Abstract
The mammalian orienting response to sounds consists of a gaze shift that can be a combination of head and eye movements. In animals with mobile pinnae, the ears also move. During head movements, vision is stabilized by compensatory rotations of the eyeball within the head because of the vestibulo-ocular reflex (VOR). While studying the gaze shifts made by cats to sounds, a previously uncharacterized compensatory movement was discovered. The pinnae exhibited short-latency, goal-directed movements that reached their target while the head was still moving. The pinnae maintained a fixed position in space by counter-rotating on the head with an equal but opposite velocity to the head movement. We call these compensatory ear movements the vestibulo-auricular reflex (VAR) because they shared many kinematic characteristics with the VOR. Control experiments ruled out efference copy of head position signals and acoustic tracking (audiokinetic) of the source as the cause of the response. The VAR may serve to stabilize the auditory world during head movements.
INTRODUCTION
A critical function of the auditory system is to determine the location of sound sources so that the eyes, which have higher spatial acuity, can be directed to the source for further inspection (Heffner and Heffner 1992). When presented with a sound, animals often make orienting responses consisting of coordinated movements of the head and eyes, which are called gaze shifts, as well as orienting movements of the pinnae and body toward the sound (Frens and van Opstal 1994; Knudsen 1981).
During movements of the head, the visual world is stabilized by compensatory rotations of the eyeball via the vestibulo-ocular reflex (VOR) (Lorente de No 1933). For example, if the head is rotated 20° to the right, the VOR reflexively moves the eyes 20° to the left, so that the eyes continue pointing to the same location in space. Without the VOR, there would be a blur of images zipping across the retina whenever the head moved. Given its importance and the fairly simple neural mechanisms that mediate it, the VOR has been extensively studied and is a standard model for neural plasticity and learning (du Lac et al. 1995; Ito 1982).
While the visual world is stabilized by the VOR, what about the auditory world, which would be equally affected by head and pinna movements? For a fixed sound in space, movements of the head and pinnae will alter the set of acoustical cues to location (Young et al. 1996), which profoundly changes sound localization performance (Vliegen et al. 2004). Suzuki and Cohen (1964) reported stereotyped and orderly pinna movements to semicircular canal stimulation in cats, and Schaefer et al. (1971) found postrotatory pinna nystagmus in phase with eye nystagmus, as well as pinna movements associated with head rotation in rabbits. This evidence for linkage between head and pinna movements led us to study the sound-evoked orienting movements of the eyes, head, and the pinna in the cat. Like humans, cats make accurate and precise gaze shifts to sounds (Tollin et al. 2005), but they have highly mobile pinnae. In head-fixed cats, the pinnae make consistent, goal-directed, and sustained movements to both auditory and visual targets primarily to the ipsilateral side (Populin and Yin 1998b). We hypothesized that there is a compensatory pinna movement in response to head movement that would stabilize the acoustic world as the VOR does for the visual environment. We report here evidence for a newly discovered compensatory movement of the pinna, which we call the vestibulo-auricular reflex (VAR).
METHODS
General
Four adult female cats were outfitted with a head holder and movement detection coils for the eyes, head, and pinnae. Ear coils were implanted subcutaneously in a caudal position relative to the pinna to maximize linear voltage output of the coil (Populin and Yin 1998b). We usually implanted both ears, but could only record the output from one ear coil at a time along with head and eye coils because of technical limitations. Eye (Tollin et al. 2005), pinna (Populin and Yin 1998b), and head (Tollin et al. 2005) coils were calibrated as previously described. During experimental sessions, the cats were placed in the center of a dimly lit, sound-attenuating chamber facing a bank of loudspeakers arranged horizontally, vertically, and diagonally along an arc either 62 or 81.6 cm from the center of the head. The two viewing distances were caused by a change in the recording chamber in the midst of this experiment. In this study, only speakers on the horizontal meridian were used so elevational components will be disregarded. Positive angles correspond to rightward in azimuth. Speakers were hidden from view by a black translucent cloth. A 2.0-mm-diameter red light emitting diode (LED, subtending ∼0.2° visual angle) was suspended over the center of each speaker and, when illuminated, could be easily seen through the cloth. Cats were trained using operant conditioning to indicate via gaze shift the apparent two-dimensional location of various auditory and visual targets placed within ±45° along the horizontal plane. For simplicity we only studied the horizontal component of the VAR. The positions of the eye, head, and pinna in space were recorded using the scleral search coil technique. All procedures used were approved by the University of Wisconsin Animal Care and Use Committee and also complied with the National Institutes of Health guidelines for animal use.
Acoustic stimuli
The acoustic stimuli for these experiments consisted of a broadband (∼1.5–25 kHz) noiseburst that was 1 s in duration and gated by a trapezoid window (rise-fall time = 7 ms). The overall level of each acoustic stimulus was varied from the baseline level from trial-to-trial by ±4–6 dB in 2-dB steps.
Psychophysical procedure
The dependent variables were the horizontal position of the eye, head, and one pinna during gaze shifts to the apparent location of the acoustic target. We usually began by training the cat with its head restrained and then with the head unrestrained. All data reported here were taken from head-unrestrained animals. All trials reported here with the exception of the passive head movement experiments began with the cat fixating a visual LED target directly ahead at 0° to ensure that the head was facing forward with the eyes centered in the orbit and that the pinnae were in a standard “ready” position (Populin and Yin 1998a,b) centered on the head. To initiate a trial, the cats visually fixated the initial LED within ±4° for a variable time period (∼500–1,000 ms) whereupon the LED was extinguished and the acoustic target presented simultaneously. The cats were required to make a gaze shift to the apparent location of the target. If the cat maintained a gaze position for 600–900 ms within a square electronic acceptance window of approximately ±8–16°, it was presented with a food reward. There was a variable intertrial interval of 10–15 s between each trial to allow time to lick the reward tube.
Data analysis
We used a velocity criterion (Populin and Yin 1998a) for gaze to determine when the gaze movements began and ended by determining the time at which the magnitude of the velocity exceeded 2 SD of the mean velocity computed during the fixation of the initial LED over a window spanning from 100 ms before to 30 ms after the onset of the acoustic target. A similar velocity criterion was used to determine the onset of the head and pinna movements, but over a different time window, spanning 100 ms before the onset of the acoustic target because some pinna latencies were shorter than 30 ms. In all cases, velocity was smoothed using the SigmaPlot (v. 6.00) “low-pass” smoothing function.
Passive head movements
As a control experiment to test whether the VAR could be caused by an efference copy signal, we examined the VAR when the body and head of the cat were rotated passively, rather than during an active head movement by the cat. Passive movements were induced with a motor linked with a pulley to the apparatus that supported the animal, which allowed the whole body to be rotated in the horizontal direction (yaw) at angular velocities of 60–90 deg/s. Because the motor made a noise when turned on, we first acclimated the cats to the motor noise for many days by having the motor turn on for random trials without the pulley connected so the cats got used to hearing the motor. Although initially the cats would orient toward the motor, they soon discovered that such behavior was not rewarded and on most, but not all, occasions ignored the motor. However, despite the adaptation, some cats still occasionally reacted when the motor was turned on. The goal was to rotate the animal on random trials during a period of time that it was actively localizing a sound source. While the cat was working on its usual array of visual and auditory saccade and fixation tasks (Tollin et al. 2005), we turned on the motor on some trials with low probability (∼10%) to rotate the animal either to the left or to the right. These trials began by turning on an acoustic target. Because the cats had had many months and in some cases years of daily training, they were accustomed to the task that usually began with fixation of the LED straight ahead. Consequently, they were usually looking and oriented forward in anticipation of the trial beginning when the acoustic target came on. After the cat had shifted its head and gaze to the target, the rotation began ∼350–450 ms afterward while the target remained on. The platform was rotated randomly in either direction and its position was recorded by a potentiometer.
