Abstract
An obligatory role for estrogen in growth, development, and functions of the mammary gland is well established, but the roles of the two estrogen receptors remain unclear. With the use of specific antibodies, it was found that both estrogen receptors, ERα and ERβ, are expressed in the rat mammary gland but the presence and cellular distribution of the two receptors are distinct. In prepubertal rats, ERα was detected in 40% of the epithelial cell nuclei. This decreased to 30% at puberty and continued to decrease throughout pregnancy to a low of 5% at day 14. During lactation there was a large induction of ERα with up to 70% of the nuclei positive at day 21. Approximately 60–70% of epithelial cells expressed ERβ at all stages of breast development. Cells coexpressing ERα and ERβ were rare during pregnancy, a proliferative phase, but they represented up to 60% of the epithelial cells during lactation, a postproliferative phase. Western blot analysis and sucrose gradient centrifugation confirmed this pattern of expression. During pregnancy, the proliferating cell nuclear antigen was not expressed in ERα-positive cells but was observed in 3–7% of ERβ-containing cells. Because more than 90% of ERβ-bearing cells do not proliferate, and 55–70% of the dividing cells have neither ERα nor ERβ, it is clear that the presence of these receptors in epithelial cells is not a prerequisite for estrogen-mediated proliferation.
Estrogens are potent mitogens in the mammary gland, where they are obligatory for normal development as well as for induction and progression of mammary carcinoma (1). The female mammary gland undergoes a surge of cell division during puberty, and throughout adult life there is cyclical proliferation and involution during estrous cycles (2). Despite the clear role for estrogen in proliferation of the mammary epithelium, the role of estrogen receptors in this process remains unclear. During pubertal growth and during the estrous cycle, the majority of proliferating cells, both in terminal end buds and in ducts, do not contain estrogen receptor α (ERα). This has been observed in both rodent (3) and human (4, 5) mammary glands. Progesterone receptor (PR) is localized in those cells that contain ERα, and induction of PR by estrogen occurs at much lower plasma levels of estrogen than are required for cell proliferation (5). These observations have led to the concept (6) of two distinct types of responses to estrogen in the breast: (i) an indirect action of estradiol in the mammary epithelium by inducing estrogen receptor-containing stromal cells to produce growth factors that stimulate epithelial cells to divide and (ii) a direct effect on ERα-containing cells that occurs at low estrogen concentrations and results in induction of PR.
Dependence of epithelium on stroma for estrogen-mediated induction of PR has been demonstrated in experiments in which epithelial and stromal components were cultured together (7–9). Stroma from responsive (mature) glands can confer estrogen sensitivity on epithelium from insensitive (immature) glands. However, once the epithelium has matured in vivo it maintains its estrogen responsiveness regardless of the maturity of the stroma with which it is cocultured (10).
It has been estimated that less than 20% of the epithelial cells in the adult human mammary gland contain ERα (4). The lactating gland has been described as “estrogen insensitive” because estrogen does not elicit either proliferation or induction of PR during lactation (3, 11). The reason for insensitivity is not clear. In contrast to the findings described here, the level of ERα in the rat mammary gland has been reported to decrease by 55% during lactation (12), but this much reduction seems unlikely to account for complete insensitivity to estrogen.
In none of the previously published observations on estrogen receptor in the mammary gland was there consideration of the second estrogen receptor, ERβ (13). Reverse transcription–PCR has provided evidence for the presence of ERβ in normal human breast (14) as well as in breast cancer (15–19). Using specific antibodies to either ERα or ERβ, we have determined the presence and localization of ERβ in the rat breast. The results indicate that present concepts of the mechanism by which estrogen regulates mammary growth and differentiation may need reevaluation.
Materials and Methods
Animals and Collection of Tissues.
Sprague–Dawley rats were purchased from Mollegaard (Ejby, Denmark), housed in a controlled environment on an illumination schedule of 12 h light/12 h dark, and fed a standard pellet diet with water provided ad libitum. Virgin cycling 6-week-old female rats were mated during proestrus with 9-week-old males. The day when the vaginal plug was evident was designated day 0 of pregnancy. Pregnant females were placed in separate cages and a group of three was killed on each of days 2, 7, and 14 of pregnancy. The day of parturition was designated day 0 of lactation. Groups of three lactating animals were killed on days 7, 14, and 21. In another group, pups were removed from their mothers on day 22 of lactation. These mothers were killed 7 days after weaning, and female pups were killed at 1, 4, and 6 weeks of age. The inguinal mammary glands were excised, frozen immediately, and stored at −80°C until used for immunodetection, sucrose gradient sedimentation, and Western blotting.
Antibodies Purchased.
