YFP showed strong colocalization with Tfn-AF647 in a perinuclear location as shown in the merged image (Fig. 5C, inset). These findings provide additional evidence that flavocytochrome b recycles from the plasma membrane to the endocytic recycling compartment.
A recent model of endosome sorting (49) indicates Rab5-positive early endosomes branching into one of three pathways: 1) Rab4-positive endosomes, which rapidly recycle to the plasma membrane without entering the Rab11-positive compartment; 2) the Rab11-positive endocytic recycling compartment for slow trafficking back to the plasma membrane; or 3) Rab7-positive late endosomes that subsequently fuse with lysosomes for cargo degradation. To determine whether flavocytochrome b also accumulated on compartments in the degradative pathway, we evaluated the extent of flavocytochrome b colocalization with Rab7 and with lysosomes preloaded with dextran. To this end, 22RED and Rab7GFP were transiently coexpressed in CHO-91 cells, using only a small amount of each plasmid (1 μg). 22RED localized to the plasma membrane in CHO-91 cells and had increased expression in the perinuclear compartment (supplemental Fig. S3), similar to 22YFP in CHO-91-22YFP cells (Fig. 5). However, 22RED did not colocalize with Rab7GFP (supplemental Fig. S3). Colocalization of flavocytochrome b with lysosomes was analyzed in CHO-91-22YFP cells after a 50-min incubation with fluorescently tagged dextran (Dex-AF647) to label lysosomes. 22YFP did not colocalize with Dex-AF647 (supplemental Fig. S3), further indicating that flavocytochrome b does not accumulate in organelles of the degradative pathway.
C-terminal deletion of p22phox does not affect localization of flavocytochrome heterodimer to the plasma membrane and recycling endosomes
Thus far, there are no identified motifs within gp91phox or p22phox that regulate trafficking of the flavocytochrome b heterodimer. A current model of p22phox places both its N- and C-terminal domains in the cytoplasm, anchored in the membrane by a hairpin formed by two hydrophobic domains (23). The C-terminal cytoplasmic domain of p22phox is hydrophilic and also contains multiple proline residues, including a Pro-Xaa-Xaa-Pro SH3-binding motif around Pro156 that is a target of the tandem SH3 domains of p47phox during assembly of the active NADPH oxidase (1). Since deletion of C-terminal aa 142–195 of p22phox does not affect formation of the flavocytochrome b heterodimer (33), we tested whether the Pro-Xaa-Xaa-Pro motif or other signals in the p22phox C terminus were required for targeting of flavocytochrome b to the plasma membrane and/or recycling endosomes.
YFP-tagged p22phox derivatives lacking aa 131–195 (131YFP), 149–195 (149YFP), or 172–195 (172YFP) were transiently expressed in CHO-WT and CHO-91 cells to evaluate their subcellular distribution in the absence of gp91phox and in the presence of excess gp91phox expression. Flow cytometry and immunoblotting with an anti-YFP Ab showed that the YFP-tagged proteins were expressed at similar levels (Fig. 6, A and B) and had increasing mobility in a SDS-PAGE gel with progressive removal of the C terminus (Fig. 6B). Increased cell surface expression and maturation of gp91phox, consistent with heterodimer formation, was seen with coexpression of 22YFP, 172YFP, or 149YFP, but not 131YFP (Fig. 6, A and B), as previously seen using untagged p22phox truncations (33).
FIGURE 6.
p22phox C-terminal aa 149–195 are not required for trafficking of the heterodimer to the plasma membrane or to the perinuclear recycling compartment. CHO-WT or CHO-91 cells were transfected with vectors for 22YFP, 172YFP, 149YFP, or 131YFP and evaluated 24 h posttransfection (n = 2 experiments where YFP-tagged p22phox C-terminal deletion proteins were evaluated). A, Top panel, Flow cytometry analysis of YFP shows similar transfection efficiency of all YFP-tagged p22phox constructs when transiently expressed in CHO-91 cells. Bottom panel, Analysis of gp91phox cell surface expression (mAb 7D5) by flow cytometry reveals increased gp91phox surface expression with coexpression of 22YFP, 172YFP, and 149YFP, but not 131YFP (bottom panel, black lines). CHO-91 cells were used as the control (gray). B, Cell lysates were evaluated for gp91phox (mAb 54.1), YFP (anti-GFP), and β-actin protein expression. C, Left column, Live imaging of YFP-tagged p22phox derivatives transiently expressed in CHO-WT cells. Right columns, CHO-WT cells transiently expressing YFP-tagged p22phox derivatives were fixed, permeabilized, and stained for calnexin. D, Left column, Live imaging of YFP-tagged p22phox derivatives transiently expressed in CHO-91 cells. Arrows show that 22YFP, 172YFP, and 149YFP associate with the endocytic recycling compartment, but 131YFP did not. Z-stack slices collected from the middle of the cell are shown. Right columns, CHO-91 cells transiently expressing YFP-tagged p22phox derivatives were fixed, permeabilized, and stained for gp91phox (mAb 54.1). 22YFP, 172YFP, 149YFP, but not 131YFP, colocalized with gp91phox at the plasma membrane (arrowheads).
Confocal microscopy showed that all YFP-tagged p22phox mutants, in the absence of gp91phox, localized to the ER (Fig. 6C). When coexpressed with excess gp91phox, 172YFP and 149YFP were present in both the perinuclear recycling compartment, best seen in live images (Fig. 6D, arrows), and in the plasma membrane, where they colocalized with gp91phox, similar to full-length 22YFP (Fig. 6D, arrowheads in merge panels). In contrast, 131YFP remained in the ER when coexpressed with gp91phox (Fig. 6D), as expected from failure to form heterodimers. Thus, C-terminal aa 149–195 in p22phox do not appear to contain localization signals required for trafficking of flavocytochrome b to the plasma membrane or to the perinuclear endocytic recycling compartment.
