Abstract
The pharyngeal swallow can be elicited as an isolated event but, in normal animals, it occurs within the context of rhythmic tongue and jaw movement (RTJM). The response includes activation of the multifunctional geniohyoid muscle, which can either protract the hyoid or assist jaw opening; in conscious nonprimate mammals, two bursts of geniohyoid EMG activity (GHemg) occur in swallow cycles at times consistent with these two actions. However, during experimentally elicited pharyngeal swallows, GHemg classically occurs at the same time as hyoglossus and mylohyoid activity (short latency response) but, when the swallow is elicited in the decerebrate in the absence of RTJM, GHemg occurs later in the swallow (long latency response). We tested the hypothesis that it was not influences from higher centers but a brain stem mechanism, associated with RTJM, which caused GHemg to occur earlier in the swallow. In 38 decerebrate piglets, RTJM occurred sporadically in seven animals. Before RTJM, GHemg had a long latency, but, during RTJM, swallow related GHemg occurred synchronously with activity in hyoglossus and mylohyoid, early in the swallow. Both early and late responses were present during the changeover period. During this changeover period, duplicate electrodes in the geniohyoid could individually detect either the early or the late burst in the same swallow. This suggested that two sets of geniohyoid task units existed that were potentially active in the swallow and that they were differentially facilitated or inhibited depending on the presence or absence of rhythmic activity originating in the brain stem.
INTRODUCTION
Swallowing is an action that classically has oral, pharyngeal, and esophageal phases. The second of these phases is described as reflexive and, in decerebrate animals, can be elicited in isolation by delivering fluid to the posterior tongue (Miller and Sherrington 1916; Thexton et al. 2007). In the decerebrate piglet, these pharyngeal swallows can be elicited in isolation and are not usually associated with rhythmic tongue and jaw movement (RTJM), although rhythmic oral movements do occur in decerebrates and anencephalics (Bremer 1923; Hall 1833; Monnier and Willi 1953; Thexton et al. 1982). In contrast, in normal intact animals, swallowing occurs within the context of RTJM, the two activities being considered to be largely under cerebral control (Huang et al. 1989; Martin and Sessle 1993; Sumi 1977). The movements of the reflex pharyngeal swallow in the decerebrate animal are a subset of those occurring during the swallow cycles of intact animals (Thexton and Crompton 1998). However, how or to what extent the reflex swallow is changed when incorporated into rhythmic feeding movements is unknown. Furthermore, the role of functioning higher centers in the pharyngeal swallow is also unclear.
Swallowing involves the action of a large number of different muscles (Cunningham and Jones 2003). Some of these have different actions, depending on the activity in the other oropharyngeal muscles. The geniohyoid muscle, connecting the mandible to the hyoid, is of particular significance; if it contracts at a time when the jaw closing muscles are active, there is an upward/forward movement of the hyoid, i.e., of the base of the tongue. In contrast, when the jaw closing muscles are relatively inactive, geniohyoid contraction, acting in series with the sternohyoid muscle, will aid jaw opening. In the swallowing cycle of some intact animals (e.g., rat, cat, pig), the geniohyoid exhibits two bursts of EMG activity (Thexton and McGarrick 1994; Thexton et al. 1998; Travers and Jackson 1992). One of these bursts of activity occurs at or near minimum gape, consistent with producing hyoid protraction, whereas the other occurs later, in jaw opening (Thexton et al. 1998), consistent with it aiding jaw depression.
In the isolated pharyngeal swallow, i.e., in a reflex swallow not associated with RTJM, the pattern of EMG activity differs in a number of respects from the EMG pattern when rhythmic activity is not specifically excluded. The most noticeable difference is in the timing of geniohyoid activity. In a range of animals, Doty and Bosma (1956) reported that geniohyoid activity is part of a leading complex of muscle activities—the earliest EMG signals in the swallow that include the superior constrictor, palatopharyngeus, palatoglossus, posterior intrinsic muscles of the tongue, styloglossus, stylohyoid, and mylohyoid. In contrast, Thexton et al. (2007) reported that, in the isolated pharyngeal swallow in the decerebrate piglet, peak geniohyoid activity occurs ≥120 ms after the leading complex. Such a difference in timing is significant in a swallow cycle that, in the intact young pig, has a duration of the order of 280 ms (Thexton et al. 1998).
The time difference between the “early” activity pattern of the geniohyoid (Doty and Bosma 1956; Lang et al. 2002) and the “late” activity pattern (Thexton et al. 2007) could be caused by experimental differences. Studies reporting the short latency response were carried out primarily in animals with intact descending pathways from cerebral structures, whereas this condition was explicitly excluded in the study reporting the late geniohyoid response (Thexton et al. 2007). Another significant difference was that the study finding the longer latency response explicitly excluded any association with rhythmic activity. Rhythmic activity was not specifically excluded from the study by Doty and Bosma (1956), and it was either specifically or implicitly included in other studies (Lang et al. 2002; Thexton et al. 1998). Finally, the various studies of intact animals included different species and developmental stages.
To avoid these confounding factors, we compared swallowing data from infant decerebrate pigs exhibiting RTJM with published data from infant decerebrate pigs that had not exhibited RTJM. This allowed us to test the hypothesis that, during the pharyngeal swallows in the two states, the timing of EMG activity in most muscles was comparable. Because the comparison excluded higher centers as a factor, it allowed us to test the hypothesis that the timing of geniohyoid EMG activity was determined by brain stem mechanisms and that the timing differed as a function of the presence or absence of RTJM.
METHODS
The data for this study came from a retrospective search of an extended database that included both video-radiographic tapes and EMG records. These data had been previously obtained during a study of swallowing in decerebrate preweaning infant pigs. Use of these data avoided species, ontogenetic, or methodological differences when comparing data from decerebrates exhibiting RTJM with that from decerebrates not exhibiting RTJM. The data had been accumulated over ∼6 years and included data previously excluded from a study of the isolated pharyngeal swallow (Thexton et al. 2007) because of the presence of episodes of RTJM. All decerebrates were prepared in the same way, and all experiments used the same methodology. The exception was that, whereas there was always radiological evidence of swallowing occurring, absence of epiglottal markers meant that radiological evidence of epiglottal flexion was not always available as a time marker for the swallow (Crompton et al. 2008). The timing of the swallow was consequently derived from the EMG signals in the hyoglossus.
In all cases, the stimulus for swallowing was fluid delivery to the valleculae. Whether delivered directly by an intraoral catheter positioned under video-radiographic control (Thexton et al. 2007) or by an artificial teat, the fluid accumulated in the valleculae, thus providing the critical stimulus for the swallow (German et al. 1997, 2004).
Because of the possibility of eliciting the laryngeal chemoreflex (Lee et al. 1977) in the decerebrate piglet during feeding and because of the thermal sensitivity of that reflex (Curran et al. 1985), all decerebrates were routinely maintained at 1–2°C below normal body temperature. This minimized the possibility of liquid induced reflex laryngeal spasm that could otherwise be exacerbated by postdecerebration activation of temperature heat gain mechanisms (Connor and Crawford 1969; Liu 1973; Rothwell et al. 1983).
The videoradiographic tapes and EMG records of 38 decerebrate infant pigs were re-examined for any evidence of rhythmic jaw and tongue movements. The data for animals exhibiting RTJM were further examined for evidence of swallows occurring in close temporal association with rhythmic oral EMG activity, i.e., where the EMG activity of the swallow was continuous with immediately preceding or succeeding rhythmic EMG signals having the same periodicity as RTJM. A second condition was that the swallow and the rhythmic EMG activity occurred within the same bout of experimental feeding as radiographically evident and overt RTJM, normally within 30 s.
