Abstract
Motor cortex (M1) activity between postnatal weeks 5 and 7 is essential for normal development of the corticospinal tract (CST) and visually guided movements. Unilateral reversible inactivation of M1, by intracortical muscimol infusion, during this period permanently impairs development of the normal dorsoventral distribution of CST terminations and visually guided motor skills. These impairments are abrogated if this M1 inactivation is followed by inactivation of the contralateral, initially active M1, from weeks 7 to 11 (termed alternate inactivation). This later period is when the M1 motor representation normally develops. The purpose of this study was to determine the effects of alternate inactivation on the motor representation of the initially inactivated M1. We used intracortical microstimulation to map the left M1 1 to 2 mo after the end of left M1 muscimol infusion. We compared representations in the unilateral inactivation and alternate inactivation groups. Alternate inactivation converted the sparse proximal M1 motor representation produced by unilateral inactivation to a complete and high-resolution proximal–distal representation. The motor map was restored by week 11, the same age that our present and prior studies demonstrated that alternate inactivation restored CST spinal connectivity. Thus M1 motor map developmental plasticity closely parallels plasticity of CST spinal terminations. After alternate inactivation reestablished CST connections and the motor map, an additional 3 wk was required for motor skill recovery. Since motor map recovery preceded behavioral recovery, our findings suggest that the representation is necessary for recovering motor skills, but additional time, or experience, is needed to learn to take advantage of the restored CST connections and motor map.
INTRODUCTION
The primary motor cortex (M1) motor representation develops postnatally. In kittens, the representation, which is examined using intracortical microstimulation (ICMS), is expressed by postnatal week 7 (Bruce and Tatton 1980; Chakrabarty and Martin 2000). Between weeks 7 and 11, the number of sites where ICMS produces a response (i.e., effective sites) increases, ICMS response thresholds decrease, and forelimb joint diversity increases (Chakrabarty and Martin 2000). Motor map development occurs after the critical period for establishing the normal pattern of corticospinal tract (CST) connectivity with spinal circuits, which is between weeks 5 and 7 (Martin 2005). This is when M1 activity is essential for normal development of the CST and visually guided movements. Unilateral reversible inactivation of M1, by intracortical muscimol infusion, during this period affects development of the CST on both the silenced and active sides. The silenced CST fails to develop the normal pattern of connections with spinal circuits—instead, terminating dorsally (Friel and Martin 2005). The forelimb contralateral to the infusion develops an endpoint control impairment in which the forepaw overshoots the target during reaching and oversteps during visually guided locomotion (Friel et al. 2007; Martin et al. 2000). The active side develops normal contralateral terminations but also aberrant ipsilateral projections, which terminate where the silenced terminations would have normally (Martin 2005).
Left untreated, unilateral M1 inactivation results in permanent changes in CST connectivity (Friel and Martin 2005) and permanent motor impairments on the affected side of the spinal cord (Friel and Martin 2007; Martin et al. 2000). However, we recently showed that inactivating untreated M1 immediately after the earlier inactivation (i.e., inactivate left M1 between weeks 5 and 7 and then right M1 between weeks 7 and 11; alternate inactivation) restored CST connections in the spinal cord and skilled motor function (Friel and Martin 2007). The alternate inactivation period is when the M1 motor representation normally develops (Chakrabarty and Martin 2000). The results were consistent with a synaptic competition model. Limiting CS system activity on the previously active side reduced its ability to compete for connections with local and downstream targets, thereby allowing the previously silenced side to further develop appropriate connections and function (Friel and Martin 2007).