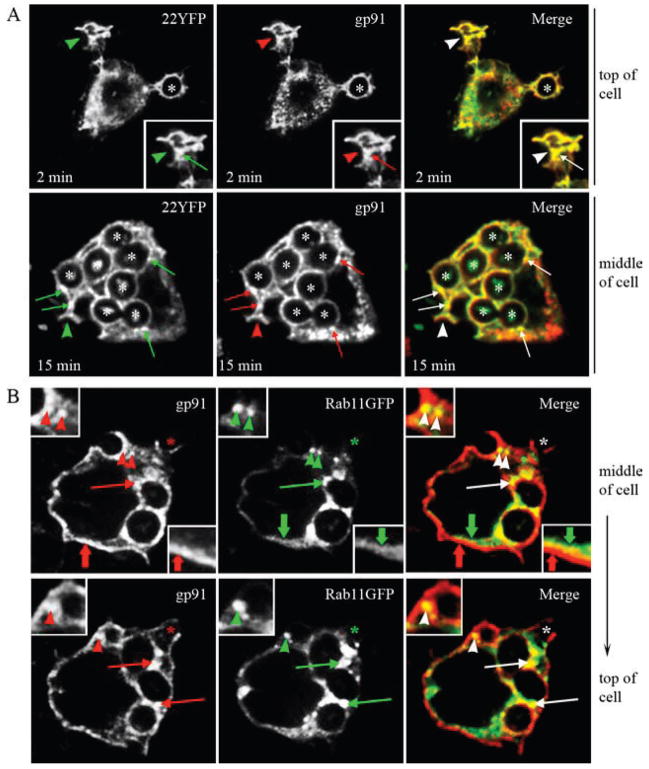
Flavocytochrome b localizes to Rab11-positive recycling endosomes in a murine macrophage cell line
We next investigated the distribution of flavocytochrome b in murine macrophages using Abs specific for endogenous gp91phox, and we determined whether this included Rab11-positive recycling endosomes, as seen in the CHO cell model. In wild-type RAW264.7 macrophages, transiently expressed Rab11GFP colocalized with endogenous gp91phox on vesicles near the plasma membrane (Fig. 7A, top panel, arrowheads) and near the nucleus (Fig. 7A, bottom panel, insets). Of note, the distribution of gp91phox was similar in adjacent cells that did not express Rab11GFP (Fig. 7A, bottom panel, arrows), indicating that overexpression of Rab11GFP did not alter gp91phox targeting. The subcellular distribution of endogenous Rab11 detected by immunofluorescence microscopy was similar to Rab11GFP (not shown).
FIGURE 7.
In macrophages, flavocytochrome b associates with Rab11-positive recycling endosomes. A, RAW-WT cells transiently expressing Rab11GFP were fixed, permeabilized, and stained for gp91phox (mAb 54.1) 48 h posttransfection. Colocalization of gp91phox and Rab11GFP is shown in vesicles near the plasma membrane (top panel, arrowheads) and in the endocytic recycling compartment (bottom panel, insets). Localization of gp91phox to the endocytic recycling compartment was not dependent on expression of Rab11GFP (top panel, arrows). Single-plane z-stack slices taken from the bottom and middle of the cell are shown. B, RAW-WT transfected with Rab11GFP were incubated with Alexa Fluor 647-conjugated transferrin (TfnAF647, 5 μg/ml) for 5 min at 37°C followed by a 25-min chase at 37°C to label the endocytic recycling compartment. Cells were fixed, permeabilized, and stained for gp91phox. Colocalization of gp91phox and Rab11GFP (top panel) and gp91phox and Tfn (bottom panel) was observed at the perinuclear compartment (arrowheads) and near the plasma membrane (arrows).
As a second approach to examine whether flavocytochrome b was associated with the endocytic recycling compartment in macrophages, we performed a pulse-chase experiment using fluorescently tagged transferrin (Tfn-AF647, 5 μg/ml) and RAW-WT cells transiently expressing Rab11GFP. After a 5-min pulse with Tfn-AF647 followed by a 25-min chase, cells were fixed, permeabilized, and stained to detect endogenous gp91phox. As shown in Fig. 7B, Rab11GFP, gp91phox, and Tfn-AF647 colocalized in the perinuclear region (arrowheads) and in vesicles near the plasma membrane (insets, arrows). Taken together with the colocalization with Rab11, these data demonstrate for the first time that a portion of macrophage flavocytochrome b localizes to the endocytic recycling compartment.
Macrophage flavocytochrome b also colocalizes with the plasma membrane and early (sorting) endosomes, but not late endosomes
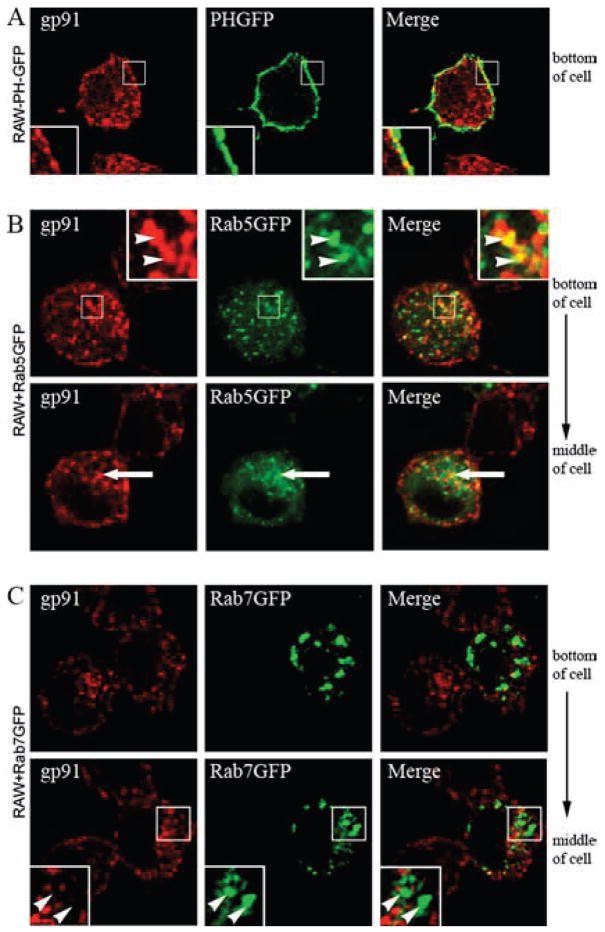
Flavocytochrome b was also present at the periphery of RAW 264.7 cells (Fig. 7). To verify that this represented targeting to the plasma membrane, we stably expressed a GFP-labeled PH domain of phospholipase C (50) to mark the inner leaflet of the plasma membrane of RAW 264.7 cells. As shown in Fig. 8A, PH-GFP colocalized with endogenous gp91phox at the cell surface.
FIGURE 8.