ERα mouse mAb, 6F11, was obtained from NovoCastra (Newcastle, U.K.); ERα rabbit polyclonal antibody, MC-20, was obtained from Santa Cruz Biotechnology; and proliferating cell nuclear antigen (PCNA) mouse mAb, PC10, and actin mouse mAb, Ab-1, were obtained from Oncogene Research Products (Cambridge, MA). Secondary antibodies, all raised in donkeys, were obtained from Jackson ImmunoResearch. These were FITC-conjugated anti-mouse and anti-rabbit IgG and Amersham fluorescent protein label (Cy3)-conjugated anti-mouse and anti-chicken IgG. For simplicity, these four fluorescing antibodies are designated here as FITC-m, FITC-r, Cy3-m, and Cy3-c, respectively.
Preparation of Antibodies to ERβ.
Chicken polyclonal ERβ 503 IgY.
ERβ 503 is human ERβ1, modified in its ligand-binding domain by insertion of the rat 18-aa sequence described by Ogawa et al. (20). Thus, this modified protein is the equivalent of human ERβ2. It was expressed in SF9 cells by KaroBio (Huddinge, Sweden) and used as a standard to test the specificity of antibodies raised against various epitopes, including those in the 18-aa sequence. The protein was used to immunize laying hens, and the IgY was isolated from egg yolks by polyethylene glycol precipitation and purified further by DE52 cellulose chromatography. Preadsorbed antibodies were prepared by incubating ERβ 503 IgY for 12 h at 4°C with ERβ protein coupled to activated Sepharose. The ERβ protein used was either the ERβ 503 employed as antigen or recombinant human ERβ obtained from Panvera (Madison, WI). As a control for preadsorption experiments, BSA was coupled to activated Sepharose.
Rabbit polyclonal anti-ERβ ligand-binding domain (LBD).
The LBD of human ERβ1 (amino acids 320–527), expressed in SF9 cells and purified to homogeneity by KaroBio, was used to immunize rabbits by Genosys (Cambridge, U.K.). The IgG obtained was purified by Protein A chromatography.
Immunohistochemistry.
The cellular presence of ERα, ERβ, and PCNA was detected by standard immunofluorescence procedure (21) in which tissue sections were incubated first with an antibody to the component to be determined and then with a fluorescent antibody prepared against the IgG of the species used for production of the primary antibody. Frozen sections of 12-μm thickness were cut and air-dried for 30 min. After washing with ice-cold methanol and acetone each for 3 min, sections were fixed for 10 min at room temperature in 4% paraformaldehyde. For reaction with PCNA antibody it was necessary to expose occult antigen epitopes by a modified antigen retrieval procedure in which sections, after fixation, were heated for 15 min without boiling in 10 mM citrate buffer, pH 6.0, in a microwave oven. All sections were treated with 0.5% Triton X-100 in PBS for 5 min, incubated with 10% normal donkey serum in PBS for 1 h at 4°C to reduce unspecific binding of secondary antibody, and exposed to a sequence of antibodies.
For double detection of ERα and ERβ, sections were incubated sequentially with ERα antibody 6F11 (1:100 dilution), FITC-m (1:50), ERβ antibody 503 (1:750), and Cy3-c (1:200). For PCNA with ERα, the sequence was PC10 (1:50), Cy3-m (1:200), MC-20 (1:200), and FITC-r (1:50), and for PCNA with ERβ, it was PC10 (1:50), FITC-m (1:50), 503 (1:750), and Cy3-c (1:200). For evaluation of the ERβ 503 antibody specificity, prostate sections were incubated with either antibody 503 (1:750) or preadsorbed antibodies (1:500), followed by Cy3-c (1:200). All incubations with primary antibodies were done overnight at 4°C in PBS buffer containing 3% BSA, except for PC10, for which 1 h at room temperature was used. All incubations with secondary antibodies were done for 1 h at room temperature in PBS buffer containing 2% normal rat serum. After incubation, the sections were dipped in 4′,6-diamidino-2-phenylindole (DAPI) solution (0.1 μg/ml in PBS) for 20 sec at room temperature to delineate nuclei and mounted in Vectashield antifading medium (Vector Laboratories).
The sections were examined under a Zeiss fluorescence microscope by using suitable filters for selectively detecting the fluorescence of FITC, Cy3, and DAPI. In this system, nuclei that bind FITC-labeled secondary antibodies emit green fluorescence and those binding Cy3-labeled antibody fluoresce red. Those that recognize both secondary antibodies appear yellow in the “overlayered” images. The images were recorded as computer files via a Hamamatsu digital camera (Hamamatsu Photonics, Hamamatsu City, Japan) and analyzed by using an openlab 2.0.3 program (Improvision, Coventry, U.K.). The results were calculated as the number of fluorescing epithelial cell nuclei relative to the total number labeled with DAPI. Cells were confirmed as epithelial by morphologic examination under a light microscope. All analyses used tissues from three individual rats for each category and were analyzed with at least three staining slides for each individual tissue (i.e., a total of nine slides were examined for one target protein for one category of animal).
Preparation of Cytosol for Sucrose Density Gradient Centrifugation.