The purpose of this study was to determine the effects of alternate inactivation on the M1 motor representation, both in relation to controlling different joints of the forelimb and in the context of the changing pattern of spinal connectivity that we know occurs during the alternate inactivation (Friel and Martin 2007). We compared the M1 motor representation in the unilateral and alternate inactivation groups. In both groups, we used ICMS to map the left M1 1 to 2 mo after the end of left M1 muscimol infusion. The difference between the two groups was that in animals receiving the alternate inactivation, the right M1 was inactivated during the period the motor map normally develops. Alternate inactivation further allowed us to examine two questions related to a broader role for map connections and functions during development. First, does the timing of representational changes in M1 coincide with the timing of CST termination plasticity in the spinal cord or is one delayed with respect to the other? Second, when does motor performance improve after alternate inactivation in relation to changes in the M1 representation? We found that alternate inactivation resulted in a somatotopically more complete motor map, and with a higher spatial resolution, than that after unilateral inactivation. M1 representational plasticity and the ventral redistribution of CST terminations occurred concurrently, but an additional 3 wk were required for motor skill recovery (Friel and Martin 2007). This suggests that whereas the map is necessary for skilled movement control, additional time, or experience, is needed to learn to take advantage of the restored CST connections and representation.
METHODS
Animals were obtained from a supplier accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (Liberty Laboratories, Vestal, NY). Kittens were delivered along with a lactating mother at postnatal day 28. All experiments were conducted with the approval of the New York State Psychiatric Institute and Columbia University IACUC.
General surgical procedures
A mixture of acepromazine (0.03 mg/kg, administered intramuscularly [im]) and Ketamine hydrochloride (32 mg/kg, im) was given to induce anesthesia. For implantation of the infusion cannula and the osmotic pump for delivering muscimol (see following text), animals were administered atropine (0.04 mg/kg, im). Cats were intubated after anesthesia was induced, and were maintained in an areflexive condition during surgery using 1–2% isofluorane. Animals were given a broad-spectrum antibiotic at the time of surgery (cephalozine; 25 mg/kg, im) and an analgesic after surgery (burprenorphine; 0.03 mg/kg, im). Animals were placed in a conventional stereotaxic frame. Body temperature was maintained at 39°C by a heating pad. A craniotomy was made over the lateral portion of the frontal lobe to expose the forelimb areas of primary motor cortex (M1). Animals resumed nursing on recovery from anesthesia, and were given supplemental milk (KMR feline milk replacement) as needed to ensure adequate weight gain.
M1 activity blockade
To block neuronal activity, the γ-aminobutyric acid type A agonist muscimol (10 mM in sterile saline; Sigma) was continuously infused using an osmotic minipump (0.5 μL/h; Alzet, model 2002) into the center of the motor cortex forelimb representation, located primarily in the lateral sigmoid gyrus (Chakrabarty and Martin 2000), as in our prior studies (Friel and Martin 2005, 2007; Friel et al. 2007; Martin et al. 1999). A 28-gauge hypodermic needle cannula (Alzet), beveled at the tip, was connected with vinyl tubing (size 4; Scientific Commodities) to the pump. For the initial inactivation, between weeks 5 and 7, the cannula was inserted into the left M1. It was lowered to a depth of 2 mm below the pial surface. The cannula was fixed to the skull with screws and dental acrylic cement. The unilateral inactivation group received only this inactivation (between weeks 5 and 7). In the alternate inactivation group, neuronal activity was blocked in left M1 from weeks 5 to 7, and then in right M1 from weeks 7 to 11. At 7 wk, we removed the cannula in the left M1, and associated osmotic pump, and implanted a new cannula into the homotopic site in right forelimb M1 and a new pump for drug delivery. Since the osmotic pump delivered muscimol for 2 wk, during the inactivation of right M1 from weeks 7 to 11, it was necessary to replace the pump at week 9.
ICMS protocol
For intracortical microstimulation (ICMS) experiments, anesthesia was induced with a mixture of acepromazine (0.03 mg/kg, im) and ketamine hydrochloride (32 mg/kg, im). Anesthesia was maintained using intravenous ketamine infusion (10 mg·kg−1·h−1; adjusted to maintain an areflexive state) as previously (Chakrabarty and Martin 2000; Martin et al. 2005). Animals were also administered atropine (0.04 mg/kg, im). Animals were placed in a stereotaxic frame. The cannula was removed if necessary and an 8- to 10-mm craniotomy was made, centered over the lateral tip of the cruciate sulcus, over M1 on each side for unilateral inactivated animals, and over the left side for the alternate inactivation animals. The dura over the lateral pericruciate cortex was incised and reflected, exposing a radius of ≥3–5 mm of cortex around the lateral margin of the cruciate sulcus.