One difficulty in these experiments was that the passive rotation of the animal often elicited a vestibulo-colic reflex (VCR) or cervico-colic reflex (CCR), i.e., as the animal's body was passively rotated in one direction, its head would reflexively rotate in the opposite direction so there was little or no passive head movement. Because the VCR/CCR usually only happened on a subset of trials, we analyzed trials in which it was not present or at least was minimal. The incidence of the VCR/CCR was higher in some animals than others, so this experiment was more effective in certain cats.
Unless we specified otherwise, statistical tests of significance were assessed using an unpaired two-sided t-test. Population data are reported as mean ± SD. Linear regression analysis was used to test for significance of relationships. Normality of the underlying distributions was not tested directly; however, the large sample sizes (n = ∼150) allows for the safe use of parametric tests (Hays 1988). For all statistical tests, a 0.05 level of significance was used.
RESULTS
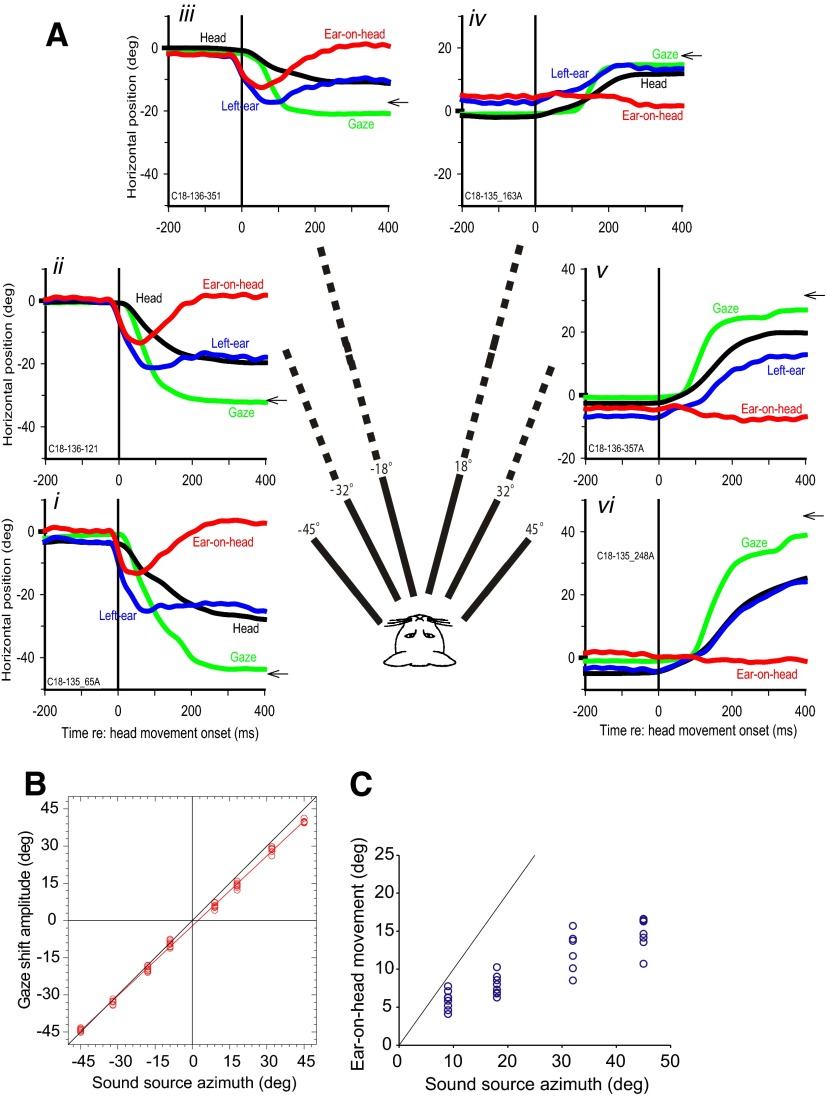
Results are based on data from four animals. Figure 1A shows gaze shifts, along with the corresponding movements of the head and the left pinna, made by a cat to auditory targets located at six different azimuths plotted relative to the onset of head movement for each trial. The cat made accurate gaze shifts to each auditory target as described earlier (Tollin et al. 2005); the slope of the linear regression line relating gaze shift amplitude to target azimuth was 0.94 (r2 = 0.998; P < 0.0001 n = 58 trials; Fig. 1B).
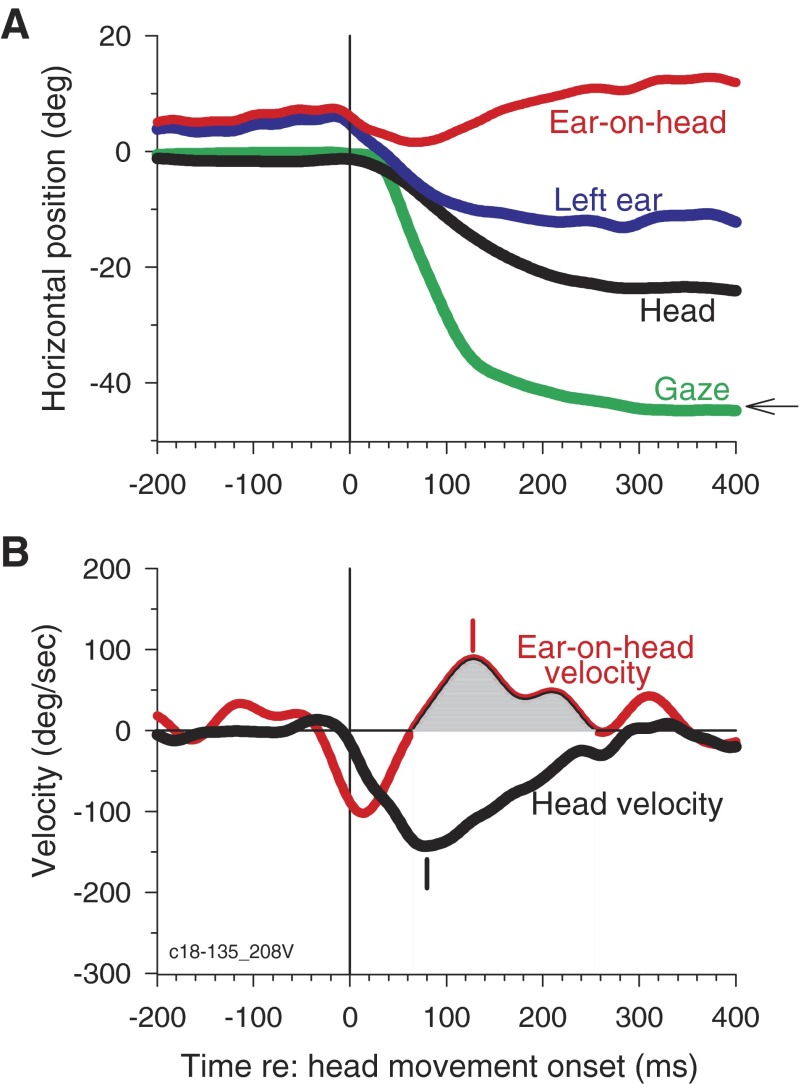
FIG. 1.
