Abstract
The striatum and hippocampus are widely held to be components of distinct memory systems that can guide competing behavioral strategies. However, some electrophysiological studies have suggested that neurons in both structures encode spatial information and may therefore make similar contributions to behavior. In rats well trained to perform a win-stay radial maze task, we recorded simultaneously from dorsal hippocampus and from multiple striatal subregions, including both lateral areas implicated in motor responses to cues and medial areas that work cooperatively with hippocampus in cognitive operations. In each brain region, movement through the maze was accompanied by the continuous sequential activation of sets of projection neurons. Hippocampal neurons overwhelmingly were active at a single spatial location (place cells). Striatal projection neurons were active at discrete points within the progression of every trial—especially during choices or following reward delivery—regardless of spatial position. Place-cell–type firing was not observed even for medial striatal cells entrained to the hippocampal theta rhythm. We also examined neural coding in earlier training sessions, when rats made use of spatial working memory to guide choices, and again found that striatal cells did not show place-cell–type firing. Prospective or retrospective encoding of trajectory was not observed in either hippocampus or striatum, at either training stage. Our results indicate that, at least in this task, dorsal hippocampus uses a spatial foundation for information processing that is not substantially modulated by spatial working memory demands. By contrast, striatal cells do not use such a spatial foundation, even in medial subregions that cooperate with hippocampus in the selection of spatial strategies. The progressive dominance of a striatum-dependent strategy does not appear to be accompanied by large changes in striatal or hippocampal single-cell representations, suggesting that the conflict between strategies may be resolved elsewhere.
INTRODUCTION
Mammals display multiple, dissociable forms of learning that involve distinct computational algorithms (Doya 2000; White and McDonald 2002), performed by parallel and largely independent neural circuits (Eichenbaum 2001). Plasticity in the striatum is thought to be important for aspects of procedural learning, especially the use of reinforcement to bias subsequent action selection (for reviews, see Berke 2009; Schultz 1998). This approach—choosing what to do next based on what has worked well in the past—is most effective when an agent has had many prior experiences in a relatively unchanging world. By contrast, the hippocampus is especially important for recording particular, distinct episodes of one's experience: this involves the preservation of what happened, when it happened, and where it happened (Eichenbaum 2004; Morris 2001).
Investigations using radial maze tasks have revealed that these memory systems can produce conflicting influences over behavior. In the win-stay task rats are rewarded (“win”) for selecting maze arms that are illuminated with a light cue (i.e., visual discrimination), regardless of their recent history of choices. Damage to dorsal/lateral (sensorimotor) parts of the striatum retards the progressive acquisition of the win-stay strategy (McDonald and White 1993; Packard et al. 1989), but animals with damage to dorsal hippocampus and related structures learn more rapidly than normal (Packard et al. 1989). Conversely, hippocampal damage (but not dorsal/lateral striatal damage) greatly impairs performance of win-shift tasks, in which rats are rewarded for avoiding (“shifting” from) recently visited arms (i.e., spatial working memory; Becker et al. 1980; McDonald and White 1993).
Striatum and hippocampus also appear to provide competing frames of reference for action selection. Dorsal/lateral striatum is more important for learning and choosing actions in body-centered (egocentric) coordinates, such as a left-turn response (e.g., Brasted et al. 1997; Cook and Kesner 1988), whereas the hippocampus is more important for remembering particular spatial locations defined by arrays of external cues (allocentric coordinates; Morris et al. 1982). When a behavioral task forces an animal to make use of one form of information over the other, inactivation of striatum increases use of a hippocampal-dependent “place” strategy, whereas inactivation of hippocampus increases use of a striatum-dependent egocentric “response” strategy (Packard and McGaugh 1996). Such experiments have also shown that hippocampal-dependent strategies tend to dominate early in learning, whereas dorsal/lateral striatal-dependent strategies progressively dominate in late stages of learning. This shift in strategy does not involve the erasure of the hippocampal-based strategy, since it can still be unmasked by striatal inactivation (Packard and McGaugh 1996). Rather, it appears to reflect a change in which brain system “controller” is dominating the behavior (Daw et al. 2006).
Given these behavioral dissociations, one might reasonably expect that individual neurons in striatum and hippocampus would display correspondingly distinct forms of information processing during maze-task performance. Although we have previously found nonspatial forms of neural coding in the hippocampus during maze tasks (e.g., Tanila et al. 1997; Young et al. 1994), the most obvious forms of neural representation in hippocampus and related structures are indeed allocentric, including “place cells,” CA1 and CA3 pyramidal neurons that are preferentially active when the animal is in one spatial location (reviewed in Buzsáki 2005). It is not well understood how such hippocampal representations support spatial memories; however, several studies have found that place cell activity can be strongly modulated by past or future trajectories (e.g., Ferbinteanu and Shapiro 2003; Frank et al. 2000; Wood et al. 2000).
By contrast, previous studies of the activity patterns of single neurons in the striatum of behaving rats have not revealed representations that are clearly connected to striatum-dependent maze choices. In fact, some studies have reported “spatial” firing of striatal neurons (e.g., Schmitzer-Torbert and Redish 2008; Shibata et al. 2001) and have even suggested that striatal neurons show allocentric coding very similar to that of hippocampal neurons (Yeshenko et al. 2004). This has been put forward as evidence against the hypothesis that striatum and hippocampus are components of distinct memory systems (Mizumori et al. 2004). However, these studies have not typically recorded in tasks that distinguish allocentric coding from other aspects of task performance and/or have not recorded from striatal areas implicated in nonspatial strategies.
Here we directly compare firing-rate changes in simultaneously recorded striatal and hippocampal neurons during win-stay performance, while using several approaches to increase our understanding of such activity. First, we used a task geometry that readily distinguishes different coding schemes and obtained a large number of trials in each session to reliably assess such coding. Second, we recorded in a range of striatal locations, including both dorsal/lateral regions that are most associated with win-stay and egocentric performance, and more ventral/medial areas that are instead implicated in win-shift behavior (Seamans and Phillips 1994). Third, we made use of recent developments that now allow rat striatal projection neurons to be separated from striatal fast-spiking interneurons (Berke 2008; Berke et al. 2004; Mallet et al. 2005; Schmitzer-Torbert et al. 2008), to increase our confidence that we were recording selectively from projection neurons. Finally, we examined the relationship between functional coding properties of striatal neurons and their entrainment to the hippocampal theta rhythm (Berke et al. 2004).
METHODS
Animals
Adult male Long–Evans rats (>300 g) were housed on a 12-h:12-h light/dark cycle, with experiments performed during the light phase. All procedures were approved by the Boston University and University of Michigan Institutional Animal Care and Use Committees. Animals were motivated by water restriction, but in addition to water obtained during task performance they were given continuous access to water after each training/recording session (for ≥30 min) and on weekends. Body weights were monitored to ensure ≥85% of preexperimental levels.
