Abstract
Purpose
Epithelial wound healing is, at least in part, mediated in an autocrine fashion by epidermal growth factor (EGF) receptor (EGFR)–ligand interactions. This study sought to identify the endogenous EGFR ligand and the mechanism by which it is generated in response to wounding in cultured porcine corneas and human corneal epithelial cells.
Methods
Epithelial debridement wounds in cultured porcine corneas and scratch wounds in an epithelial monolayer of SV40-immortalized human corneal epithelial (THCE) cells were allowed to heal in the presence of tyrphostin AG1478 (an EGFR inhibitor), GM6001 (a matrix metalloproteinase [MMP] inhibitor), or CRM197 (a diphtheria toxin mutant), with or without HB-EGF. The activation of EGFR and extracellular signal-regulated kinase (ERK) was analyzed by immunoprecipitation using EGFR antibodies and Western blot analysis with phosphotyrosine antibody. Wound induced HB-EGF shedding was assessed by isolation of secreted HB-EGF from wounded THCE cells and by measuring the release of alkaline phosphatase (AP) in THCE stable cell lines expressing HB-EGF-AP.
Results
In THCE cells, wound-induced EGFR phosphorylation and ERK activation. In both organ and cell culture models, epithelial wounds were healed in basal media and inhibition of EGFR activation by AG1478 blocked wound closure with or without exogenously added HB-EGF. GM6001 delayed wound closure. Its effects diminished in the presence of exogenous EGF or HB-EGF, suggesting that the MMP inhibitor primarily blocks the release of EGFR ligands. CRM197, a highly specific antagonist of HB-EGF, impaired epithelial wound closure, suggesting that HB-EGF is an endogenous ligand released on epithelial wounding. Consistent with the effects on epithelial migration, all inhibitors as well as HB-EGF function-blocking antibodies retarded wound-induced EGFR phosphorylation in cultured THCE cells. The release of HB-EGF in response to wounding was demonstrated by the fact that heparin-binding proteins isolated from wounded, but not control, THCE-conditioned medium stimulated EGFR and ERK phosphorylation and by the expression of HB-EGF-AP in THCE cells, in which wounding induced the release of AP activity in an MMP-inhibitor–sensitive manner.
Conclusions
HB-EGF released on wounding acts as an autocrine–paracrine EGFR ligand. HB-EGF shedding and EGFR activation represent a critical event during corneal epithelial wound healing, suggesting a possible manipulation of wound healing during the early phases.
Corneal epithelial cells respond rapidly to injury, resulting in a healing process of cell migration as a sheet to cover the defect and to reestablish its barrier function.1 Shortly after injury, the basal epithelial cells at the wound margin begin to lose their hemidesmosome attachment sites and to change shape from a columnar to a more elongated morphology as they begin to send out lamellipodia and to move across the wound bed.2 Successful wound healing involves a number of processes including cell migration, cell proliferation, matrix deposition, and tissue remodeling.3 Critical are cell migration and proliferation, which are driven by growth factors and cytokines released coordinately into the injured bed. During corneal wound healing, epithelia play a central role, not only as a key cell type in repair, but also as the source of a number of growth factors. Prominent among these epithelium-derived factors are ligands for the epidermal growth factor receptor (EGFR), the EGF family.1 Numerous studies have shown that epithelial wound healing is, at least in part, mediated in an autocrine fashion by EGFR-ligand interactions.1,4,5
The EGF family is composed of at least six members including the EGF,6 transforming growth factor (TGF)-α,7 amphiregulin,8 heparin-binding EGF-like growth factor (HB-EGF),9 β-cellulin,10 and epiregulin.11 All members of the EGFR ligand family are synthesized as membrane-anchored forms, which are then processed to give bioactive soluble factors. These factors act through the stimulation of specific cell-surface receptors.12,13 Four related receptor tyrosine kinases have been identified (reviewed in Refs. 14–16). These are EGFR/erbB1/HER1, erbB2/HER2/neu, erbB3/HER3, and erbB4/HER4.12 Three of them, erbB1-3, have been detected in corneal epithelium.17,18 The EGF ligands bind to the erbBs with a degree of specificity. EGF, TGF-α, and amphiregulin, bind exclusively to erbB1. The neuregulins bind to erbB3 and/or to erbB4. HB-EGF and epiregulin bind to both erbB1 and erbB4.16,19 No ligand has yet been identified for erbB2, a potent oncogene. The EGF receptor tyrosine kinases can heterodimerize, resulting in transactivation of receptors, which expands the signaling potential of the EGF-like ligands. Using EGFR inhibitors, studies from Zeiske et al.18 and Nakamura et al.20 have shown that EGFR activation is essential for proper epithelial wound healing in vivo. However, the endogenous EGFR ligand(s) that is released on wounding and acts in an autocrine fashion to activate EGFR in epithelial cells remains to be determined.
Recently, ectodomain shedding is recognized as a novel pathway to control the availability of EGFR ligands, including HB-EGF and TGF-α, in a variety of cells.21,22 Like other members of the EGF family,23,24 HB-EGF is synthesized as a type-1 transmembrane protein that can be shed enzymatically to release a soluble 14- to 20-kDa growth factor. The process has been termed ectodomain shedding.25–27 Whereas the transmembrane form of HB-EGF acts in a juxtacrine manner to signal neighboring cells,28 the soluble form of HB-EGF is a potent mitogen and chemoattractant for many cell types, including keratinocytes and epithelial cells.29,30 Ectodomain shedding of transmembrane HB-EGF has been shown to participate in transactivation of EGFR by G-protein–coupled receptors31 and has been implicated in keratinocyte migration in cutaneous wound healing.32 More recently, this process has been linked to cardiac hypertrophy33,34 and to the lung epithelial cell response to Gram-positive bacterial challenge.35 Thus, it is of interest to determine whether HB-EGF shedding also plays a role in mediating the epithelial response to wounding.
In this study, we evaluated the effects of HB-EGF on epithelial wound healing of cultured porcine corneas and cultured human corneal epithelial cells. We also investigated the role of HB-EGF shedding in regulating epithelial wound closure in these two wound models. Based on the study, we propose that shedding of HB-EGF, as an early signaling step, plays a role in cornea epithelial cell migration and wound closure.
Materials and Methods
Materials
Porcine eye bulbs were obtained from a local abattoir, transported to the laboratory on ice in a moist chamber, and processed for culture the next day. Minimum essential medium (MEM) and nonessential amino acid solution were purchased from Invitrogen (Grand Island, NY). Agarose was obtained from ICN Biomedicals (Aurora, OH). Rat tail tendon collagen was from Collaborative Biomedical (Medford, MA). Keratinocyte basic medium (KBM) and keratinocyte growth medium (KGM, KBM-supplemented with bovine pituitary extract, epinephrine, hydrocortisone, transferring, insulin and EGF) were from BioWhittaker (Walkersville, MD). Human recombinant HB-EGF and its function-blocking antibody were obtained from R&D System (Minneapolis, MN). GM6001, a hydroxamic acid matrix metalloproteinase (MMP) inhibitor (3-(N-hydroxy-carbamoyl)-2-(R)-isobutylpropionyl-L-tryptophan methylamide), was from Calbiochem (La Jolla, CA) and Immunex Compound 3 (IC-3), a peptide hydroxamate that inhibits ADAM17 (and other MMPs) by binding to its active site and chelating a zinc ion required for catalytic activity,36 was prepared at Immunex Corp., (now Amgen, Inc., Thousand Oaks, CA).37 CRM197 and tyrphostin AG 1478 were from Sigma-Aldrich (St. Louis, MO). Rabbit anti-human EGFR (agarose conjugate), mouse extracellular signal-regulated kinase (ERK)-2 and phosphor-ERK1/2, and mouse anti-human PY99 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
Corneal Wounding and Organ Culture
An epithelial wound was made by demarcating an area on the central cornea with a 4-mm trephine and then removing the epithelium within the circle with a small scalpel, leaving an intact basement membrane.38 The corneas were then processed for organ culture.39 Corneal–scleral rims, with approximately 4 mm of the limbal conjunctiva present, were excised and rinsed in sterilized phosphate-buffered saline (PBS). The excised corneas were placed epithelial side down into a sterile cup containing MEM. The endothelial corneal cavity was then filled with MEM containing 1% agarose and 1 mg/mL rat tail tendon collagen maintained at 42°C. This mixture was allowed to gel. The cornea, along with its supporting gel, was inverted and then transferred to a 35-mm dish. Approximately 2 mL of MEM was added drop-wise to the surface of the central cornea until the limbal conjunctiva was covered, leaving the epithelium exposed to the air. The corneas were allowed to heal for 48 hours in MEM containing HB-EGF (50 ng/mL), AG1478 (1 μM), GM6001 (100 μM), or CRM197 (10 μg/mL) in 5% CO2 incubator at 37 °C.