Gaze, head, pinna, and ear-on-head movements to 6 different sound sources. A: gaze (green), head (black), left pinna (blue), and ear-on-head (red) positions in space for 1 cat as a function of time with respect to the onset of the head movement for 6 acoustic targets along the horizontal plane (i–vi). Ear-on-head position was computed by subtracting the head from the pinna movement. The location of the target is indicated by the small arrow on the right axis. Ear-on-head movement compensates for the head movement and returns to a centered position on the head at the end of the gaze shift. B: final gaze shift amplitude as a function of sound source azimuth. Gaze shifts to auditory targets are accurate to within a few degrees. C: maximum ear-on-head movement amplitude for 4 ipsilateral targets as a function of sound source azimuth. The line is at unity gain and represents what would be expected if the pinna movements were identical to the target position. Pinna movements are goal directed though they undershoot the target considerably.
Active VAR
The interesting findings from this study concern the sound-evoked movements of the pinnae. First, the pinna ipsilateral to the sound source (e.g., Figures 1A, i–iii) made rapid “pinna saccades” toward the target with very short latency, usually before the onsets of either gaze shift or head movement. Mean ± SD of pinnae movement latency for the ipsilateral targets was 33.7 ± 11.5 ms (n = 179), whereas the mean head latency (49.5 ± 25.5 ms, n = 179) was significantly slower (paired t178 = −8.64, P < 0.0001). The pinna moved on average 15.8 ms before the onset of the head. Second, the contralateral pinna (e.g., Figs 1A, iv–vi) typically did not move on the head toward the source, but rather was carried by the head so that head and the pinna movement were nearly identical in magnitude and latency. Third, for ipsilateral sources, pinna movement was goal directed (Fig. 1C) in that the magnitude and direction of movements of the ear-on-head varied directly with source location, although it undershot the target. Note, however, that in Fig. 1A, the pinna and the head ultimately are oriented at approximately the same location in space toward the target. All of these results are in agreement with our previous study of pinna movements to sounds in head-restrained cats (Populin and Yin 1998a). These general characteristics of pinna movement were seen in all of the other cats in this study to varying degrees. Thus goal-directed sound-evoked pinna movements are also present in the more ethologically natural, head unrestrained condition and are not affected by an impending head movement.
The focus of this paper is on the pinna movements to ipsilateral acoustic targets that occurred after the head began to move. For these targets, the pinna (Fig. 1A, blue traces) reached its final position in space by 60–80 ms after head movement onset and maintained its position even though the head continued to move toward the target. Because the ear is positioned on the head, for the pinna to remain at a fixed location in space despite movements of the head, it must counter-rotate on the head with the same, but opposite velocity as the head movement. This compensatory mechanism for the pinna on the head is similar in function to the VOR, and we will refer to it as the VAR for convenience. In later sections, we will provide additional evidence in support of a vestibular origin. We refer to the compensatory pinna movements in Fig. 1 as the active VAR because they are triggered by the cats actively moving their heads, whereas later we will examine the VAR following passive head movements. Figure 1A shows the VAR by plotting the ear-on-head position. For all ipsilateral targets, the ear-on-head position traces (red) in Fig. 1A, i–iii, shows that the pinna moved before the head and counter-rotated as the head continues to move. For the contralateral targets (Fig. 1A, iv–vi), pinna movements were quite different: there was no pinna saccade toward the target, but instead the pinna movement matched the head movement (ear-on-head movement is flat) showing that in these cases the pinna was passively moved by the head.
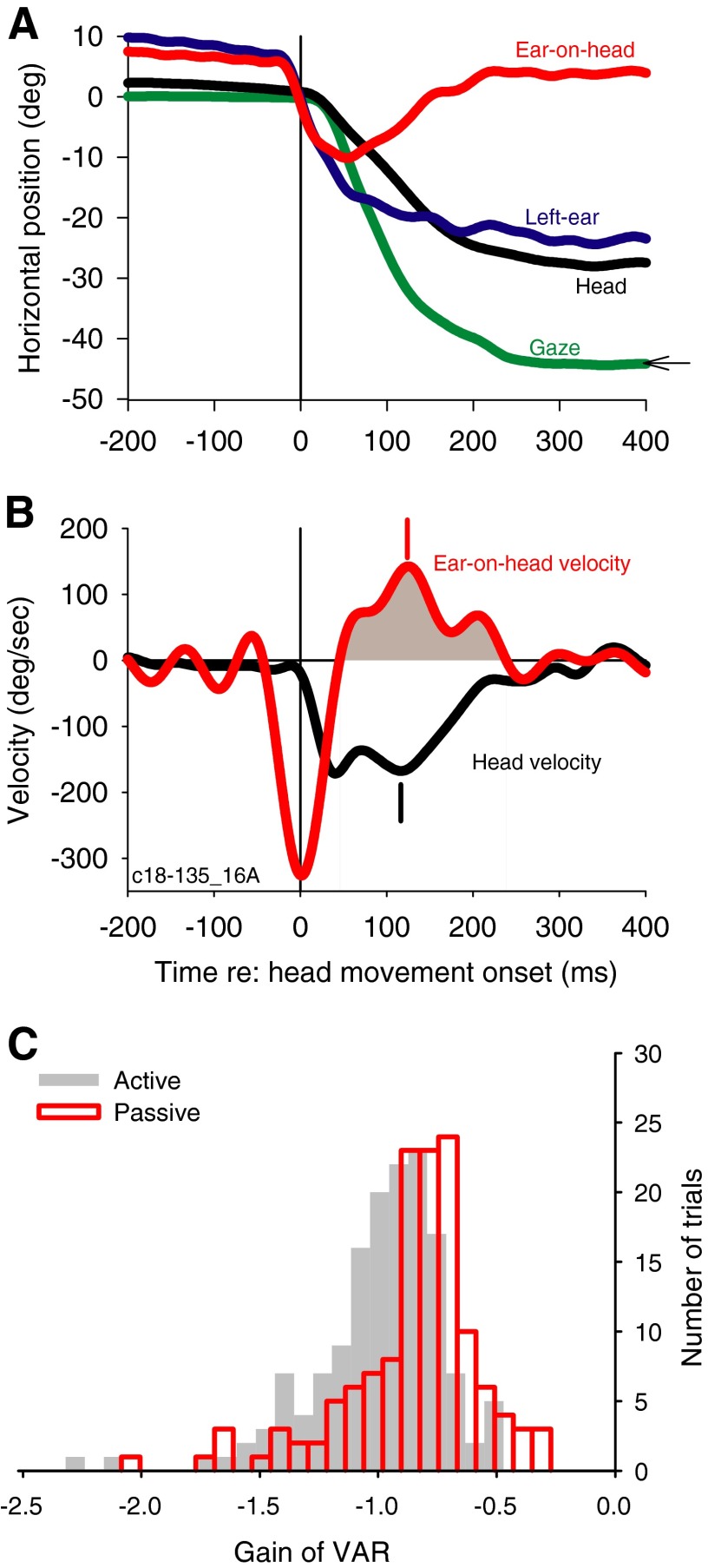
To link the compensatory pinna movements to the kinematics of the head movements, we calculated the gain of the VAR with a method similar to that typically used to study the VOR (Huterer and Cullen 2002). Figure 2A shows similar plots as Fig. 1A for a different trial from the same cat, whereas Fig. 2B shows the instantaneous velocities of the head and the ear-on-head movements. During the VAR, the pinna moves in the opposite direction to the head (Fig 2B, shaded). If pinna rotation completely compensated for head rotation, the ratio of the ear-on-head (Fig. 2B, red) and the head velocity (Fig. 2B, black) should be −1.0. To estimate the VAR, we computed the VAR gain, which is the ratio of the peak ear-on-head and peak head velocities (142.5/−167.7 deg/s = −0.85); peak velocities occurred at 124 and 116 ms, respectively (Fig 2B, vertical dashes).