Microstimulation was applied through tungsten microelectrodes (0.5-mΩ impedance; Microprobe). Stimuli (45-ms-duration train, 330 Hz, 0.2-ms biphasic pulses) were delivered once every 3 s using a commercial constant-current stimulator (AM Systems). These are the same parameters we used in prior studies (Chakrabarty and Martin 2000; Ghosh 1997). Electrode penetrations were made orthogonal to the cortical surface, at 1-mm intervals, similar to other studies mapping multiple cortical areas in the same animal (e.g., Ghosh 1997). Furthermore, we verified that using a 1-mm grid did not bias our results by randomly sampling a reduced number of penetrations in each animal and recalculating map parameters (see results). To minimize animal state-dependent effects, especially due to anesthesia level, in the unilateral inactivation animals we alternately stimulated one then the other side after every 5–10 penetrations. For a given animal, approximately the same number of penetrations was made in each motor cortex.
Motor effects produced by ICMS occurred at the lowest stimulus currents at depths where we recorded multiunit activity with the largest amplitude spikes. This was typically between 1.2 and 1.5 mm from the pial surface and likely corresponded to layer 5. We examined only the superficial cortex, not the cortex within the cruciate sulcus. We made penetrations within the lateral portion of area 4γ (Ghosh 1997; Hassler and Muhs-Clement 1964), a zone extending from about 2 mm caudal, 5 mm rostral, and 2 mm lateral to the cruciate sulcus. This is the forelimb representation (Armstrong and Drew 1985; Chakrabarty and Martin 2000; Keller 1993; Pappas and Strick 1981) and it projects densely to the cervical enlargement in kittens and adult cats (Ghosh 1997; Li and Martin 2000; Martin 1996). To ensure that we thoroughly explored the forelimb zone, in all experiments we examined the region until we encountered a consistent band of ineffective sites or nonforelimb sites, indicating the limits of excitable forelimb motor cortex. These sites were noted during the experiment (see Fig. 3, top row). In some experiments, we mapped to the bony rostral or rostrolateral limit of the craniotomy, which was at or close to the area 4γ boundary (Hassler and Muhs-Clement 1964). As discussed in results, we restricted sites for quantitative analyses to those where the threshold for evoking a response was ≤ 60 μA (see Fig. 3, bottom row). This value was computed on the basis of the population threshold distributions (see results and Fig. 2). Although our analysis strategy may have resulted in eliminating some forelimb sites, we minimized the risk of including sites out of the motor cortex forelimb limb zone. We were careful to sample homotopic regions of M1 within or between animals. This was ensured on the basis of landmarks and by stimulating only the surface cortex.
In determining the threshold and type of motor effects evoked by ICMS, we kept the limb in a posture in which the shoulder was slightly extended, the elbow was approximately halfway between flexion and extension, and the wrist was plantar flexed. The fixed limb position was necessary to prevent afferent (i.e., mechanoreceptor) facilitation from reducing current thresholds. Sometimes it was necessary to stabilize a proximal joint during stimulation to verify that distal joint movement was not due to an inertial interjoint interaction. When this was done, or whenever the limb was moved, we waited several seconds before stimulating to minimize the effect of mechanical limb stimulation on current threshold. We characterized changes at the shoulder (extension, flexion, abduction, adduction), elbow (flexion, extension), wrist (flexion, extension, supination, pronation), and at digit joints (flexion/digit closure, extension). Commonly, ICMS evoked movements about multiple forelimb joints (termed multijoint sites) at slightly suprathreshold currents (≤1.5-fold threshold value). These sites were noted and distinguished from single-joint sites. Mutijoint sites may encode simple motor synergies and, in the cat and monkey, increase in frequency with motor training (Martin et al. 2005; Nudo et al. 1996).
When ICMS produced a motor effect, we determined the current threshold, defined as the lowest current that produced a motor effect in 50% or more of the stimuli, and the kinds of effects produced by stimulation at threshold and 1.5-fold threshold value. At first, we quickly raised the current to suprathreshold values, then reduced the current to below threshold, noting the lowest current at which the effect was present. Next we increased the current from a subthreshold value to ≥1.5-fold the threshold value and noted when the effect reappeared. We typically repeated this procedure until we were confident of the threshold value and particular motor effects. Each threshold value corresponds to the average current threshold measured over at least one descending and one ascending stimulation run.
