Abstract
The classical model of visual processing emphasizes the lateral geniculate nucleus (LGN) as the major intermediary between the retina and visual cortex. Yet, anatomical findings inspired Francis Crick to suggest an alternative model in which the thalamic reticular nucleus, which envelops the LGN, acts as the “guardian” of visual cortex by modulating LGN activity. Recent work by McAlonan and colleagues supports Crick's hypothesis, thereby enhancing our understanding of the early stages of visual processing.
One of the primary visual pathways begins in the retina, continues through the lateral geniculate nucleus (LGN) of the thalamus, and enters the first cortical way station in primary visual cortex (V1). This geniculostriate pathway is a fundamental part of early visual processing. Data from the LGN constitute the primary source of information for our understanding of thalamic processing of visual stimuli. However, anatomical and functional studies show that the LGN is only one among many thalamic nuclei involved in visual processing (e.g., Berman and Wurtz 2008; Sommer and Wurtz 2006; Tanaka 2006; Fig. 1A). Far less is known about the function of these nuclei outside the LGN, especially in awake behaving animals. A recent study by McAlonan et al. (2008) directly addressed this issue by comparing neuronal activity in the LGN to that of a reciprocally connected area, the thalamic reticular nucleus (TRN).
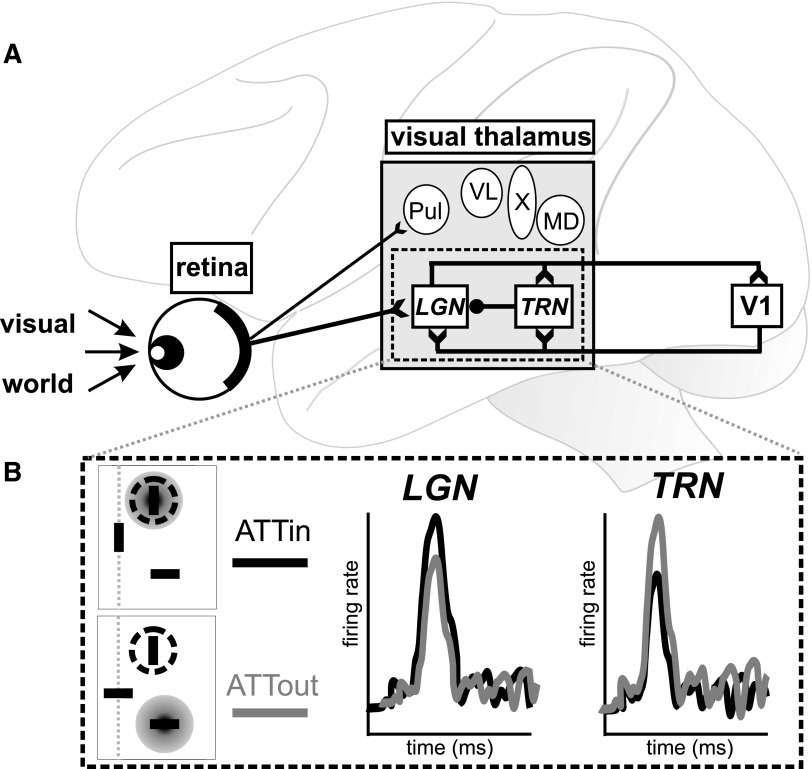
FIG. 1.
Simplified illustration of visual pathway from retina to visual cortex. A: visual information enters the retina and passes through visual thalamus (gray box), including LGN and TRN (italics). Excitatory (open arrow tails) and inhibitory (closed circle) connections are shown for retinal output and the LGN–TRN–V1 circuit. Other nuclei (circles) also likely influence our global visual percept. Note that only Pul and LGN are likely recipients of direct retinal input, as shown, and not all visual nuclei project directly to V1. LGN, lateral geniculate nucleus; MD, mediodorsal nucleus; Pul, pulvinar; TRN, thalamic reticular nucleus; V1, primary visual cortex; VL, ventrolateral nucleus; X, area X. B: key physiological results from McAlonan et al. (2008). Top left: in the ATTin condition, the focus of attention (gray disc) is aligned with the receptive field (dashed circle). Bottom left: in the ATTout condition, attention is fixed on the stimulus outside the receptive field. Right: schematic representation of neuronal responses in LGN and TRN. LGN neurons showed greater activity when attention was focused at the receptive field location (ATTin; black). In contrast, TRN neurons showed relatively larger responses when the focus of attention was outside the receptive field (ATTout; gray).