Determination of Epithelial Wound Healing in Cultured Corneas
Epithelial wound repair was monitored by fluorescein sodium (0.25%) staining and photographed under a dissecting microscope (Nikon, Tokyo, Japan) with the attached camera (MDS290; Eastman Kodak, Rochester, NY). After a 48-hour incubation, the corneas were treated with Richardson’s staining40 to mark the remaining wound area. The corneas were photographed, and the wound area was quantified by weighing the excised Richardson’s staining spots from the photograph reprints. Three corneas were used for each treatment: At least two independent experiments were performed (six or more corneas for each treatment). MEM treated alone was used as control for spontaneous healing and MEM in the presence of HB-EGF for enhanced healing. The extent of healing over time was defined as the ratio of the area difference between the original and the remaining wound after 48 hours to the original wound area.
Means of the remaining wound areas of treated and untreated corneas were analyzed statistically using a Student’s t-test. P < 0.05 was considered statistically significant.
Cell Culture and Migration Studies
SV40-immortalized human corneal epithelial (THCE) cells, an accepted corneal epithelial cell line, were generously provided by Kaoru Araki-Sasaki.41 THCE cells were grown in KGM in a humidified 5% CO2 incubator at 37°C. For wounding experiments, cells were seeded on 12-well plates or 100-mm culture dishes coated with fibronectin collagen coating mix (Biological Research Faculty and Facility, Ljamsville, MD). After reaching subconfluence, the cells were starved with KBM overnight and wounded in either of the following ways.
For migration assay, THCE cells were grown to 80% confluence in 12-well tissue culture plates. Cells were then starved in KBM overnight and wounded with a sterile 0.1- to 10-μL pipet tip (TipOne; USA Scientific, Ocala, FL) to remove cells by two perpendicular linear scrapes. After suspended cells were washed away, the cells were refed with KBM-plus (KBM + epinephrine, hydrocortisone, and transferrin, but no growth factors) in the presence of HB-EGF (50 ng/mL), HB-EGF–neutralizing antibody (10 μg/mL), AG1478 (0.5 μM), GM6001 (50 μM), or CRM197 (10 μg/mL). The progress of migration was photographed immediately or 24 hours after wounding near the crossing point with an inverted microscope equipped with a digital camera (SPOT; Diagnostic Instruments, Sterling, MI).
Determination of EGFR and ERK Phosphorylation
To determine EGFR tyrosine phosphorylation in wounded THCE cells, growth-factor–starved cell monolayers on 100-mm dishes were pretreated with HB-EGF–neutralizing antibody (10 μg/mL), AG1478 (0.5 μM), GM6001 (50 μM), or CRM197 (10 μg/mL) for 20 minutes and then wounded by multiple linear scratches with a cut of 48-well sharkstooth comb from a DNA sequencing gel (Bio-Rad, Hercules, CA) going from one side to the other of the dish. The dish was then rotated and scrapes were made the same way at 45°, 90°, and 135° to the original scrapes. Cells with no scrape wound were used as the control. After wounding, the cells were incubated for 15 minutes (time point chosen after an initial time course experiment) in the presence of the same inhibitors. Damaged cells were washed away before the cells were incubated in fresh KBM. After incubation, cells were lysed with lysis buffer (150 mM NaCl, 100 mM Tris-HCl [pH 7.5], 1% deoxy-cholate, 0.1% sodium dodecyl sulfate, 1% Triton X-100, 50 mM NaF, 100 mM sodium pyrophosphate, 3.5 mM sodium orthovanadate, proteinase inhibitor cocktails, and 0.1 mM phenylmethylsulfonyl fluoride). For each reaction, cell lysates of 600 μg proteins were immunoprecipitated with 10 μg antibody against EGFR (agarose conjugate) and immunoblotted with mouse anti-PY99 antibody (1:1000). ERK2 and ERK1/2 phosphorylation were determined using monoclonal antibodies against ERK2 (1:20,000) or phosphor-ERK1/2 (1:500 dilution).
Purification of Heparin-Binding Proteins
Heparin-binding proteins were purified by heparin-affinity chromatography, using a chromatography system (Primer; Pharmacia Upjohn, Uppsala, Sweden).42 Growth-factor–starved THCE cells were extensively injured, cell debris rapidly removed, and fresh medium added to the culture. Conditioned media (CM) were collected at the indicated time, and the cell-surface–associated proteins were extracted briefly with 2 M NaCl in KBM. The extraction solution was diluted to 12.5× with KBM and mixed with collected CM. This mixture was centrifuged and filtered through a 0.45-mm filter. Each sample of mixture was applied to an affinity column (HiTrap Heparin HP; Amersham Biosciences, Piscataway, NJ) that preequilibrated with 10 mM Tris·HCl (pH 7.4) containing 0.2 M NaCl and 1 mM benzamidine. After an extensive wash with equilibration buffer, bound proteins were eluted with 2.0 M NaCl in 10 mM Tris·HCl (pH 7.4). The isolation was performed using a liquid chromatography system (AktaPrime; Amersham Biosciences). The eluent was dialyzed first against 10 mM Tris-HCl and then 1 mM Tris-HCl (pH 7.4) for more than 48 hours. The dialyzed eluent was lyophilized and tested for biological activity.
Cell Lines Expressing HB-EGF-AP Fusion Proteins and Measurement of HB-EGF-AP Shedding
We were unable to detect the release of endogenous HB-EGF using several approaches. We then established cells stably transfected with pHB-EGF-AP43 and examined the release of HB-EGF by measuring alkaline phosphatase (AP) activity in incubation medium.43,44 THCE cells were transfected with expression plasmid pHB-EGF-AP43 using transfection reagent (Lipofectamine; Invitrogen-Life Technology, Gaithersburg, MD). This construct had AP inserted into the heparin binding region of HB-EGF.43 Transfected cells were selected with 0.5 μg/mL puromycin. Three stable cell lines with PMA-inducible release of AP activity in the incubation medium were selected. To measure wound-induced AP-HB-EGF release, cells were extensively injured, cell debris rapidly removed, and fresh KBM added to the culture. The injured cells and controls were continually cultured in KBM, and at the indicated times 15 μL CM were transferred into a 96-well plate. Before AP measurement, the collected media were heated at 65°C for 30 minutes in a water bath. AP activity in the collected media was measured by chemiluminescence detection (Great EscAPe SEAP Chemiluminescence Detection Kit; BD Biosciences, Palo Alto, CA) according to the manufacturer’s protocol. Chemiluminescence was quantitated on a fluorometer (GENios; Phenix Research Products, Asheville, NC). The values, after background absorbance in the medium of nontransfected THCE cells was subtracted, were normalized against cellular protein concentration, using bovine serum albumin (BSA) as a standard (Pierce, Rockford, IL) and were shown as relative light units.