FIG. 2.
Pinna movements compensate for head movements via the vestibulo-auricular reflex (VAR). A: head (black), left ear (blue), gaze (green), and ear-on-head (red) traces for a different trial from the same cat as shown in Fig. 1 to an acoustic target at −45°. B: instantaneous velocities of the ear-on-head (red) and the head (black) for the trial shown in A. All times are plotted relative to the onset of head movement. C: histograms of the active (gray bars) and passive VAR gains (red). Active VAR was computed by the ratio of peak ear-on-head and head velocities while passive VAR gain was computed by the ratio of mean of ear-on-head and mean head velocity during the time of the VAR. Gain of −1.0 indicates that the pinna counter-rotates with an equal but opposite velocity as the head. Mean active VAR is −1.03 ± 0.28 (n = 149) and mean passive VAR is −0.89 ± 0.29 (n = 135).
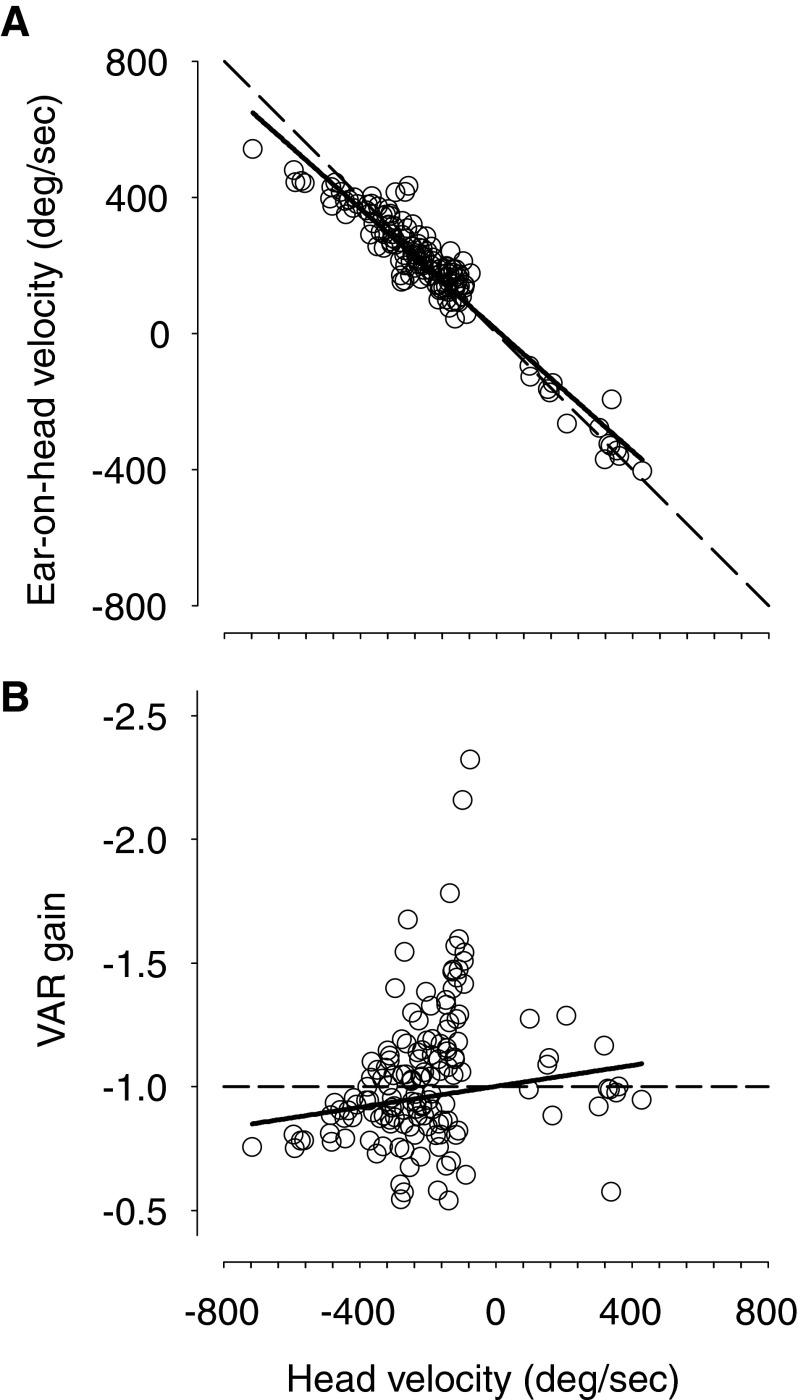
Across the four animals, the gain of the VAR for ipsilateral targets had a value of approximately −1.0 during the compensatory movement of the ear on the head (Fig. 2B, shaded). On average, the peak ear-on-head velocity occurred 9.7 ± 29.2 ms (n = 149) after the peak head velocity. Hence, the pinna movements seem to compensate for the head movement. To summarize active VARs across all subjects for different ipsilateral head movements, Fig. 2C shows a histogram of the active VAR gains (gray) with a mean of −1.03 ± 0.28 (SD; n = 149). The theoretical VAR gain of −1.0 falls within the 99% CI (−0.97 to −1.09) of the distribution of active VAR gains. Figure 3A shows that the peak ear-on-head velocity is highly correlated with peak head velocity (r = −0.94, P < 0.0001; slope = −0.89, n = 149), and VAR gain is largely independent of the head velocity (r = 0.16, P = 0.0506; Fig. 3B); the slope of the regression was very small [−0.0002 (deg/s)−1], although still significantly different from 0.0 (P = 0.6028), and VAR gain is reduced somewhat for very large head velocities. The active VAR seems to operate in a similar way as the classical VOR.
FIG. 3.
A: peak ear-on-head velocity as a function of peak head velocity, which varies with target eccentricity. Regardless of the eccentricity of the target, the counter-rotation of the pinna-on-head nearly completely compensates for the ongoing head movements resulting in a stable pinna position in space (Fig. 1A). For each trial and for each target, the gain of the VAR is near −1.0. Slope of regression line (solid line) is −0.89 and correlation index is −0.94, n = 149. The theoretical VAR gain of −1.0 is shown by the dashed line. Most of the peak ear-on-head velocity values are positive because most of our measurements were made in the left ear. B: VAR gain plotted as a function of peak head velocity. The active VAR gain is largely independent of head velocity [slope of −0.0002 (deg/s)−1].
Unlike the VOR, however, the counter-rotation of the ear on the head for active VAR is not seen for targets on the side contralateral to the ear, at least not for the angles of deviation of the head tested here (e.g., 18° and larger in Fig. 1A). An analysis of head velocity and ear-on-head velocity similar to that shown in Fig. 2B (not shown) indicated the ear-on-head velocity hovers near 0.0 for contralateral targets.