Behavioral task
Rats were trained to perform a continuous win-stay task under computer control in a wooden plus maze, painted matte black, elevated 71 cm from the floor, and surrounded by dark curtains. Dim illumination was provided by diffuse light sources mounted near the ceiling above each maze corner. The maze consisted of a central octagon (25.5-cm diameter), four arms (46 × 9.5 cm), and four goal boxes at the end of the arms (30 × 15 cm). Each component had 3-cm-high walls. A CCD camera mounted on the ceiling tracked the position of infrared light-emitting diodes (LEDs) on the rats' headstage at 30 Hz and also recorded video for off-line analysis. A pair of cue lights (green LEDs on stalks, located 12 cm into each arm) indicated the rewarded arm on each trial; normally off, they began flashing (125 ms on; 75 ms off) as the rat approached the central octagon (triggered at 32.2 cm from the maze center) and kept flashing until the rat entered a goal box. Cue lights were carefully arranged to be visible as soon as they started flashing, regardless of which other arm the rat was on. The cued arm was selected pseudorandomly on each trial, with two constraints: 1) the arm that the rat was currently on was never the selected arm and 2) if the rat had made the same egocentric (e.g., left turn) choice on the previous two trials, then the rat was not cued to make the same movement a third time in a row. Since on each trial the cue was randomly chosen to appear on one of three arms (left, right, or straight ahead), on about one third of trials the cue appeared on the arm that the rat had most recently visited, prior to the current arm. These were termed “conflict trials” because they placed cue-approach (win-stay) and foraging (win-shift) strategies in conflict. The goal boxes contained liquid dispensers that automatically rewarded correct (i.e., win-stay) performance with droplets (100–150 μl) of slightly sweetened water (the water was dispensed as the rat entered the correct goal box). The task was continuous in that the goal box for each trial immediately became the start box for the next trial and no barriers were ever present to limit the rat's movement. Rats were allowed to run freely until they showed a prolonged pause without initiating another trial (typically, this produced session durations of ∼40 min). The very first session (in which rats generally explored the new environment without drinking) and sessions in which ≤36 trials were performed were excluded from the behavioral and neural analyses.
Electrophysiology
Neuronal spikes and local field potentials were recorded using standard methods, as described in Berke et al. (2004). The set of neurons presented here overlaps with those described in that earlier study (which was not concerned with behavioral correlates). Eleven of the 14 included rats were implanted with tetrodes (12.5-μm wire) into both the dorsal hippocampal CA1 pyramidal layer and the striatum and the remaining three rats received bundles of stereotrodes (25-μm wire) into multiple striatal subregions. Animals had ≥1 wk to recover from surgery before behavioral testing. Extracellular spikes were amplified and filtered at 600–6,000 or 300–6,000 Hz by custom-built amplifiers, digitized at 30 kHz and stored using the software program Spike (by Loren Frank). At the end of recording, electrode locations were verified by passing current (15–25 μA, 10 s) through each tetrode to create a small lesion that was visible after Nissl staining. In cases where electrode positions were outside the intended targets or could not be identified with confidence, data from those electrodes were not used. The relative proportion of striatal and hippocampal projection neurons varied between recording sessions; however, these two populations were sampled at equivalent learning stages since overall behavioral performance was similar for each cell population (mean % correct for striatal neurons = 88.0; for hippocampal neurons = 90.8; performance in each session was counted once for each neuron in that session). The additional cell population recorded at an earlier stage of learning likely overlapped with the population recorded late in learning, although we did not attempt to match up neurons that were often separated by many days of recordings (and in some cases electrode depth adjustments).
Data analysis and statistics
To avoid counting neurons more than once, spikes from a given electrode were measured from only a single behavioral session, unless the electrode had been moved ≥80 μm between sessions. Spikes were manually clustered using Off-line Sorter (Plexon). To be included in the analyses, neurons had to show stable waveform size and shape over the course of the maze session, fire a minimum of 100 spikes, and show a response pattern in the first half of performed trials similar to that of the second half. Consistent with previous work showing that striatal projection neurons typically have low firing rates and diverse behavioral correlates, these conservative criteria excluded many striatal neurons that fired only a handful of spikes within a behavioral session and so were not likely to be involved in this behavioral task. It is also possible that we excluded a small number of neurons encoding factors that may change systematically across the session, such as thirst. For the hippocampus we confined our analysis to presumed pyramidal projection neurons, using standard waveform length and phasic firing criteria. For the striatum we used the exact same criteria as in Berke (2008): starting by plotting the widths of each unit's average (filtered) waveform peak and valleys (at half-maximum). Six units were excluded from this classification because unusually complex waveform shapes made it hard to unequivocally assign “peak” and “valley” labels to waveform components. Cells with valley width >0.35 ms and mean firing rate <10 Hz were presumed medium spiny projection neurons (MSNs). No included cells had the very wide valleys and wide autocorrelogram gap characteristic of monkey “tonically active neurons.”
All other analyses were performed in MATLAB. Local field potential analyses, including assessment of theta entrainment of striatal neurons and the detection of epochs of slow-wave sleep, were performed as described (Berke et al. 2004). Analyses did not distinguish between correct and error trials. All major analysis results were robust over variations in parameters (such as bin sizes and cutoff values). To better compare neuronal population firing rates to running speed, the mean instantaneous running speed across all sessions was calculated by weighting each individual session's mean instantaneous running speed by the total number of included cells from that session. Spatial firing maps were constructed by binning position values into a 128 × 128 pixel grid and averaging the firing rates across all visits to each pixel, followed by spatial smoothing by convolution with a Gaussian kernel (5 × 5 bins, sigma = 2 bins). For the spatial maps we excluded moments in which the rat's speed was ≲4 cm/s (to remove task-unrelated behaviors such as grooming) and those within 1 s of an “out-of-bounds” position value (these were occasionally produced through occlusion of the infrared tracking LEDs by the tether cable). Pixels with fewer than three visits are shown in black. To calculate the spatial asymmetry index, we first expressed the position at which each spike fired as a vector from the maze center. We took the vector sum of all the individual vectors and divided by the sum of their lengths. If a neuron's spikes occur approximately evenly across the four arms, then this vector sum will be close to zero because individual vectors arising from spikes on one arm will tend to be cancelled out by vectors from the diametrically opposite arm. To compensate for bias introduced by the rat spending different amounts of time on the four arms, each vector was weighted by the relative proportion of time spent on that arm. We noted that this asymmetry measure was not fully satisfactory for a few hippocampal units that did not have comparable firing on all four arms but in which activity on one arm was approximately balanced by activity on the opposite arm. We therefore expressed the final asymmetry index as the higher of two values: calculated as just described or with the positions for the lower half of the firing map “reflected” about the y-axis (which in effect swaps two adjacent arms).
To produce rasters of firing by position we linearized the path taken on each trial. We broke these paths into 20 bins (e.g., see Supplemental Fig. S2)1 and used ANOVA to separately test whether the number of spikes in each bin significantly varied 1) with left/right/straight turn behavior (this is the basis of the “egocentricity score” in Fig. 3), 2) with starting location (after entering the chosen arm; retrospective spatial coding), and 3) with end location (before entering the chosen arm; prospective spatial coding). This analysis is vulnerable to false positives arising from the rat having a different spatial position within a bin during left, right, straight trials, so we separately tested for this (ANOVA, P < 0.05) and excluded such bins from retrospective and prospective testing. For sorted plots of firing by position along a path (Fig. 4A, right), linearized paths were divided into 100 bins and activity was smoothed (Gaussian kernel, sigma = 6.25 bins). For sorted perievent time histograms (Fig. 4A, left and middle), the firing rate was calculated in 1/30-s bins and activity was smoothed (Gaussian kernel, sigma = 3 bins). The time of “reward” was defined as the moment at which the rat's nose reached the baited water port. This was directly measured using frame-by-frame (30/s) video analysis for every reward event in 59 sessions (including 11 of the 39 stable task performance sessions for which cells were analyzed) and found to correspond very closely to the moment at which the rat crossed a fixed radius from the maze center; this moment was taken as the reward moment for the remaining analyzed sessions. To assess the directionality of neuronal firing fields we spilt each goal box-to-goal box trajectory in half and summed the spike counts in each half. The arm with the highest mean spike counts (either on inbound or outbound half-trajectories) was used as the comparison arm and a t-test was used to test the hypothesis that mean spike counts were different in the two directions. This is a conservative measure of directionality because it disregards differences in firing patterns along the arms.