All animals were prepared according to one protocol (Thexton et al. 2007) (Harvard University IACUC 23-05). Briefly, under general anesthesia (O2 and halothane or isoflurane) and aseptic conditions, the cerebral hemispheres and diencephalic structures were removed by suction down to a midcollicular level (Fig. 1). Hemostasis was obtained with Gelfoam (Pharmacia and Upjohn). The animals were allowed to stabilize ≥12 h. Again under general anesthesia and aseptic conditions, the supra- and infra-hyoid muscles were exposed, and bipolar wire recording electrodes were implanted into selected muscles; patch electrodes (Loeb and Gans 1986) were sutured onto the ventral surface of the mylohyoid muscle.
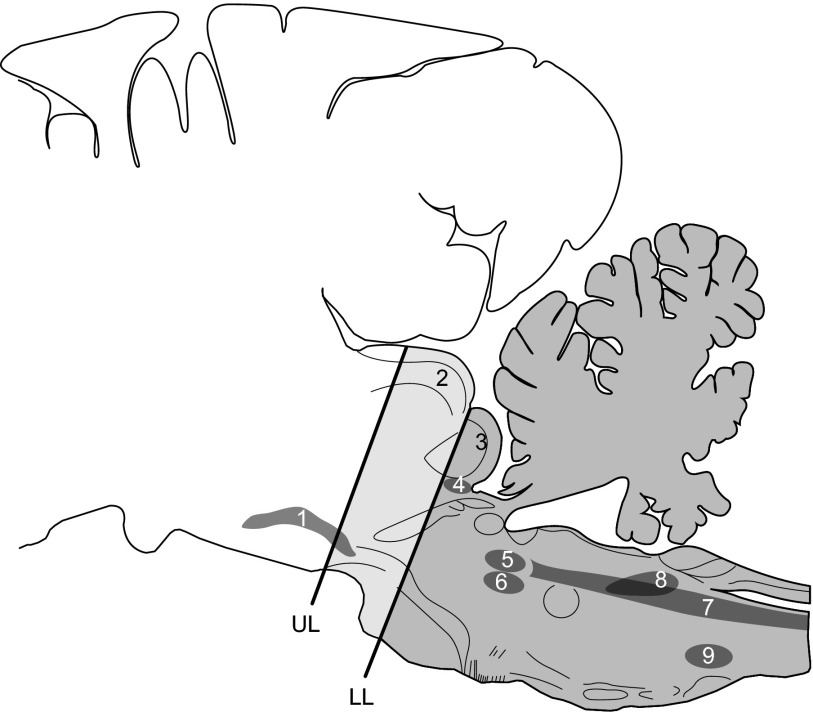
FIG. 1.
Sagittal map of the pig brain (modified L5.6 from Felix et al. 1997 to include selected structures from L5.0 to L7.4). The uppermost (UL) and lowermost (LL) limits of the levels of suction decerebration in different animals, as determined by the postmortem appearance of the sagittally sectioned heads, are shown on the diagram. 1, substantia nigra; 2, superior colliculus; 3, inferior colliculus; 4, trigeminal mesencephalic nucleus; 5, main trigeminal sensory nucleus; 6, trigeminal motor nucleus; 7, trigeminal spinal sensory nucleus; 8, nucleus of the tractus solitarius; 9, nucleus ambiguus.
As in our previous study of the decerebrate pig (Thexton et al. 2007), we followed a standard methodology for the bipolar wire electrode construction and insertion (Basmajian 1962). Duplicate electrodes in muscle were inserted to be laterally displaced from each other by 3–5 mm relative to the long axis of the muscle fibers. In the case of geniohyoid, one electrode was also inserted 3–4 mm deeper along the axis of the muscle than the other. Placement of electrodes was confirmed in postmortem dissections by a researcher who had not originally placed the electrodes.
After the final surgery, the animals were left for a minimum of 5 h to eliminate the inhalational anesthetic agent before delivering milk by catheter or artificial teat and recording swallows. In four animals, the order of surgery was reversed, so that, if any swallows occurred immediately after recovery from decerebration, the associated EMG activity might be recorded. The experimental periods lasted 1–3 days, after which the animals were killed. The levels of brain stem section were checked postmortem; in eight frozen specimens, photographs were taken of the sagittally hemisected heads for transfer of the level of brain stem transection to a stereotaxic map (Felix et al. 1999).
The bipolar electrodes in this study recorded selectively from only a small sample of all the motor units within the muscle (Loeb and Gans 1986). They also discriminated strongly against activity in muscle fibers that were >1 mm or so from the recording surfaces (Buchthal and Schmalbruch 1980; Ertas et al. 1995; Thexton et al. 2007). Cross-correlation tests, on the raw EMG signals recorded from duplicate electrodes in the same muscle, indicated that no significant signals were detected simultaneously by the two electrodes (Thexton et al. 2007). The implication was that, when duplicate electrodes were inserted, they recorded from separate selections of motor units present at different sites in the same muscle and thus permitted simultaneous comparisons of intramuscle activities.
All swallows were identified radiographically and correlated with the associated pattern of EMG activity in the cricothyroid, hyoglossus, mylohyoid, omohyoid, sternothyroid, and thyrohyoid muscles. For technical reasons relating to image quality (e.g., head rotation, epiglottal marker obscured or lost), it was not always possible to establish the precise time of epiglottal flexion from the videotape data as a time marker for the swallow (Crompton et al. 2008). Consequently, because of the scarcity of the data, swallow timing was routinely derived from the onset of EMG activity in muscles of the leading complex, specifically hyoglossus. These EMG signals had clear times of onset unlike those previously encountered in decerebrate piglets not exhibiting RTJM (Thexton et al. 2007). EMG data were recorded in 800-ms blocks, from 200 ms before the time of onset of leading complex EMG activity to 600 ms after this time. It was not possible to determine whether any quantitative differences existed between swallowing movements in the arrhythmic and the rhythmically active groups because there were no radio-opaque markers in the tongue and none attached to the uncalcified hyoid to make either adequately visible.
The period of EMG activity associated with each swallow was processed in exactly the same way as described for the pharyngeal swallow in the infant pig (Thexton et al. 2007). Briefly, the band-pass filtered and amplified EMG activities were processed by rectification and 10-ms reset integration, a noise threshold was defined statistically, and background activity was rejected (Thexton 1996; Thexton et al. 1998). These processes were combined in a computer program that also presented a display of the quantified EMG signals and allowed swallow-related sections of the data to be indexed for further analysis.
The 800-ms period of recorded EMG activity, associated with each swallow, had the same time register (the onset of hyoglossal EMG activity). Consequently, for each muscle, the periods of reset integration of the EMG activity in successive swallows corresponded to each other; for convenience, they were linearly interpolated to generate 100 time bins each of 8-ms duration. The data for each of the 100 corresponding periods in each swallow were used to generate individual median and quartile values so that representative profiles of EMG activity could be produced (Thexton et al. 2007). This allowed the timing of activity in one muscle to be related to that of other muscles studied (geniohyoid, cricothyroid, hyoglossus, mylohyoid, omohyoid, sternothyroid, and thyrohyoid) across successive swallows in each animal and again across animals to generate ensemble median profiles of activity.
Thyrohyoid EMG activity is a constant feature of the swallow but one that is slightly delayed relative to the leading complex of muscles (McFarland and Lund 1993; Uchida 1994). Geniohyoid activity coincident with the leading complex would consequently precede the thyrohyoid peak, whereas geniohyoid activity occurring 120 ms later than the leading complex would have a peak that occurred after the peak of thyrohyoid activity. Consequently, when EMG records included clear thyrohyoid activity, the temporal relationship of the peaks of activity in geniohyoid and thyrohyoid could be used to classify geniohyoid activity as early or late.