RESULTS
EGFR Phosphorylation in THCE Cells in Response to Wounding
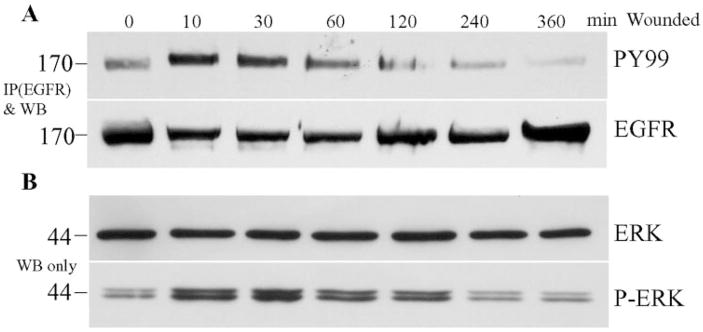
To study wound-induced activation of signaling pathways, we developed a method of creating extensive injury of THCE cell monolayer by using a sharkstooth sequencing gel comb to make scratch wounds. Figure 1A shows the time course of tyrosine phosphorylation of EGFR, an indication of its activation in cells, in the extensively wounded THCE cells. There was a low but detectable EGFR phosphorylation in THCE cells cultured in KBM. EGFR phosphorylation induced by an extensive scratch wound can be observed as early as 5 minutes (not shown, the earliest time tested), peaked at 10 minutes, and started to decline after 30 minutes, but was still higher than control at 60 minutes after wounding (Fig. 1A, top). The increase in EGFR phosphorylation in wounded THCE cells was not related to the total amount of EGFR precipitated (Fig. 1A, bottom).
Figure 1.
Time course of tyrosine phosphorylation of EGFR and ERK in wounded THCE cells. THCE cells were cultured in 100-mm dishes and starved in KBM overnight. Cells were extensively injured by sequential comb scratching and incubated for different time points in KBM. (A) Wounded THCE cells were lysed and 600 μg protein of each reaction was immunoprecipitated (IP) with 10 μg agarose-conjugated rabbit anti-EGFR, subjected to SDS-PAGE, and immuno-blotted with mouse anti-PY99 antibody (top). After stripping, the membrane was reprobed with EGFR antibody (bottom) to assess the amount of protein precipitated. (B) To assess ERK phosphorylation, 10 μg cell lysates of the same samples were subjected to Western blot analysis with either anti-ERK2 (ERK2) or anti-phospho-ERK1 and 2 (pERK). The figure is a representative of three independent experiments. Left: molecular mass (kDa).
Activation of EGFR is known to elicit an array of intracellular-signaling pathways including ERK. Phosphorylation of ERK is indicative of activation of mitogen-activated protein kinase (MAPK) pathway. Figure 1B shows the time course of wound-induced ERK phosphorylation in THCE cells. There was basal ERK phosphorylation in growth-factor–starved THCE cells. Wounding induced ERK phosphorylation, the intensity peaked at 10 minutes, and the elevated levels were observed 2 hours after wounding. The time course of ERK phosphorylation suggested that the MAPK pathway was elicited by EGFR in wounded corneal epithelial cells.
Involvement of EGFR Ligand Release and EGFR Activation in Corneal Epithelial Wound Healing
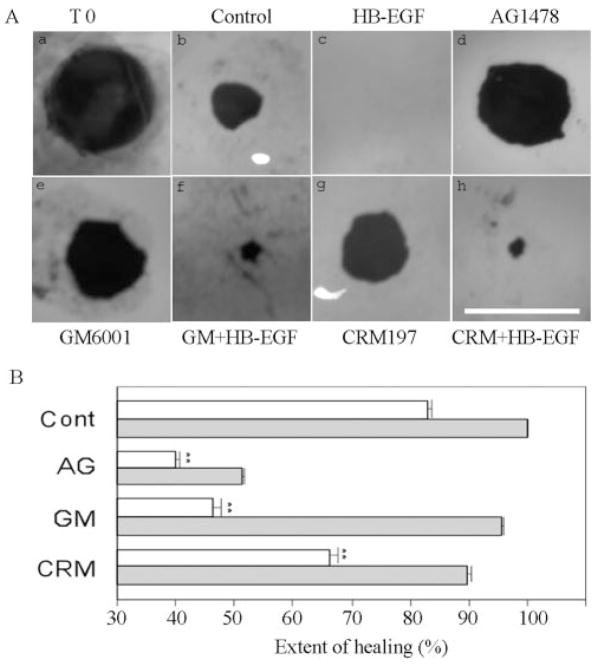
To study the role of EGFR signaling in epithelial wound healing, we used a corneal organ culture model.39,45 We created an epithelial debridement wound of 4 mm in diameter in the center of the porcine corneas (Fig. 2Aa) and cultured the wounded corneas in an air-lifted corneal organ culture setting first described by Foreman et al.39 This air-lifted corneal organ culture seems to maintain the epithelial cell morphology, while decreasing the intercellular edema usually observed in conventional submerged models.39,46,47 Corneal epithelial wound closure of 4 mm in diameter was observed in less than 3 days in a serum- and growth-factor–free medium. After 48 hours, the wound was approximately 83% covered (Fig. 2Ab). This natural healing process was termed spontaneous healing. With recombinant human HB-EGF (50 ng/mL), the wound recovered completely in approximately 48 hours (Fig. 2Ac). In the presence of 1 μM AG1478, an inhibitor of EGFR, epithelial wound closure was blocked, with or without exogenous HB-EGF (Figs. 2Ad, 2B), suggesting that EGFR activation accounts for spontaneous and HB-EGF–enhanced epithelial wound closure.
Figure 2.
Corneal epithelial wound healing in cultured porcine corneas. (A) Representative epithelial wound closure in cultured porcine corneas treated with different reagents. Porcine corneas were injured, and one cornea was stained immediately with Richardson staining solution to show the area of initial wound (T0) (a). Injured corneas were cultured for 48 hours in MEM (control, b), MEM containing 50 ng/mL HB-EGF (c), 1 μM AG1478 (d), 100 μM GM6001 (e), 100 μM GM6001 + 50 ng/mL HB-EGF (f), 10 μg/mL CRM197 (g), or 10 μg/mL CRM197 + 50 ng/mL HB-EGF (h). Bar, 4 mm. (B) Changes in the extent of healing in cultured porcine corneas treated with different reagents. (□) Treatment with inhibitors alone; ( ) treatments with in the presence of HB-EGF. The coverage of the wound (0% for no migration and 100% for a complete covering of the wound bed) was then calculated. Statistically, there are significant decreases of corneal epithelial healing rate in AG1478-, GM6001-, and CRM197-treated groups compared with MEM-treated (cont, **P < 0.01). Data are the mean ± SE of at least six corneas from two or more independent experiments.
) treatments with in the presence of HB-EGF. The coverage of the wound (0% for no migration and 100% for a complete covering of the wound bed) was then calculated. Statistically, there are significant decreases of corneal epithelial healing rate in AG1478-, GM6001-, and CRM197-treated groups compared with MEM-treated (cont, **P < 0.01). Data are the mean ± SE of at least six corneas from two or more independent experiments.
The release of EGFR ligands has been shown to be sensitive to MMP inhibitors. To determine the effects of MMP activity on corneal wound healing, injured porcine corneas were incubated with GM6001, a hydroxamate MMP inhibitor. A substantial inhibition of epithelial wound closure was observed (46.4% wound covered, Figs. 2Ae, 2B) in comparison with control (Fig. 2Ab). Addition of recombinant HB-EGF significantly overcame the GM6001 effect and promoted epithelial wound closure (95.8% wound covered, Figs. 2Af, 2B). Thus, the wound-induced release of endogenous EGFR ligands requires MMP activity.