To generate rough overall classifications for each cell that we could use to compare striatal and hippocampal coding (for Fig. 4E), we determined the mean and SD of firing rate for each bin on an arm, for time periods in which the animal's position was changing by ≥1 cm/s. Cells in which the firing rate in any bin was significantly different between left turn, right turn, and straight trials (using ANOVA with a threshold of P = 0.0001) were classified as “egocentric.” For cells that did not meet this criterion, we considered bins with a firing rate that was >2.5 Hz above the mean of all bins, in which the SD was <1.4× the mean, to be firing fields. If a cell had a firing field and 80% of all spikes occurred within 25 cm of the maze center, it was classified as a “center field.” We next calculated the arm selectivity ratio as the firing rate in the arm bin with the highest firing rate divided by the lowest firing rate in the corresponding bins on the other arms. Cells with an arm selectivity ratio of ≥8 were classified as “place cells.” No cells met the criteria for both egocentric and place cell categories. If a cell with a defined firing field did not fall into one of the above-cited categories it was classified as “task-stage.” For cells without a firing field we tested whether, within the 2 s following the reward, there were any 0.25-s time bins with an average firing rate that was >2.5 Hz above the sessionwide mean. If so, the cell was classified as “reward” responsive; otherwise, the cell was “unclassified.”
RESULTS
Competing strategies for task performance
We recorded from 16 chronically implanted rats during 270 sessions in which they were trained to perform a continuous win-stay (visual discrimination) task in a plus-maze (Fig. 1A). Performance progressively improved over the first 10 sessions (Fig. 1B). Although smooth learning curves derived from multiple animals often mask sudden individual performance jumps (Gallistel et al. 2004), in this case all individual animals showed incremental improvements consistent with the gradual strengthening of a response tendency (Supplemental Fig. S1). We next examined how performance depended on whether animals were, by chance, cued to return to the arm they had visited on the immediately preceding trial (“conflict trials”; ∼1/3 of all trials). We found clear behavioral evidence for conflict between win-stay and win-shift strategies early in learning, in that all subjects were less likely to choose the cued arm on conflict compared with nonconflict trials (Fig. 1B and Supplemental Fig. S1; planned comparison for conflict vs. nonconflict on day 1, Wilcoxon ranks test, P = 0.012). Despite this initial dominance of a win-shift, spatial foraging strategy (Olton and Samuelson 1976), analysis of conflict trials shows that rats progressively switched to nearly exclusive use of the win-stay strategy (Fig. 1B).
FIG. 1.
Win-stay task and behavioral performance. A: plus-maze arrangement (to scale). Thirsty rats ran continuously between water ports at the arm ends. As the rat approached the central zone on each trial, one pair of lights began flashing to indicate which arm choice would be rewarded. B: acquisition of continuous win-stay task. Solid black line indicates mean (±SE) overall performance for each of the first 10 training days. Gray and dashed lines show the same data divided into “conflict trials” (in which rats were cued to return to the arm they came from on the immediately preceding trial) and the rest (“nonconflict”). Note that rats were initially reluctant to quickly revisit locations, but after extended training they ignored their recent choice history; n = 8 (all rats for which conflict/nonconflict data were recorded; see Supplemental Fig. S1 for individual animal data).
We first examined neural representations during stable task performance, in which the (presumed hippocampal-dependent) win-shift spatial strategy “loses” this competition with the (presumed striatal-dependent) win-stay strategy. We present single-unit data from the 14 rats that learned to perform the task accurately, rapidly, and smoothly, during 39 sessions (mean % correct = 88.6, range 73.5–99.1; mean number of trials performed = 103.5, range 68–154). Although many other sessions had comparably high levels of performance and neural activity, to avoid counting neurons more than once we included neurons for a given electrode location from only a single session. We identified 175 striatal neurons and 106 hippocampal neurons that were sufficiently active and stable to analyze further (see methods). These included 109 striatal units that had waveforms and firing patterns characteristic of MSNs (Berke et al. 2004) and 95 dorsal CA1 units that had the corresponding characteristics for hippocampal pyramidal neurons. In this report we focus exclusively on projection cells (but see Berke 2008 for descriptions of presumed striatal interneurons).
Place coding in hippocampus but not striatum
The symmetrical plus-maze design allows us to assess spatial (allocentric) encoding by testing whether neurons fired differently on the four arms. We assessed this, first, by calculating a spatial asymmetry index (see methods). As expected, hippocampal neurons almost invariably had high asymmetry (Fig. 2, A and B). The rare cases with lower asymmetry values were neurons active in the center of the maze (e.g., Fig. 2A, panel h) or a few examples with multiple firing fields at different positions on distinct maze arms (which may reflect incomplete neuronal isolation). By contrast, most striatal neurons showed extremely similar patterns of firing across the four arms and the corresponding asymmetry values for this population were much lower (Fig. 2, A and B). We next tested specifically for allocentric “place cell”-type coding. We looked for “place fields” by considering the subset of neurons that displayed spatially focused increases in firing rate on at least one arm, then calculating the ratio of firing rate within this field on the most active to the least active arm (“arm selectivity ratio”). Most hippocampal neurons were exclusively active on one arm (Fig. 2C) and symmetrically located firing fields on all four arms were never observed. By contrast, no striatal units were exclusively active on one arm (Fig. 2C) and those with focal increases in activity fired in the equivalent location on all four arms. Therefore the striatum does not appear to contain hippocampal-style place cells, at least during well-practiced performance of our radial maze win-stay task.
FIG. 2.
Place cells in hippocampus but not striatum. A: examples of projection neurons in striatum (panels a–g) and dorsal CA1 (panels h–n). Striatal locations are indicated with an asterisk on the corresponding coronal atlas section (Swanson 1992). For each cell, the “north” arm is shown at top right in the firing map. The number above the firing map to the left is the spatial asymmetry index (0–1; higher = more asymmetrical) and the number to the right is the maximum of the firing-rate color scale (in Hz; unvisited locations are shown in black). Examples were chosen to illustrate the full range of the asymmetry values for each population. Below each firing map is a raster plot showing activity on each trial as a function of position on the linearized path between trial start and trial end. Dashed redline = point of cue light onset. Trials are sorted by trajectory: {n, e, w, s} indicate {north, east, west, south} arms, respectively, for both start and end locations. B: histogram of spatial asymmetry index values for all unique striatal (n = 109) and hippocampal (n = 95) projection cells recorded during well-practiced performance. C: histogram of arm selectivity ratios (see methods) for all striatal and hippocampal neurons with firing fields on an arm; bin size = 10. Inset: distribution for ratios <10; bin size = 1. Note that all striatal neurons had low arm selectivity ratios. D: spatial asymmetry vs. location within striatum. No significant relationship was observed between asymmetry and distance from midline (left; P = 0.262, 95% confidence limits for r = [−0.289 0.270]) or distance from the anterior-medial-ventral tip of the striatum (right; “origin” = AP 3.13 mm, ML 0; DV 8.0 mm relative to bregma; P = 0.545; 95% confidence limits for r = [−0.243 0.132]). E: no significant correlation (P = 0.695) between the spatial asymmetry of striatal medium spiny projection neurons (MSNs) and the precision of their entrainment to hippocampal theta rhythm (measured as the length of the mean phase vector r, range 0–1).
The observation that striatal projection neurons fired similarly on all four arms and often in a specific relative position along the arms suggested that they might be sensitive to the sequential progress of the rats within each trial (Schmitzer-Torbert and Redish 2004; Shidara et al. 1998). Alternatively, they might simply encode a specific position on the maze, defined relative to generic intramaze landmarks—for example, a spot that is halfway between the maze center and (any) goal box. That could be considered a (nonallocentric) form of “spatial” coding. To help distinguish these possibilities we examined whether neurons fired similarly while moving inbound (toward the maze center) versus outbound (toward the goal boxes), for the subsets of neurons that had clear firing fields on maze arms. In the hippocampus, such direction-specific firing of CA1 place cells is well established for situations in which trajectories are limited by discrete tracks or behavioral parameters (e.g., Gothard et al. 1996; McNaughton et al. 1983; Wiener et al. 1989) and is considered important for the internal processing of event sequences (Eichenbaum et al. 1999). Of 28 hippocampal cells with clear firing fields on maze arms in our win-stay task, 20 (71%) fired to significantly different extents during inbound versus outbound passes through the firing field (using a threshold of P < 0.01, t-test on spike counts) and this directionality was usually obvious by inspection of trajectory-sorted raster plots (e.g., see Fig. 2A, panel l). The remaining 8 (29%) fired similarly in both directions and therefore seemed to reflect spatial position regardless of the rats' stage within a trial. For striatal neurons, 11/12 (92%) fired very different numbers of spikes inbound versus outbound (P < 0.01, t-test) and the remaining cell fired on different portions of the arm in the two directions. Thus both structures contained neurons that were sensitive to the sequence of events within a task, although a substantial fraction of hippocampal cells behaved as “pure” place cells. For neither population was there an obvious bias toward inbound or outbound portions of the trajectory (significantly directional cells: striatum: 6 preferred inbound, 5 preferred outbound; hippocampus: 11 preferred inbound, 9 preferred outbound).