The minimum requirement for including data, from any animal, in the statistical analysis was that swallow-related EMG recordings included activity from geniohyoid, hyoglossus, and mylohyoid muscles. A minimum of seven swallows was set as the criterion for inclusion of an animal in the statistical analysis of this study. From all the records available, only seven exhibited RTJM but only five of these decerebrates 1) generated an adequate (n > 7) number of swallows, 2) yielded clean EMG signals from the required muscles, and 3) exhibited rhythmic EMG activity that arose before or extended beyond the completion of the pharyngeal swallow (Fig. 2). The five animals generated a total of 70 swallows (range, 11–18 swallows per animal); these data were analyzed statistically.
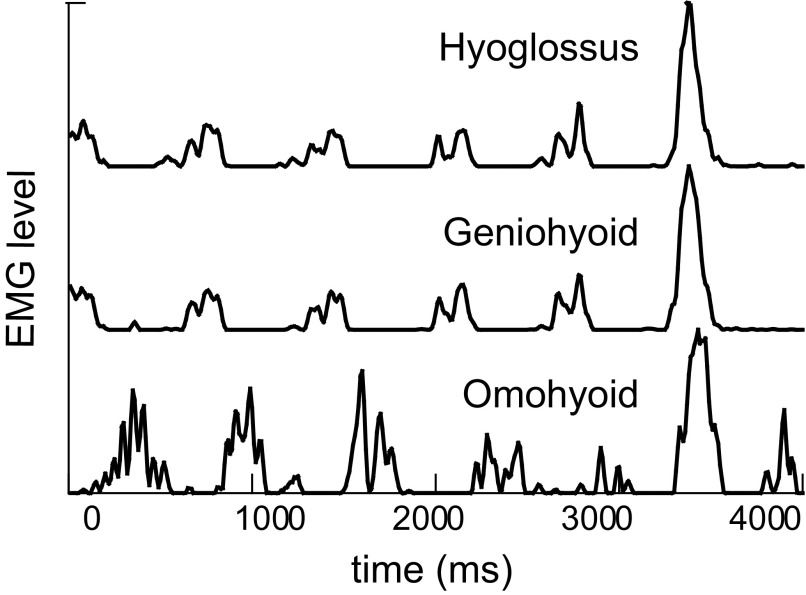
FIG. 2.
Rectified and integrated EMG activity associated with 4 cycles of rhythmic oral movements followed by a swallow cycle in a decerebrate infant pig. The amplitude of the EMG activity in the 3 muscles has been scaled to a maximum of 100 scale units in each case.
We tested our hypotheses in several ways. First, using all of the RTJM-associated data, we tested if the proportion of animals swallowing with short latency geniohyoid activity differed from random occurrence, using a single tail binomial test (http://www.graphpad.com/quickcalcs/binomial1.cf). Second, using the median profiles of EMG activity from different muscles in the swallow, we used cross-correlation analysis (CCF) to establish the differences in the timings of the bursts in the muscles of interest (Thexton et al. 2007). This function is useful for intermuscle comparisons, where the shapes of bursts are not identical. Finally, the CCF data allowed us to compare the time lag, between the activity in each muscle and in hyoglossus of the intact animal, to the equivalent data previously obtained from decerebrates not exhibiting RTJM (Thexton et al. 2007). The correlation between the timings in the two models were tested using regression and regression residual analysis.
In two animals exhibiting sequences of swallows, it was possible to follow the swallows and associated EMG signals from a quiescent period, i.e., with no rhythmic activity, through the time when rhythmic activity was present. Because these sequences were represented by very limited data from two different animals, statistical analysis of this transition was not possible. However, as case studies, such results indicate what patterns of activity are possible in a decerebrate that is sporadically capable of RTJM.
RESULTS
Rhythmic oral activity
RTJMs were unpredictable, infrequent events (<1% of total recording time). They could occur spontaneously or could follow mechanical or liquid stimulation of the oral cavity. The duration of RTJM varied from a few seconds to several minutes. The strength of the rhythmic movements was also variable. In some animals, the movements were sufficient to express milk from a feeding bottle with an artificial teat, whereas at other times, only weak movements were visible video-radiographically.
There was no obvious relationship between the occurrence of RTJM and the postnatal age of the animal (in the range 0–30 days postpartum) or with the time after decerebration (RTJM occurred any time from 4 to 53 h after decerebration). Among the animals where the level of the brain stem section had been transferred to a stereotaxic map (Fig. 1), there was only one decerebrate that exhibited RTJM. Animals with both higher and lower levels of brain stem transection failed to exhibit RTJM so that there was no obvious correlation between the level of the brain stem section and the presence of RTJM.
Only 7 of 38 decerebrate animals generated overt rhythmic oral movements at some point in their 3-day experimental period. The rhythm was generated following fluid delivered through a catheter to the posterior tongue (3/7) and by pumped delivery of milk through a teat (2/7). Occasionally a decerebrate would actively feed from a bottle (1/7) or would spontaneously exhibit rhythmic oral movements (1/7). In all cases, RTJM was associated with rhythmic EMG activity in hyoglossus, mylohyoid, or geniohyoid muscles. When the rhythmic movements were of very small amplitude, rhythmic activity was still clearly evident in the EMG signals. Periods of rhythmic EMG activity sometimes terminated with a swallow (Fig. 2), whereas in other cases, they started with a swallow. The cycle periods of 500–650 ms were longer (P < 0.001) than those seen (250–350 ms) when intact animals suckled (German et al. 1997; Thexton and Crompton 1998).
Swallows during rhythmic oral activity
The five decerebrates that met the criteria for inclusion in the statistical analysis generated a total of 70 swallows (range, 11–18 swallows per animal). In all swallows recorded in these animals, the peak of the geniohyoid EMG activity coincided with activity in muscles considered to be members of the leading complex (hyoglossus, mylohyoid). These signals had little variability, as can be seen from the median and quartile profiles of EMG activity in an example animal (Fig. 3). This pattern of activity, in which geniohyoid activity coincided with hyoglossal or mylohyoid activity in all five animals was significantly different from random (P < 0.05).
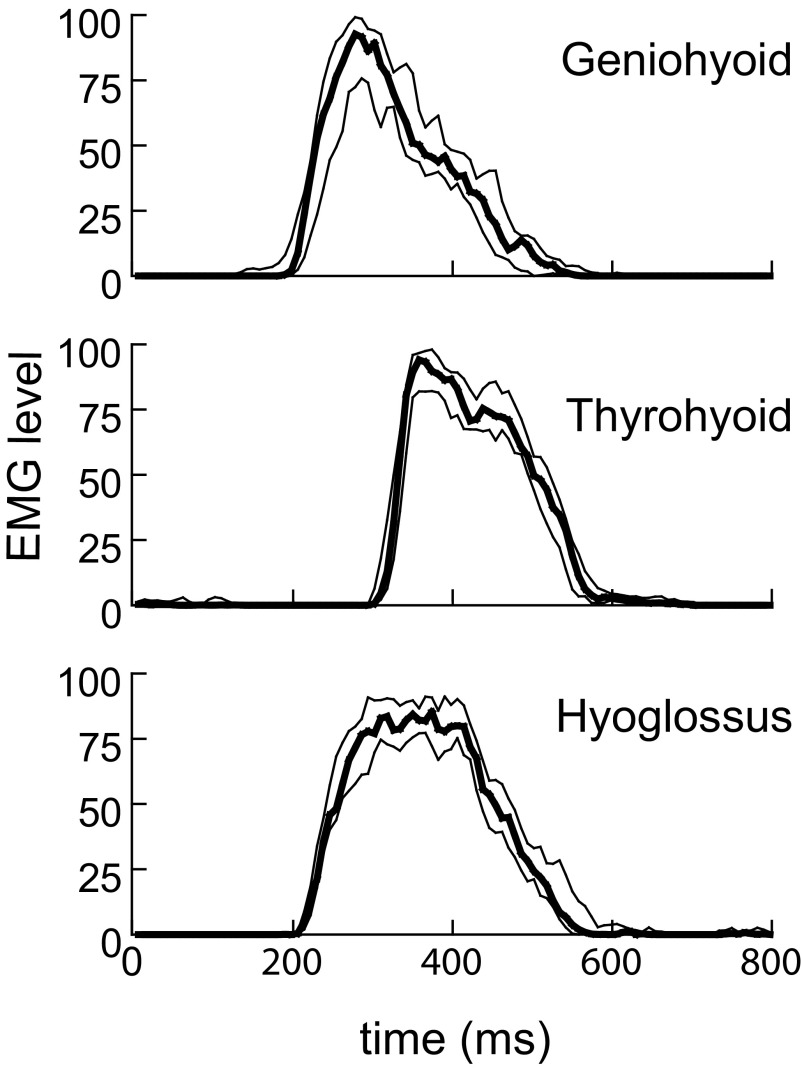
FIG. 3.