Membrane bound (pro)HB-EGF also serves as the unique high-affinity receptor for diphtheria toxin.48,49 A nontoxic and catalytically inactive [Glu-52] mutant of diphtheria toxin, CRM197, has been shown to bind extracellular HB-EGF domain specifically and potently inhibits the activity of HB-EGF.31,50 Unlike in mice and rats, the amino acid residues critical for diphtheria toxin binding, Phe-115, Leu-127, and Glu-141 in the binding domain of porcine proHB-EGF, are identical with those of the human proHB-EGF, enabling CRM197 to bind and interact with the porcine proHB-EGF.42,50–52 To determine whether HB-EGF shedding contributes to epithelial wound healing, we pretreated wounded corneas with 10 μg/mL CRM197 and assessed its effects on epithelial wound closure. When CRM197 was added to the organ culture medium, epithelial wound closure was retarded (66% wound covered, Figs. 2Ag, 2B) compared with control (Fig. 2Ab). However, CRM197 inhibition of wound closure was not as effective as AG1478 or GM6001. Furthermore, CRM 197 exhibited no effects on EGF (20 ng/mL)-enhanced epithelial wound closure (data not shown), and a high level of exogenous HB-EGF (100 ng/mL) was needed for partial restoration of the enhanced healing (89.8% wound covered, Figs. 2Ah, 2B), suggesting that CRM197 specifically targets HB-EGF in cultured porcine corneas. Thus, endogenously generated HB-EGF appears to play a role in the induction of epithelial wound closure in cultured porcine corneas.
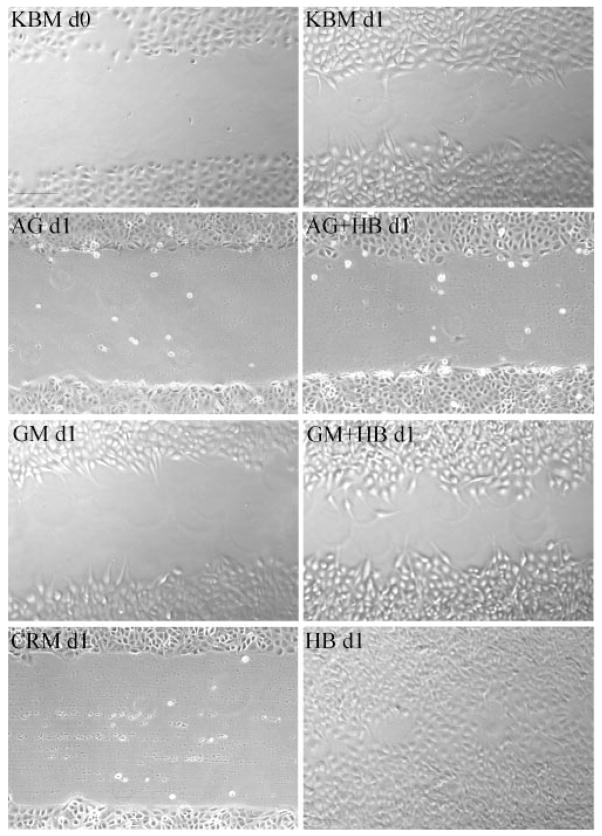
The effects of HB-EGF–shedding inhibitors were also tested in the healing of scratch wounds in THCE cells. Figure 3 shows cell migration toward the center of a scratch wound (KBM d0 [day 0]) in cultured THCE cells in the presence of KBM-plus for 24 hours (day 1) after wounding (KBM d1). The presence of HB-EGF without inhibitors resulted in complete closure of the scratch wound (HBd1). Similar to that observed in cultured porcine corneas, 0.5 μM AG1478 suppressed epithelial wound closure with (AG + HB d1) or without (AG d1) exogenously added HB-EGF. GM6001 (50 nM, GM d1) and CRM197 (10 ng/mL, CRM d1) impaired spontaneous wound healing, and the presence of HB-EGF overcame the effects of GM6001 (GM + HB d1) on epithelial wound closure. Taken together, THCE cells showed responses to wounding and to HB-EGF–shedding inhibitors similar to organ-cultured porcine corneas, indicating a similar underlying mechanism for epithelial wound closure ex vivo and in vitro.
Figure 3.
Healing of scratch wound in cultured human corneal epithelial cells. Growth factor-starved THCE cell monolayers cultured in 12-well plates were injured with a sterile 0.1- to 10-μL pipet tip (KBM d0). Cells were incubated in KBM-plus for 24 hours (KBM d1), in KBM containing 0.5 μM AG1478 (AG d1), 0.5 μM AG1478 with 50 ng/mL HB-EGF (AG+HB d1), 50 μM GM6001 (GM d1), 50 μM GM6001 with 50 ng/mL HB-EGF (GM+HB d1), 10 μg/mL CRM197 (CRM d1), or 50 ng/mL HB-EGF (HB d1). Photomicrographs represent one of four samples. Bar, 100 μm.
Effects of HB-EGF Shedding Inhibition on EGFR Activation
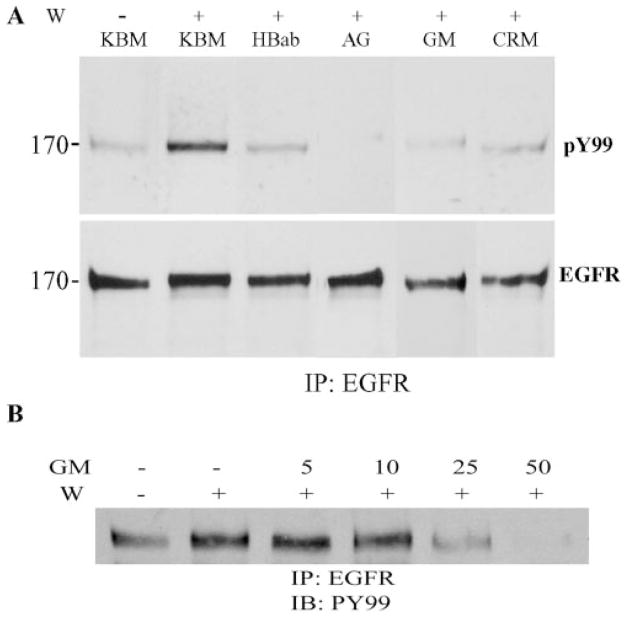
To detect the effects of reagents that inhibit corneal epithelial wound healing on EGFR activation, we pretreated cells with human HB-EGF–neutralizing antibody, AG1478, GM6001, and CRM197; made scratch wounds; and lysed samples 15 minutes after wounding. As shown in Figure 4A, the presence of 0.5 μM AG1478, 50 μM GM6001, and 10 μg/mL CRM197 abrogated wound-induced EGFR activation (Fig. 4A, top). Furthermore, incubation of human HB-EGF function-blocking antibodies inhibited wound-induced EGFR phosphorylation. Thus, HB-EGF acts as an endogenous ligand released on wounding to activate EGFR.
Figure 4.
Effects of HB-EGF and EGFR inhibitors on wound-induced EGFR phosphorylation. Growth factor-starved THCE cells were pretreated with 10 μg/mL HB-EGF neutralizing antibody, 0.5 μM AG1478, 50 μM GM6001, 10 μg/mL CRM197 (A) or 0 to 50 μM GM6001 (B) for 20 minutes and then wounded by comb-scratching, with unwounded (W −) serving as the control. Cells were lysed 15 minutes after wounding. For each reaction, 600 μg of protein was immunoprecipitated (IP) by 10 μg EGFR antibody and immunoblotted by mouse anti-PY99 (top), followed by reprobing with EGFR antibody (bottom). Data are representatives of three independent experiments. Left: molecular mass (kDa).