Our finding of limited or absent spatial coding in striatum is rather different from several prior studies (Mulder et al. 2005; Shibata et al. 2001; Wiener 1993), including some that used a radial maze design (Lavoie and Mizumori 1994; Yeshenko et al. 2004). One major difference with the present work is that we sampled from a wide range of striatal locations, whereas the prior studies focused on medial and/or ventral regions of the striatum. Medial/ventral striatal regions receive more inputs from hippocampus and associated cortical regions and this projection appears to be important for performing spatial foraging tasks (Floresco et al. 1997). We therefore examined whether there was a correlation between spatial coding and striatal location. We found no relationship between our asymmetry index and medial–lateral position of recorded striatal neurons (Fig. 2D, left). Nor was there a significant correlation between asymmetry and distance from the anterior-ventral-medial tip of the striatum (Fig. 2D, right), a measure that takes in consideration the overall gross trend in striatal functional anatomy from more emotional and cognitive functions in anterior/ventral/medial striatum to sensorimotor functions in posterior/dorsal/lateral striatum (Berke et al. 2004; Voorn et al. 2004).
Many striatal neurons in medial/ventral parts of the striatum are entrained to the approximately 8-Hz theta rhythm (Berke et al. 2004) that is found in a number of brain regions but is most associated with the hippocampus. We reasoned that striatal neurons whose firing was coherent with hippocampal theta might also be those with more hippocampal-like representations. However, tests showed no significant relationship between the precision of theta entrainment and the spatial asymmetry of spike firing rate (Fig. 2E). We therefore conclude that the entrainment of striatal neurons to hippocampal theta rhythm is unlikely to be directly related to allocentric encoding.
Motor/egocentric encoding in striatum but not hippocampus
To assess possible egocentric encoding, we compared trials in which the rats ran straight ahead, made left turns, or made right turns (regardless of which specific arms were involved or whether they had been recently visited). The path taken on each trial was linearized and divided into segments (bins) and ANOVA was used to compare firing rates in each bin as a function of trial type. An egocentricity score was taken simply as the negative log of the resulting P value—that is, a neuron with a high egocentricity score fires very differently on left, right, and straight trials. In both hippocampus and striatum most neurons had low egocentricity scores and no cells in either structure showed exclusive activity on one type of trial. However, we did observe some cases of differential firing (Fig. 3A); this was stronger and more commonly observed in striatum than in hippocampus (Fig. 3, B and C). All hippocampal neurons with significant egocentricity had place fields near the maze center (Fig. 3A, right; as another example, the hippocampal neuron in Fig. 2A, panel h had an egocentricity score of 3.8). In all such hippocampal cases the apparent egocentricity resulted from subtle spatial differences in the animals' trajectories, such as passing through the center of a place field only on straight trials. We also found high egocentricity values for some symmetric striatal neurons without central place fields (Fig. 3A, left). However, differential activity was still almost exclusively observed in the portion of the trial in which the animals' path clearly diverged between left, right, and straight trials (Fig. 3B). Thus egocentric activity in the striatum may encode aspects of ongoing movements and there did not appear to be substantial prospective or retrospective coding of turn direction in either structure.
FIG. 3.
Egocentric/motor-related activity in striatum but not hippocampus. A: examples of individual neurons that showed differential activity on straight, left, and right turn trials. Top panels show firing rate maps; middle plots show raster of spike events along the linearized path for each trial, arranged by trial type; and bottom panels show histograms of the same data, normalized to the maximum counts in any path bin. For these examples (striatal, hippocampal), total number of trials = (103, 135), firing map color scale maxima = (41, 33) Hz and egocentricity scores = (10.7, 4.6), respectively. In the hippocampal case, differential activity arises due to a place field in the center of the maze; only on select straight trials does the rat pass directly through the place field. B: significance values for all path bins for each of the striatal (left) and hippocampal (right) projection neurons, sorted by egocentricity score (indicated by color scale). Red lines indicate the approximate portion of the path for which the rat paths showed clear divergence on left, right, and straight trials. Note that the high significance bins were concentrated within this path portion. C: histogram of peak significance values for each cell. Note that the striatal distribution shows a long tail of high significance values, whereas the hippocampal distribution does not.
Absence of trajectory encoding in either striatum or hippocampus
We performed similar comparisons of activity in path bins by trial type to look for encoding of the spatial position of the (already-departed) start box or the (not-yet-encountered) goal box. In four separate analyses, we examined striatal or hippocampal projection neuron spike counts per bin, by starting or ending arm location. Only path bins in which the rat was in the same spatial position across trial types were included in the analysis (total number of bins compared: hippocampal by start arm, 2,475; hippocampal by end arm, 2,504; striatal by start arm, 2,849; striatal by end arm, 2,873). We found no convincing evidence for either prospective or retrospective coding of spatial position in either cell population. Although some individual path bins showed significant differences (for the strongest example, see Supplemental Fig. S2), none remained significant after accounting for multiple statistical comparisons. Furthermore, in every case the difference relied solely on a small number of spikes and was unimpressive on visual inspection. Although it remains possible that there are subtle forms of trajectory coding that might be revealed by other types of analysis, the robust differences in firing rate observed in some prior studies (see discussion) were clearly not present in our data.
Distinct population activity time courses in striatum and hippocampus
Many projection neurons in both striatum and hippocampus showed transient increases in firing rate while the rats ran through the maze. We next considered how such activity was distributed during each trial, as a function of either path bin or time relative to two key events: 1) the onset of the light cue that instructs the rat which arm to choose and 2) the moment at which the rat reaches the reward port. In both structures, the progression through each trial was accompanied by sequential activation of projection neurons at each moment, without obvious “gaps” (Fig. 4A). Overall, the hippocampal firing rate peaked in fairly close correspondence with running speed (Fig. 4B) and decreased dramatically as rats slowed near the reward ports then stopped to drink. By contrast, peaks in the overall striatal MSN firing rate were not coupled to running speed (Fig. 4B). The greatest number of MSNs became active as the rats detected the presence of reward and began drinking; two distinct volleys from separate groups of MSNs produced a double peak following rewards (this can be seen by close inspection of Fig. 4, A and B). Inspection of the average firing maps (Fig. 4C) indicated that an earlier hump in overall MSN firing rate corresponded to a disproportionate number of MSNs active in the central area of the maze, as rats executed their choice. Consistent with previous reports (Schmitzer-Torbert and Redish 2004), responses while moving through the maze and after reward onset were generated by mostly separate populations of MSNs (Fig. 4A; see also Supplemental Fig. S3). Although some previous studies have reported increases in striatal MSN activity either in anticipation of or in response to instruction cues (e.g., Gardiner and Kitai 1992; Lauwereyns et al. 2002a; Schultz and Romo 1992), neither form of cue-related activity was apparent in our data either at the population level or in single-unit patterns (Fig. 4, A and B).
FIG. 4.