Passive VAR
Although these data are consistent with a vestibular origin for the VAR, we also considered mechanisms other than vestibular. Given the short latency of the reflex that would rule out proprioceptive feedback, the most likely alternative is that the VAR results from an efference copy of the head movement signal that is also sent to the pinna muscles to compensate for the impending head movement. To address this possibility, we examined the VAR while passively rotating the cats during active sound localization. During passive head rotation, there is no head movement command and therefore no possible efference copy to the ears provided the head moves with the platform.
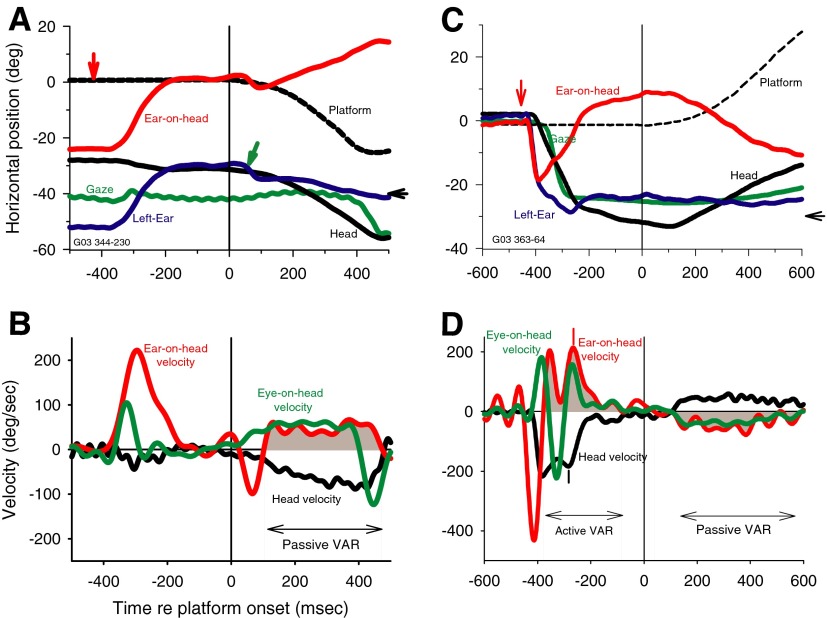
Figure 4 shows results from one cat for two trials plotted as in Fig. 2, in which we rotated the animal passively to the left (Fig. 4, A and B) and to the right (Fig. 4, C and D) during fixation of an auditory target. For the trials shown in Fig. 4, A and B, when the acoustic target was turned on (vertical red arrow) at −40°, by chance, the cat's gaze happened to be looking almost at the target and the head and ears were also turned to the left so there was little head movement until the platform moved at t = 0. In Fig. 4A, the platform rotation was to the left, and there is a small deflection (green arrow) in the pinna position in response to the noise of the motor onset. After a short delay, the head moved passively with the platform as reflected in the approximate parallel traces of platform and head movement beginning at about +100 ms. During the leftward head movement, both the gaze and ear position remained largely constant, because both the VOR and VAR were active during the passive head movement. The resemblance of the VOR and VAR is reflected in the similarities of the ear-on-head velocity (Fig. 4B, red) and eye-on-head velocity (Fig. 4B, green) traces during the passive head movement. The relatively slow and steady head movement caused by the rotating platform resulted in ill-defined peak velocities of head and ear-on-head that precluded using estimates of peak ear-on-head and head velocities to determine the gain of the VAR. Instead, we computed the passive VAR gain as the ratio of mean ear-on-head velocity/mean head velocity during the VAR (shaded). In Fig. 4B, the passive VAR gain = 45.2/−61.9 = −0.73.
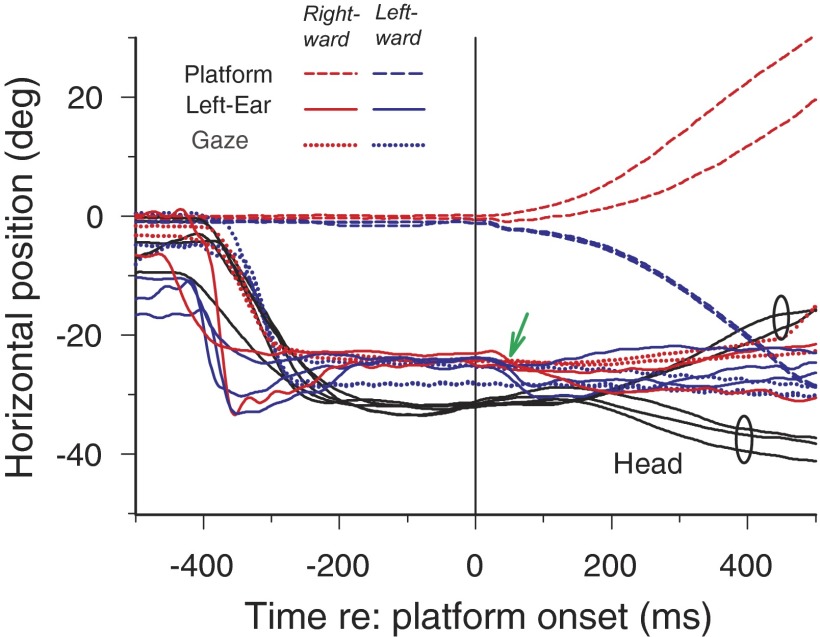
FIG. 4.
The VAR is maintained during passive head rotation. A: movements of the head (black), left ear (blue), ear-on-head (red), gaze (green), and the platform supporting the cat (dashed black) during a trial in which an acoustic target was turned on at −447 ms (red vertical arrow) and remains on for 1,000 ms at −40° (black horizontal arrow on the right axis). All traces are synchronized to the onset time of the motor that rotates the platform in the horizontal plane, in this case to the left. The small green arrow points to a small ear saccade to the left in response to the noise of the motor coming on. B: velocity traces for the head (black), ear-on-head (red), and eye-on-head (green) for the same movements in a. The leftward head movement evokes a compensatory rightward ear movement that keeps the ear roughly stable in space and shows the passive VAR (shaded). The phase of passive VAR is denoted by the shaded areas under the ear-on-head trace and by the double-headed arrow and has a gain = 45.2/−61.9 = −0.73. C and D: another trial with platform movement in the opposite direction. Same format as in A and B. The head, gaze, and ears are near 0° when the target at −30° came on so orientation to the leftward target evokes an active VAR (left shaded region) as well as a passive VAR (right shaded). Small vertical tick marks indicate the peak ear-on-head and peak head velocity values used to estimate the active VAR gain (213.9/−184 deg/s = −1.16; at −267 and −285 ms, respectively). The passive VAR gain (−39.0/39.0) = −1.0.
Figure 4, C and D, shows a trial in which the platform was moved to the right rather than to the left. In this trial, as in most of our experiments of passive VAR, there are two distinct phases: an active VAR while the cat oriented its eyes, ears, and head to fixate the acoustic target and a passive VAR during platform rotation. Figure 4C shows that the active phase of the VAR is similar to those shown earlier in Figs. 1 and 2. When the acoustic stimulus was turned on (vertical red arrow) at −30°, the pinna (blue) and head (black) were both oriented near 0°. The pinna responded to the target first, making a quick leftward movement toward the target with a latency of 24 ms, followed 16 ms later with a leftward head movement (black), which triggered the active VAR and the counter-rotation of the ear-on-head (Fig. 4D, shaded). The gain of the active VAR in Fig. 4D was −1.16 and peak ear-on-head velocity occurred 18 ms after peak head velocity (vertical red and black lines, respectively). The pinna maintained its new position during the leftward head movement by counter-rotation of the activeVAR (shaded). When the platform moved to the right, both the ear and gaze position remained stable in space because of the VAR and VOR, respectively. Given the results in Fig. 1A, which show a unilateral VAR response (i.e., no VAR response when the head rotated to the side contralateral to the ear), it was a bit surprising to see a vigorous VAR during the passive movement in the contralateral direction. Note that the bilateral response seen with passive rotation involves smaller amplitude head movements than those shown in Fig. 1.