As discussed in the following text, we used a maximal current of 100 μA during each experiment, to minimize the likelihood of missing effective sites. However, we present an analysis justifying using a 60-μA cutoff for quantitative study (see results). Moreover, 60 μA spreads about 270 μm (Asanuma and Sakata 1967), which was an acceptable distance considering the sizes of joint representation zones in cat M1 (Keller 1993). We have found in this study and previously (Chakrabarty and Martin 2000) that caudal regions in M1 had thresholds higher than those of other areas. This may be due to connectivity of caudal M1 (Alstermark and Ohlson 2000) or sampling of sites within somatic sensory cortex. To minimize this sampling effect, we limited our analysis to sites within 2 mm caudal to the cruciate sulcus.
Motor representational changes after unilateral inactivation were compared with changes produced by alternate inactivation, to show the effects of activity-dependent interactions. In addition, we used the characteristics of the motor map in the untreated M1 in unilateral inactivated animals for comparison (i.e., the side opposite the inactivated M1). The advantage of using the untreated side in unilateral inactivated animals, opposed to separate experiments in age-matched controls, is that data from the unilateral inactivated M1 and untreated M1 in unilateral inactivated animals were collected under identical anesthetic conditions. Nevertheless, we supplemented these data with summary data from controls.
Statistical and other analyses
We used the programs Statview and SAS to determine the statistical significance of differences. We conducted routine nonparametric (Kruskal–Wallis rank test; Mann–Whitney test) and parametric statistics. Unpaired t-tests and ANOVAs were used to compared results collected in different animals and a paired t-test for data in the unilateral and untreated M1 (i.e., two sides in the same animal). Data for the cumulative percentage effectiveness histogram (Fig. 2) were fitted with a third-degree polynomial using the program KaleidaGraph for the Macintosh computer (Synergy Software, Reading, PA).
RESULTS
We compared the effects of unilateral inactivation (n = 5; at the following ages: 11, 13, 14, 16, and 16 wk) with alternate inactivation (n = 5; 11, 11, 14, 16, 16 wk) on the M1 motor representation. In both groups, we inactivated left M1 between weeks 5 and 7 and used ICMS to map the left M1 1 to 2 mo after the end of the period of left M1 inactivation. The difference between groups was that in animals receiving the alternate inactivation, the right M1 was inactivated between weeks 7 and 11. In animals of each group, we determined the current thresholds for evoking movements, the percentage of sites from which stimulation evoked a response (effective sites), the forelimb joints represented, and the area of representation of each joint. Data for the initially inactivated hemisphere in the unilateral and alternate inactivation groups were also compared with M1 map data from the untreated side in unilateral inactivation animals and, when applicable, with data from age-matched controls reanalyzed from our prior study (Chakrabarty and Martin 2000).