Figure 4B shows the dose dependent effect of GM6001 on wound-induced EGFR phosphorylation. In THCE cells, 25 μM GM6001 was effective in inhibition of wound-induced EGFR phosphorylation, a concentration comparable to that needed to inhibit ADAM17-mediated ectodomain shedding.53,54
Wound-Induced HB-EGF Ectodomain Shedding
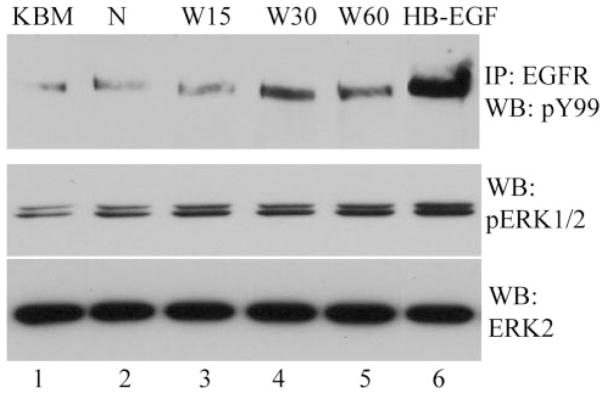
To examine whether wounding could initiate proteolytic processing of pro-HB-EGF, CM were collected from normal and wounded THCE cells and combined with 2 M NaCl extractions of cell-surface–associated proteins. Heparin-binding proteins from each condition were purified by heparin-affinity chromatography. To examine the presence of the wound-induced release of HB-EGF, we treated growth-factor–starved THCE cells, with or without the purified HB proteins from CM (Fig. 5). Compared with KBM alone (lane 1) or the eluent from nonwounded cells (lane 2), the eluents (lanes 3–5) from the CM of wounded THCE cells apparently induced EGFR tyrosine phosphorylation, especially for the eluents of 30- and 60-minute postwound cells and ERK activation in THCE cells. As expected, exogenously added HB-EGF stimulated EGFR and ERK activation (lane 6).
Figure 5.
Wound-induced release of soluble HB-EGF. CM was collected at indicated times after extensive wounding of THCE cells and combined with 2 NaCl extract of cell surface proteins. The mixture was then applied to a heparin HP affinity column to purify heparin-binding proteins. Bound proteins were eluted. The eluents were used to stimulate growth-factor–starved THCE cells and cell lysates were subjected to immunoprecipitation (IP: EGFR) and/or immunoblot analysis with pY99 antibody (WB: pY99) for EGFR phosphorylation, anti-phospho-ERK1/2 (WB: pERK1/2) for ERK phosphorylation and anti-ERK for normalization of protein loading. Lane 1: KBM; lane 2: eluent from nonwounded cells; lanes 3 to 5: the eluents from wounded, 15, 30, and 60 minutes, respectively, THCE cells; lane 6: KBM plus 50 ng/mL HB-EGF.
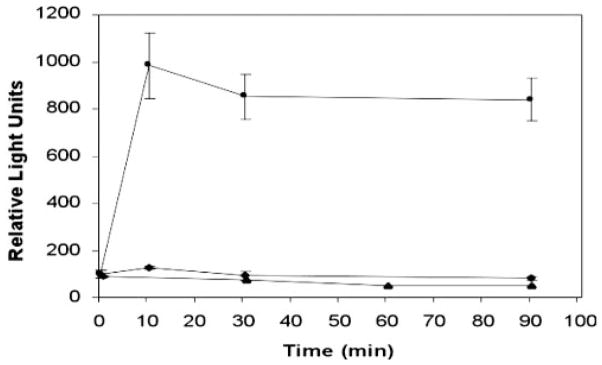
To further assess whether wounding releases HB-EGF, we established THCE cell lines transfected with plasmids containing the human AP cDNA inserted into the heparin-binding region of mature human HB-EGF protein (HB-EGF-AP).27,44 Release of HB-EGF-AP in the transfected cells in response to wounding was evaluated by measuring AP activities in culture media (Fig. 6). In nonwounded cells (filled triangles), there was little AP activity, and no accumulation of AP activity was detected up to 90 minutes, suggesting a low level of HB-EGF shedding in nonstimulated cells. AP activity is increased after wounding and reached a 7.9-fold increase at 15 minutes (filled squares). Pretreatment of 50 μM GM6001 completely abolished the wound-induced increase in AP activity (filled diamonds). Thus, wounding of epithelial cells induces HB-EGF release in an MMP-dependent manner.
Figure 6.
Wound-induced HB-EGF-AP release. THCE cells were stably transfected with plasmid pHB-EGF-AP. HB-EGF-AP–expressing cells were cultured on six-well plates and one group was pretreated with GM6001 (50 μM) for 1 hour. Cells, GM6001 treated (◆) and non-treated (■), were then scratched sequentially with a comb, washed, and cultured in KBM for up to 90 minutes, with the nonwounded cells serving as the control (▲). Fifteen microliters were taken from each group at indicated times and the AP activity in the media were measured. The results are expressed in relative light units. Data represents the mean ± SD of results in triplicate wells from a representative experiment. Similar results were obtained in two independent experiments.
Taken together, these data indicate that wounding activates EGFR and its downstream signaling by induction of pro-HB-EGF shedding.
Discussion
In this study, we used porcine corneal organ cultures and human corneal epithelial cell cultures to study the regulation of corneal epithelial wound healing. Consistent with previous findings,18,20 we showed that epithelial wounding induces EGFR activation and blocking EGFR activation impairs wound closure in both models, indicating a key role for the EGFR-mediated signal cascade in epithelial cells. Addition of GM6001 inhibited epithelial wound closure, which probably includes both cell migration and cell proliferation, in both cultured porcine corneas and in vitro scratch wound and wound-induced EGFR phosphorylation in cultured THCE cells. The effects of MMP inhibition can be overcome if recombinant soluble HB-EGF was added along with GM6001, suggesting that the inhibition was primarily affecting the proteolytic release of EGFR ligands after wounding. Furthermore, CRM197, known to bind specifically to and neutralize HB-EGF, attenuates epithelial wound closure and wound-induced EGFR phosphorylation ex vivo and/or in vitro. Together with the observation that HB-EGF–neutralizing antibody reduced wound-induced EGFR phosphorylation, our data suggest that proteolytic release of HB-EGF generates an autocrine ligand for EGFR activation on wounding. The release of HB-EGF in response to wounding was demonstrated by identifying heparin-binding protein from wounded THCE cells that stimulates EGFR phosphorylation and subsequent MAPK activation and by the release of AP activity in an MMP-inhibitor–sensitive manner. Taken together, we propose that ectodomain shedding of HB-EGF and EGFR phosphorylation constitutes initial signaling steps for corneal epithelial wound healing.
Migration and proliferation are the key events during corneal epithelial wound healing and are mediated, at least in part, by autocrine EGFR activation, as demonstrated in a cutaneous1,18,20 and corneal wound models.18,20 Phosphorylation of EGFR is an initial and essential event of EGFR ligand-induced signal transduction.55 In this study, wounding stimulated EGFR and subsequent ERK phosphorylation in cultured THCE cells. Inhibition of EGFR activation by AG1478 blocked both EGFR phosphorylation and epithelial wound closure in cultured corneas as well as in cultured THCE cells, indicating an essential role for EGFR signaling in epithelial wound healing. AG1478 also inhibited wound-induced ERK phosphorylation, suggesting that the ERK-signaling pathway in wounded corneal epithelial cells is elicited by EGFR activation. Thus, our data reaffirmed previous findings18,20 that EGFR activation is necessary for proper wound healing in the cornea. The essential role of the EGFR-signaling network was further supported by our recent study showing that depletion of erbB2, the coreceptor of EGFR, affects the intensity and duration of wound-induced ERK and phosphatidylinositol 3-kinase activation and impairs in vitro cell migration and wound closure in THCE cells (Xu K-P and Yu F-SX, manuscript in preparation). Recently, p38 MAPK has been shown to cross-talk with ERK1/2 and to selectively activate cell migration during epithelial wound healing.56 It would be of interest to determine whether EGFR activation in response to wounding induces p38 signaling.