Median and quartile amplitudes of the processed EMG recorded in 6 consecutive swallows in 1 animal. The graph shows the median (heavy line) with the upper and lower quartiles for 3 muscles. The variation was relatively low, as indicated by the narrow interquartile range.
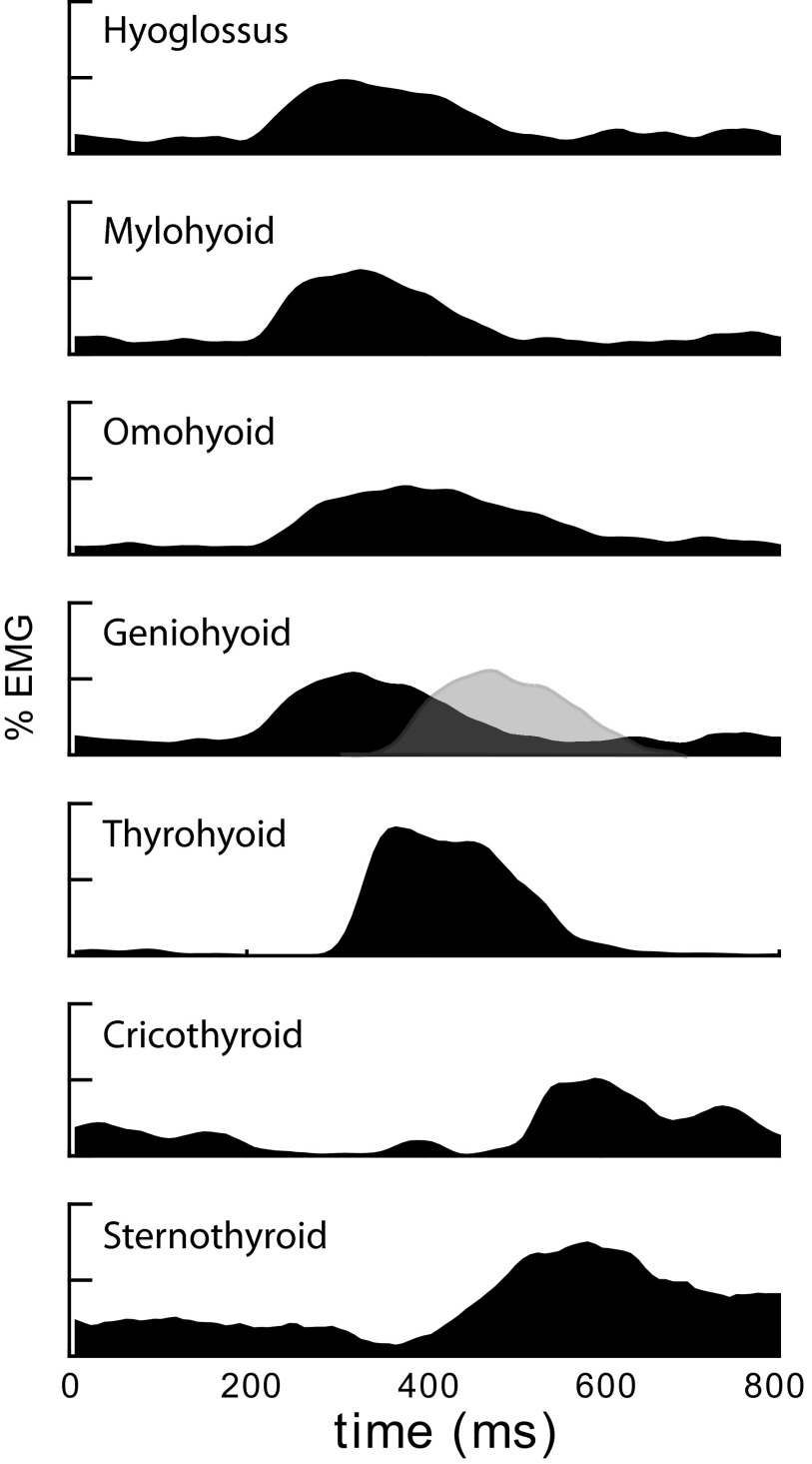
The ensemble median profiles of EMG activity for the seven target muscles, (across the 5 decerebrates with adequate data) are shown in Fig. 4. Cross-correlation analysis of the median profiles of EMG activity indicated that hyoglossus, mylohyoid, and geniohyoid activity coincided (r = 0.984). Relative to the hyoglossus, the omohyoid profile showed peak correlation with a lag of 50 ms (r = 0.943). The lag of thyrohyoid activity to the leading complex was 100 ms (r = 0.986).
FIG. 4.
Ensemble median signals derived from the pooled data of all rhythmic tongue and jaw movement (RTJM)-associated swallows in all 5 animals. The vertical axis is EMG amplitude; the units of the original processed EMG were scaled to a maximum of 100. In the process of obtaining the ensemble median, temporal variation in the profile of the burst reduces the peak of the grand median to <100. In the case of the geniohyoid graph, the gray profile outlined in a dotted line represents the approximate time at which geniohyoid activity would appear if it was timed as in an isolated pharyngeal swallow (Thexton et al. 2007).
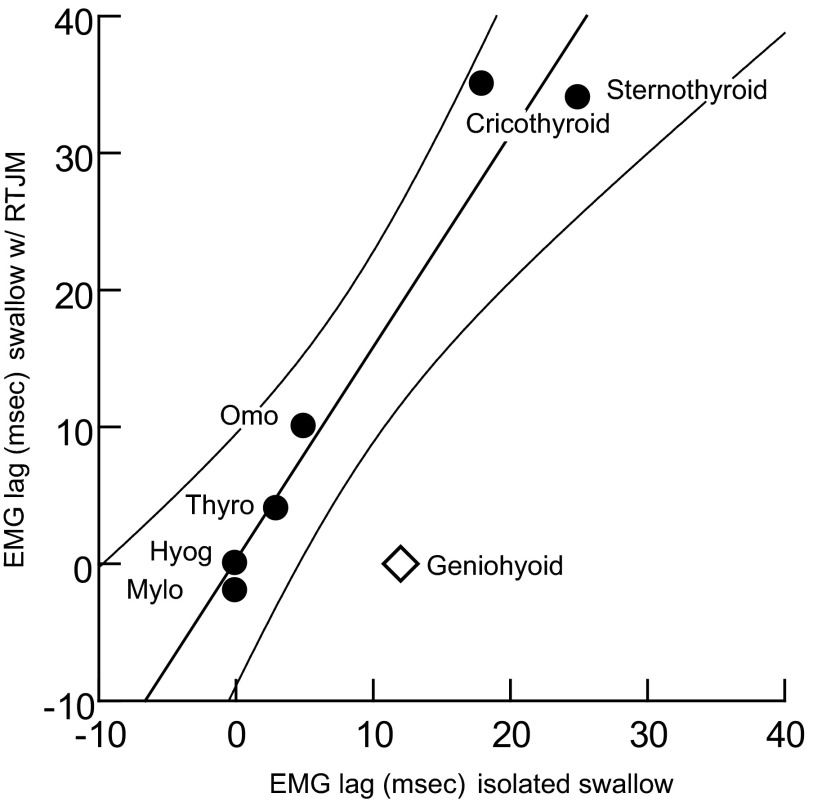
Comparison of these results with those of the isolated pharyngeal swallow (Thexton et al. 2007) was limited because relatively few muscles with adequate EMG signals were common to the two studies. However, in the seven muscles of interest, when the relative timings of the swallow-related activity were plotted against each other, six of the seven points fell within the 95% CI of the regression line between the two datasets (Fig. 5). The only muscle that fell outside the CI was the geniohyoid. The timing of the activity of this muscle was substantially different in swallows elicited as isolated events and in swallows elicited during rhythmic activity (P < 0.05).