Tissue was frozen in liquid nitrogen and pulverized in a Dismembrator (Braun, Melsungen, Germany) for 45 sec. Pulverized tissue was added to buffer composed of 10 mM Tris⋅HCl (pH 7.5), 1.5 mM EDTA, and 5 mM sodium molybdate, using 1 ml per 100 μg of tissue. Cytosol was obtained by centrifugation of the homogenate at 204,000 × g for 1 h in a 70Ti rotor at 4°C. Cytosol from MCF-7 human breast cancer cells, in which the receptor is essentially all ERα, was kindly provided by Abbott.
Sucrose Density Gradient Assay.
Sedimentation studies were carried out as described previously (22). Breast tissue cytosols were incubated for 3 h at 0°C with 10 nM [3H]estradiol-17β (Amersham) in the presence or absence of 50-fold excess of cold estradiol, and the unbound steroid was removed with dextran-coated charcoal. Sucrose density gradients [10–30% (wt/vol)] were prepared in a buffer containing 10 mM Tris⋅HCl, 1.5 mM EDTA, 1 mM α-monothioglycerol (Sigma), and 10 mM KCl. Samples of 200 μl were layered on 3.5-ml gradients and centrifuged at 4°C for 16 h at 300,000 × g in a Beckman L-70K ultracentrifuge with SW-60Ti rotor. Successive 100-μl fractions were collected from the bottom by paraffin oil displacement, using a collector of our own design, and assayed for radioactivity by scintillation counting. For Western blotting, fractions were precipitated with trichloroacetic acid, and the precipitates were resuspended in methanol. Samples were placed on dry ice for 30 min, and the proteins were recovered by centrifugation. Protein pellets were dissolved in SDS sample buffer and resolved by SDS/PAGE by using 4–20% gradient gels.
Protein Extraction for Western Blotting.
All tissue handling was done at 4°C. Tissue samples were homogenized for a few seconds with a Polytron PT3100 in 5 vol of buffer containing 600 mM Tris⋅HCl, 1 mM EDTA (pH 7.4), and two protease inhibitor mixture tablets (Boehringer Mannheim) per 50 ml. The homogenates were centrifuged for 1 h at 105,000 × g, and the protein content of the supernatant fractions was measured by a Bio-Rad Protein Assay with BSA as standard.
Western Blotting Analysis.
Proteins were resolved on SDS-polyacrylamide gels in Tris-glycine buffer, using either 9% polyacrylamide or preformed gradient gels with 4–20% acrylamide (NOVEX, San Diego). Transfer to poly(vinylidene difluoride) membranes was done by electroblotting either semidry or in Tris-glycine buffer. Molecular weight markers were Kaleidoscope prestained standards (Bio-Rad), LMW electrophoresis calibration kit (Amersham Pharmacia), and high-range protein molecular weight markers (Promega). Antibody dilutions were 1:3,000 for ERβ LBD, 1:75 for ERα 6F11, and 1:50 for actin Ab-1.
Results
Characterization of ERβ 503 Antibody.
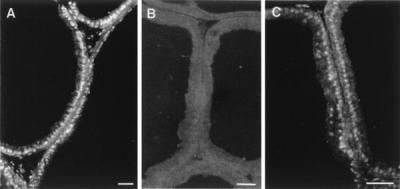
The specificity of ERβ 503 IgY used for immunofluorescent labeling was evaluated with prostate tissue from 12-week-old male rats (Fig. 1). This antibody showed strong immunoreactivity in the nuclei of prostate epithelial cells, which was almost completely eliminated when the antibodies were preadsorbed with recombinant ERβ protein coupled to activated Sepharose. In contrast, exposure to BSA coupled to Sepharose caused only a small reduction in immunoreactivity, apparently because of nonspecific adsorption of antibody, but it did not alter the specificity.
Figure 1.
Evaluation of the specificity of ERβ 503 antibody. Frozen sections of ventral prostate from 12-week-old male rats were stained by using ERβ 503 antibody (A), ERβ 503 antibody preadsorbed with recombinant ERβ protein (B), and ERβ antibody preadsorbed with BSA (C). (Bars = 50 μm.)
Detection and Colocalization of ERα and ERβ in Individual Sections of Rat Mammary Gland.
To evaluate not only expression levels of ERα and ERβ but also the colocalization of both receptors in the rat mammary gland at different stages of development, immunofluorescence detection with two kinds of secondary antibodies was used (Fig. 2). In this system, nuclei containing ERα emit bright green fluorescence and those with ERβ emit bright red-orange, easily detectable in both cases against the background of nonspecific secondary-antibody localization. Nuclei of cells expressing both receptors appeared yellow. The study examined breast tissue from rats from 1 week of age through pregnancy, lactation, and 1 week postlactation.
Figure 2.