The authors were motivated in part by the integrative thinking of geneticist-cum-neuroscientist Francis Crick. Traditionally, the LGN has been considered the “gateway” from the retina to visual cortex due to its critical position in the circuit that interconnects the two structures. The TRN receives both input from the LGN and feedback projections from the visual cortex. The TRN, in turn, projects to the LGN (Fig. 1A). Crick believed that these characteristics of the TRN made it an ideal “guardian” of the thalamic gateway to the visual cortex (Crick 1984). Crick's “guardian” metaphor was intended to emphasize the regulatory influence of the TRN on visual activity passing through the thalamus.
The guardian hypothesis made two physiological predictions: one regarding the direction of responses (i.e., excitation or inhibition) in the TRN and LGN and the other regarding the timing of activity between the two structures. In simple terms, the first prediction is that if neuronal responses are recorded in the TRN and LGN, then increased activity in the TRN should correspond to decreased activity in the LGN and vice versa. This conjecture was based on anatomical evidence showing that the TRN received excitatory input from the LGN but also sent inhibitory output back to the LGN (see McAlonan et al. 2006 for references; Fig. 1A). The second prediction logically follows from the guardian metaphor: if the TRN truly modulates LGN activity, then we would expect changes in activity to occur first in TRN and later in LGN. Thus modulation of TRN would subsequently influence activity in the LGN.
McAlonan et al. (2008) set out to test these two predictions by recording the visual responses of single neurons in the TRN and magnocellular and parvocellular layers of the LGN. In a standard cued-attention task, a monkey began the trial by looking at the center of the screen. Three stimuli then appeared, one at the center and two in the periphery. One peripheral stimulus was positioned in the receptive field of the isolated neuron under study and the other peripheral stimulus was placed well outside of the receptive field. Each of the three stimuli was either a horizontal or a vertical bar. The central cue stimulus always matched one, and only one, of the peripheral stimuli. The monkey's task was to attend to the peripheral stimulus that matched the central cue without moving its eyes away from the center location. When the matching peripheral stimulus dimmed, the monkey had to make an eye movement to it to receive a juice reward. If no dimming occurred then the animal was rewarded for continuing fixation at the center of the screen. If the eyes drifted away from the center position or if the monkey looked at the target stimulus before it dimmed, the trial was aborted and no reward was given. The central cue randomly matched one of the two peripheral stimuli on a trial-by-trial basis. For each neuron, the key comparison was between activity in those trials in which the monkey was attending to the stimulus within the receptive field (ATTin) versus activity in those trials in which attention was directed to the stimulus outside the receptive field (ATTout).
Previous studies in visual cortex using a similar paradigm found that the ATTin condition led to a relative increase in the neuron's firing rate, usually in conjunction with improved behavioral performance (for review, see Maunsell and Cook 2002). McAlonan et al. (2008) replicated this attentional enhancement in their population of LGN neurons (Fig. 1B), suggesting that at least some attentional modulation occurs before visual information reaches the cortex. Notably, TRN neurons also showed differential activity in the two conditions of the cued-attention task. However, the effect of attention on neuronal activity was reversed relative to that in the LGN (and much of the visual cortex). When the monkey attended to the stimulus within the receptive field, TRN responses were lower than responses when the monkey attended to the stimulus outside the receptive field. Thus TRN neurons showed a decrease in responsiveness when the focus of attention was aligned with the receptive field location. Importantly, these differences in neuronal activity between the ATTin and ATTout conditions were not caused by differences in receptive field eccentricity or eye position.