Stimulation sites were located within the inactivated field
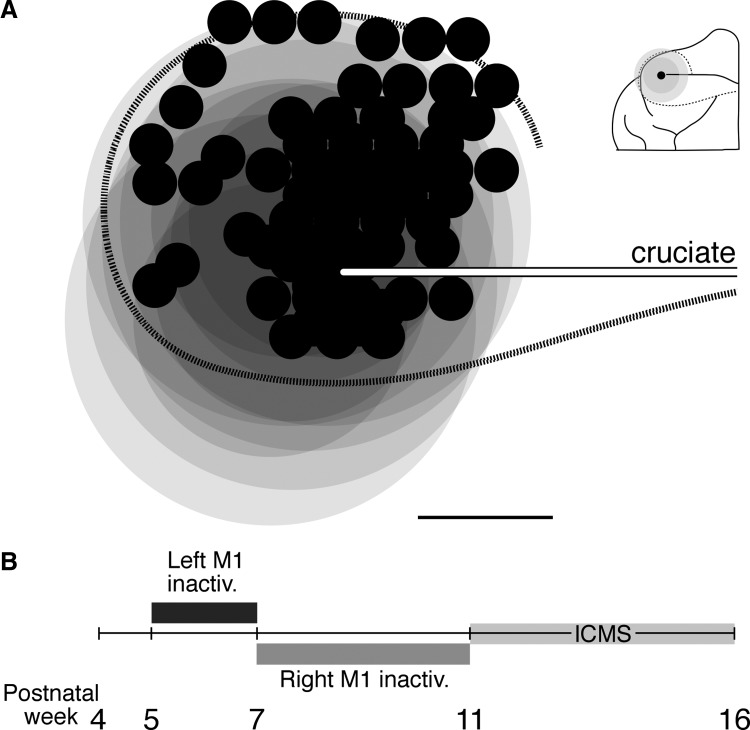
Stimulation sites were likely within the region inactivated between weeks 5 and 7. Figure 1 plots all stimulation sites in unilateral and alternate inactivated animals, overlaid in relation to the lateral tip of the cruciate sulcus (white/black line). Each of the overlapping large gray circles is an estimate of the central region of maximal inactivation (dark gray; 3-mm radius) extending into a region of reduced inactivation (light gray; 1.5 mm) for each animal. The estimates of inactivation are based on our published data showing reductions in either cytochrome oxidase staining (Martin et al. 1999) or parvalbumin immunoreactivity (Friel et al. 2007) at week 7. Note that the maps were obtained after week 11, when the cortex is larger than that at week 7. Nevertheless, this comparison shows that the stimulation sites were likely within the inactivated territory. The effects we describe are due to mapping, at or near threshold, the efferent zones that were silenced.
FIG. 1.
Stimulation sites were within the inactivated region and experimental timeline. A: all stimulation sites (small black circles) in all animals are shown, aligned according to the lateral tip of the cruciate sulcus (white line with black border). Darker gray circles correspond to the region of maximal activity reduction (3-mm radius) for each infusion and the lighter gray circles indicate region of reduced activity reduction for each infusion, based on cytochrome oxidase staining (Martin et al. 1999) and supplemented with parvalbumin immunohistochemistry (Friel et al. 2007). The dotted black line corresponds approximately to the area 4γ border. Inset shows a schematic representation of an example infusion site at the lateral tip of the cruciate sulcus and associated regions of maximal (dark gray) and reduced (light gray) inactivation. Cal. 3 mm. B: timeline showing the ages at which left primary motor cortex (M1) and right M1 inactivations took place.
Motor map of the unaffected side after unilateral inactivation is similar to that of untreated controls
The untreated side in unilateral inactivated animals has a normal contralateral projection to the spinal cord (Friel and Martin 2005). The contralateral motor representation of the untreated side in unilateral inactivated animals was similar to that of age-matched controls in terms of joints represented and laterality. Sites evoking wrist, elbow, and shoulder movement were most common. Responses were evoked alone or as a combination of responses at different joints, resembling simple synergies. Digit responses were rarely encountered and, when they were, were evoked in combination with a more proximal response. The percentage of sites evoking contralateral responses within the defined forelimb zone on the untreated side, using a maximal current of 60 μA, was not significantly different from that of age-matched controls [untreated M1: 80.1%; age-matched controls (n = 8 cats): 73.7%; Chakrabarty and Martin 2000; t = 0.816; P = 0.432; df = 11]. In one animal, we infused saline from 5 to 7 wk and mapped the infused and noninfused sides at week 13. The saline infusion had no effect on motor map development; the saline-infused and noninfused sides had a similar percentage of effective sites (at 60-μA maximal current: saline-infused side, 87.9%; noninfused side, 91.7%).
The untreated side in unilateral inactivated animals develops an aberrant ipsilateral projection (Friel and Martin 2005; Martin et al. 1999). In this study, we were particularly interested in determining whether there was an ipsilateral motor representation that paralleled the ipsilateral spinal projection. Surprisingly, out of 87 sites (n = 5 animals) examined with maximal currents of 100 μA in the untreated M1, contralateral to the unilateral inactivation, we did not observe any ipsilateral responses. Despite robust ipsilateral terminations within the premotor laminae of the spinal cord (Friel and Martin 2005, 2007), there was no representation of this aberrant projection in M1.