FIG. 5.
Times of peak EMG activity during swallows associated with RTJM (vertical axis) plotted against the times of peak activity in isolated pharyngeal swallow (horizontal axis). The tight linear relationship among 6 of 7 muscles (circles) indicates that the phase relationship between the activities, induced under the 2 conditions, is constant. Geniohyoid (diamond), however, lies significantly off the line, outside the 95% CI.
Latency changes and developing rhythmic activity
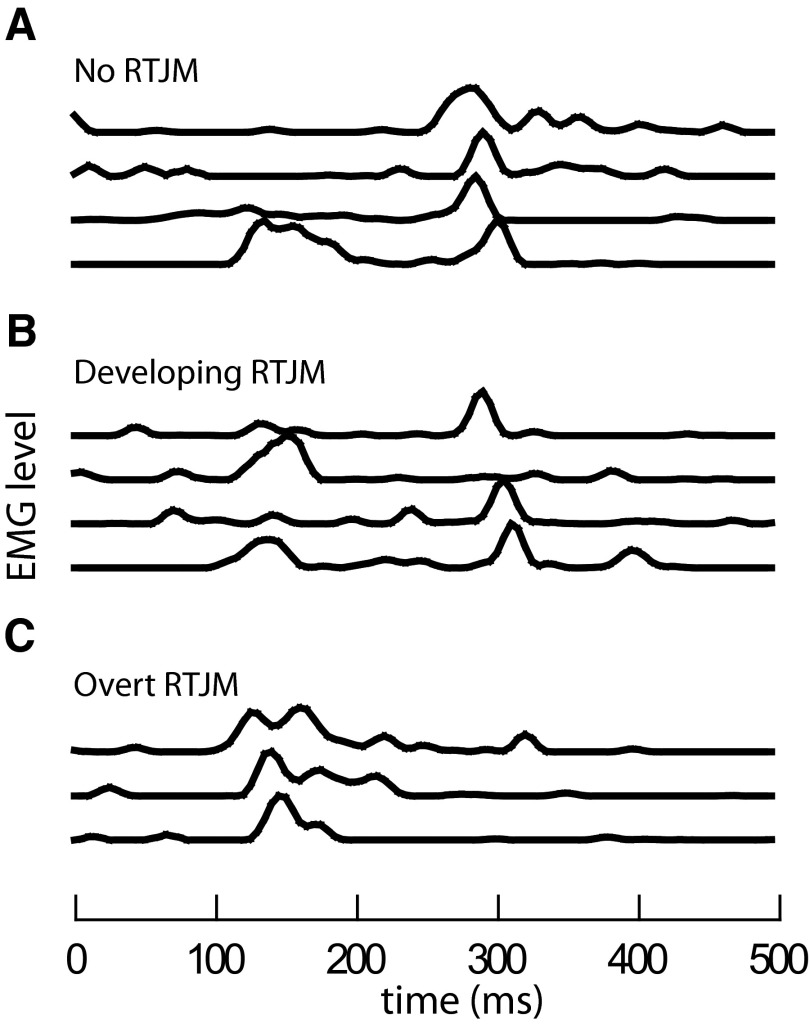
In two animals, extended recordings showed rhythmic EMG activity arising from a background of apparently random EMG noise. In one animal, at the start of one of the recording periods, there was no sign of rhythmic EMG activity. The predominant pattern of the swallow related geniohyoid EMG was of activity occurring 300 ms after the onset of hyoglossal activity, i.e., ∼300 ms after the time at which epiglottal flexion would occur (Fig. 6A). However, over the next few minutes, the EMG pattern periodically included double periods of geniohyoid activity. In these cases, an additional early response occurred at the time of activation of the muscles of the leading complex and, where radiographic evidence was available, this also corresponded to the time at which the epiglottis flexed. This type of dual EMG response became much more common over the next 5–10 min (Fig. 6B) but gave way to a third pattern (Fig. 6C). Toward the end of this recording period, the predominant pattern was of a single burst of EMG activity occurring with short latency, i.e., close to the time of epiglottal flexion. At this stage, rhythmic oral EMG activity was evident and overt RTJM appeared.
FIG. 6.
Swallowing related geniohyoid EMG activity from a single animal in 3 periods separated by ∼2 min. Data obtained 47 h after decerebration and 35 h after electrode insertion. Swallows elicited every 4–5 s. A: 4 swallows with no rhythmic EMG activity and a long latency geniohyoid EMG. B: 4 swallows while rhythmic EMG activity developed, with activity recorded by the same electrode at 2 different latencies. C: 3 swallows elicited during overt RTJM, with short latency geniohyoid EMG responses. The difference between the median times of the early and late bursts was 151 ms.
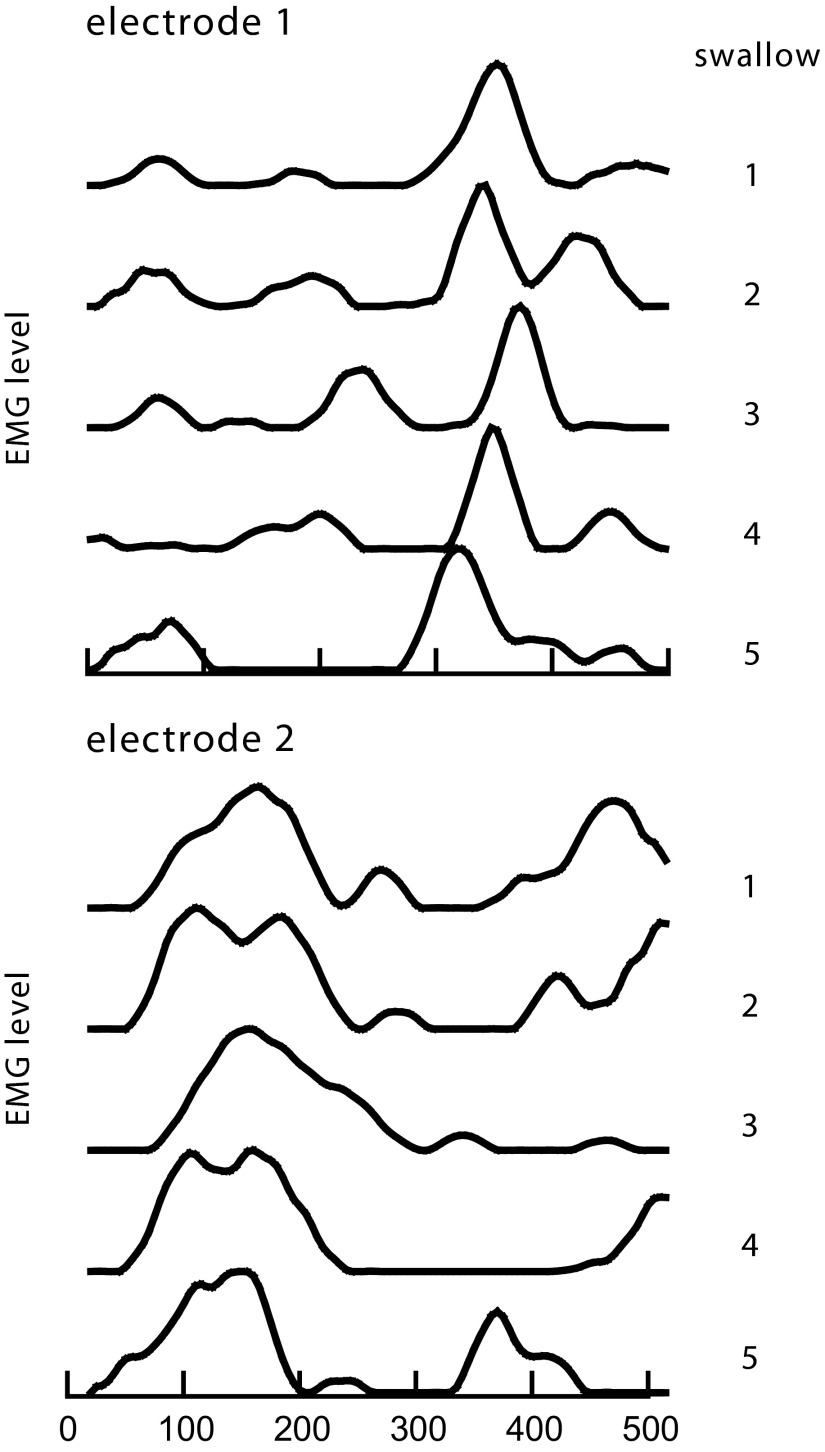
In another animal, duplicate electrodes in the geniohyoid muscle recorded the swallow-related EMG signals during the time rhythmic activity was developing. The latency of the high-amplitude EMG responses, recorded by each of the two electrodes, was significantly different (Fig. 7, A and B). One electrode (Fig. 7A) consistently detected a late response (corresponding to that in Fig. 6A), whereas the other electrode (Fig. 7B) detected primarily an early response (corresponding to that occurring in Fig. 6C).