In macrophages, flavocytochrome b is present in plasma membrane and some Rab5-positive sorting endosomes, but not in Rab7-positive late endosomes. A, RAW-PH-GFP cells were fixed, permeabilized, and stained for endogenous gp91phox (mAb 54.1). Colocalization of gp91phox and the plasma membrane marker, PH-GFP, was seen at the cell surface in RAW-PH-GFP cells (insets). A single-plane z-stack slice taken from the middle of the cell is shown (n = 2 experiments where colocalization of gp91phox and PH-GFP was evaluated). RAW-WT cells transfected with Rab5GFP (B) or Rab7GFP (C) were fixed, permeabilized, and stained for gp91phox (mAb 54.1) 48 h posttransfection. B, gp91phox and Rab5GFP showed some colocalization in vesicles near the plasma membrane (top panel, arrowheads) and near the nucleus (bottom panel, arrows). C, Rab7GFP accumulated on large vesicles dispersed throughout the cell that did not colocalize with gp91phox near the plasma membrane (top panel) or near the nucleus (bottom panel, arrowheads).
In addition to Rab11-positive structures, flavocytochrome b was detected in other vesicular populations (Fig. 7A). The extent of flavocytochrome b targeting to early (sorting) endosomes and late endosomes was therefore evaluated by comparison with Rab5GFP and Rab7GFP transiently expressed in RAW-WT cells. Flavocytochrome b exhibited modest colocalization with Rab5GFP in vesicles near the plasma membrane (Fig. 8B, top panel, insets) and near the perinuclear compartment (Fig. 8B, bottom panel, arrows). In contrast, the distribution of flavocytochrome b had little, if any, overlap with the late endosome marker Rab7GFP, which accumulated on large vesicles dispersed throughout the cell (Fig. 8C, arrowheads), but not in the perinuclear compartment (Fig. 8C, arrows).
Collectively, these data indicate that flavocytochrome b in macrophages resides in both early endosomes and the endocytic recycling compartment, suggesting that flavocytochrome b under homeostatic conditions undergoes recycling from the plasma membrane.
Fcγ receptors accumulate in the plasma membrane, but not in recycling endosomes
Although it is known that some surface receptors recycle while others are targeted for degradation in lysosomes (51), the extent to which different plasma membrane proteins traverse the endocytic recycling compartment is unclear. For comparison to flavocytochrome b, we investigated whether the Fcγ receptor was present in the endocytic recycling compartment in RAW 264.7 macrophages. Using both indirect immunofluorescence to detect endogenous Fcγ receptors in RAW-WT cells and live-cell imaging to detect stably expressed FcγRllaGFP in RAW-FcR-GFP transfectants, we detected Fcγ receptors in the plasma membrane but not in the endocytic recycling compartment, identified in a pulse-chase experiment using Tfn-AF647 (supplemental Fig. S4). These results confirm published data (52), which suggest that Fcγ receptors do not recycle and, as such, indicate selective accumulation of plasma membrane proteins in the endocytic recycling compartment.
Unassembled fluorescently tagged gp91phox and p22phox subunits localize to the ER in RAW 264.7 cells, while the heterodimer does not
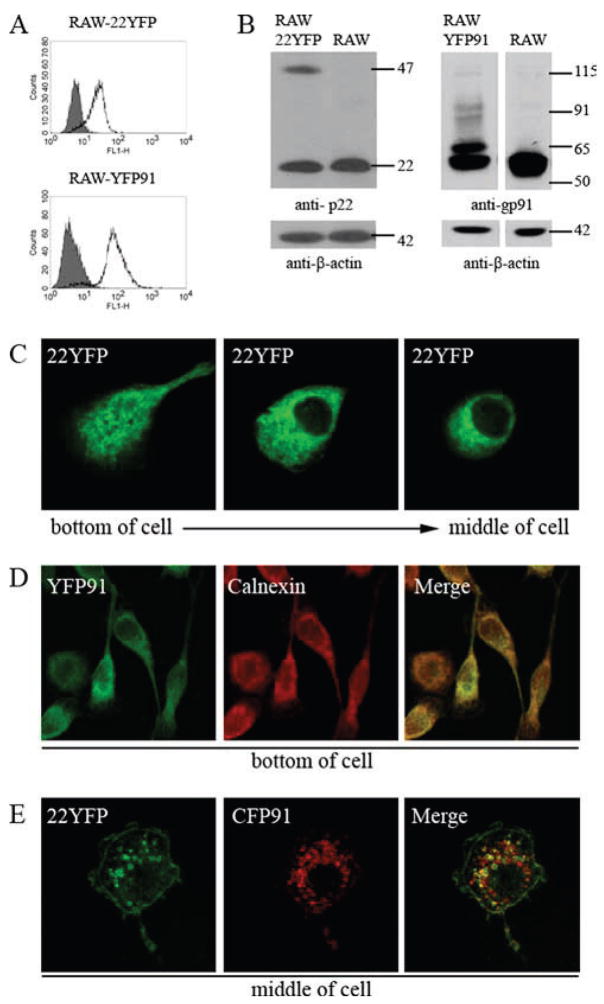
To visualize flavocytochrome b trafficking in macrophages using live cell imaging, 22YFP and YFP91 were stably expressed in RAW cells to generate RAW-22YFP and RAW-YFP91 cell lines (Fig. 9, A and B). However, the distribution of the fluorescently tagged subunit in each of these two cell lines was reticular (Fig. 9, C and D), suggesting that the fluorescently tagged subunits accumulated in the ER and were expressed in excess of their endogenous partner. Colocalization with calnexin confirmed ER localization of YFP91 in RAW-YFP91 cells (Fig. 9D). To determine whether the ER-like distribution of 22YFP reflected unassembled 22YFP in excess of endogenous gp91phox, CFP91 was transiently expressed in RAW-22YFP cells. Coexpression of CFP91 in RAW-22YFP cells resulted in loss of the reticular distribution of 22YFP seen in Fig. 9C, as well as a shift to the cell surface and intracellular vesicles that had a perinuclear distribution (Fig. 9E). Colocalization of 22YFP and CFP91 was detected in the plasma membrane and intracellular vesicles (Fig. 9E). Taken together, these data indicate that, similar to CHO cells, unassembled monomers localize to the ER in RAW 264.7 cells while p22phox/gp91phox heterodimers traffic to the plasma membrane and the endocytic recycling compartment.
FIGURE 9.
Unassembled fluorescently tagged flavocytochrome b subunits localize to the ER in RAW 264.7 macrophages, while assembled subunits localize to endosomes and the plasma membrane. Protein expression and subcellular targeting of 22YFP and YFP91 were analyzed in RAW-22YFP and RAW-YFP91 cells. 22YFP and YFP91 protein expression in RAW-22YFP and RAW-YFP91 cells was examined using flow cytometry to detect YFP (A) and using Western blotting to detect p22phox (mAb NS2) or gp91phox (mAb 54.1) (B). RAW-WT cells were used as controls for analysis by flow cytometry (filled gray) and Western blot (labeled RAW). C, Subcellular distribution of 22YFP in living RAW-22YFP cells. D, RAW-YFP91 cells were fixed, permeabilized, and stained for calnexin (n = 2 experiments where YFP91 and calnexin colocalization was evaluated). E, Live RAW-22YFP cells transfected with CFP91 were examined 24 h posttransfection. Single z-stack slices from the bottom and/or middle of the cells are shown as indicated.