Temporal evolution and classification of striatal, hippocampal activity. A: individual activity of all projection neurons (one per line) in hippocampus (top) and striatum (bottom). Firing rates are shown with a normalized color scale (zero to peak rate for that neuron), as a function of time relative to instruction cue onset (left) or arrival at the reward port (middle), or as a function of position along the linearized path between trial start and end (right). Neurons are sorted by point of peak activity. The time between cue and reward varied between approximately 2 and 4 s; the mean of the session means was 3.09 s. Note the kink in the striatal distributions at the reward point, where many MSNs increased firing rate; no such kink is seen at the cue onset. B: population activity around the same events, measured as the average firing rate of all projection cells (with each cell's rate normalized to its own mean; shaded area shows overall SE). Top panel shows corresponding average running speed. C: average firing rate maps for the hippocampal (left) and striatal (right) projection cell populations. Color scale is normalized for each population, with maximum values of 1.7 Hz (hippocampus) and 2.3 Hz (striatum). D: no significant correlation (r2 = 0.0227) between the location along the linearized path of peak MSN firing and location within striatum, measured as the distance from the same anterior/ventral/medial origin point as in Fig. 2. E: functional classification of striatal and hippocampal projection neurons (see methods). The same classification criteria were applied to both neuronal populations. “Task-stage” refers to cells with symmetrical firing fields on all 4 arms (that were not also egocentric). Numbers in italics indicate absolute numbers of cells assigned to each category. F: proportion of each class of striatal MSN that was entrained (P < 0.01) to theta rhythm (recorded in hippocampal CA1 pyramidal layer). Colors are as in E. Numbers indicate entrained/nonentrained cells in each class; numbers are lower than those in E because not all striatal neurons were recorded simultaneously with CA1 theta rhythm.
Based on the patterns of activity observed across all analyses of striatal and hippocampal projection neurons, we classified them into several rough categories as shown in Fig. 4E (classification criteria were identical for both structures; see methods). The great majority of hippocampal units, but no striatal units, were “place cells”; by contrast, substantial fractions of striatal units, but no hippocampal units, met the criteria for “egocentric,” “reward,” or “task-stage” (discrete firing fields on all four arms) coding. This categorization serves to highlight the very different forms of activity in hippocampus and striatum. However, it did not do justice to the variety of striatal cell firing, since even within each category diverse firing patterns were observed (Supplemental Fig. S3) and many cells remained unclassified. There was no clear relationship between MSN functional classification and location with striatum (data not shown), nor was there any significant correlation between the point of peak MSN activity within a trial and striatal location (measured as distance from the anterior-ventral-medial tip of the striatum; Fig. 4D). We did notice that all striatal (but no hippocampal) neurons with selective firing in the maze center were egocentric (compare Fig. 2Aa–2Ah, see also Supplemental Fig. S3). This suggests that egocentric coding dominates in striatum during this portion of the trial (and is also why no striatal units were classified as “center-field”).
We next asked whether there was a relationship between this functional classification of striatal neurons and their entrainment to the hippocampal theta rhythm. Of 83 MSNs for which we simultaneously recorded from the CA1 pyramidal layer, 15 were theta-entrained (at P < 0.01) and the majority of these (9) were task-stage cells (Supplemental Fig. S4). The proportion of task-stage cells entrained was thus higher than that for other classes (Fig. 4E). Although this comparison is made more difficult by small numbers and the behavior-dependence of theta power, it does suggest that the information encoded by MSNs may be reflected in their relationship to hippocampal activity, even if that information is not itself spatial.
Neural coding earlier in learning
The fact that striatal cells showed no evidence of spatial coding even in medial striatum was somewhat surprising, given both prior reports and the known cooperation between medial striatum and hippocampus in cognition. Similarly surprising was the absence of prospective/retrospective coding by hippocampal neurons. We next considered the possibility that these results might reflect the late stage of task learning, at which spatial working memory is no longer having a strong influence on the pattern of choices. We therefore identified earlier sessions in which many “conflict trials” were resolved in favor of the win-shift strategy (14 sessions from 11 rats; mean win-shift percentage = 58.6, range 42.6–72.5; average number of prior training days = 3.2, range 2–7) that also had good recordings of striatal (n = 55) and hippocampal (n = 54) projection neurons. This stage of learning was chosen because, unlike the very first sessions of training, rats were no longer performing frequent investigatory behavior of maze components; average trial duration and speed profiles were very similar to those later in learning (data not shown). We performed the same neural coding analyses as described earlier on this smaller data set.
Overall, neural coding at this earlier stage of learning strongly resembled coding at the later stage, in both striatum and hippocampus. Once again, place fields dominated the responses of dorsal CA1 cells while striatal cells almost invariably had symmetrical activity on the four arms (for examples, see Fig. 5, panels a–e). Exceptions included a few striatal MSNs in which apparent place fields actually arose from bursts of activity on just single trials (Fig. 5, panel f). In neither structure did we find path bins that showed significant prospective or retrospective coding of spatial position (at P < 0.05), after correcting for multiple comparisons (data not shown). Once again, significant egocentricity was found for some striatal neurons (e.g., the cell in Fig. 5, panel a fired less on right-turn trials) producing a distribution of egocentricity values with a long tail in striatum compared with hippocampus (Supplemental Fig. S4). The cell classification procedure gave results very similar to those later in learning, with exclusively place cells in hippocampus and no place cells in striatum. The exception was one striatal MSN that was classified as a “place cell” due to a high arm selectivity ratio; inspection of the firing pattern of this cell (Fig. 5, panel g) showed that it actually fired in three of the four goal boxes, always on the “northern” edge. This striatal cell therefore was not a true place cell, but instead appeared to have head-direction selectivity (e.g., Ragozzino et al. 2001; Wiener 1993). Inspection of all firing maps and raster plots produced no other projection cells with similar activity, at either training stage (one presumed striatal interneuron also had head-direction selectivity; not shown). In summary, no substantial differences in neural representations were observed in a comparison between sessions earlier in learning and sessions with asymptotic behavioral performance.
FIG. 5.
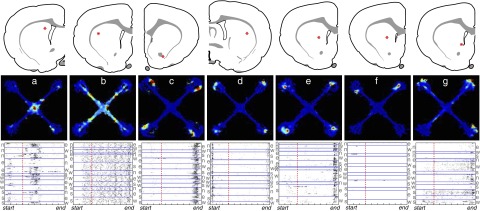
Striatal single-unit representations are similar at early to later stages of learning. Layout is like that in Fig. 2. Panels a–g: examples of individual neuron firing patterns and their corresponding locations within striatum, recorded when rats were using both win-stay and win-shift strategies (see main text). Panels a–e are representative units; panels f and g show notable exceptions. As seen at later stages of learning, all parts of the maze were associated with the activity of subpopulations of striatal neurons. As for other striatal neurons that were preferentially active in the maze center, the neuron shown in panel a showed significant egocentricity—in this case firing less on right-turn trials (n-w, e-n, w-s, s-e) than that on other trials. Panel f: illustrates how apparently place-specific activity can arise when the variability between trials is not considered. Candidate “place fields” are visible on 2 of the maze arms, but inspection of the raster plots indicates that each arose from a strong burst of activity occurring only on a single trial. The neuron shown in panel g was the only striatal neuron to be classified as a “place cell” by the classification algorithm, but it is in fact active in 3 of the 4 arms.