FIG. 7.
Simultaneous recordings of swallow-related EMG activity at 2sites (A and B) in the same geniohyoid muscle, showing 2 latency responses detected independently by the 2 electrodes at the same time. This set of 5 swallows was recorded as rhythmic EMG activity developed. The difference, between the median times of the late bursts (electrode 1) and the median times of the early bursts (electrode 2) was 219 ms.
DISCUSSION
Rhythmic oral activity
Some anencephalic human infants and decerebrate animals are capable of swallowing and of generating rhythmic oral activity (Bremer 1923; Hall 1833; Monnier and Willi 1953; Thexton et al. 1982). However, despite numerous previous attempts, neither RTJM nor its effects could be studied systematically in the decerebrate infant pig because of the unpredictable occurrence of the rhythmic activity. Similarly, studies of locomotor function have found that, following decerebration, rhythmic limb movements may occur only sporadically (Brozek et al. 1996).
The levels of brain stem transection in this study did not directly involve the primary brain stem circuits known to be involved in the premotoneuronal generation of rhythmic oral activities or of swallowing, e.g., nucleus reticulogigantocellularis, nucleus parvocellularis, subnucleus oralis spinalis trigemini, nucleus tractus solitarius plus peri-ambigual, and other areas in the ventromedial medulla (Amirali et al. 2001; Brozek et al. 1996; Jean 2001; Westberg et al. 2000). Nevertheless, the pathways descending from cerebral hemispheres to those centers were clearly transected. The levels of section spared variable amounts of the superior colliculi, substantia nigra, and hypothalamus, all of which have been reported to influence oral function (Adachi et al. 2002, 2003; Schwartzbaum 1988). However, there was no obvious link, between the macroscopically determined levels of brain section and the presence of RTJM. The appearance and disappearance of rhythmic EMG activity over periods of minutes also indicated that there were factors, other than simply the level of brain stem transection, that determined the occurrence of RTJM. One possibility is that the sporadically occurring rhythmic activity reflected the paradoxical “sleep/wakefulness” changes of the decerebrate brain stem (Villablanca 1966).
The cycle period of decerebrate animals exhibiting RTJM (500–600 ms) differed considerably from that of the intact conscious animal (200–250 ms) when suckling (German et al. 1997; Thexton et al. 1998). The periodicity of a central pattern generator can vary with the strength of its excitation, with more excitation favoring a shorter period (Jung et al. 1996; Roberts et al. 1995). Consequently, removal of sources of excitation by decerebration might be the cause of the longer period we found in the RTJM of the decerebrates. An additional factor in cycle length may be the maintenance of the decerebrate at a lower than normal body temperature to avoid possible laryngeal spasm during feeding.
Reflex/rhythm interaction
Rhythmic oral or locomotor activity can modulate reflexes of cutaneous or mucosal origin in a cyclical fashion, with task-specific modulations (Forssberg 1979; Lamont and Zehr 2006; Seki and Yamaguchi 1997; Thexton and McGarrick 1987). Consequently, it was not surprising that, in the swallows of decerebrates with RTJM, the pattern of geniohyoid activity should differ from the pattern described for arrhythmic decerebrates (Thexton et al. 2007). In contrast, the relative timings of a number of other muscles in the pharyngeal swallow were unchanged by their incorporation into rhythmic oral activity (Fig. 5). This finding supports the first hypothesis and is discussed further below.
These results also supported our initial hypothesis that, in the decerebrate exhibiting rhythmic oral activity, the timing of geniohyoid EMG activity in the swallow would change from late to early. A geniohyoid EMG signal, statistically synchronous with the EMG activity in other muscles of the leading complex, was consequently not dependent on the presence of higher centers. This early timing was consistent with the reports by Doty and Bosma (1956) and Lang et al. (2002) but differed from the timing of geniohyoid activity in the isolated reflex pharyngeal swallow. In that case, the activity occurs ∼120 ms after the leading complex of muscles and after the peak activity of the thyrohyoid activity (Thexton et al. 2007). Because this comparison was made using the same model system and identical experimental parameters, the latency difference was not caused by experimental method but by the presence or absence of rhythmic oral activity.
Whether the swallows were elicited in the arrhythmic state or during RTJM, they were effective actions in terms of video-radiographically assessed movement of the bolus from oropharynx to esophagus. Thus the precise timing of geniohyoid activity seemed not to be critical to the basic performance of the swallow. However, during fluid swallowing, two bursts (or 2 peaks with intervening weak activity) of swallow-related geniohyoid activity are also found in rats (Travers and Jackson 1992), in opossums (Thexton and Crompton 1998), and in intact weaning pigs (Thexton et al. 1998); the first burst occurs 30–40% of the way through the cycle from the onset of jaw elevator activity, and the second burst coincides with digastric activity and the completion of jaw opening. In weaning pigs, as well as in two other species (hyraxes and cats), two separate stages of forward movement of the hyoid can be identified during swallowing (Franks et al. 1985; Thexton and McGarrick 1988; Thexton et al. 1998). We hypothesize that the two bursts of geniohyoid activity are related to the two stages of hyoid protraction, the second of which may be reduced in amplitude by other muscles acting on the hyoid. Although the loss of either burst of EMG activity may well compromise swallowing efficiency, that aspect was not quantified in this study; it seemed that the entire ensemble of hyoid-related muscle activity still provided sufficient forward movement of the hyoid to enable swallowing to be completed.