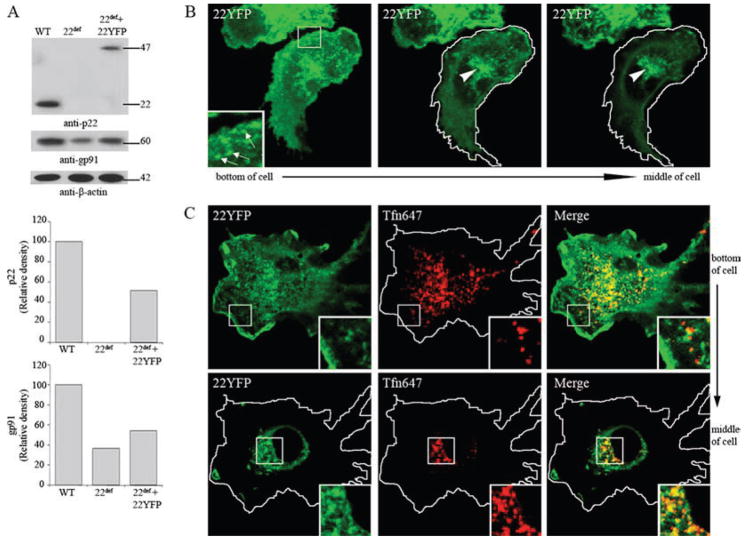
Flavocytochrome b localizes to recycling endosomes in primary murine BMDM
We next examined the localization of flavocytochrome b in BMDM by confocal microscopy of live cells. To minimize potential problems in balancing expression of a fluorescently tagged subunit with endogenous flavocytochrome b subunits, we utilized the recently characterized p22phox-deficient nmf333 mouse (34) to express 22YFP in BMDM. The nmf333 mouse harbors a missense mutation in the p22phox gene that prevents expression of the p22phox subunit. nmf333 BM progenitor cells were transduced with a retroviral vector for expression of 22YFP and then differentiated into macrophages. Western blot analysis of BMDM (Fig. 10A) confirmed the absence of p22phox in mock-transduced nmf333 BMDM, and that 22YFP was expressed. The relative level of the YFP-tagged p22phox was 43 ± 14% of endogenous p22phox in mock-transduced wild-type C57BL/6J BMDM (n = 3 experiments where densitometry was performed). As previously mentioned, formation of flavocytochrome b in phagocytic leukocytes increases the stability of each of its constituent subunits (1). A small amount of residual gp91phox expression was detected in nmf333 BMDM, which increased upon expression of 22YFP by 39 ± 12% (n = 3 experiments where densitometry was performed) (Fig. 10A), consistent with the stabilization of endogenous gp91phox by heterodimer formation.
FIGURE 10.
22YFP expressed in p22phox-deficient BMDM localizes to the plasma membrane and endocytic recycling compartment. p22phox-deficient (p22def) BM cells were transduced with MSCV-22YFP and differentiated into macrophages for 6 –7 days. A, Lysates of WT, p22def, and 22YFP-expressing p22def BMDM were probed to detect p22phox (mAb NS2), gp91phox (mAb 54.1), and β-actin (mAb). Top bar graph shows the relative density of p22phox protein, normalized to β-actin. Bottom bar graph shows the relative density of gp91phox protein normalized to β-actin. Data shown are representative of three independent experiments. B, 22YFP expressed in p22phox-deficient BMDM was imaged in living cells. 22YFP localizes to vesicles near the plasma membrane (insets, arrows) and vesicles that cluster near the nucleus (arrowheads). C, 22YFP-expressing p22phox-deficient BMDM were incubated with Alexa Fluor 647-conjugated transferrin (TfnAF647, 5 μg/ml) for 5 min at 37°C followed by a 25-min chase at 37°C to label the endocytic recycling compartment. 22YFP and Tfn colocalized in vesicles near the plasma membrane (top panel, insets) and near the nucleus (bottom panel, insets).
We utilized confocal microscopy to localize flavocytochrome b in nmf333 BMDM transduced with 22YFP. As shown in Fig. 10B, much of 22YFP in living macrophages was associated with intracellular vesicles (arrows) that appeared to cluster near the nucleus (arrowheads). In fixed cells, 22YFP and endogenous gp91phox showed a very similar distribution and colocalized at the plasma membrane, on vesicles near the plasma membrane, and on perinuclear vesicles that clustered in the middle of the cell (supplemental Fig. S5A). A similar distribution was observed for endogenous gp91phox in wild-type BMDM (supplemental Fig. S5B). To verify that flavocytochrome b localized to the endocytic recycling compartment in primary macrophages, recycling endosomes were labeled with Tfn-AF647, as described above. Imaging of live p22phox-deficient BMDM transduced with 22YFP revealed colocalization of 22YFP and transferrin in vesicles near the plasma membrane (Fig. 10C, top panel, insets) and in a perinuclear distribution (Fig. 10C, bottom panel, insets). These data indicate that flavocytochrome b is present in the endocytic recycling compartment of primary BMDM.
Flavocytochrome b trafficking during phagocytosis in BMDM
We next investigated trafficking of flavocytochrome b during phagocytosis. While use of YFP- and CFP-tagged p22phox and gp91phox would have allowed simultaneous imaging of both heterodimer subunits during phagocytosis, the CFP-tagged proteins were only weakly fluorescent and not suitable for collecting a series of images over time. Thus, we evaluated colocalization of 22YFP and endogenous gp91phox, visualized by indirect immunofluorescence, in p22phox-deficient BMDM expressing 22YFP after 2 and 15 min of phagocytosis of SOZ particles. 22YFP and gp91phox showed a very similar distribution in the phagocytic cup (Fig. 11A, top panel, arrowheads) and nascent phagosomes (Fig. 11A, top and bottom panels, asterisks). We also observed the presence of 22YFP and gp91phox in discrete patches near the phagocytic cup and near phagosomes (Fig. 11A, arrows), which appeared vesicular. We hypothesize that these regions might be endosomes involved in trafficking of flavocytochrome b between phagosomes and internalized membranes.
FIGURE 11.