The fact that in vitro18 or ex vivo wounding stimulates EGFR activation indicates autocrine production of EGFR ligands. Constitutive gene expression of EGFR ligands such as EGF, HB-EGF, TGF-α, and their receptors have also been demonstrated in corneal epithelial cells.18,57–60 Members of the EGF family are synthesized as membrane-anchored forms and are then processed by proteolytic cleavage to give bioactive soluble factors.61,62 Although EGF processing appears to be catalyzed by a serine proteinase,63 the release of other members such as HB-EGF, TGF-α, and amphiregulin is stimulated by phorbol esters and is sensitive to MMP inhibitors.25,29,64 Zieske and Bukusoglu65 reported that the MMP inhibitor, 1,10-phenanthroline, completely blocked epithelial migration and proliferation in cultured rat corneas. Consistent with their study, we observed that GM6001 as well as IC-3 inhibited epithelial wound closure in cultured porcine corneas and in THCE cells after a scraping wound, suggesting a role for MMP activity in corneal epithelial wound healing. Furthermore, the effect of GM6001 on epithelial migration can be overcome if recombinant soluble HB-EGF was added along with GM6001, suggesting that the inhibitor primarily affected the release of EGFR ligands after wounding. Thus, we concluded that MMP-mediated release of EGFR ligand(s) is required for epithelial wound closure.
Which member of the EGF family acts as the endogenous ligand generated after wounding? We showed that CRM197 effectively inhibited epithelial wound closure in both ex vivo and in vitro systems. CRM197, a nontoxic mutant protein of diphtheria toxin,49 has been shown specifically to bind to human or porcine HB-EGF and, thus, neutralizing the activity of HB-EGF but not other EGF ligands.50 It has been used in numerous systems to investigate the role of HB-EGF in vitro.31,42,66 The specificity of CRM197 in porcine corneas and in THCE cells was verified by the observation that HB-EGF, but not EGF, is sensitive to CRM197 in enhancing wound closure. Thus, the release of HB-EGF contributes to the activation of EGFR and subsequent intracellular signaling pathways in response to epithelial wounding in the cornea. However, because CRM197 inhibition of wound closure is not as effective as AG1478 or GM6001, it is likely that MMP-dependent shedding of other EGFR ligands such as TGF-αand/or amphiregulin may provide additional ligands for EGFR activation.18,67,68 Taken together, we conclude that shedding of HB-EGF and/or other EGFR ligands such as TGF-α and amphiregulin induced by wounding provide soluble EGFR ligand(s) that is a critical event for autocrine–paracrine stimulation of corneal epithelial wound healing.
Although all three inhibitors, CRM197, GM6001, and AG1478, are targeting the wound-induced EGFR activation, they appear to have different effects on cell shape at wound edge (Fig. 3). How these changes in cell shape are related to the effects of different reagents on wound closure remains to be determined.
Among a large body of literature showing HB-EGF ectodomain shedding as a major pathway mediating EGFR transactivation, only a few studies demonstrated directly the release of HB-EGF from cells containing high levels of proHB-EGF.42,69 We initially used several approaches including Western blot analysis of concentrated culture media and heparin affinity purification of conditioned media followed by Western blot analysis and were unable to detect the release of endogenous HB-EGF from wounded THCE cells, as reported by other laboratories.44,66,70 This finding may be due to the low concentration of HB-EGF released from cells and due to the binding of HB-EGF to its receptors and/or heparin-containing molecules such as CD4471,72 at the cell surface, or both. Indeed, by stripping cell-surface–associated proteins with 2 M NaCl, we were able to identify biological activity of HB-EGF from cell surface protein extracts of wounded, but not control, THCE cells. We also used cells expressing HB-EGF-AP to demonstrate the release of HB-EGF in THCE cells in response to wounding. These biochemical and genetic approaches demonstrated the release of HB-EGF from corneal epithelial cells in response to wounding.
HB-EGF was first identified in the CM of macrophages.9 Whereas the transmembrane form of HB-EGF acts in a juxtacrine manner to signal neighboring cells,28 the soluble form of HB-EGF is a potent mitogen and chemoattractant for many cell types, including keratinocytes and epithelial cells.29 The availability of MMP inhibitors and CRM197 has greatly facilitated the research into HB-EGF shedding in recent years.31 Ectodomain shedding of transmembrane HB-EGF has recently been shown to play an important role in cardiac hypertrophy,33,34 in the lung epithelial cell response to bacterial challenge35,73 and in TGF-β–mediated fibronectin expression in mesangial cells.44 Our study presents evidence that HB-EGF shedding is also a key regulatory event for corneal epithelial wound healing. Recently, molecular mechanisms for the ectodomain shedding of EGFR ligands including HB-EGF and TGF-αhave been vigorously studied, showing the involvement of the ADAM (a disintegrin and MMP) family of proteinases.22,31,74 We recently found that all four types of ADAMs—ADAM-9, -10, -12, and -17—which possess MMP activity and are implicated as the catalyst in the ectodomain shedding of HB-EGF22,74 in other cells, are expressed in human corneal epithelial cells (Yu F-SX, et al. IOVS 2003;44:ARVO E-Abstract 3817). Given the importance of ectodomain shedding in corneal wound healing, it is clear that shedding enzymes and their involvement in wound healing deserve further investigation.
In summary, HB-EGF released after wounding provides a major ligand for EGFR and ectodomain shedding of HB-EGF ligands is a critical event in regulating corneal wound healing. Further studies aimed at the underlying mechanisms of ectodomain shedding of EGFR ligands may suggest possible targets for an effective clinical treatment in the early phases of corneal wound healing.
Acknowledgments
Supported by Grants R01EY10869 and EY14080 from the National Eye Institute.
The authors thank Michael Klagsbrun (Children’s Hospital and Harvard Medical School, Boston, MA) for providing the HB-EGF-AP expression vector, Eisuke Mekada (Department of Cell Biology, Research Institute for Microbial Diseases, Osaka University, Japan) for providing HB-EGF extracting protocol, and Rhea-Beth Markowitz (Institute of Molecular Medicine and Genetics, Medical College of Georgia) for critical reading of the manuscript.