Changes in geniohyoid latency
As rhythmic behavior developed, the changes in geniohyoid EMG activity over successive swallows provided an insight into the two different latency bursts of activity. In the absence of rhythmic oral activity, the latency of the geniohyoid EMG was comparable to the timing of the activity in the isolated pharyngeal swallow. During subsequent rhythmic oral activity, the signals detected by the same electrode had a shorter latency, comparable to that reported by Doty and Bosma (1956) and Lang et al. (2002). However, during the changeover from arrhythmia to rhythmic activity, both patterns of EMG activity could periodically be detected in the same swallow and the pattern also differed with the electrode site within the muscle. The early and the late EMG responses were therefore consistent with the activity of two different, independently controlled, sets of motor units, i.e., the occurrence of the early signal was not associated with inhibition of the later signal or vice versa, nor did the existence of one signal depend on the existence of the other. This suggested the existence of different task units within the geniohyoid motor unit pool, consistent with the findings of van Lunteren and Dick (2000).
In the case of extended recording of successive swallows, the time difference between the early and the late bursts of geniohyoid EMG activity was of the order of 150–220 ms, which is ∼30% of the duration of RTJM cycles; this is comparable to the same relative timings in an intact animal (Thexton et al. 1998). The 150- to 220-ms separation was nevertheless longer than the 120-ms gap between the time of leading complex activity and the time of geniohyoid activity in the arrhythmic state (Thexton et al. 2007). One possible explanation for the increased latency of the later burst, in the presence of RTJM, is that the neural mechanisms generating rhythmic activity not only facilitated an early geniohyoid EMG response but also entrained and thus delayed the later EMG response, bringing it more in phase with the ongoing cyclical activity.
By definition, the EMG activity of the isolated or reflex pharyngeal swallow lacked any rhythmic components. Nevertheless, the relative timings of EMG signals in such swallows were highly correlated with their timing in swallows elicited during RTJM (Fig. 5). This indicated that some part of the isolated pharyngeal swallow was included within a rhythmic swallow cycle with little relative temporal change to the inserted components, thus supporting the first hypothesis. This concordance, across a range of muscles, emphasized the unusual status of geniohyoid activity within the pharyngeal swallow. Geniohyoid activity did not conform to the linear time scaling of other muscles but was temporally altered by the presence of RTJM.
In different species, the arrangement of muscle fibers and their subtypes in the geniohyoid muscle has been described as “mosaic” (Cobos et al. 2001) or septate, i.e., with different fiber subtype composition in different muscle compartments defined by a transverse septum (Lakars and Herring 1987; Mu and Sanders 1998). On morphological and histological grounds, geniohyoid is not homogeneous and on biomechanical grounds it is bifunctional, acting either as a jaw opener or as a hyoid protractor. During infant swallow cycles, geniohyoid activation concurrent with jaw elevator activation would consequently produce just hyoid protraction, whereas activation concurrent with sternohyoid activation would also assist jaw opening. The possibility that different motor units might be recruited for the different functions is supported by these findings. The two potential activation periods for the geniohyoid in this study corresponded to the two functions described as operating within normal cyclical swallowing in the infant. The fact that geniohyoid EMG activity, detected by a single bipolar electrode, occurred early in the swallow during RTJM but occurred later during arrhythmic periods, suggests that the electrode was sampling signals in an area of the muscle that was not homogeneous with respect to motor units.
In the animals in this study, the change in timing of geniohyoid activity occurred in the absence of any cerebral mechanisms. The brain stem pattern generator for rhythmic oral activity (or some neural circuitry predisposing to or associated with that activity) must therefore be independently capable of producing differential inhibition or excitation of one or more sets of geniohyoid motor units, thus changing the pattern of swallow-related EMG activity.
GRANTS
This work was funded by National Institute of Deafness and Other Communication Disorders Grant DC-03604.
Acknowledgments
We thank C. Musinsky and C. Sullivan for assistance with data collection.
Present address of T. Owerkowicz: Center for Comparative and Evolutionary Physiology, University of California, Irvine, CA.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Adachi et al. 2002.Adachi K, Hasegawa M, Fujita S, Sato M, Miwa Y, Ikeda H, Koshikawa N, Cools AR. Dopaminergic and cholinergic stimulation of the ventrolateral striatum elicit rat jaw movements that are funnelled via distinct efferents. Eur J Pharmacol 442: 81–92, 2002. [DOI] [PubMed] [Google Scholar]
- Adachi et al. 2003.Adachi K, Hasegawa M, Ikeda H, Sato M, Koshikawa N, Cools AR. The superior colliculus contains a discrete region involved in the control of jaw movements: role of GABAA receptors. Eur J Pharmacol 464: 147–154, 2003. [DOI] [PubMed] [Google Scholar]
- Amirali et al. 2001.Amirali A, Tsai G, Schrader N, Weisz D, Sanders I. Mapping of brain stem neuronal circuitry active during swallowing. Ann Otol Rhinol Laryngol 110: 502–513, 2001. [DOI] [PubMed] [Google Scholar]
- Basmajian and Stecko 1962.Basmajian J, Stecko G. A new bipolar electrode for electromyography. J Appl Physiol 17: 849, 1962. [Google Scholar]
- Bremer 1923.Bremer F Physiologie nerveuse de la mastication chez le chat et le lapin. Arch Int Physiol 21: 309–352, 1923. [Google Scholar]
- Brozek et al. 1996.Brozek G, Zhuravin IA, Megirian D, Bures J. Localization of the central rhythm generator involved in spontaneous consummatory licking in rats: functional ablation and electrical brain stimulation studies. Proc Natl Acad Sci USA 93: 3325–3329, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchthal and Schmalbruch 1980.Buchthal F, Schmalbruch H. Motor unit of mammalian muscle. Physiol Rev 60: 901–942, 1980. [DOI] [PubMed] [Google Scholar]
- Cobos et al. 2001.Cobos AR, Segade LA, Fuentes I. Muscle fibre types in the suprahyoid muscles of the rat. J Anat 198: 283–294, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor and Crawford 1969.Connor JD, Crawford IL. Hyperthermia in midpontine lesioned cats. Brain Res 15: 590–593, 1969. [DOI] [PubMed] [Google Scholar]
- Crompton et al. 2008.Crompton AW, Thexton AJ, German RZ. Development of the movement of the epiglottis in infant and juvenile pigs. Zoology 111: 339–349, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham and Jones 2003.Cunningham ET, Jones B. Anatomical and physiological overview. In: Normal and Abnormal Swallowing: Imaging in Diagnosis and Therapy, edited by Jones B. New York: Springer, 2003, p. 11–34.