DISCUSSION
The striatum and hippocampus region share a notable common property: they both have sets of projection neurons that are selectively active, in sequence, as rats run through a maze. Both structures have been proposed to be involved in encoding sequences of events (e.g., Berns and Sejnowski 1998; Eichenbaum 2004; Fukai 1999) and, in both cases, such sequences may be used in the (implicit or explicit) prediction of rewards. Nonetheless, we found that these two structures use very different representational schemes. Overwhelmingly, hippocampal CA1 pyramidal cells were active in specific spatial locations defined by extramaze cues; in most cases this firing was also modulated by the direction in which the rat was traversing the place field. We found no evidence that hippocampal neurons represented any other features of this task, including egocentric turn, reward, or origins and destinations that define a complete route through the maze. Although some studies have described disproportionate clustering of hippocampal place fields around salient events such as instruction cues and rewards (e.g., Hollup et al. 2001), in the present study hippocampal population activity closely matched the animals' running speed. By contrast, no striatal neurons resembled hippocampal “place cells” and striatal population activity did not track running speed. Instead, MSNs were most commonly active at specific moments during the progression through a trial sequence, with the greatest number of cells active around the central area in which rats executed different choices and at the reward ports. This striking difference between striatal and hippocampal representations is consistent with theories that these structures play different roles in learning and choosing. At the same time, our results suggest that important aspects of those theories may need to be reconsidered (see following text).
Although we took care to record many neurons from a range of striatal subregions, it is not possible to prove that there are no striatal neurons whose firing is comparable to that of hippocampal place cells, and it might be that an important form of spatial coding is achieved through the sparse activity of very few cells (see also Tang et al. 2007). However, our results clearly demonstrate that in this task, place fields are certainly not characteristic of a substantial proportion of the striatal population, irrespective of whether a spatial strategy is being used by the animal. This finding stands in sharp contrast to some previous reports. For example, Mizumori and colleagues (2004) concluded: “In summary, striatum contains neurons whose location-selective firing is remarkably similar to that of hippocampal place fields.” One important methodological difference is that the data presented here are from long sessions of fluid, well-practiced performance, each with many trials. Neuronal activity needed to be quite consistent to show up in our analyses, as was the case for spatial coding in the hippocampus. Fluid performance also reduced the rate of extraneous behaviors such as pausing to investigate maze elements or groom, which could be associated with firing at specific spatial locations on a small number of trials. Indeed, in several striatal cases from the sessions earlier in learning we observed what appeared to be strongly spatially selective firing; however, inspection of raster plots showed that this arose from a burst of activity on a single trial. Such observations highlight the importance of showing individual trial data.
Another possibly critical difference to prior work concerns the demands of the behavioral task. Rewarded win-stay performance does not require allocentric representations, so if the striatum makes use of coding schemes that are only actually being used for the current behavioral strategy, one might not expect to see substantial striatal spatial coding in well-trained rats. We attempted to address this issue by examining sessions earlier in learning, for which spatial working memory was still having a considerable impact on behavior. Although the number of recorded cells was smaller, striatal neurons again did not show substantial allocentric coding and none behaved remotely like hippocampal place cells. We therefore found no evidence for broad strategy-dependent shifts in neural representations (in either structure), again in contrast to some prior work (Eschenko and Mizumori 2007). Another difference that might help to account for our divergent results is that rewards were never given specifically for spatially dependent performance (although win-shift performance would be rewarded on about one third of trials, by chance). If striatal spatial representations develop through reinforcement learning of spatially dependent behavior, we would not necessarily have observed them in this task. This would be in line with the suggestion that “striatal spatial representations depend on the degree to which spatial task parameters can be unambiguously associated with goals” (Schmitzer-Torbert and Redish 2008).
We also cannot rule out other, more subtle means of achieving spatial coding in striatum. For example, we did observe some striatal neurons that appeared to have a spatial bias to their task-related activity (e.g., Fig. 2A, far right). However, although we endeavored to make the task as symmetrical between the four arms as possible, we cannot force the rats to treat the task as perfectly symmetrical. Indeed, we occasionally noticed biases to choose or avoid certain arms and one might expect to see corresponding neural coding differences in striatum associated with different degrees of reward expectation (Kawagoe et al. 1998; Lauwereyns et al. 2002b; but see also Mulder et al. 2004).
The dorsal hippocampus did maintain strong spatial coding, even though this information is unhelpful for obtaining rewards in the win-stay task. However, we did not observe prospective or retrospective coding of the animal's trajectory, unlike several previous hippocampal studies (Ainge et al. 2007; Bower et al. 2005; Ferbinteanu and Shapiro 2003; Frank et al. 2000; Griffin et al. 2007; Lee et al. 2006; Wood 2000). Once again, this may reflect our particular task demands. Acquisition of win-stay performance in maze tasks is accelerated in rats with hippocampal lesions, most likely because the conflicting win-shift (foraging) strategy requires hippocampal representations of recently visited locations. Performing the win-stay task at a high level may require the omission, or even active suppression, of information about specific past/future trajectories in hippocampus. Our results during well-learned performance are consistent with a model in which a core framework of spatial encoding is consistently maintained in hippocampus, with additional, mnemonic aspects of coding enabled or suppressed, depending on task requirements (Lenck-Santini et al. 2001). However, arguing against this idea is the apparent lack of substantial prospective/retrospective coding—albeit in a more limited data set—earlier in learning, when animals were frequently using spatial working memory. One possible factor is the presence of the light signal in the midst of each route through the maze. In a previous study, the presence of intermediate salient events prevented trajectory coding in comparison to the same task without intermediate events (Bower et al. 2005).
In contrast to dorsal/lateral striatal circuits that are important for win-stay performance, lesion studies indicate that medial parts of striatum cooperate with hippocampus in spatial/cognitive processing (Devan and White 1999; Whishaw et al. 1987; Yin and Knowlton 2004). This is consistent with our finding that medial striatal neurons are much more likely to be entrained to the hippocampal theta rhythm (Berke et al. 2004). However, even theta-entrained medial striatal neurons did not show hippocampal-style allocentric coding. Again, this argues against the idea that hippocampus and striatum “process similar types of information in parallel” (Mizumori et al. 2004). Rather, it appears that the medial striatum makes use of hippocampal inputs in decision making without itself performing similar information processing. This is consistent with observations that rats with medial striatal damage remain capable of using spatial strategies, but tend not to do so—“the deficit, which has features of a neglect rather than a loss of ability per se, suggests that medial caudate-putamen neural systems are involved in the selection of alternative strategies in spatial navigation tasks” (Whishaw et al. 1987). Large-scale loop circuits that include both medial striatal subregions and medial prefrontal cortical areas have been implicated in strategy shifting in maze tasks (Ragozzino et al. 2002, 2003) and may thus be important for resolving the competition between win-shift and win-stay performance in the present task.
Although projection neurons in both striatum and hippocampus appear to provide complete coverage of each moment through trial progression (Schmitzer-Torbert and Redish 2004), the two populations showed distinct overall activity time courses. Hippocampal activity peaked in the middle of the inbound and outbound arms, as the rats ran fastest. Striatal MSN population activity instead was strongest in the central area of the maze, as rats slowed down and made their choice, then showed sharp peaks following rewards. These changes in overall MSN activity arose predominantly from distinct subpopulations of individual MSNs, consistent with prior studies that have found actions and rewards to be separately encoded in striatum (Schmitzer-Torbert and Redish 2004). Reward-responsive neurons were broadly distributed through the striatum, rather than being clustered in nucleus accumbens, and did not show differential activity depending on which (egocentric or allocentric) choice resulted in reward (Lau and Glimcher 2007). An important challenge for future physiological studies is to determine whether these functionally defined subpopulations correspond to known subclasses of striatal MSNs, such as the striatonigral versus striatopallidal or patch/striosome versus matrix neurons. Conversely, a challenge for modelers is to show how these separate MSN populations serve as components of reinforcement learning and sequence generation architectures (Fukai 1999). One intriguing possibility is that intermixed MSN subpopulations are serving distinct computational roles in the prediction and assessment of states and rewards, respectively (Kawato and Samejima 2007).
Increased MSN population activity in the central part of the maze is consistent with ongoing striatal involvement in the selection and implementation of choices in the win-stay task, although our results differ from prior work that suggested a shift in population activity away from choice points as actions become overtrained and presumably habitual (Jog et al. 1999). Although rats were highly trained in the present task, having performed many hundreds or thousands of trials, an important limitation of both this and prior maze studies of striatal activity is that the habitual versus goal-directed nature of task performance was not tested, e.g., through reinforcer devaluation (Sage and Knowlton 2000).