Footnotes
Disclosure: K.-P. Xu, None; Y. Ding, None; J. Ling, None; Z. Dong, None; F.-S.X. Yu, None
References
- 1.Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med. 2001;226:653–664. doi: 10.1177/153537020222600711. [DOI] [PubMed] [Google Scholar]
- 2.Gipson I, Sugrue S. Cell Biology of the Corneal Epithelium. In: Albert D, Jakobiec F, editors. Principles and Practice of Ophthalmology. Philadelphia: WB Saunders; 1994. pp. 3–16. [Google Scholar]
- 3.Martin P. Wound healing: aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 4.Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 2000;19:113–129. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 5.Wells A. Tumor invasion: role of growth factor-induced cell motility. Adv Cancer Res. 2000;78:31–101. doi: 10.1016/s0065-230x(08)61023-4. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S. Isolation and biological effects of an epidermal growth-stimulating protein. Natl Cancer Inst Monogr. 1964;13:13–27. [PubMed] [Google Scholar]
- 7.Derynck R, Roberts AB, Winkler ME, Chen EY, Goeddel DV. Human transforming growth factor: precursor structure and expression in E. coli. Cell. 1984;38:287–297. doi: 10.1016/0092-8674(84)90550-6. [DOI] [PubMed] [Google Scholar]
- 8.Shoyab M, Plowman GD, McDonald VL, Bradley JG, Todaro GJ. Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science. 1989;243:1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- 9.Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 10.Shing Y, Christofori G, Hanahan D, et al. Betacellulin: a mitogen from pancreatic beta cell tumors. Science. 1993;259:1604–1607. doi: 10.1126/science.8456283. [DOI] [PubMed] [Google Scholar]
- 11.Toyoda H, Komurasaki T, Uchida D, et al. Epiregulin: a novel epidermal growth factor with mitogenic activity for rat primary hepatocytes. J Biol Chem. 1995;270:7495–500. doi: 10.1074/jbc.270.13.7495. [DOI] [PubMed] [Google Scholar]
- 12.Hynes NE, Horsch K, Olayioye MA, Badache A. The ErbB receptor tyrosine family as signal integrators. Endocr Relat Cancer. 2001;8:151–159. doi: 10.1677/erc.0.0080151. [DOI] [PubMed] [Google Scholar]
- 13.Carpenter G, Wahl M. The epidermal growth factor family. In: Sporn M, Roberts AB, editors. Peptides, Growth Factors and Their Receptors I. New York: Springer-Verlag; 1991. pp. 69–171. [Google Scholar]
- 14.Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198:165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 15.Riese DJ, II, Komurasaki T, Plowman GD, Stern DF. Activation of ErbB4 by the bifunctional epidermal growth factor family hormone epiregulin is regulated by ErbB2. J Biol Chem. 1998;273:11288–11294. doi: 10.1074/jbc.273.18.11288. [DOI] [PubMed] [Google Scholar]
- 16.Olayioye M, Neve R, Lane Ha, Hynes N. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z, Carvajal M, Carraway CA, Carraway K, Pflugfelder SC. Expression of the receptor tyrosine kinases, epidermal growth factor receptor, ErbB2, ErbB3, in human ocular surface epithelia. Cornea. 2001;20:81–85. doi: 10.1097/00003226-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Zieske JD, Takahashi H, Hutcheon AE, Dalbone AC. Activation of epidermal growth factor receptor during corneal epithelial migration. Invest Ophthalmol Vis Sci. 2000;41:1346–1355. [PubMed] [Google Scholar]
- 19.Elenius K, Corfas G, Paul S, et al. A novel juxtamembrane domain isoform of HER4/ErbB4: isoform-specific tissue distribution and differential processing in response to phorbol ester. J Biol Chem. 1997;272:26761–26768. doi: 10.1074/jbc.272.42.26761. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura Y, Sotozono C, Kinoshita S. The epidermal growth factor receptor (EGFR): role in corneal wound healing and homeostasis. Exp Eye Res. 2001;72:511–517. doi: 10.1006/exer.2000.0979. [DOI] [PubMed] [Google Scholar]
- 21.Lee DC, Sunnarborg SW, Hinkle CL, et al. TACE/ADAM17 processing of EGFR ligands indicates a role as a physiological convertase. Ann NY Acad Sci. 2003;995:22–38. doi: 10.1111/j.1749-6632.2003.tb03207.x. [DOI] [PubMed] [Google Scholar]
- 22.Schlondorff J, Blobel CP. Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J Cell Sci. 1999;112:3603–3617. doi: 10.1242/jcs.112.21.3603. [DOI] [PubMed] [Google Scholar]
- 23.Massagué J, Pandiella A. Membrane-anchored growth factors. Annu Rev Biochem. 1993;62:515–541. doi: 10.1146/annurev.bi.62.070193.002503. [DOI] [PubMed] [Google Scholar]
- 24.Henikoff S, Henikoff J. Protein family classification based on searching a database of blocks. Genomics. 1994;19:97–107. doi: 10.1006/geno.1994.1018. [DOI] [PubMed] [Google Scholar]
- 25.Raab G, Higashiyama S, Hetelekidis S, et al. Biosynthesis and processing by phorbol ester of the cells surface-associated precursor form of heparin-binding EGF-like growth factor. Biochem Biophys Res Commun. 1994;204:592–597. doi: 10.1006/bbrc.1994.2500. [DOI] [PubMed] [Google Scholar]
- 26.Goishi K, Higashiyama S, Klagsbrun M, et al. Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factor: conversion from juxtacrine to paracrine growth factor activity. Mol Biol Cell. 1995;6:967–980. doi: 10.1091/mbc.6.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gechtman Z, Alonso JL, Raab G, Ingber DE, Klagsbrun M. The shedding of membrane-anchored heparin-binding epidermal-like growth factor is regulated by the Raf/mitogen-activated protein kinase cascade and by cell adhesion and spreading. J Biol Chem. 1999;274:28828–28835. doi: 10.1074/jbc.274.40.28828. [DOI] [PubMed] [Google Scholar]
- 28.Higashiyama S, Iwamoto R, Goishi K, et al. The membrane protein CD9/DRAP 27 potentiates the juxtacrine growth factor activity of the membrane-anchored heparin-binding EGF-like growth factor. J Cell Biol. 1995;128:929–938. doi: 10.1083/jcb.128.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwamoto R, Mekada E. Heparin-binding EGF-like growth factor: a juxtacrine growth factor. Cytokine Growth Factor Rev. 2000;11:335–344. doi: 10.1016/s1359-6101(00)00013-7. [DOI] [PubMed] [Google Scholar]
- 30.Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim Biophys Acta. 1997;1333:F179–F199. doi: 10.1016/s0304-419x(97)00024-3. [DOI] [PubMed] [Google Scholar]
- 31.Prenzel N, Zwick E, Daub H, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 32.Tokumaru S, Higashiyama S, Endo T, et al. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J Cell Biol. 2000;151:209–220. doi: 10.1083/jcb.151.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asakura M, Kitakaze M, Takashima S, et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med. 2002;8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- 34.Liao JK. Shedding growth factors in cardiac hypertrophy. Nat Med. 2002;8:20–21. doi: 10.1038/nm0102-20. [DOI] [PubMed] [Google Scholar]
- 35.Lemjabbar H, Basbaum C. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat Med. 2002;8:41–46. doi: 10.1038/nm0102-41. [DOI] [PubMed] [Google Scholar]
- 36.Maskos K, Fernandez-Catalan C, Huber R, et al. Crystal structure of the catalytic domain of human tumor necrosis factor-alpha-converting enzyme. Proc Natl Acad Sci USA. 1998;95:3408–3412. doi: 10.1073/pnas.95.7.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 38.Zieske J, Gipson I. Protein synthesis during corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 1986;27:1–7. [PubMed] [Google Scholar]
- 39.Foreman DM, Pancholi S, Jarvis EJ, McLeod D, Boulton ME. A simple organ culture model for assessing the effects of growth factors on corneal re-epithelialization. Exp Eye Res. 1996;62:555–564. doi: 10.1006/exer.1996.0065. [DOI] [PubMed] [Google Scholar]