- Curran et al. 1985.Curran AK, Xia L, Leiter JC, Bartlett D Jr. Elevated body temperature enhances the laryngeal chemoreflex in decerebrate. J Appl Physiol 98: 780–786, 1985. [DOI] [PubMed] [Google Scholar]
- Doty and Bosma 1956.Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol 19: 44–60, 1956. [DOI] [PubMed] [Google Scholar]
- Ertas et al. 1995.Ertas M, Stalberg E, Falck B. Can the size principle be detected in conventional EMG recordings? Muscle Nerve 18: 453–459, 1995. [DOI] [PubMed] [Google Scholar]
- Felix et al. 1999.Felix B, Leger ME, Albe-Fessard D, Marcilloux JC, Rampin O, Laplace JP. Stereotaxic atlas of the pig brain. Brain Res Bull 49: 1–137, 1999. [DOI] [PubMed] [Google Scholar]
- Forssberg 1979.Forssberg H Stumbling corrective reaction: a phase-dependent compensatory reaction during locomotion. J Neurophysiol 42: 936–953, 1979. [DOI] [PubMed] [Google Scholar]
- German et al. 1997.German RZ, Crompton AW, Hertweck DW, Thexton AJ. Determinants of rhythm and rate in suckling. J Exp Zool 278: 1–8, 1997. [DOI] [PubMed] [Google Scholar]
- German et al. 2004.German RZ, Crompton AW, Owerkowicz T, Thexton AJ. Volume and rate of milk delivery as determinants of swallowing in an infant model (Sus scrofia). Dysphagia 19: 147–154, 2004. [DOI] [PubMed] [Google Scholar]
- Hall 1833.Hall M On the reflex function of the medulla oblongata and medulla spinalis. Philos Trans 123: 635–665, 1833. [Google Scholar]
- Huang et al. 1989.Huang C-S, Hiraba H, Sessle BJ. Topographical distribution and functional properties of cortically induced rhythmical jaw movements in the monkey (Macaca fascicularis). J Neurophysiol 61: 635–650, 1989. [DOI] [PubMed] [Google Scholar]
- Jean 2001.Jean A Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev 81: 929–969, 2001. [DOI] [PubMed] [Google Scholar]
- Jung et al. 1996.Jung R, Kiemel T, Cohen A. Dynamic behavior of a neural network model of locomotor control in the lamprey. J Neurophysiol 75: 1074–1086, 1996. [DOI] [PubMed] [Google Scholar]
- Lakars and Herring 1987.Lakars TC, Herring SW. Polymorphous geniohyoid muscles of mice, rats and hamsters. Arch Oral Biol 32: 421–427, 1987. [DOI] [PubMed] [Google Scholar]
- Lamont and Zehr 2006.Lamont EV, Zehr EP. Task-specific modulation of cutaneous reflexes expressed at functionally relevant gait cycle phases during level and incline walking and stair climbing. Exp Brain Res 173: 185–192, 2006. [DOI] [PubMed] [Google Scholar]
- Lang et al. 2002.Lang IM, Dana N, Medda BK, Shaker R. Mechanisms of airway protection during retching, vomiting and swallowing. Am J Physiol 283: G529–G536, 2002. [DOI] [PubMed] [Google Scholar]
- Lee et al. 1977.Lee J, Stoll B, Downing S. Properties of the laryngeal chemoreflex in neonatal piglets. Am J Physiol 233: R30–R36, 1977. [DOI] [PubMed] [Google Scholar]
- Liu 1973.Liu JC Extrahypothalamic heat gain responses in the decerebrate and spinal monkey. Chin J Physiol 22: 79–92, 1973. [PubMed] [Google Scholar]
- Loeb and Gans 1986.Loeb G, Gans C. Electromyography for Experimentalists. Chicago, IL: University of Chicago Press, 1986, p. 119–120.
- Martin and Sessle 1993.Martin RE, Sessle BJ. The role of the cerebral cortex in swallowing. Dysphagia 8: 195–202, 1993. [DOI] [PubMed] [Google Scholar]
- McFarland and Lund 1993.McFarland DH, Lund JP. An investigation of the coupling between respiration, mastication, and swallowing in the awake rabbit. J Neurophysiol 69: 95–108, 1993. [DOI] [PubMed] [Google Scholar]
- Miller and Sherrington 1916.Miller RF, Sherrington CS. Some observations on the bucco-pharyngeal stage of reflex deglutition in the cat. Q J Exp Physiol 9: 147–186, 1916. [Google Scholar]
- Monnier and Willi 1953.Monnier M, Willi H. The integrative activity of the nervous system of a mesorhombencephalic anencephalus. I. Clinicophysiologica. Monatsschrift fur Psychiatrie und Neurologie 126: 239–258, 1953. [PubMed] [Google Scholar]
- Mu and Sanders 1998.Mu L, Sanders L. Neuromuscular specializations of the pharyngeal dilator muscles: I. Compartments of the canine geniohyoid muscle. Anat Rec 250: 146–153, 1998. [DOI] [PubMed] [Google Scholar]
- Roberts et al. 1995.Roberts A, Tunstall M, Wolf E. Properties of networks controlling locomotion and significance of voltage dependency of NMDA channels: stimulation study of rhythm generation sustained by positive feedback. J Neurophysiol 73: 485–495, 1995. [DOI] [PubMed] [Google Scholar]
- Rothwell et al. 1983.Rothwell NJ, Stock MJ, Thexton AJ. Decerebration activates thermogenesis in the rat. J Physiol 342: 15–22, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzbaum 1988.Schwartzbaum JS Electrophysiology of taste, feeding and reward in lateral hypothalamus of rabbit. Physiol Behav 44: 507–526, 1988. [DOI] [PubMed] [Google Scholar]
- Seki and Yamaguchi 1997.Seki K, Yamaguchi T. Cutaneous reflex activity of the cat forelimb during fictive locomotion. Brain Res 753: 56–62, 1997. [DOI] [PubMed] [Google Scholar]
- Sumi 1977.Sumi T Modification of cortically evoked rhythmic jaw movements by reflex deglutition in rabbits. Jpn J Physiol 27: 391–398, 1977. [DOI] [PubMed] [Google Scholar]
- Thexton 1996.Thexton AJ A randomisation method for discriminating between signal and noise recordings of rhythmic electromyographic activity. J Neurosci Methods 66: 93–98, 1996. [DOI] [PubMed] [Google Scholar]
- Thexton and Crompton 1998.Thexton AJ, Crompton AW. Control of Swallowing. Basel: Karger, 1998, p. 168–222.
- Thexton et al. 1998.Thexton AJ, Crompton AW, German RZ. Transition from suckling to drinking at weaning: a kinematic and electromyographic study in miniature pigs. J Exp Zool 280: 327–343, 1998. [DOI] [PubMed] [Google Scholar]
- Thexton et al. 2007.Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol 102: 587–600, 2007. [DOI] [PubMed] [Google Scholar]
- Thexton et al. 1982.Thexton AJ, Griffiths C, McGarrick JD. Brainstem mechanisms underlying variations in the occurrence of experimentally elicited rhythmic oral movements in the rat. Arch Oral Biol 27: 411–415, 1982. [DOI] [PubMed] [Google Scholar]
- Thexton and McGarrick 1987.Thexton AJ, McGarrick J. Effect of experimentally elicited rhythmic oral activity on the linguodigastric reflex in the lightly anesthetized rabbit. Exp Neurol 96: 104–117, 1987. [DOI] [PubMed] [Google Scholar]
- Thexton and McGarrick 1998.Thexton AJ, McGarrick JD. Tongue movement of the cat during lapping. Arch Oral Biol 33: 331–339, 1998. [DOI] [PubMed] [Google Scholar]
- Thexton and McGarrick 1994.Thexton AJ, McGarrick JD. The electromyographic activities of jaw and hyoid musculature in different. Arch Oral Biol 39: 599–612, 1994. [DOI] [PubMed] [Google Scholar]
- Travers and Jackson 1992.Travers JB, Jackson LM. Hypoglossal neural activity during licking and swallowing in the awake rat. J Neurophysiol 67: 1171–1184, 1992. [DOI] [PubMed] [Google Scholar]
- Uchida et al. 1994.Uchida K, Yamada Y, Sato T. The coordination of rhythmical drinking behavior with swallowing in rabbits. Physiol Behav 55: 795–801, 1994. [DOI] [PubMed] [Google Scholar]
- van Lunteren and Dick 2000.van Lunteren E, Dick TE. Heterogeneity within geniohyoid motor unit subpopulations in firing patterns during breathing. Resp Physiol 124: 23–33, 2000. [DOI] [PubMed] [Google Scholar]
- Villablanca 1966.Villablanca J Behavioral and polygraphic study of “sleep” and “wakefulness” in chronic decerebrate cats. Electroencephalogr Clin Neurophysiol 21: 562–577, 1966. [DOI] [PubMed] [Google Scholar]
- Westberg et al. 2000.Westberg KG, Kolta A, Clavelou P, Sandstrom G, Lund JP. Evidence for functional compartmentalization of trigeminal muscle spindle. Eur J Neurosci 12: 1145–1154, 2000. [DOI] [PubMed] [Google Scholar]