Habits or not, a striking common feature of both our results and previous related maze studies (Jog et al. 1999) was the lack of strong striatal responses to instruction cue onset. The striatum, especially dorsal/lateral portions, has been repeatedly conceptualized as the substrate of the stimulus–response (S-R) associations (reviewed in Berke 2009). The nature of the “stimulus” is often unspecified in S-R theorizing and the win-stay task is psychologically complex because it has both instrumental and Pavlovian (cue-approach) aspects. Nonetheless, the flashing light cue here is clearly strongly directing behavior and one might therefore expect it to evoke correspondingly large changes in neural activity. The meager cue response is particularly surprising, given that much of the evidence for striatal involvement in stimulus–response associations comes from lesions and drug manipulations in the very win-stay task on which the current experiment was based (Packard and McGaugh 1996; Packard et al. 1989). Nor did we observe a clear pattern of anticipatory activity before a predictable instruction cue, as seen in some monkey striatal studies (Apicella et al. 1992; Hikosaka et al. 1989; Lauwereyns et al. 2002a). Theoretical accounts that frame striatal function in terms of learned associations (e.g., White and McDonald 2002; Yin and Knowlton 2006) may therefore need to be further developed to account for this paucity of cue-related responses.
GRANTS
This work was supported by National Institutes of Health Grants R01 DA-014318 and F32 MH-12908 to J. D. Berke.
Supplementary Material
Acknowledgments
Technical assistance was provided by J. Skurski, L. McDougal, and V. Hetrick. M. Okatan and P. Alvarez contributed to computer programming and data analysis routines. We thank T. Robinson and K. Berridge for helpful comments on a prior version of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Ainge et al. 2007.Ainge JA, Tamosiunaite M, Woergoetter F, Dudchenko PA. Hippocampal CA1 place cells encode intended destination on a maze with multiple choice points. J Neurosci 27: 9769–9779, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella et al. 1992.Apicella P, Scarnati E, Ljungberg T, Schultz W. Neuronal activity in monkey striatum related to the expectation of predictable environmental events. J Neurophysiol 68: 945–960, 1992. [DOI] [PubMed] [Google Scholar]
- Becker et al. 1980.Becker JT, Walker JA, Olton DS. Neuroanatomical bases of spatial memory. Brain Res 200: 307–320, 1980. [DOI] [PubMed] [Google Scholar]
- Berke 2008.Berke JD Uncoordinated firing rate changes of striatal fast-spiking interneurons during behavioral task performance. J Neurosci 28: 10075–10080, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke 2009.Berke JD Procedural learning: striatum. In: Encyclopedia of Neuroscience, edited by Squire LR. Oxford: Academic, 2009.
- Berke et al. 2004.Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron 43: 883–896, 2004. [DOI] [PubMed] [Google Scholar]
- Berns and Sejnowski 1998.Berns GS, Sejnowski TJ. A computational model of how the basal ganglia produce sequences. J Cogn Neurosci 10: 108–121, 1998. [DOI] [PubMed] [Google Scholar]
- Bower et al. 2005.Bower MR, Euston DR, McNaughton BL. Sequential-context-dependent hippocampal activity is not necessary to learn sequences with repeated elements. J Neurosci 25: 1313–1323, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasted et al. 1997.Brasted PJ, Humby T, Dunnett SB, Robbins TW. Unilateral lesions of the dorsal striatum in rats disrupt responding in egocentric space. J Neurosci 17: 8919–8926, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki 2005.Buzsáki G Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus 15: 827–840, 2005. [DOI] [PubMed] [Google Scholar]
- Cook and Kesner 1988.Cook D, Kesner RP. Caudate nucleus and memory for egocentric localization. Behav Neural Biol 49: 332–343, 1988. [DOI] [PubMed] [Google Scholar]
- Daw et al. 2006.Daw ND, Niv Y, Dayan P. Actions, policies, values and the basal ganglia. In: Recent Breakthroughs in Basal Ganglia Research, edited by Bezard E. Hauppauge, NY: Nova Science, 2006, p. 91–106.
- Devan and White 1999.Devan BD, White NM. Parallel information processing in the dorsal striatum: relation to hippocampal function. J Neurosci 19: 2789–2798, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doya 2000.Doya K Complementary roles of basal ganglia and cerebellum in learning and motor control. Curr Opin Neurobiol 10: 732–739, 2000. [DOI] [PubMed] [Google Scholar]
- Eichenbaum 2004.Eichenbaum H Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron 44: 109–120, 2004. [DOI] [PubMed] [Google Scholar]
- Eichenbaum and Cohen 2001.Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the Brain. New York: Oxford Univ. Press, 2001.
- Eichenbaum et al. 1999.Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron 23: 209–226, 1999. [DOI] [PubMed] [Google Scholar]
- Eschenko and Mizumori 2007.Eschenko O, Mizumori SJY. Memory influences on hippocampal and striatal neural codes: effects of a shift between task rules. Neurobiol Learn Mem 87: 495–509, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbinteanu and Shapiro 2003.Ferbinteanu J, Shapiro ML. Prospective and retrospective memory coding in the hippocampus. Neuron 40: 1227–1239, 2003. [DOI] [PubMed] [Google Scholar]
- Floresco et al. 1997.Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci 17: 1880–1890, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank et al. 2000.Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron 27: 169–178, 2000. [DOI] [PubMed] [Google Scholar]
- Fukai 1999.Fukai T Sequence generation in arbitrary temporal patterns from theta-nested gamma oscillations: a model of the basal ganglia-thalamo-cortical loops. Neural Networks 12: 975–987, 1999. [DOI] [PubMed] [Google Scholar]
- Gallistel et al. 2004.Gallistel CR, Fairhurst S, Balsam P. The learning curve: implications of a quantitative analysis. Proc Natl Acad Sci USA 101: 13124–13131, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard et al. 1996.Gothard KM, Skaggs WE, Moore KM, McNaughton BL. Binding of hippocampal CA1 neural activity to multiple reference frames in a landmark-based navigation task. J Neurosci 16: 823–835, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin et al. 2007.Griffin AL, Eichenbaum H, Hasselmo ME. Spatial representations of hippocampal CA1 neurons are modulated by behavioral context in a hippocampus-dependent memory task. J Neurosci 27: 2416–2423, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka et al. 1989.Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. III. Activities related to expectation of target and reward. J Neurophysiol 61: 814–832, 1989. [DOI] [PubMed] [Google Scholar]
- Hollup et al. 2001.Hollup SA, Molden S, Donnett JG, Moser MB, Moser EI. Accumulation of hippocampal place fields at the goal location in an annular watermaze task. J Neurosci 21: 1635–1644, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jog et al. 1999.Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science 286: 1745–1749, 1999. [DOI] [PubMed] [Google Scholar]
- Kawagoe et al. 1998.Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nat Neurosci 1: 411–416, 1998. [DOI] [PubMed] [Google Scholar]
- Kawato and Samejima 2007.Kawato M, Samejima K. Efficient reinforcement learning: computational theories, neuroscience and robotics. Curr Opin Neurobiol 17: 205–212, 2007. [DOI] [PubMed] [Google Scholar]
- Lau and Glimcher 2007.Lau B, Glimcher PW. Action and outcome encoding in the primate caudate nucleus. J Neurosci 27: 14502–14514, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwereyns et al. 2002a.Lauwereyns J, Takikawa Y, Kawagoe R, Kobayashi S, Koizumi M, Coe B, Sakagami M, Hikosaka O. Feature-based anticipation of cues that predict reward in monkey caudate nucleus. Neuron 33: 463–473, 2002a. [DOI] [PubMed] [Google Scholar]