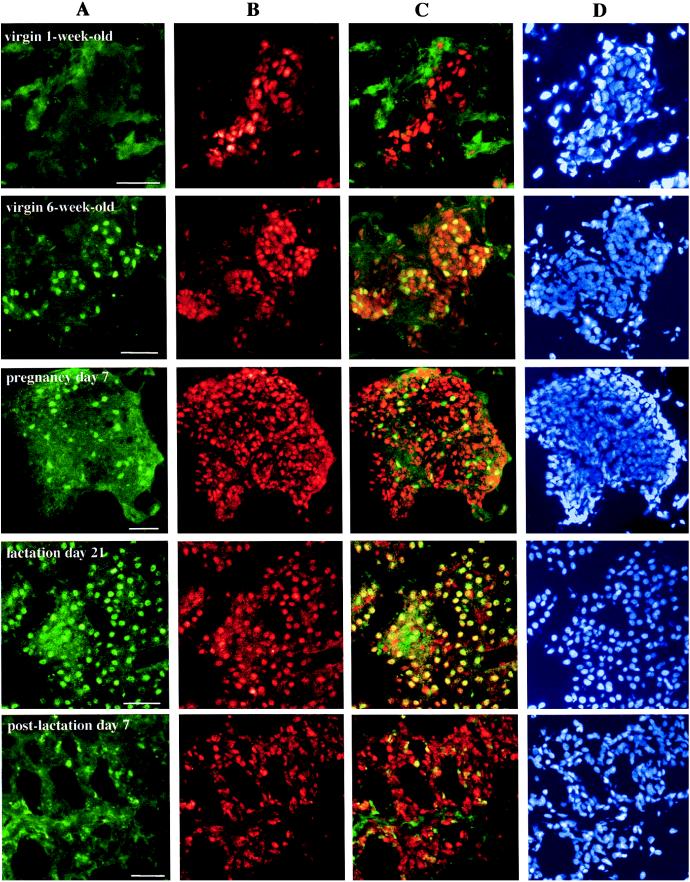
Detection and localization by immunofluorescent labeling of ERα and ERβ proteins in rat mammary glands at different stages of development. Tissue sections from female rats at indicated intervals were stained by sequential exposure of each section to antibodies 6F11, FITC-m, 503, and Cy3-c, followed by DAPI. The green fluorescence spots indicate nuclei containing ERα (A), and the red spots indicate those with ERβ (B). The yellow nuclei observed in overlayered images, especially from lactating glands, indicate that there is coexpression of both receptors in a single epithelial cell (C). Lane D shows the total nuclei in the same section. Pictures are representative of nine slides from three individual rats for each stage. (Bars = 50 μm.)
As shown in Fig. 2, ERβ protein was evident in the mammary gland at all stages. Staining was mainly in the nuclei of epithelial cells and in some stromal cells. Unlike the continuous expression of ERβ, that of ERα in mammary epithelial cells varied during the period studied. ERα was present in some epithelial cells in the 6-week-old rat breast. This expression decreased during pregnancy, increased during lactation, and decreased again in the first week postlactation. Overlayered images revealed that some cells express either ERα or ERβ whereas others express both.
Detailed Analysis of ERα and ERβ Expression Levels During Pregnancy and Lactation.
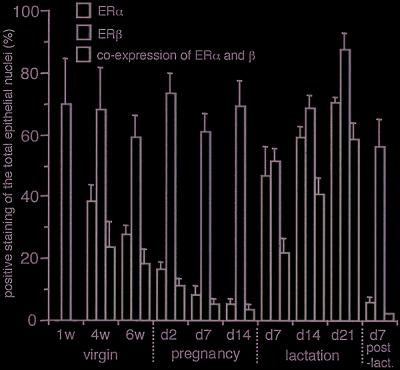
Nuclei of all cells were visualized with DAPI (lane D, Fig. 2). Cells positive for ERα, ERβ, or both were counted and expressed as a percentage of the total epithelial cell nuclei. As shown in Fig. 3, the relative number of cells with each ER phenotype changed with the endocrine status of the mammary gland. The percentage of cells expressing ERα during pregnancy was less than 20%. During lactation this increased to between 45% in the first week to 70% by the third week. Throughout the period studied, ERβ protein remained relatively constant, being present in more than half of the population of epithelial cells. By counting the number of yellow spots on the overlayered image (lane C, Fig. 2), the dominant cells during the lactation period were shown to be those expressing both receptors. It is noteworthy that during pregnancy, considered to be a highly proliferative period for the mammary gland, the majority of cells expressed only ERβ. However, during lactation, a nonproliferating period, the ratio of cells expressing only ERβ was reduced, and cells expressing both receptors dominated. The percentage of cells coexpressing ERα and ERβ was as high as 60% in late lactation.
Figure 3.
Detailed analysis of ERα and ERβ expression as indicated by immunofluorescent labeling. Cell nuclei positive for ERα, ERβ, or both were counted and the numbers were expressed as the percentage of total epithelial cell nuclei. Results are mean ± SD of three determinations for each of three individual tissues. Green bars indicate the percentage of cells expressing ERα, red bars indicate those expressing ERβ, and yellow bars indicate those expressing both receptors.
Western Blot Analysis of ERα and ERβ.