Additional evidence that the VAR is present with passive head movement in both directions is shown in Fig. 5, which superimposes five additional trials with platform movement in both directions. As in Fig. 4, C and D, all five trials show the characteristics of the active VAR after the onset of the auditory target at approximately −400 ms. The head, eyes, and ears are all directed toward the target when the platform begins to move at 0 ms. In some trials, there is a small leftward movement of the pinna (small green arrow) after the motor turns on in response to the noise of the motor, which is situated to the cat's left. Three trials with passive movement to the left and two to the right are plotted. The trials corresponding to leftward and rightward passive rotations (2 ellipses) can be easily distinguished by the divergence of the head traces starting at ∼160 ms, whereas the ear and gaze traces in the two directions do not diverge as much as the head. Thus in these trials, the vestibulo-colic reflex was relatively small after the onset of the motor rotation, i.e., the head was rotated passively by the motor.
FIG. 5.
Five additional superimposed trials with the target at −30° and with rotation of the platform both to the right and left. During the passive phase of the VAR, the head movement traces diverge (small ellipses), corresponding to the 2 directions of platform movement, but the ear (blue) and gaze (green) positions remain relatively stable as reflected in the lack of divergence of the those traces.
Figure 2C shows the distribution of passive VAR gains (red) across the three cats for which passive head rotation data were collected. The mean passive VAR gain ± SD was −0.89 ± 0.29 (n = 135), which compares with the active VAR gain computed above of −1.03. Although the magnitude of the difference between the active and passive VAR gains was small (0.14, or 13.4%), it was significant (unpaired t283 = 4. 06, P < 0.0001). Note that the metrics used to compute the gains of the active VAR and passive VAR were not the same.
Another way to quantify the VAR is to measure the compensatory movement of the ear relative to the head movement rather than using velocities. For the data shown in Figs. 4 and 5, we measured the range of head, ear, and eye movement from maximum to minimum over the 100- to 400-ms time period of the seven trials to avoid the quick pinna saccade toward the motor (Figs. 4A and 5). The mean range of head movement was 9.74°, whereas the mean range of ear and eye movement was 3.0 and 2.0°, respectively. This corresponds to a ratio of pinna movement/head movement for a VAR gain of −0.69 [(3.0 − 9.74)/9.74] compared with the ratio of eye movement/head movement for the VOR gain of −0.79. Thus the VAR and VOR stabilize both the ear and eye, respectively, despite passive movements of the head.
It is also conceivable that the VAR results because the auditory system tracks the spatial position of the sound source, and the information is fed back to the pinna musculature to maintain pinna orientation toward the source during head movements. The rapidity and smoothness, or nonsaccadic nature, of the VAR strongly argues against this possibility: peak ear-on-head velocity lags peak head velocity on average by 9.7 ms (n = 149), which is much too short a time for such feedback to be operative, and the pinna movements during the VAR do not seem saccade like. Because it seems that there is no smooth pursuit of auditory targets (Boucher et al. 2004), it is unlikely that the pinna tracks the acoustic target. Another finding arguing against this hypothesis is the observation that the VAR was also present in cats making gaze shifts to purely visual stimuli, because the visual latencies are even longer than acoustic latencies. Figure 6 shows results for one trial in the same format at Fig. 2 to a visual target at −45°. Figure 6A shows that the pinna reached its final position by ∼100 ms before the head reached its target. The pinna maintained this position by counter-rotating on the head from 100 to ∼300 ms while the head completed its movement (Fig. 6B, shaded). In Fig. 6, the gain of the VAR was −0.62, which is on the low end of the gains observed for visual or auditory targets. Although the magnitude of the pinna movement toward comparable source locations was approximately one third less for the visual targets than for the auditory (see Populin and Yin 1998b), the gains of the VAR still hovered around −1.0. In one cat, for visual targets ipsilateral to the pinna being monitored, the mean VAR gain ± SD was −0.87 ± 0.25 (n = 8). These data show that the VAR was still present for visual gaze shifts, ruling out audiokinetic, or acoustic tracking, influences. The mean and SD of the latency of pinna movements to ipsilateral visual targets was 92.7 ± 38.5 ms (n = 65), whereas the head moved significantly (paired t64 = −2.94, P = 0.0046) later with a latency of 104.2 ± 34.6 ms (n = 65). On average, the head moved 11.5 ms after the pinna.
FIG. 6.
The vestibulo-auricular reflex is not caused by audiokinetic tracking of an acoustic target. A: gaze, head, left pinna, and ear-on-head movements to a visual target located at (−45°,0°). Ear-on-head position shows that the pinna moves at virtually the same time but with a greater velocity than the head. B: compensatory pinna-on-head movements show that the VAR is present also for visual stimuli with a gain in this trial of −0.62.
DISCUSSION
These results show evidence for a newly recognized reflex, the VAR, which is in many ways similar to the well-known VOR. Like the VOR, the VAR seems to compensate for movements of the head by counter-rotation of the pinna on the head. The presence of the VAR even for passive head movement rules out efference copy as the source of the signal driving it, and its rapidity suggest that it is vestibular in origin.
Aside from humans, most mammals can and do move their pinnae individually and quite extensively. In the cat, a nocturnal predator with outstanding sound localization capabilities (May and Huang 1996; Tollin et al. 2005), >22 muscles are dedicated to moving the pinnae (Crouch 1969; Populin and Yin 1995). In contrast, the position of each eye is controlled by only six muscles. Although there is little direct evidence on the function of mobile pinnae, behavioral and physiological studies have suggested possible advantages of pinna movements for sound localization. First, the pinnae orient with extraordinarily short latency toward sounds (Fig. 1A), allowing animals to attend to those sounds without looking directly at the source, even before the head can move (Heffner and Heffner 1982; Middlebrooks and Knudsen 1987; Populin and Yin 1998b). Second, movements of the pinnae systematically change the acoustical cues to sound source location (Calford and Pettigrew 1984; Middlebrooks and Knudsen 1987; Young et al. 1996). Physiologically, pinna movements can systematically change the responses of neurons that are believed to be involved in sound localization, which is likely a consequence of the changes in the acoustical cues (Middlebrooks and Knudsen 1987; Sun and Jen 1987).
Interestingly, stereotyped pinna movements are evoked regardless of the modality of the stimulus, under both restrained (Populin and Yin 1998b) and unrestrained (Figs. 1A, 2A, and 6) head conditions. Although we expect the pinnae to orient to acoustic stimuli, their movements to strictly visual stimuli probably reflects the fact that most stimuli that the cat would ordinarily encounter would have both a visual and acoustic component. The major difference between the responses to the two modalities lies in the latency of the response: pinna movements to acoustic targets are much shorter than to visual targets (Populin and Yin 1998b).