Flavocytochrome b colocalizes with Rab11GFP in or near phagocytic cups and nascent phagosomes. A, Colocalization of 22YFP and gp91phox, detected by immunofluorescence, in p22phox-deficient BMDM following 2 min (top panels) and 15 min (bottom panels) of synchronized phagocytosis. A series of z-stack planes (0.16 μm) were collected. Five single planes (total of 0.8 μm) were merged using the z-stack projection tool in ImageJ. Arrowheads denote phagocytic cups, and asterisks indicate nascent phagosomes. Arrows indicate discrete foci of increased 22YFP and gp91phox colocalization near phagocytic cups and nascent phagosomes. Representative images from two independent experiments in which at least 10 cells were evaluated at each time point. B, RAW 264.7 cells transiently expressing Rab11GFP were fixed, permeabilized, and stained to detect gp91phox (red) 15 min after synchronized phagocytosis of opsonized zymosan. Arrowheads indicate colocalization of gp91phox and Rab11GFP near a forming phagocytic cup (merged image). Arrows show colocalization on or near nascent phagosomes. Wide arrows indicate gp91phox in the plasma membrane with subjacent to Rab11GFP, and asterisks mark a phagocytic cup that is gp91phox-positive, but Rab11GFP-deficient.
Flavocytochrome b colocalizes with Rab11GFP at vesicles near the phagocytic cup and sealed phagosomes
Rab11-positive recycling endosomes can be detected in the vicinity of nascent phagosomes and forming phagocytic cups (46), and they can deliver proteins such as TNF-α to forming phagosomes (53). Our finding that flavocytochrome b localizes to the Rab11 endocytic recycling compartment in both RAW 264.7 and BMDM and that flavocytochrome b appeared to accumulate in discrete foci near the phagosome at different time points during phagocytosis suggested that recycling endosomes may serve as an intracellular reservoir from which additional flavocytochrome b can be delivered to the plasma membrane or phagosome. Therefore, we examined the localization of endogenous gp91phox and Rab11GFP during phagocytosis of SOZ particles in RAW 264.7 cells. Staining of gp91phox was evident on the plasma membrane, the phagocytic cup, and nascent phagosomes, as well as on intracellular vesicles (Fig. 11B, left column), similar to BMDM. Some gp91phox also appeared to be subjacent to the plasma membrane in colocalization with Rab11GFP (Fig. 11B, right column, block arrows), consistent with the role of Rab11-positive endosomes in trafficking intracellular membrane to the cell surface (42). Interestingly, gp91phox was also detected on phagocytic cups that lacked Rab11GFP (Fig. 11B, asterisks), suggesting that some flavocytochrome b was targeted directly to phagocytic cups as they formed from the plasma membrane. Concordant with published data (54), Rab11GFP was also detected on vesicles near the base of forming phagocytic cups and on the membranes of newly formed phagosomes, where it colocalized with gp91phox (Fig. 11B, right column, arrowheads and arrows, respectively). These data thus also suggest that Rab11-positive recycling endosomes may be involved in trafficking of flavocytochrome b between intracellular membranes and forming or nascent phagosomes.
Discussion
In these studies, we show for the first time that the NADPH oxidase flavocytochrome b is localized to both the plasma membrane and the endocytic recycling compartment in macrophages. We used a combination of fluorescently tagged probes for gp91phox and p22phox and indirect immunofluorescence of the corresponding endogenous proteins to examine the subcellular distribution of each subunit in living and fixed cells. While our study is the first to show localization of macrophage flavocytochrome b to recycling endosome compartments, human peripheral blood monocytes also contain intracellular vesicles enriched in flavocytochrome b, but the nature of these structures and whether they recycle remains to be determined (17, 18). Of note, Fcγ receptors did not accumulate in recycling compartments, thereby indicating selectivity in this process despite the fact that these receptors decline in the membranes of nascent phagosomes following ingestion of IgG-coated particles (55). Additional results from our experiments demonstrate that individual subunits of flavocytochrome b, either when expressed alone or when one subunit is expressed in excess of its partner, remain in the ER, thereby confirming previous studies in neutrophils that demonstrated that heterodimer formation is an essential prerequisite of, and provides an important signal for, efficient trafficking to more peripheral membrane compartments (10, 13, 33). Similar data were obtained using murine RAW 264.7 macrophages, primary BMDM, and the CHO cell model system. Our demonstration that a portion of the flavocytochrome b in macrophages is present in Rab-11-positive endosomes suggests that the endocytic recycling compartment may act as a reservoir of flavocytochrome b that cycles between this compartment and the cell surface.
This study is also the first to indicate a relationship between relative expression levels of gp91phox and p22phox and their accumulation in different subcellular compartments. In CHO cells, where each flavocytochrome b subunit is relatively stable in the absence of its partner, both gp91phox and p22phox accumulated in the ER when expressed individually. Additionally, a small fraction of gp91phox is present in its mature 91-kDa glycosylated form and traffics to the cell surface (32, 33). These data suggest that some maturation and trafficking to the cell surface of unassembled gp91phox occurs as an inefficient default pathway or, alternatively, it is possible that another protein is capable of directing some gp91phox to the cell surface in CHO cells in the absence of p22phox. Titration experiments in CHO cells showed that when coexpressed at similar levels, p22phox and gp91phox no longer accumulate in the ER, and instead colocalize at the cell surface and in the endocytic recycling compartment. However, in both CHO cells and macrophages, if one subunit is overexpressed compared with its partner, the “excess” subunit remains in the ER. Previous studies examining localization of p22phox and gp91phox in transfected cells have not addressed the importance of the ratio of the two subunits or clearly shown that the location of the heterodimer may be distinct from the location of the individual subunits (56, 57).
In neutrophils, specific granules carrying flavocytochrome b can serve as a storage pool and a mechanism by which flavocytochrome b is recruited to the plasma membrane and phagosomes. Our findings localizing flavocytochrome b to Rab11-positive and Rab5-positive endosomes in macrophages suggest that recycling endosomes may serve a similar function. Macrophages have the capacity to rapidly ingest many large particles or microbes, and it has long been known that cell surface area increases during phagocytosis (58–60). Membrane compartments mobilized for expansion of the cell surface include but are not limited to Vamp3-and/or Rab11-positive endosomes (46, 53, 54, 61–63). However, the nature of the compartments mobilized in response to any one stimulus remains controversial, and the extent to which vesicles fuse directly with forming phagosomes as compared with the “uninvolved” plasma membrane is not well defined (60, 64). Thus, it is also possible that fusion of recycling endosomes with the cell surface provides a mechanism to replenish plasma membrane flavocytochrome b during phagocytosis or macrophage activation, as has been described for TNF-α (53, 62). Alternatively, it is also possible that recycling endosomes remove flavocytochrome b from the membranes of phagosomes as they mature. In either case, it is interesting to hypothesize that macrophages, which unlike neutrophils are long-lived cells, may utilize this compartment to retain and conserve the use of flavocytochrome b.