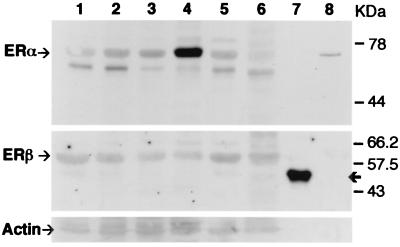
LBD antibodies generated against the LBD of ERβ protein were used for the detection of ERβ in homogenized tissue lysates (Fig. 4). This antibody also reacted with the recombinant 503 protein used as the antigen for the 503 antibody. Therefore, both ERβ 503 and LBD antibody can detect the same ERβ protein in the tissues. On Western blots, the endogenous ERβ protein of rat tissues had a molecular mass of about 62 kDa. These results indicate that ERβ expressed in rat mammary gland and prostate is translated from the first and/or second of the three possible ATG codon start sites on rat ERβ sequence. The recombinant 503 protein was expressed from a transcript encoding the shortest ERβ isoform, i.e., from the third ATG. Because of the 18-aa insert, this protein had a molecular mass of 55 kDa. The expression levels of ERα and ERβ in this experiment were consistent with the immunofluorescence data. The highest expression of ERα was during lactation whereas ERβ remained constant throughout the various endocrine states of the breast.
Figure 4.
Detection of ERα and ERβ by Western blot analysis. Similar amounts of homogenized tissue lysates were separated by 9% SDS/PAGE and immunoblotted with either ERα antibody 6F11, ERβ antibody LBD, or actin antibody Ab-1. Lanes: 1, virgin 6 week old; 2, pregnancy day 7; 3, pregnancy day 14; 4, lactation day 21; 5, postlactation day 7; 6, ventral prostate; 7, ERβ 503 recombinant protein; 8, ERα recombinant protein. The arrows at the top, middle, and bottom sections indicate ERα, ERβ, and actin proteins, respectively. The bold arrow at the middle section indicates recombinant ERβ 503 protein. The results are representative of triplicate experiments.
Detection of ERα and ERβ in Cytosols from Rat Mammary Gland by Sucrose Density Gradient Centrifugation.
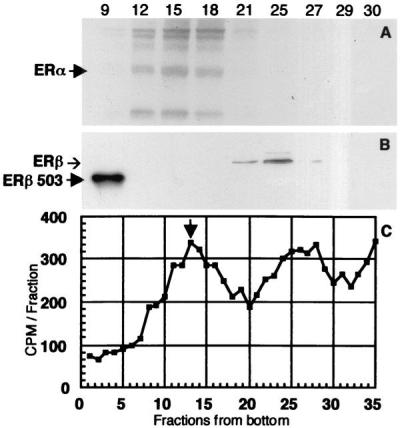
In low-salt sucrose gradients, the cytosol receptor of the lactating gland showed two broad peaks of tritiated estradiol binding of about the same magnitude (Fig. 5C). The faster peak, like that seen with MCF-7 cell cytosol, sedimented at 8S and was shown by Western blotting to consist of ERα (Fig. 5A), whereas the peak in the 4S region represents ERβ (Fig. 5B). Binding in both regions was very low in mammary cytosol from 4- and 9-week-old virgin rats (data not shown), and both peaks were increased significantly during lactation.
Figure 5.
(C) Sedimentation analysis of tritiated estradiol binding by receptors in cytosol from lactating rat mammary gland. Arrowhead shows the position of the 8S peak of the receptor present in MCF-7 cell cytosol (ERα). (A and B) Determination of the ERα and ERβ content of representative gradient fractions of both peaks by Western blotting. The arrowhead in A indicates the position of ERα on the blot, and the arrowhead as well as the band in the left lane of B indicate that of ERβ 503.
Evaluation of Proliferating Cells and Receptor Expression.
To determine which estrogen receptor was expressed in proliferating epithelial cells, double staining for PCNA and either ERα or ERβ was performed. PCNA staining in the nucleus indicates that these cells are in the late S phase of the cell cycle. PCNA-expressing cells were rarely observed (<0.01%) in the sections from lactating and postlactating rats (data not shown). However, in pregnant rats, more than 10% of the cells were PCNA-positive (Fig. 6 and Table 1). As indicated in the overlayered images and table, cells expressing PCNA did not contain ERα, whereas 30–47% of the proliferating cells contained ERβ. From the data in Fig. 3 and Table 1, it can be calculated that only a small percentage of ERβ-containing cells are proliferating, i.e., 6%, 7%, and 3% on days 2, 7, and 14 of pregnancy, respectively.
Figure 6.