Stimulus–motor response relationship in M1
We examined the relationship between the ICMS current used and the percentage of effective sites within M1. We conducted this analysis both as an assay for the efficiency of activation of spinal motor circuits by ICMS and as a method for determining the optimal current range for quantifying the effects of unilateral and alternate inactivation.
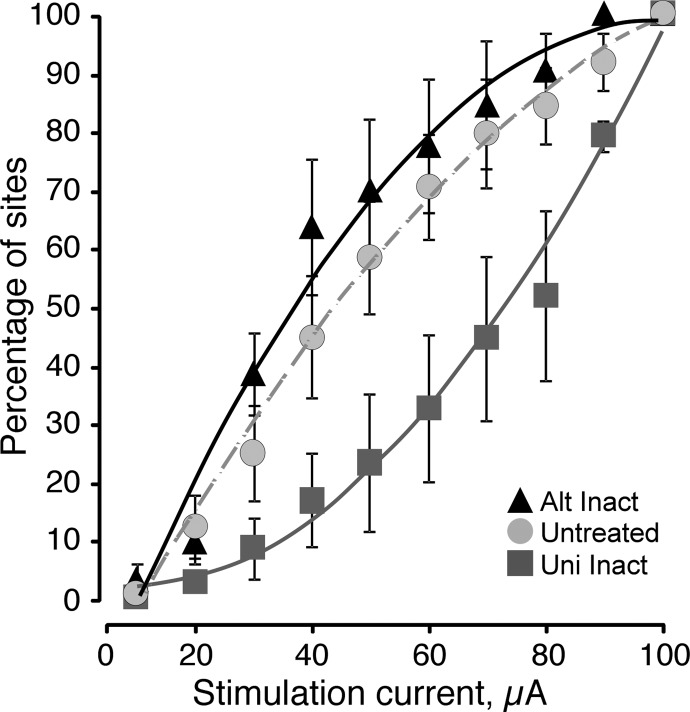
During each experiment we determined the effects of ICMS using a maximal current of 100 μA. However, stimulation at many sites in animals of each group evoked movement at substantially lower currents. For each animal, we combined thresholds into 10-μA bins and constructed a cumulative distribution histogram of percentage of effective sites for each animal group (unilateral inactivation M1 only: n = 5 cats; 58 sites; initially inactivated M1 followed by contralateral inactivation; termed alternate inactivation: n = 5 cats; 86 sites; untreated M1: n = 5 cats; 87 sites; Fig. 2). The relation for M1 subjected to unilateral inactivation was accelerating (solid gray line; r = 0.989); with each 10-μA increment, a greater number of additional responses were evoked. This relation suggests the presence of sparse high-threshold sites, each recruited with increasing stimulus amplitude. By contrast, the stimulus–response relation for alternate inactivation (solid black line; r = 0.978) shows a saturating function. With each 10-μA increment, fewer additional responses were evoked after about 60 μA. This characterizes a high-resolution map, with a preponderance of low-threshold effective sites, as we have reported for developing M1 (Chakrabarty and Martin 2000; Martin et al. 2005). The relation for alternate inactivation is identical in its form to that of the untreated M1 in unilateral inactivation animals (dashed gray line; r = 0.994), also a saturating function. There was a significant difference among these groups (two-way ANOVA; F = 31.46, df = 2, P < 0.0001). Unilateral inactivation was significantly lower than alternate inactivation (F = 58.07, P < 0.0001, df = 1) and the untreated side (F = 32.69, df = 1, P < 0.0001), whereas alternate inactivation and the untreated side were not significantly different (F = 3.62, df = 1, P = 0.06). The positively accelerating relation for unilateral inactivation is specifically related to the muscimol infusion because in the saline control we observed the same saturating function as with untreated side and alternate inactivation (data not shown). These changes in the stimulus–response relations suggest that alternate inactivation improved the efficiency of spinal motor circuit activation by ICMS, like that of untreated M1 (Chakrabarty and Martin 2000).
FIG. 2.