The difference in TRN and LGN attentional responses confirmed the first premise of the “guardian” hypothesis of the TRN. After establishing the direction of attentional modulation, McAlonan et al. (2008) went on to analyze the timing of visual and attentional signals in both areas. To complete the second part of the “guardian” formulation, TRN neuronal modulation should occur before modulation of LGN neurons despite LGN′s direct retinal input. In this way, inhibitory connections from TRN to LGN could regulate visual attention in the retina–LGN–V1 pathway. In agreement with this idea, attentional modulation occurred significantly earlier in TRN than in both portions of the LGN.
Intriguingly, the effect of attention was significant for the TRN and LGN during the first 100 ms after stimulus onset, i.e., during the time of the visual response. After this initial epoch, TRN attentional modulation returned to baseline level for the remainder of the trial, whereas LGN modulation decreased and showed a late rebound in activity.
The authors operationally defined attentional modulation as significantly different activity in the ATTin and ATTout conditions. Consequently, the timing of attentional modulation was not necessarily equivalent to the timing of visual responses within each area. The visual response could occur prior to or at the same time as the influence of attention. Indeed, visual responses and attentional modulation did not progress across areas in the same order. The earliest visual responses occurred in magnocellular LGN, followed by the TRN, and finally parvocellular LGN. In contrast, the earliest evidence of attentional modulation occurred in the TRN, followed by magnocellular LGN and then parvocellular LGN. These differences in neuronal latencies across areas were not simply due to differences in baseline firing rates, response latencies, or response durations in each area. However, as the authors point out, the difference between the timing of visual responses and attentional modulation across areas may help elucidate the circuit-level mechanisms governing visual attention. In their model, the initial volley of visual information from the retina first arrives in magnocellular LGN via direct retinal projections. This activity drives the subsequent visual response in TRN via excitatory collaterals (see McAlonan et al. 2006 for references). Shortly afterward, efferent inhibitory connections back from TRN modulate the ongoing LGN visual activity.
Such an interpretation accounts for the order of visual and attentional responses in the TRN and LGN, but seems to blur the fact that only the fast-response magnocellular LGN showed differing visual (∼21 ms) and attentional (∼26 ms) median latencies. That is, visual responses and attentional modulation occurred at the same time within the TRN (∼22 ms) and parvocellular LGN (∼37 ms), but attentional effects occurred, on average, 5 ms later than the visual response in magnocellular LGN. This pattern of responsiveness suggests an immediate effect of attentional modulation (i.e., attentional modulation cooccurs with the visual response) in TRN and parvocellular LGN. Such a differential effect of visual attention on magnocellular and parvocellular LGN provides a potentially useful dissociation for future experiments.
Future work might also investigate visual and attentional responses in the TRN as a function of stimulus size and receptive field size. Visual studies tend to present stimuli entirely within a neuron's receptive field to avoid suppressive effects caused by extrareceptive field stimulation (e.g., Solomon et al. 2002). McAlonan et al. (2008) scaled the size of their stimuli according to receptive field eccentricity because receptive fields tend to increase in diameter at more eccentric locations. However, the average diameter of TRN receptive fields at eccentricities <20° was 0.83°, whereas the bar stimuli were ≥1.5 by 0.6°. The visual stimuli therefore completely covered the receptive field and extended outside it along one dimension. Given the subtle and often sophisticated dynamics of neuronal activity near and beyond the receptive field border, follow-up work should determine whether these border phenomena alter the basic attentional findings.