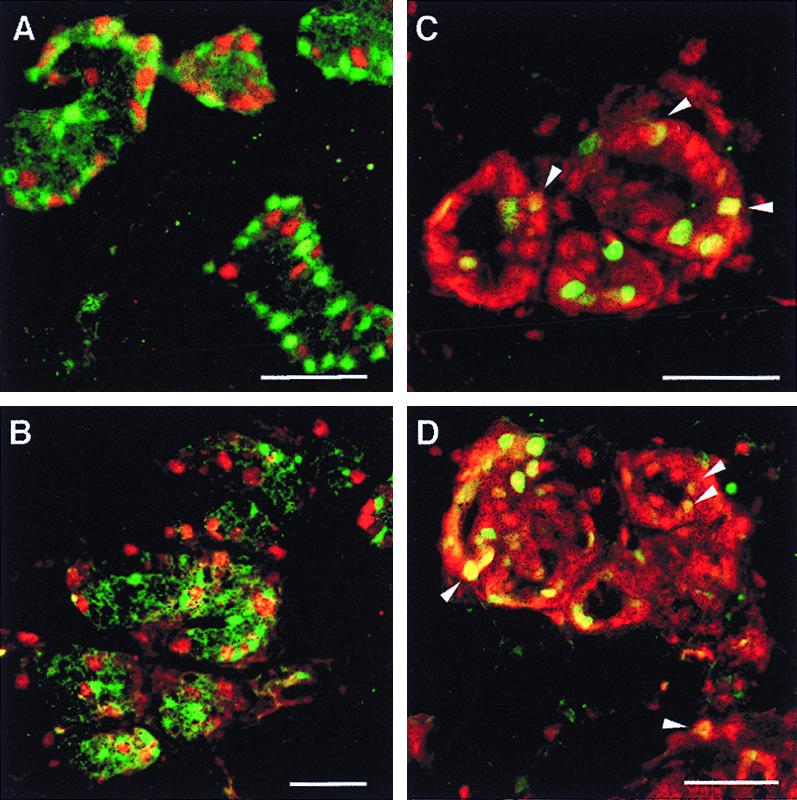
Colocalization of PCNA with ERα or ERβ. Mammary gland sections from 6-week-old (A and C) and pregnancy day 7 (B and D) female rats were doubly stained with antibodies to PCNA (PC10) and ERα (MC20) (A and B) or to PCNA (PC10) and ERβ (503) (C and D). In A and B, green nuclei indicate cells expressing ERα and red nuclei indicate those containing PCNA. In C and D, green nuclei contain PCNA and red nuclei contain ERβ. The yellow nuclei, some of them marked with arrowheads, indicate coexpression of receptor and PCNA. (Bars = 50 μm.)
Table 1.
Percentage of proliferating cells expressing ERα or β in developing mammary gland
| Stage | PCNA-positive, % | ERα-positive/ PCNA-positive, % | ERβ-positive/ PCNA- positive, % |
|---|---|---|---|
| Virgin 6-week-old | 14.7 ± 1.6 | 1.5 ± 0.5 | 37.5 ± 2.8 |
| Pregnancy day 2 | 14.3 ± 4.3 | 0.3 ± 0.2 | 29.7 ± 4.5 |
| Pregnancy day 7 | 10.6 ± 2.2 | 0.2 ± 0.2 | 38.9 ± 7.8 |
| Pregnancy day 14 | 4.3 ± 0.6 | 0.0 ± 0.0 | 46.7 ± 9.2 |
Results are represented as means ± SD.
Discussion
The foregoing studies show that during most phases of development and endocrine stimulation, there are more cells in the rodent breast expressing ERβ than ERα. ERβ is always present, and the percentage of nuclei containing this receptor remains constant at around 70%. What does change during development and hormone responsiveness is the percentage of nuclei that are ERα-positive and the percentage of nuclei that contain both receptors. Four distinct stages of the breast can be defined on the basis of the estrogen receptor profile. These are: (i) prepuberty, when both ERα and ERβ are present (at this stage, ERα-positive cells account for 40% and coexpressing cells account for 25% of epithelial cell nuclei); (ii) pregnancy, when ERβ is present in the majority of epithelial cells, ERα-expression is low, and few cells express both receptors; (iii) lactation, when ERα and ERβ are both expressed in the majority of epithelial cells; and (iv) postlactation, when ERα is extremely low and there is little colocalization of the two receptors. These different stages as defined by estrogen receptor profile also represent stages in breast development where estradiol elicits very different actions. In stage i, before 3 weeks of age, estrogen does not induce proliferation in the mammary epithelium (23). In stage ii, pregnancy, estrogen causes rapid growth and maturation of the mammary gland and the concentration of PR is high. In stage iii, lactation, the breast is insensitive to proliferative and PR-inducing effects of estradiol. In stage iv, postlactation, the breast is undergoing remodeling, plasma estrogen levels are higher than they were during lactation, and PR levels in the breast increase.
In previous studies involving ERα-knockout (ERKO) mice (24) it was shown that ERβ, in the absence of ERα, does not mediate estrogen-dependent growth and development of the mammary gland. In reconstitution experiments with ERKO mice (25) it was found that the presence of ERα in breast stroma, but not in epithelium, is sufficient for estrogen-dependent ductal growth. As clearly indicated in Fig. 6 and Table 1, proliferating epithelial cells rarely express ERα, whereas 30–47% of proliferating cells contain ERβ. From these data two important calculations can be made. These are the percentage of ERβ-containing cells that do not proliferate and the percentage of proliferating cells that do not express either estrogen receptor. At day 7 of pregnancy, when more than 70% of the total epithelial cell population express ERβ (Fig. 3), only 4% had both PCNA and ERβ. This indicates that more than 90% of ERβ-bearing cells do not proliferate and, even more surprising, 53–70% of the proliferating cells do not contain either receptor.