- 40.Richardson K, Jarett L, Finke E. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- 41.Araki-Sasaki K, Ohashi Y, Sasabe T, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614–621. [PubMed] [Google Scholar]
- 42.Chen J-K, Capdevila J, Harris RC. Heparin-binding EGF-like growth factor mediates the biological effects of P450 arachidonate epoxy-genase metabolites in epithelial cells. Proc Natl Acad Sci USA. 2002;99:6029–6034. doi: 10.1073/pnas.092671899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raab G, Kover K, Paria BC, Dey SK, Ezzell RM, Klagsbrun M. Mouse preimplantation blastocysts adhere to cells expressing the transmembrane form of heparin-binding EGF-like growth factor. Development. 1996;122:637–645. doi: 10.1242/dev.122.2.637. [DOI] [PubMed] [Google Scholar]
- 44.Uchiyama-Tanaka Y, Matsubara H, Mori Y, et al. Involvement of HB-EGF and EGF receptor transactivation in TGF-beta-mediated fibronectin expression in mesangial cells. Kidney Int. 2002;62:799–808. doi: 10.1046/j.1523-1755.2002.00537.x. [DOI] [PubMed] [Google Scholar]
- 45.Xu KP, Li XF, Yu FS. Corneal organ culture model for assessing epithelial responses to surfactants. Toxicol Sci. 2000;58:306–314. doi: 10.1093/toxsci/58.2.306. [DOI] [PubMed] [Google Scholar]
- 46.Richard NR, Anderson JA, Weiss JL, Binder PS. Air/liquid corneal organ culture: a light microscopic study. Curr Eye Res. 1991;10:739–749. doi: 10.3109/02713689109013868. [DOI] [PubMed] [Google Scholar]
- 47.Collin HB, Anderson JA, Richard NR, Binder PS. In vitro model for corneal wound healing; organ-cultured human corneas. Curr Eye Res. 1995;14:331–339. doi: 10.3109/02713689508999930. [DOI] [PubMed] [Google Scholar]
- 48.Naglich JG, Metherall JE, Russell DW, Eidels L. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell. 1992;69:1051–1061. doi: 10.1016/0092-8674(92)90623-k. [DOI] [PubMed] [Google Scholar]
- 49.Uchida T, Pappenheimer A, Greany R. Diphtheria toxin and related proteins. I. Isolation and properties of mutant proteins serologically related to diphtheria toxin. J Biol Chem. 1973;248:3838–3844. [PubMed] [Google Scholar]
- 50.Mitamura T, Higashiyama S, Taniguchi N, Klagsbrun M, Mekada E. Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. J Biol Chem. 1995;270:1015–1019. doi: 10.1074/jbc.270.3.1015. [DOI] [PubMed] [Google Scholar]
- 51.Abraham JA, Damm D, Bajardi A, Miller J, Klagsbrun M, Ezekowitz RAB. Heparin-binding EGF-like growth factor: characterization of rat and mouse cDNA clones, protein domain conservation across species, and transcript expression in tissues. Biochem Biophys Res Commun. 1993;190:125–133. doi: 10.1006/bbrc.1993.1020. [DOI] [PubMed] [Google Scholar]
- 52.Mitamura T, Umata T, Nakano F, et al. Structure-function analysis of the diphtheria toxin receptor toxin binding site by site-directed mutagenesis. J Biol Chem. 1997;272:27084–27090. doi: 10.1074/jbc.272.43.27084. [DOI] [PubMed] [Google Scholar]
- 53.Contin C, Pitard V, Itai T, Nagata S, Moreau J-F, Dechanet-Merville J. Membrane-anchored CD40 is processed by the tumor necrosis factor-α-converting Enzyme: implications for CD40 signaling. J Biol Chem. 2003;278:32801–32809. doi: 10.1074/jbc.M209993200. [DOI] [PubMed] [Google Scholar]
- 54.Garton KJ, Gough PJ, Blobel CP, et al. Tumor necrosis factor-α-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) J Biol Chem. 2001;276:37993–38001. doi: 10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- 55.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 56.Sharma GD, He J, Bazan HE. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: evidence of cross-talk activation between MAP kinase cascades. J Biol Chem. 2003;278:21989–21997. doi: 10.1074/jbc.M302650200. [DOI] [PubMed] [Google Scholar]
- 57.Wilson S, Lloyd S, He Y. EGF, basic FGF, and TGF beta-1 messenger RNA production in rabbit corneal epithelial cells. Invest Ophthalmol Vis Sci. 1992;33:1987–1989. [PubMed] [Google Scholar]
- 58.Wilson SE, Chen L, Mohan RR, Liang Q, Liu J. Expression of HGF, KGF, EGF and receptor messenger RNAs following corneal epithelial wounding. Exp Eye Res. 1999;68:377–397. doi: 10.1006/exer.1998.0603. [DOI] [PubMed] [Google Scholar]
- 59.Li D, Tseng S. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cel Physiol. 1995;163:61–79. doi: 10.1002/jcp.1041630108. [DOI] [PubMed] [Google Scholar]
- 60.Zieske JD, Wasson M. Regional variation in distribution of EGF receptor in developing and adult corneal epithelium. J Cell Sci. 1993;106:145–152. doi: 10.1242/jcs.106.1.145. [DOI] [PubMed] [Google Scholar]
- 61.Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer. 2001;8:11–31. doi: 10.1677/erc.0.0080011. [DOI] [PubMed] [Google Scholar]
- 62.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 63.Journe F, Wattiez R, Piron A, et al. Renal epidermal growth factor precursor: proteolytic processing in an in vitro cell-free system. Biochim Biophys Acta. 1997;1357:18–30. doi: 10.1016/s0167-4889(97)00012-8. [DOI] [PubMed] [Google Scholar]
- 64.Harano T, Mizuno K. Phorbol ester-induced activation of a membrane-bound candidate pro-transforming growth factor-alpha processing enzyme. J Biol Chem. 1994;269:20305–20311. [PubMed] [Google Scholar]
- 65.Zieske J, Bukusoglu G. Effect of protease inhibitors on corneal epithelial migration. Invest Ophthalmol Vis Sci. 1991;32:2073–2078. [PubMed] [Google Scholar]
- 66.Lin J, Freeman MR. Transactivation of ErbB1 and ErbB2 receptors by angiotensin II in normal human prostate stromal cells. Prostate. 2003;54:1–7. doi: 10.1002/pros.10160. [DOI] [PubMed] [Google Scholar]
- 67.Schultz G, Rotatori DS, Clark W. EGF and TGF-alpha in wound healing and repair. J Cell Biochem. 1991;45:346–352. doi: 10.1002/jcb.240450407. [DOI] [PubMed] [Google Scholar]
- 68.Schultz G, Chegini N, Grant M, Khaw P, MacKay S. Effects of growth factors on corneal wound healing. Acta Ophthalmol Suppl. 1992;202:60–66. doi: 10.1111/j.1755-3768.1992.tb02170.x. [DOI] [PubMed] [Google Scholar]
- 69.Takenobu H, Yamazaki A, Hirata M, Umata T, Mekada E. The stress-and inflammatory cytokine-induced ectodomain shedding of heparin-binding epidermal growth factor-like growth factor is mediated by p38 MAPK, distinct from the 12-O-tetradecanoylphorbol-13-acetate- and lysophosphatidic acid-induced signaling cascades. J Biol Chem. 2003;278:17255–17262. doi: 10.1074/jbc.M211835200. [DOI] [PubMed] [Google Scholar]
- 70.Kalmes A, Vesti BR, Daum G, Abraham JA, Clowes AW. Heparin blockade of thrombin-induced smooth muscle cell migration involves inhibition of epidermal growth factor (EGF) receptor transactivation by heparin-binding EGF-like growth factor. Circ Res. 2000;87:92–98. doi: 10.1161/01.res.87.2.92. [DOI] [PubMed] [Google Scholar]
- 71.Yu WH, Woessner JF, Jr, McNeish JD, Stamenkovic I. CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev. 2002;16:307–323. doi: 10.1101/gad.925702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu FX, Guo J, Zhang Q. Expression and distribution of adhesion molecule CD44 in healing corneal epithelia. Invest Ophthalmol Vis Sci. 1998;39:710–717. [PubMed] [Google Scholar]
- 73.Wallasch C, Crabtree JE, Bevec D, Robinson PA, Wagner H, Ullrich A. Helicobacter pylori-stimulated EGF receptor transactivation requires metalloprotease cleavage of HB-EGF. Biochem Biophys Res Commun. 2002;295:695–701. doi: 10.1016/s0006-291x(02)00740-4. [DOI] [PubMed] [Google Scholar]
- 74.Blobel CP. Functional and biochemical characterization of ADAMs and their predicted role in protein ectodomain shedding. Inflamm Res. 2002;51:83–84. doi: 10.1007/BF02684007. [DOI] [PubMed] [Google Scholar]