More generally, the findings of McAlonan et al. (2008) may help explain a perplexing result from human psychophysics. Unlike most aspects of spatial vision, visual attention does not appear to assist in discriminating time-dependent stimuli. Numerous experiments suggest that attention may actually hinder our ability to judge brief time intervals or rapidly changing stimuli (e.g., Rolke et al. 2008; Yeshurun and Levy 2003). This behavioral result has been difficult to reconcile with the physiological literature on attention, which consistently demonstrates increased firing correlated with increased performance when attention is directed to the receptive field (Maunsell and Cook 2002). At the neuronal level, how might attention facilitate the processing of spatial vision while hampering its fine-tuned temporal resolution? Hypotheses so far depend critically on differential effects of attention on fast-response magnocellular and slow-response parvocellular LGN neurons (Yeshurun 2004; Yeshurun and Levy 2003). To date, however, there is no direct physiological evidence for this differential “parvo-magno inhibition” (Yeshurun and Levy 2003). Results presented by McAlonan et al. (2008) now provide evidence of such contrasting attentional effects in magnocellular and parvocellular LGN and even suggest a potential neural source in the TRN. Thus behavioral results showing a decrement in temporal discrimination as a result of attention may be directly linked to the dynamics of differential inhibitory control exerted by the TRN on the LGN. If true, such a unification of the spatial and temporal domains of attention would be a major contribution to the fields of visual neurophysiology and psychophysics.
The findings of McAlonan et al. (2008) add an exciting new piece to the enduring puzzle of visual attention. With a general understanding of the role of the TRN in attentional selection now in place, more sophisticated experimental techniques can be used to flesh out the detailed interaction between the TRN and LGN. One possibility is to carry out identified recordings using ortho- and antidromic stimulation to determine direct, single-synapse connections between the areas (e.g., Sommer and Wurtz 2004). Such an approach may tease apart the causal role of the TRN without resorting to the more difficult manipulations of microstimulation or reversible inactivation, which would be particularly problematic given the thin anatomy of the TRN. In all, these findings expand our view of thalamic circuitry and open the door to a comprehensive circuit-level understanding of visual attention.
GRANTS
This work was supported by an Alfred P. Sloan Foundation grant and National Eye Institute Grant R01 EY-017592 to M. A. Sommer, the Center for the Neural Basis of Cognition, the Center for Neuroscience at the University of Pittsburgh, and the Department of Neuroscience.
Acknowledgments
I thank M. A. Sommer and A. R. Clause for helpful comments.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Berman and Wurtz 2008.Berman RA, Wurtz RH. Exploring the pulvinar path to visual cortex. Prog Brain Res 171: 467–473, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick 1984.Crick F Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci USA 81: 4586–4590, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell and Cook 2002.Maunsell JHR, Cook EP. The role of attention in visual processing. Philos Trans R Soc Lond B Biol Sci 357: 1063–1072, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan et al. 2006.McAlonan K, Cavanaugh J, Wurtz RH. Attentional modulation of thalamic reticular neurons. J Neurosci 26: 4444–4450, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan et al. 2008.McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature 456: 391–394, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolke et al. 2008.Rolke B, Dinkelbach A, Hein E, Ulrich R. Does attention impair temporal discrimination? Examining non-attentional accounts. Psychol Res 72: 49–60, 2008. [DOI] [PubMed] [Google Scholar]
- Solomon et al. 2002.Solomon SG, White AJR, Martin PR. Extraclassical receptive field properties of parvocellular, magnocellular, and koniocellular cells in the primate lateral geniculate nucleus. J Neurosci 22: 338–349, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer and Wurtz 2004.Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. J Neurophysiol 91: 1381–1402, 2004. [DOI] [PubMed] [Google Scholar]
- Sommer and Wurtz 2006.Sommer MA, Wurtz RH. Influence of the thalamus on spatial visual processing in frontal cortex. Nature 444: 374–377, 2006. [DOI] [PubMed] [Google Scholar]
- Tanaka 2006.Tanaka M Inactivation of the central thalamus delays self-timed saccades. Nat Neurosci 9: 20–22, 2006. [DOI] [PubMed] [Google Scholar]
- Yeshurun 2004.Yeshurun Y Isoluminant stimuli and red background attenuate the effects of transient spatial attention on temporal resolution. Vision Res 44: 1375–1387, 2004. [DOI] [PubMed] [Google Scholar]
- Yeshurun and Levy 2003.Yeshurun Y, Levy L. Transient spatial attention degrades temporal resolution. Psychol Sci 14: 225–231, 2003. [DOI] [PubMed] [Google Scholar]