If ERβ is not involved in proliferative effects of estrogen, what role does it have in the breast? One hint may be our observation that the highest degree of colocalization of ERα and ERβ occurs during lactation, when the breast is insensitive to estradiol (3, 11). This insensitivity occurs despite the presence of normal ERα (12). Immunohistochemistry, sucrose gradient sedimentation, and Western blots all show that lactation is the time when the concentration of ERα in the breast epithelium is highest. It could be speculated that one function of ERβ is to antagonize the effects of ERα in the epithelium. Because one of the main functions of ERα in the epithelium is the induction of PR, it is possible that an ERα-ERβ heterodimer can inhibit this action. This is consistent with the fact that the level of PR in the breast is extremely low during lactation (26), when colocalization of the two receptors is highest.
There are several alternative explanations for the estrogen insensitivity of cells despite the presence of ERα. These include the presence of limiting concentrations of coactivators or excessive amounts of corepressors (27) and rapid inactivation of the hormone through metabolism (28). In the case of ERβ, another important consideration is the presence of ERβ isoforms that do not respond to physiological concentrations of estradiol. ERβ2 contains 18 additional amino acids in its LBD, and this insertion renders the receptor 100-fold less sensitive to estradiol (16). ERβCx has an alternative C terminus and does not bind estradiol (20). One explanation for estrogen insensitivity in the neonatal period and during lactation may be that variant forms of ERβ, such as ERβ2 or ERβCx, are expressed at these times. The high level of PR during pregnancy, when the ERα level is low, suggests that, under certain conditions, ERβ may regulate PR. Support for this possibility comes from the studies of ERKO mammary glands that do maintain some PR and can respond to progesterone (24).
The sucrose gradient centrifugation experiments show that, in low-salt sucrose gradients, the native ERβ present in cytosol from rat mammary glands sediments in the 4S region whereas ERα sediments as an 8S peak as expected (22). This has been observed in several tissues (prostate, lung, uterus) in which ERα and ERβ are present (unpublished data). Because in most tissues native ERα exists as an 8S complex of the receptor protein associated with heat shock proteins and other chaperones (29), it appears that native ERβ, at least in rat mammary gland, does not form such complexes. So far, the binding of estradiol to ERα and ERβ as observed on sucrose density gradients appears to correlate well with the immunohistochemical studies. During lactation, the ERα and ERβ content of the breast is similar and the binding at 4S and 8S is roughly equivalent.
Until now, evidence for the presence of ERβ in the normal human breast and in breast cancer has been provided from reverse transcription–PCR (14–19) and from in situ hybridization of mRNA (30), but no information has been available concerning the actual ERβ protein in these tissues. The present studies demonstrate the ERβ protein itself in breast epithelial cells of the rat. The precise role of ERβ in the breast remains to be defined, and this should become clearer as the mammary glands of ERβ-knockout mice are studied and specific ERβ agonists and antagonists are developed (31, 32).
Acknowledgments
This research was supported by grants from the Swedish Cancer Fund, from KaroBio AB, and from the Deutsche Krebshilfe. We gratefully acknowledge Christina Thulin-Andersson for skillful technical assistance and Ylva Ekendahl for immunizing hens and collecting eggs.