Salmonella enterica serovar Typhimurium (S. typhimurium) is a facultative intracellular pathogen of macrophages. An essential aspect of virulence is the ability of Salmonella to introduce bacterial effector proteins into the cytosol of host cells using a type III secretion system that disrupts membrane trafficking and prevents phagosome maturation. Previous studies by us and others have shown that effectors of the Salmonella pathogenicity island 2-encoded type III secretion system are also required to prevent accumulation of NADPH oxidase components, including flavocytochrome b on Salmonella compartments in primary murine and human macrophages (6, 7). Although the mechanisms of action of type III secretion system effectors are not well defined, it is attractive to predict that targeting Rab11 may allow selective depletion of flavocytochrome b from the Salmonella phagosome, as has recently been reported for CD44 (65).
In summary, these studies show that much of the NADPH oxidase flavocytochrome b in macrophages is present in the recycling endocytic compartment. Future studies will investigate the trafficking between this intracellular “pool” of flavocytochrome b and phagosomes and/or the plasma membrane during microbial infection, thus examining a potential mechanism by which superoxide production can be regulated.
Supplementary Material
Acknowledgments
We thank Natalie Stull for generating MSCV-22YFP supernatant, Christophe Marchal for transduction of murine bone marrow progenitors, and Cliff Babbey for helpful discussions.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants R01 HL45635 (to M.C.D.), TK32 DKI07519 (to A.J.C.), R01 AI073835 (to L.A.A.), and R01 DK51098 (to K.W.D.); a Veterans Affairs Merit Review Grant (to L.A.A.); NIH Grant P30 CA082709 to the Indiana University Simon Cancer Center, which provides partial support for the Indiana University Flow Cytometry and Indiana Center for Biological Microscopy Cores; and funding from the Riley Children’s Foundation (to M.C.D.).
Abbreviations used in this paper: ER, endoplasmic reticulum; BM, bone marrow; BMDM, bone marrow-derived macrophage; CFP, cyan fluorescent protein; CHO, Chinese hamster ovary; SOZ, serum-opsonized zymosan; YFP, yellow fluorescent protein.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Nauseef WM. Assembly of the phagocyte NADPH oxidase. Histochem Cell Biol. 2004;122:277–291. doi: 10.1007/s00418-004-0679-8. [DOI] [PubMed] [Google Scholar]
- 2.Dinauer M. The phagocyte system and disorders of granulopoiesis and granulocyte function. In: Nathan DG, Orkin SH, Ginsburg D, Look AT, editors. Nathan and Oski’s Hematology of Infancy and Childhood. 6. Saunders; Philadelphia, PA: 2003. pp. 923–1010. [Google Scholar]
- 3.IJdo JW, Mueller AC. Neutrophil NADPH oxidase is reduced at the Anaplasma phagocytophilum phagosome. Infect Immun. 2004;72:5392–5401. doi: 10.1128/IAI.72.9.5392-5401.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCaffrey RL, Allen LA. Francisella tularensis LVS evades killing by human neutrophils via inhibition of the respiratory burst and phagosome escape. J Leukocyte Biol. 2006;80:1224–1230. doi: 10.1189/jlb.0406287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lodge R, Diallo TO, Descoteaux A. Leishmania donovani lipophosphoglycan blocks NADPH oxidase assembly at the phagosome membrane. Cell Microbiol. 2006;8:1922–1931. doi: 10.1111/j.1462-5822.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- 6.Gallois A, Klein JR, Allen LA, Jones BD, Nauseef WM. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J Immunol. 2001;166:5741–5748. doi: 10.4049/jimmunol.166.9.5741. [DOI] [PubMed] [Google Scholar]
- 7.Vazquez-Torres A, Fantuzzi G, Edwards CK, 3rd, Dinarello CA, Fang FC. Defective localization of the NADPH phagocyte oxidase to Salmonella-containing phagosomes in tumor necrosis factor p55 receptor-deficient macrophages. Proc Natl Acad Sci USA. 2001;98:2561–2565. doi: 10.1073/pnas.041618998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen LA, Beecher BR, Lynch JT, Rohner OV, Wittine LM. Helicobacter pylori disrupts NADPH oxidase targeting in human neutrophils to induce extracellular superoxide release. J Immunol. 2005;174:3658–3667. doi: 10.4049/jimmunol.174.6.3658. [DOI] [PubMed] [Google Scholar]
- 9.Parkos CA, Allen RA, Cochrane CG, Jesaitis AJ. Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J Clin Invest. 1987;80:732–742. doi: 10.1172/JCI113128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu L, DeLeo FR, Biberstine-Kinkade KJ, Renee J, Nauseef WM, Dinauer MC. Biosynthesis of flavocytochrome b558.gp91(phox) is synthesized as a 65-kDa precursor (p65) in the endoplasmic reticulum. J Biol Chem. 1999;274:4364–4369. doi: 10.1074/jbc.274.7.4364. [DOI] [PubMed] [Google Scholar]
- 11.Yu L, Zhen L, Dinauer MC. Biosynthesis of the phagocyte NADPH oxidase cytochrome b558: role of heme incorporation and heterodimer formation in maturation and stability of gp91phox and p22phox subunits. J Biol Chem. 1997;272:27288–27294. doi: 10.1074/jbc.272.43.27288. [DOI] [PubMed] [Google Scholar]
- 12.Porter CD, Parkar MH, Verhoeven AJ, Levinsky RJ, Collins MK, Kinnon C. p22-Phox-deficient chronic granulomatous disease: reconstitution by retrovirus-mediated expression and identification of a biosynthetic intermediate of gp91-phox. Blood. 1994;84:2767–2775. [PubMed] [Google Scholar]
- 13.DeLeo FR, Burritt JB, Yu L, Jesaitis AJ, Dinauer MC, Nauseef WM. Processing and maturation of flavocytochrome b558 include incorporation of heme as a prerequisite for heterodimer assembly. J Biol Chem. 2000;275:13986–13993. doi: 10.1074/jbc.275.18.13986. [DOI] [PubMed] [Google Scholar]