- Lauwereyns et al. 2002b.Lauwereyns J, Watanabe K, Coe B, Hikosaka O. A neural correlate of response bias in monkey caudate nucleus. Nature 418: 413–417, 2002b. [DOI] [PubMed] [Google Scholar]
- Lavoie and Mizumori 1994.Lavoie AM, Mizumori SJ. Spatial, movement- and reward-sensitive discharge by medial ventral striatum neurons of rats. Brain Res 638: 157–168, 1994. [DOI] [PubMed] [Google Scholar]
- Lee et al. 2006.Lee I, Griffin AL, Zilli EA, Eichenbaum H, Hasselmo ME. Gradual translocation of spatial correlates of neuronal firing in the hippocampus toward prospective reward locations. Neuron 51: 639–650, 2006. [DOI] [PubMed] [Google Scholar]
- Lenck-Santini et al. 2001.Lenck-Santini PP, Save E, Poucet B. Place-cell firing does not depend on the direction of turn in a Y-maze alternation task. Eur J Neurosci 13: 1055–1058, 2001. [DOI] [PubMed] [Google Scholar]
- Mallet et al. 2005.Mallet N, Le Moine C, Charpier S, Gonon F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci 25: 3857–3869, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald and White 1993.McDonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav Neurosci 107: 3–22, 1993. [DOI] [PubMed] [Google Scholar]
- McNaughton et al. 1983.McNaughton BL, Barnes CA, O'Keefe J. The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp Brain Res 52: 41–49, 1983. [DOI] [PubMed] [Google Scholar]
- Mizumori et al. 2004.Mizumori SJ, Yeshenko O, Gill KM, Davis DM. Parallel processing across neural systems: implications for a multiple memory system hypothesis. Neurobiol Learn Mem 82: 278–298, 2004. [DOI] [PubMed] [Google Scholar]
- Morris 2001.Morris RG Episodic-like memory in animals: psychological criteria, neural mechanisms and the value of episodic-like tasks to investigate animal models of neurodegenerative disease. Philos Trans R Soc Lond B Biol Sci 356: 1453–1465, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris et al. 1982.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature 297: 681–683, 1982. [DOI] [PubMed] [Google Scholar]
- Mulder et al. 2005.Mulder AB, Shibata R, Trullier O, Wiener SI. Spatially selective reward site responses in tonically active neurons of the nucleus accumbens in behaving rats. Exp Brain Res 163: 32–43, 2005. [DOI] [PubMed] [Google Scholar]
- Mulder et al. 2004.Mulder AB, Tabuchi E, Wiener SI. Neurons in hippocampal afferent zones of rat striatum parse routes into multi-pace segments during maze navigation. Eur J Neurosci 19: 1923–1932, 2004. [DOI] [PubMed] [Google Scholar]
- Olton and Samuelson 1976.Olton DS, Samuelson RJ. Remembrance of places passed: spatial memory in rats. J Exp Psych Anim Behav Process 2: 97–115, 1976. [Google Scholar]
- Packard et al. 1989.Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci 9: 1465–1472, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard and McGaugh 1996.Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem 65: 65–72, 1996. [DOI] [PubMed] [Google Scholar]
- Ragozzino et al. 2001.Ragozzino KE, Leutgeb S, Mizumori SJ. Dorsal striatal head direction and hippocampal place representations during spatial navigation. Exp Brain Res 139: 372–376, 2001. [DOI] [PubMed] [Google Scholar]
- Ragozzino et al. 2003.Ragozzino ME, Kim J, Hassert D, Minniti N, Kiang C. The contribution of the rat prelimbic-infralimbic areas to different forms of task switching. Behav Neurosci 117: 1054–1065, 2003. [DOI] [PubMed] [Google Scholar]
- Ragozzino et al. 2002.Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci 116: 105–115, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage and Knowlton 2000.Sage JR, Knowlton BJ. Effects of US devaluation on win-stay and win-shift radial maze performance in rats. Behav Neurosci 114: 295–306, 2000. [DOI] [PubMed] [Google Scholar]
- Schmitzer-Torbert and Redish 2004.Schmitzer-Torbert N, Redish AD. Neuronal activity in the rodent dorsal striatum in sequential navigation: separation of spatial and reward responses on the multiple T task. J Neurophysiol 91: 2259–2272, 2004. [DOI] [PubMed] [Google Scholar]
- Schmitzer-Torbert and Redish 2008.Schmitzer-Torbert NC, Redish AD. Task-dependent encoding of space and events by striatal neurons is dependent on neural subtype. Neuroscience 153: 349–360, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz and Romo 1992.Schultz W, Romo R. Role of primate basal ganglia and frontal cortex in the internal generation of movements. I. Preparatory activity in the anterior striatum. Exp Brain Res 91: 363–384, 1992. [DOI] [PubMed] [Google Scholar]
- Seamans and Phillips 1994.Seamans JK, Phillips AG. Selective memory impairments produced by transient lidocaine-induced lesions of the nucleus accumbens in rats. Behav Neurosci 108: 456–468, 1994. [DOI] [PubMed] [Google Scholar]
- Shibata et al. 2001.Shibata R, Mulder AB, Trullier O, Wiener SI. Position sensitivity in phasically discharging nucleus accumbens neurons of rats alternating between tasks requiring complementary types of spatial cues. Neuroscience 108: 391–411, 2001. [DOI] [PubMed] [Google Scholar]
- Shidara et al. 1998.Shidara M, Aigner TG, Richmond BJ. Neuronal signals in the monkey ventral striatum related to progress through a predictable series of trials. J Neurosci 18: 2613–2625, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang et al. 2007.Tang C, Pawlak AP, Prokopenko V, West MO. Changes in activity of the striatum during formation of a motor habit. Eur J Neurosci 25: 1212–1227, 2007. [DOI] [PubMed] [Google Scholar]
- Tanila et al. 1997.Tanila H, Shapiro M, Gallagher M, Eichenbaum H. Brain aging: changes in the nature of information coding by the hippocampus. J Neurosci 18: 5155–5166, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn et al. 2004.Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci 27: 468–474, 2004. [DOI] [PubMed] [Google Scholar]
- Whishaw et al. 1987.Whishaw IQ, Mittleman G, Bunch ST, Dunnett SB. Impairments in the acquisition, retention and selection of spatial navigation strategies after medial caudate-putamen lesions in rats. Behav Brain Res 24: 125–138, 1987. [DOI] [PubMed] [Google Scholar]
- White and McDonald 2002.White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem 77: 125–184, 2002. [DOI] [PubMed] [Google Scholar]
- Wiener 1993.Wiener SI Spatial and behavioral correlates of striatal neurons in rats performing a self-initiated navigation task. J Neurosci 13: 3802–3817, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener et al. 1989.Wiener SI, Paul CA, Eichenbaum H. Spatial and behavioral correlates of hippocampal neuronal activity. J Neurosci 9: 2737–2763, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood et al. 2000.Wood ER, Dudchenko PR, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron 27: 623–633, 2000. [DOI] [PubMed] [Google Scholar]
- Yeshenko et al. 2004.Yeshenko O, Guazzelli A, Mizumori SJ. Context-dependent reorganization of spatial and movement representations by simultaneously recorded hippocampal and striatal neurons during performance of allocentric and egocentric tasks. Behav Neurosci 118: 751–769, 2004. [DOI] [PubMed] [Google Scholar]
- Yin and Knowlton 2004.Yin HH, Knowlton BJ. Contributions of striatal subregions to place and response learning. Learn Mem 11: 459–463, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin and Knowlton 2006.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci 7: 464–476, 2006. [DOI] [PubMed] [Google Scholar]
- Young et al. 1994.Young BJ, Fox GD, Eichenbaum H. Correlates of hippocampal complex-spike cell activity in ras performing a nonspatial radial maze task. J Neurosci 14: 6553–6583, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