Abbreviations
- Cy3
Amersham fluorescent protein label
- DAPI
4′,6-diamidino-2-phenylindole dihydrochloride
- ERα and ERβ
estrogen receptors α and β
- LBD
ligand-binding domain
- PCNA
proliferating cell nuclear antigen
- PR
progesterone receptor
References
- 1.Russo J, Russo I H. Medicina. 1997;57, Suppl 2:81–91. [PubMed] [Google Scholar]
- 2.Prall O W, Rogan E M, Sutherland R L. J Steroid Biochem Mol Biol. 1998;65:169–174. doi: 10.1016/s0960-0760(98)00021-1. [DOI] [PubMed] [Google Scholar]
- 3.Zeps N, Bentel J M, Papadimitriou J M, D'Antuono M F, Dawkins H J. Differentiation. 1998;62:221–226. doi: 10.1046/j.1432-0436.1998.6250221.x. [DOI] [PubMed] [Google Scholar]
- 4.Clarke R B, Howell A, Potten C S, Anderson E. Cancer Res. 1997;57:4987–4991. [PubMed] [Google Scholar]
- 5.Clarke R B, Howell A, Anderson E. Breast Cancer Res Treat. 1997;45:121–133. doi: 10.1023/a:1005805831460. [DOI] [PubMed] [Google Scholar]
- 6.Wiesen J F, Young P, Werb Z, Cunha G R. Development. 1999;126:335–344. doi: 10.1242/dev.126.2.335. [DOI] [PubMed] [Google Scholar]
- 7.Cooke P S, Buchanan D L, Lubahn D B, Cunha G R. Biol Reprod. 1998;59:470–475. doi: 10.1095/biolreprod59.3.470. [DOI] [PubMed] [Google Scholar]
- 8.Haslam S Z, Counterman L J. Endocrinology. 1991;129:2017–2023. doi: 10.1210/endo-129-4-2017. [DOI] [PubMed] [Google Scholar]
- 9.Humphreys R C, Lydon J, O'Malley B W, Rosen J M. Mol Endocrinol. 1997;11:801–811. doi: 10.1210/mend.11.6.9891. [DOI] [PubMed] [Google Scholar]
- 10.Haslam S Z, Counterman L J, Nummy K A. J Cell Physiol. 1993;155:72–78. doi: 10.1002/jcp.1041550110. [DOI] [PubMed] [Google Scholar]
- 11.Shyamala G, Ferenczy A. Endocrinology. 1982;110:1249–1256. doi: 10.1210/endo-110-4-1249. [DOI] [PubMed] [Google Scholar]
- 12.Shyamala G, Schneider W, Schott D. Endocrinology. 1990;126:2882–2889. doi: 10.1210/endo-126-6-2882. [DOI] [PubMed] [Google Scholar]
- 13.Kuiper G G J M, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-Å. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crandall D L, Busler D E, Novak T J, Weber R V, Kral J G. Biochem Biophys Res Commun. 1998;248:523–526. doi: 10.1006/bbrc.1998.8997. [DOI] [PubMed] [Google Scholar]
- 15.Vladusic E A, Hornby A E, Guerra-Vladusic F K, Lupu R. Cancer Res. 1998;58:210–214. [PubMed] [Google Scholar]
- 16.Dotzlaw H, Leygue E, Watson P H, Murphy L C. J Clin Endocrinol Metab. 1997;82:2371–2374. doi: 10.1210/jcem.82.7.4212. [DOI] [PubMed] [Google Scholar]
- 17.Dotzlaw H, Leygue E, Watson P H, Murphy L C. Cancer Res. 1999;59:529–532. [PubMed] [Google Scholar]
- 18.Leygue E, Dotzlaw H, Watson P H, Murphy L C. Cancer Res. 1999;59:1175–1179. [PubMed] [Google Scholar]
- 19.Speirs V, Parkes A T, Kerin M J, Walton D S, Carleton P J, Fox J N, Atkin S L. Cancer Res. 1999;59:525–528. [PubMed] [Google Scholar]
- 20.Ogawa S, Inoue S, Watanabe T, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. Nucleic Acids Res. 1998;26:3505–3512. doi: 10.1093/nar/26.15.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shevach E M. In: Current Protocols in Immunology. Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. New York: Wiley; 1998. , Chap. 5. [Google Scholar]
- 22.Greene G L, Closs L E, Fleming H, DeSombre E R, Jensen E V. Proc Natl Acad Sci USA. 1977;74:3681–3685. doi: 10.1073/pnas.74.9.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haslam S Z. Endocrinology. 1989;125:2766–2772. doi: 10.1210/endo-125-5-2766. [DOI] [PubMed] [Google Scholar]
- 24.Couse J F, Korach K S. Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 25.Cunha G R, Young P, Hom Y K, Cooke P S, Taylor J A, Lubahn D B. J Mammary Gland Biol Neoplasia. 1997;2:393–402. doi: 10.1023/a:1026303630843. [DOI] [PubMed] [Google Scholar]
- 26.Sharoni Y, Feldman B, Teuerstein I, Levy J. Endocrinology. 1984;115:1918–1924. doi: 10.1210/endo-115-5-1918. [DOI] [PubMed] [Google Scholar]
- 27.Horwitz K B, Jackson T A, Bain D L, Richer J K, Takimoto G S, Tung L. Mol Endocrinol. 1996;10:1167–1177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]
- 28.Hellmold H R, Lamb J G, Wyss A, Gustafsson J-Å, Warner M. Mol Pharmacol. 1995;48:630–638. [PubMed] [Google Scholar]
- 29.Pratt W B, Toft D O. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 30.Sasano H, Suzuki T, Matsuzaki Y, Fukaya T, Endoh M, Nagura H, Kimura M. J Clin Endocrinol Metab. 1999;84:781–785. doi: 10.1210/jcem.84.2.5435. [DOI] [PubMed] [Google Scholar]
- 31.Katzenellenbogen J A, Katzenellenbogen B S. Endocrinology. 1999;140:800–804. doi: 10.1210/endo.140.2.6480. [DOI] [PubMed] [Google Scholar]
- 32.Meyers M J, Sun J, Carlson K E, Katzenellenbogen B S, Katzenellenbogen J A. J Med Chem. 1999;42:2456–2468. doi: 10.1021/jm990101b. [DOI] [PubMed] [Google Scholar]