- 14.Borregaard N, Heiple JM, Simons ER, Clark RA. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jesaitis AJ, Buescher ES, Harrison D, Quinn MT, Parkos CA, Livesey S, Linner J. Ultrastructural localization of cytochrome b in the membranes of resting and phagocytosing human granulocytes. J Clin Invest. 1990;85:821–835. doi: 10.1172/JCI114509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 17.Ginsel LA, Onderwater JJ, Fransen JA, Verhoeven AJ, Roos D. Localization of the low-Mr subunit of cytochrome b558 in human blood phagocytes by immunoelectron microscopy. Blood. 1990;76:2105–2116. [PubMed] [Google Scholar]
- 18.Calafat J, Kuijpers TW, Janssen H, Borregaard N, Verhoeven AJ, Roos D. Evidence for small intracellular vesicles in human blood phagocytes containing cytochrome b558 and the adhesion molecule CD11b/CD18. Blood. 1993;81:3122–3129. [PubMed] [Google Scholar]
- 19.Johansson A, Jesaitis AJ, Lundqvist H, Magnusson KE, Sjolin C, Karlsson A, Dahlgren C. Different subcellular localization of cytochrome b and the dormant NADPH-oxidase in neutrophils and macrophages: effect on the production of reactive oxygen species during phagocytosis. Cell Immunol. 1995;161:61–71. doi: 10.1006/cimm.1995.1009. [DOI] [PubMed] [Google Scholar]
- 20.Vazquez-Torres A, Xu Y, Jones-Carson J, Holden DW, Lucia SM, Dinauer MC, Mastroeni P, Fang FC. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- 21.Burritt JB, Quinn MT, Jutila MA, Bond CW, Jesaitis AJ. Topological mapping of neutrophil cytochrome b epitopes with phage-display libraries. J Biol Chem. 1995;270:16974–16980. doi: 10.1074/jbc.270.28.16974. [DOI] [PubMed] [Google Scholar]
- 22.Baniulis D, Nakano Y, Nauseef WM, Banfi B, Cheng G, Lambeth DJ, Burritt JB, Taylor RM, Jesaitis AJ. Evaluation of two anti-gp91phox antibodies as immunoprobes for Nox family proteins: mAb 54.1 recognizes recombinant full-length Nox2, Nox3 and the C-terminal domains of Nox1–4 and cross-reacts with GRP 58. Biochim Biophys Acta. 2005;1752:186–196. doi: 10.1016/j.bbapap.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Taylor RM, Burritt JB, Baniulis D, Foubert TR, Lord CI, Dinauer MC, Parkos CA, Jesaitis AJ. Site-specific inhibitors of NADPH oxidase activity and structural probes of flavocytochrome b: characterization of six monoclonal antibodies to the p22phox subunit. J Immunol. 2004;173:7349–7357. doi: 10.4049/jimmunol.173.12.7349. [DOI] [PubMed] [Google Scholar]
- 24.Yamauchi A, Yu L, Potgens AJ, Kuribayashi F, Nunoi H, Kanegasaki S, Roos D, Malech HL, Dinauer MC, Nakamura M. Location of the epitope for 7D5, a monoclonal antibody raised against human flavocytochrome b558, to the extracellular peptide portion of primate gp91phox. Microbiol Immunol. 2001;45:249–257. doi: 10.1111/j.1348-0421.2001.tb02614.x. [DOI] [PubMed] [Google Scholar]
- 25.Dinauer MC, Pierce EA, Erickson RW, Muhlebach TJ, Messner H, Orkin SH, Seger RA, Curnutte JT. Point mutation in the cytoplasmic domain of the neutrophil p22-phox cytochrome b subunit is associated with a nonfunctional NADPH oxidase and chronic granulomatous disease. Proc Natl Acad Sci USA. 1991;88:11231–11235. doi: 10.1073/pnas.88.24.11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamashiro DJ, Tycko B, Fluss SR, Maxfield FR. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984;37:789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- 27.Zhen L, King AA, Xiao Y, Chanock SJ, Orkin SH, Dinauer MC. Gene targeting of X chromosome-linked chronic granulomatous disease locus in a human myeloid leukemia cell line and rescue by expression of recombinant gp91phox. Proc Natl Acad Sci USA. 1993;90:9832–9836. doi: 10.1073/pnas.90.21.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY. Reducing the environmental sensitivity of yellow fluorescent protein: mechanism and applications. J Biol Chem. 2001;276:29188–29194. doi: 10.1074/jbc.M102815200. [DOI] [PubMed] [Google Scholar]
- 29.Price MO, McPhail LC, Lambeth JD, Han CH, Knaus UG, Dinauer MC. Creation of a genetic system for analysis of the phagocyte respiratory burst: high-level reconstitution of the NADPH oxidase in a nonhematopoietic system. Blood. 2002;99:2653–2661. doi: 10.1182/blood.v99.8.2653. [DOI] [PubMed] [Google Scholar]
- 30.Babbey CM, Ahktar N, Wang E, Chen CC, Grant BD, Dunn KW. Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 2006;17:3156–3175. doi: 10.1091/mbc.E05-08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang E, Pennington JG, Goldenring JR, Hunziker W, Dunn KW. Brefeldin A rapidly disrupts plasma membrane polarity by blocking polar sorting in common endosomes of MDCK cells. J Cell Sci. 2001;114:3309–3321. doi: 10.1242/jcs.114.18.3309. [DOI] [PubMed] [Google Scholar]
- 32.Biberstine-Kinkade KJ, Yu L, Stull N, LeRoy BA, Bennett S, Cross AR, Dinauer M. Mutagenesis of p22phox histidine 94. J Biol Chem. 2002;277:30368–30374. doi: 10.1074/jbc.M203993200. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Y, Marchal CC, Casbon AJ, Stull N, von Lohneysen K, Knaus UG, Jesaitis AJ, McCormick S, Nauseef WM, Dinauer MC. Deletion mutagenesis of p22phox subunit of flavocytochrome b558: identification of regions critical for gp91phox maturation and NADPH oxidase activity. J Biol Chem. 2006;281:30336–30346. doi: 10.1074/jbc.M607191200. [DOI] [PubMed] [Google Scholar]
- 34.Nakano Y, Longo-Guess CM, Bergstrom DE, Nauseef WM, Jones SM, Banfi B. Mutation of the Cyba gene encoding p22phox causes vestibular and immune defects in mice. J Clin Invest. 2008;118:1176–1185. doi: 10.1172/JCI33835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamauchi A, Marchal CC, Molitoris J, Pech N, Knaus U, Towe J, Atkinson SJ, Dinauer MC. Rac GTPase isoform-specific regulation of NADPH oxidase and chemotaxis in murine neutrophils in vivo: role of the C-terminal polybasic domain. J Biol Chem. 2005;280:953–964. doi: 10.1074/jbc.M408820200. [DOI] [PubMed] [Google Scholar]