Although the neural mechanisms responsible for the VAR are unknown, evidence suggests that, like the VOR, it uses a three neuron arc consisting of the semicircular canal input to the vestibular nucleus and a projection to the ipsilateral facial nucleus (Shaw and Baker 1983), which contains the motoneurons that drive pinna movements (Henkel 1981; Kume et al. 1978; May et al. 1990; Populin and Yin 1995). In addition to our observations here, there is experimental evidence that inputs from the vestibular system can give rise to compensatory pinna movements. Electrical stimulation of the vestibular nerves produces pinna movements parallel to the plane of the canal whose nerve was stimulated and compensatory to the movements of the head that were induced by the stimulation (Suzuki and Cohen 1964). In other words, electrical stimulation of the canal nerves can induce VAR-like pinna movements. Moreover, Schaefer et al. (1971) showed that a postrotational nystagmus could be produced in the pinna of rabbits that both mimicked and was in phase with the traditional eye nystagmus. The eye nystagmus relies on the vestibular system (Lorente de No 1933), so it is likely that the pinna nystagmus also results from similar mechanisms. Our experiments showed that the VAR was present for gaze shifts to visual stimuli, which rules out audiokinetic influences. Schaefer et al. (1971) also indicated that during the initial moment of acceleration of passive rotation in blindfolded rabbits, the pinnae made a compensatory rotation against the direction of rotation. In agreement with their finding, and ruling out an efference copy of the head movement as the driver of the VAR, the VAR was apparent during passive rotation of the cat (Fig. 4).
The relative latencies of the peak head and ear-on-head velocities during the active VAR also implicate the vestibular system in generating the VAR. The peak ear-on-head velocity lagged the peak head velocity by ∼10 ms, averaged across animals. This value is comparable to the time lags found in traditional VOR, where the peak eye velocity lags the peak head velocity (induced by passive rotation) by ∼7–14 ms in monkeys (Lisberger 1984). Although we do not know the latency of auditory following by the pinna, it is known that the latency of visual following is on the order of 74 ms (Gellman et al. 1990). Sound-evoked pinna movements in head-fixed cats average ∼25–35 ms (Populin and Yin 1998b). Purely auditory following by pinna movements would be expected to have at least this latency and possibly larger. Moreover, there is no evidence for visual smooth pursuit of auditory targets (Boucher et al. 2004).
Although the VAR shares many characteristics of the well-known VOR, there are clearly some differences. Presumably both reflexes arise because of the directional nature of the peripheral end organs: visual acuity is maximal at the fovea, and the pinna has an acoustic axis where the gain is highest (Calford and Pettigrew 1984; Middlebrooks and Knudsen 1987). In addition, both the eye and ear are movable, yet they are themselves located on a movable head; therefore the need for a VOR and VAR arises to compensate for the head movements by counter-rotating the eyes and ears, respectively. However, a striking difference between eye and ear movements is that the two eyes move conjugately, whereas the ears move independently. Thus the VOR results in conjugate movements of both eyes in both directions, whereas the active VAR is largely only for the ipsilateral ear and for ipsiversive movement, i.e., the left ear shows an active VAR during an active leftward head turn but not for a rightward one (Fig. 1A). However, the results from the passive head turn experiment show that the situation is not quite that simple. When the head is pointing in the direction of the sound source, as it is during the latter phase of the active VAR (Fig. 4C), the VAR is activated for passive head rotation in both directions. Presumably, this is because the sound source is closer to the midline than the more peripheral (18°) location of the central targets used in Fig. 1A. The anatomical projections of the vestibular nuclei to the abducens and facial nuclei reflect a possible directional bias. Specifically, the vestibular nuclei project to the contralateral abducens nucleus (Lorente de No 1933) to turn the contralateral eye laterally (the ipsilateral medial rectus motoneurons are activated by the crossed projection of the internuclear abducens neurons), whereas the vestibular nuclei project primarily to the ipsilateral facial nucleus (Shaw and Baker 1983) to move the ipsilateral ear laterally.
The short latency of pinna movements relative to head movements facilitates the analysis of the VAR compared with the VOR where the head and eye latencies are usually closely matched and where the action of the VOR during a gaze shift is controversial. When the head and eyes begin to move at about the same time during a gaze shift, the VOR would be counterproductive because it is designed to keep the eyes stabilized in space. The early work of Bizzi and colleagues (Bizzi et al. 1971; Morasso et al. 1973) reported that gaze saccades in the monkey were similar under head free or head restrained conditions and preceded head movements. They suggested that the VOR operated at full gain during gaze shifts in their “linear summation hypothesis.” Others have argued that the VOR is turned off or attenuated during a gaze shift (Laurutis and Robinson 1986; Pare and Guitton 1998; Pelisson et al. 1988; Roy and Cullen 1998; Sparks 1999). The differences in these results are most likely caused by the relative timing of head and eye movements. When the gaze latency does not lead head latency, the VOR would oppose the gaze shift if it were not attenuated. In our experiments during active head movements, the pinna consistently moved before the head and reached its final position well before the head movement ended, so the counter-rotation of the pinna on the head was clear (Figs. 1–3). Thus, although the action of the VOR is counterproductive during a gaze shift, there is no such ambiguity for the VAR.
Our experiments with the passive whole body rotation showed that, unlike the VOR, the VAR is fully active during both the active and the passive phases of the trial. The presence of the VAR during the passive component strongly implicates the vestibular system as the origin of the reflex and eliminates the possibility that an efference copy of the head movement command drives the pinnae. Because the head is moving approximately parallel to the platform, i.e., the head is not turning on the neck, it also suggests that proprioceptive signals from the neck are unlikely to be involved.
We believe that pinna movements are an important part of the mammalian acoustic orientation response. However, at present, it is not known why some animals, like cats, have independently mobile pinnae that can be moved extensively and in complex ways, whereas others, like humans, do not. The usual explanation is that the pinnae act as acoustic “antennae” and by positioning them independently, animals are able to squelch noisy or uninteresting sounds while boosting sounds of interest. That is, the pinnae allow the animal to maximize the signal-to-noise ratio. However, there is no experimental evidence for this. We propose here that compensatory movements like the VAR allow the auditory worlds of animals to be stabilized during head movements in the same way that the VOR stabilizes the visual world. In this capacity, the VAR may function to keep the spatial direction of an animal's attention fixated at a particular acoustic target of interest regardless of movements of the head. Like the classical VOR, the VAR would play an important function to stabilize the auditory world of predators like the cat, despite the rapid movements of the head that occur during the pursuit of prey.
GRANTS
This work was supported by National Institutes of Deafness and other Communicative Disorders Grants DC-00116, DC-07177, and DC-02840 to T.C.T. Yin and an Individual National Research Service Award (NIDCD DC-000376) to D. J. Tollin.
Acknowledgments
We thank R. Kochhar and J. Sekulski for designing and implementing the analysis software and the data collection software, respectively.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Bizzi et al. 1971.Bizzi E, Kalil RE, Tagliasco V. Eye-head coordination in monkeys: evidence for centrally patterned organization. Science 173: 452–454, 1971. [DOI] [PubMed] [Google Scholar]
- Boucher et al. 2004.Boucher L, Lee A, Cohen YE, Hughes HC. Ocular tracking as a measure of auditory motion perception. J Physiol (Paris) 98: 235–248, 2004. [DOI] [PubMed] [Google Scholar]
- Calford and Pettigrew 1984.Calford MB, Pettigrew JD. Frequency dependence of directional amplification at the cat's pinna. Hear Res 14: 13–19, 1984. [DOI] [PubMed] [Google Scholar]
- Crouch 1969.Crouch JE Text-Atlas of Cat Anatomy. Philadelphia, PA: Lea and Febiger, 1969.