- 36.Racoosin EL, Swanson JA. Macrophage colony-stimulating factor (rM-CSF) stimulates pinocytosis in bone marrow-derived macrophages. J Exp Med. 1989;170:1635–1648. doi: 10.1084/jem.170.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quinn MT, Deleo F, Bokoch GM. Neutrophil Methods and Protocols. Humana; Totowa, NJ: 2007. [DOI] [PubMed] [Google Scholar]
- 38.Allen LA. Rate and extent of Helicobacter pylori phagocytosis. Methods Mol Biol. 2008;431:147–157. doi: 10.1007/978-1-60327-032-8_12. [DOI] [PubMed] [Google Scholar]
- 39.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 40.Rasband WS. Image J. National Institutes of Health; Bethesda, MD: 1997–2004. [Google Scholar]
- 41.Zhen L, Yu L, Dinauer MC. Probing the role of the carboxyl terminus of the gp91phox subunit of neutrophil flavocytochrome b558 using site-directed mutagenesis. J Biol Chem. 1998;273:6575–6581. doi: 10.1074/jbc.273.11.6575. [DOI] [PubMed] [Google Scholar]
- 42.Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biberstine-Kinkade KJ, DeLeo FR, Epstein RI, LeRoy BA, Nauseef WM, Dinauer MC. Heme-ligating histidines in flavocytochrome b558: identification of specific histidines in gp91phox. J Biol Chem. 2001;276:31105–31112. doi: 10.1074/jbc.M103327200. [DOI] [PubMed] [Google Scholar]
- 44.Saraste J, Goud B. Functional symmetry of endomembranes. Mol Biol Cell. 2007;18:1430–1436. doi: 10.1091/mbc.E06-10-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 46.Cox D, Lee DJ, Dale BM, Calafat J, Greenberg S. A Rab11-containing rapidly recycling compartment in macrophages that promotes phagocytosis. Proc Natl Acad Sci USA. 2000;97:680–685. doi: 10.1073/pnas.97.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urbe S, Huber LA, Zerial M, Tooze SA, Parton RG. Rab11, a small GTPase associated with both constitutive and regulated secretory pathways in PC12 cells. FEBS Lett. 1993;334:175–182. doi: 10.1016/0014-5793(93)81707-7. [DOI] [PubMed] [Google Scholar]
- 48.Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seachrist JL, Ferguson SS. Regulation of G protein-coupled receptor endocytosis and trafficking by Rab GTPases. Life Sci. 2003;74:225–235. doi: 10.1016/j.lfs.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 50.Botelho RJ, Teruel M, Dierckman R, Anderson R, Wells A, York JD, Meyer T, Grinstein S. Localized biphasic changes in phosphatidyl-inositol-4,5-bisphosphate at sites of phagocytosis. J Cell Biol. 2000;151:1353–1368. doi: 10.1083/jcb.151.7.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lodish H, Berk A, Kaiser C, Krieger M, Scott M, Bretscher A, Ploegh H, Matsudaira P. Vesicular traffic, secretion and endocytosis. In: Lodish, Berk, Kaiser, Krieger, Scott, Bretscher, Ploegh, Matsudaira, editors. Molecular Cell Biology. Freeman; New York: 2008. pp. 279–620. [Google Scholar]
- 52.Amigorena S, Bonnerot C, Drake JR, Choquet D, Hunziker W, Guillet JG, Webster P, Sautes C, Mellman I, Fridman WH. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes. Science. 1992;256:1808–1812. doi: 10.1126/science.1535455. [DOI] [PubMed] [Google Scholar]
- 53.Murray RZ, Kay JG, Sangermani DG, Stow JL. A role for the phagosome in cytokine secretion. Science. 2005;310:1492–1495. doi: 10.1126/science.1120225. [DOI] [PubMed] [Google Scholar]
- 54.Leiva N, Pavarotti M, Colombo MI, Damiani MT. Reconstitution of recycling from the phagosomal compartment in streptolysin O-permeabilized macrophages: role of Rab11. Exp Cell Res. 2006;312:1843–1855. doi: 10.1016/j.yexcr.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 55.Pitt A, Mayorga LS, Stahl PD, Schwartz AL. Alterations in the protein composition of maturing phagosomes. J Clin Invest. 1992;90:1978–1983. doi: 10.1172/JCI116077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 57.Murillo I, Henderson LM. Expression of gp91phox/Nox2 in COS-7 cells: cellular localization of the protein and the detection of outward proton currents. Biochem J. 2005;385:649–657. doi: 10.1042/BJ20040829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cox D, Tseng CC, Bjekic G, Greenberg S. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J Biol Chem. 1999;274:1240–1247. doi: 10.1074/jbc.274.3.1240. [DOI] [PubMed] [Google Scholar]
- 59.Hackam DJ, Rotstein OD, Sjolin C, Schreiber AD, Trimble WS, Grinstein S. v-SNARE-dependent secretion is required for phagocytosis. Proc Natl Acad Sci USA. 1998;95:11691–11696. doi: 10.1073/pnas.95.20.11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di A, Nelson DJ, Bindokas V, Brown ME, Libunao F, Palfrey HC. Dynamin regulates focal exocytosis in phagocytosing macrophages. Mol Biol Cell. 2003;14:2016–2028. doi: 10.1091/mbc.E02-09-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bajno L, Peng XR, Schreiber AD, Moore HP, Trimble WS, Grinstein S. Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J Cell Biol. 2000;149:697–706. doi: 10.1083/jcb.149.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manderson AP, Kay JG, Hammond LA, Brown DL, Stow JL. Subcompartments of the macrophage recycling endosome direct the differential secretion of IL-6 and TNFα. J Cell Biol. 2007;178:57–69. doi: 10.1083/jcb.200612131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allen LA, Yang C, Pessin JE. Rate and extent of phagocytosis in macrophages lacking vamp3. J Leukocyte Biol. 2002;72:217–221. [PMC free article] [PubMed] [Google Scholar]
- 64.Rogers LD, Foster LJ. Contributions of proteomics to understanding phagosome maturation. Cell Microbiol. 2008;10:1405–1412. doi: 10.1111/j.1462-5822.2008.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith AC, Cirulis JT, Casanova JE, Scidmore MA, Brumell JH. Interaction of the Salmonella-containing vacuole with the endocytic recycling system. J Biol Chem. 2005;280:24634–24641. doi: 10.1074/jbc.M500358200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.