- du Lac et al. 1995.du Lac S, Raymond JL, Sejnowski TJ, Lisberger SG. Learning and memory in the vestibulo-ocular reflex. Annu Rev Neurosci 18: 409–441, 1995. [DOI] [PubMed] [Google Scholar]
- Frens and van Opstal 1994.Frens MA, van Opstal AJ. A quantitative study of auditory-evoked saccadic eye movements in two dimensions. Exp Brain Res 107: 103–117, 1994. [DOI] [PubMed] [Google Scholar]
- Gellman et al. 1990.Gellman RS, Carl JR, Miles FA. Short latency ocular following responses in humans. Vis Neurosci 5: 107–122, 1990. [DOI] [PubMed] [Google Scholar]
- Hays 1988.Hays WL Statistics (4th ed.). Fort Worth, TX: Holt, Rinehart and Winston, 1988.
- Heffner and Heffner 1982.Heffner RS, Heffner HE. Hearing in the elephant (Elephas maximus): absolute sensitivity, frequency discrimination, and sound localization. J Comp Physiol Psychol 96: 926–944, 1982. [PubMed] [Google Scholar]
- Heffner and Heffner 1992.Heffner RS, Heffner HE. Visual factors in sound localization in mammals. J Comp Neurol 317: 219–232, 1992. [DOI] [PubMed] [Google Scholar]
- Henkel 1981.Henkel CK Afferent sources of a lateral midbrain tegmental zone associated with the pinnae in the cat as mapped by retrograde transport of horseradish peroxidase. J Comp Neurol 203: 213–226, 1981. [DOI] [PubMed] [Google Scholar]
- Huterer and Cullen 2002.Huterer M, Cullen KE. Vestibuloocular reflex dynamics during high-frequency and high-acceleration rotations of the head on body in rhesus monkey. J Neurophysiol 88: 13–28, 2002. [DOI] [PubMed] [Google Scholar]
- Ito 1982.Ito M Cerebellar control of the vestibulo-ocular reflex –around the flocculus hypothesis. Annu Rev Neurosci 5: 275–296, 1982. [DOI] [PubMed] [Google Scholar]
- Knudsen 1981.Knudsen E The hearing of the barn owl. Sci Am 245: 82–91, 1981. [Google Scholar]
- Kume et al. 1978.Kume M, Uemura M, Matsuda K, Matsushima R, Mizuno N. Topographical representation of peripheral branches of the facial nucleus within the facial nucleus: a HRP study in the cat. Neurosci Lett 8: 5–8, 1978. [DOI] [PubMed] [Google Scholar]
- Laurutis and Robinson 1986.Laurutis VP, Robinson DA. The vestibulo-ocular reflex during human saccadic eye movements. J Physiol 373: 209–233, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger 1984.Lisberger SG The latency of pathways containing the site of motor learning in the monkey vestibulo-ocular reflex. Science 225: 74–76, 1984. [DOI] [PubMed] [Google Scholar]
- Lorente de No 1933.Lorente de No R The vestibulo-ocular reflex arc. Arch Neurol Psychiatry Chicago 33: 245–291, 1933. [Google Scholar]
- May 1996.May BJ, Huang AY. Sound orientation behavior in cats. I. Localization of broadband noise. J Acoust Soc Am 100: 1059–1069, 1996. [DOI] [PubMed] [Google Scholar]
- May et al. 1990.May PJ, Vidal P-P, Baker R. Synaptic organization of tectal-facial pathways in the cat. II. Synaptic potentials following midbrain tegmentum stimulation. J Neurophysiol 64: 381–402, 1990. [DOI] [PubMed] [Google Scholar]
- Middlebrooks and Knudsen 1987.Middlebrooks JC, Knudsen EI. Changes in external ear position modify the spatial tuning of auditory units in the cat's superior colliculus. J Neurophysiol 57: 757–781, 1987. [DOI] [PubMed] [Google Scholar]
- Morasso et al. 1973.Morasso P, Bizzi E, Dichgans J. Adjustment of saccade characteristics during head movements. Exp Brain Res 16: 492–500, 1973. [DOI] [PubMed] [Google Scholar]
- Pare and Guitton 1998.Pare M, Guitton D. Brain stem omnipause neurons and the control of combined eye-head gaze saccades in the alert cat. J Neurophysiol 79: 3060–3076, 1998. [DOI] [PubMed] [Google Scholar]
- Pelisson et al. 1988.Pelisson D, Prablanc C, Urquizar C. Vestibuloocular reflex inhibition and gaze saccade control characteristics during eye-head orientation in humans. J Neurophysiol 59: 997–1013, 1988. [DOI] [PubMed] [Google Scholar]
- Populin and Yin 1995.Populin LC, Yin TCT. Topographical organization of the motoneuron pools that innervate the muscles of the pinna of the cat. J Comp Neurol 363: 600–614, 1995. [DOI] [PubMed] [Google Scholar]
- Populin and Yin 1998a.Populin LC, Yin TCT. Behavioral studies of sound localization in the cat. J Neurosci 18: 2147–2160, 1998a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Populin and Yin 1998b.Populin LC, Yin TCT. Pinna movements of the cat during sound localization. J Neurosci 18: 4233–4243, 1998b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy and Cullen 1998.Roy JE, Cullen KE. A neural correlate for vestibulo-ocular reflex suppression during voluntary eye-head gaze shifts. Nat Neurosci 1: 404–410, 1998. [DOI] [PubMed] [Google Scholar]
- Schaefer et al. 1971.Schaefer K-P, Meyer DL, Schott D. Optic and vestibular influences on ear movements. Brain Behav Evol 4: 323–333, 1971. [DOI] [PubMed] [Google Scholar]
- Shaw and Baker 1983.Shaw MD, Baker R. Direct projections from vestibular nuclei to facial nucleus in cats. J Neurophysiol 50: 1265–1280, 1983. [DOI] [PubMed] [Google Scholar]
- Sparks 1999.Sparks DL Conceptual issues related to the role of the superior colliculus in the control of gaze. Curr Opin Neurobiol 9: 698–707, 1999. [DOI] [PubMed] [Google Scholar]
- Sun and Jen 1987.Sun XD, Jen PH. Pinna position affects the auditory space representation in the inferior colliculus of the FM bat, Eptesicus fuscus. Hear Res 27: 207–219, 1987. [DOI] [PubMed] [Google Scholar]
- Suzuki and Cohen 1964.Suzuki J-I, Cohen B. Head, eye, body and limb movements from semicircular canal nerves. Exp Neurol 10: 393–405, 1964. [DOI] [PubMed] [Google Scholar]
- Tollin et al. 2005.Tollin DJ, Populin LC, Moore JM, Ruhland JL, Yin TCT. Sound localization performance in the cat: the effect of restraining the head. J Neurophysiol 93: 1223–1234, 2005. [DOI] [PubMed] [Google Scholar]
- Vliegen et al. 2004.Vliegen J, Van Grootel TJ, van Opstal AJ. Dynamic sound localization during rapid eye-head gaze shifts. J Neurosci 24: 9291–9302, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young et al. 1996.Young ED, Rice JJ, Tong SC. Effects of pinna position on head-related transfer functions in the cat. J Acoust Soc Am 99: 1–13, 1996. [DOI] [PubMed] [Google Scholar]