Stimulus–motor response relations are affected by unilateral and alternate inactivation. Data are from the inactivated M1 in unilateral inactivation animals, the initially inactivated M1 in alternate inactivation animals, and M1 on the untreated side in the animals subjected to unilateral inactivation (i.e., no inactivation). Data from all animals (weeks 11–16) are pooled. Maximal current for this analysis was 100 μA. The plot shows cumulative percentage of sites that were effective in relation to increasing intracortical microstimulation (ICMS) current. (r values for line fits. Untreated: r = 0.994; unilateral inactivation: r = 0.989; alternate inactivation: r = 0.978).
We also used the stimulus–response relation to determine the optimal current range for assessing the motor representation across the different M1 groups, rather than using an arbitrary maximal current limit, as in most ICMS studies. Different outcomes could occur depending on the current cutoff we used. For example, using a very low current cutoff would reveal few motor responses overall, at only the strongest and most optimized M1 sites. By contrast, a high current cutoff would reveal responses at most, if not all, M1 sites, through direct effects of both the strong stimulus and the current spread. Thus the higher the maximal current used to assess the motor map, the less sensitive will be the measure. We therefore chose 60 μA as the cutoff; using a higher cutoff would have been less sensitive for distinguishing the effects of inactivation because the relation between the inactivated and control sides is small.
Alternate inactivation increases total effective sites and distal sites
Figure 3 plots all sites in all animals where ICMS evoked a movement using currents ≤100 μA (top row) and 60 μA (bottom row). Maps from individual animals were aligned with the lateral tip of the cruciate sulcus and superimposed. Each point is plotted as a semitransparent circle. The top row plots the boundaries of the forelimb zone (black outlines), defined by no response at 100 μA or the presence of a nonforelimb effect. Caudally, thresholds rose as penetrations became close to the area 3a–4 border. This resulted in a consistent caudal boundary. Caudomedially, we encountered trunk or hind limb sites. Rostromedially, we mapped to within approximately 4 mm of the midline, which is close to or at the area 4γ–6 border (Ghosh 1997; Hassler and Muhs-Clement 1964). In some experiments, we mapped to the rostral or rostrolateral bony margin of the brain, which is also close to the area 4γ border (Hassler and Muhs-Clement 1964). Thus we examined most, if not all, of the forelimb representation. Importantly, the impact of missing a region on our conclusions was minimized by our experimental design, which focused on the forelimb zone within the same anatomical region in all animal groups. Comparison of A and B shows that restricting our quantitative analyses to sites at which effects were produced at ≤60 μM tended to eliminate more sites at the representation border for all the groups and more sites overall in the animals with the unilateral inactivation.
FIG. 3.
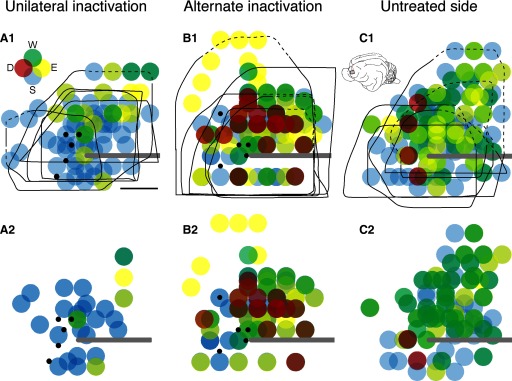
Changes in M1 motor representation after unilateral and alternate inactivation. Each colored circle plots the joint effect obtained by stimulation. Data from all experiments are superimposed, with effects obtained at <100 μA shown in the top row and <60 μA, in the bottom row. The black lines in the top row mark the boundaries of the forelimb representation. The dashed portion of the lines correspond to the bony limit of the craniotomy, which was at or very close to the area 4γ border in that experiment. The inset in A1 shows the color coding used (D, digits; W, wrist; E, elbow; S, shoulder) and, in C1, the cat brain and the region explored. Each dot was plotted as 50% transparent, with distal shown as the most superficial graphical layer and proximal, the deepest. A: unilateral inactivated (left) M1. B: alternate inactivation (i.e., the left side, which received the initial inactivation between weeks 5–7, but examined after inactivation of the other M1 from weeks 7–11). C: untreated M1 (i.e., right; contralateral to the M1 that received unilateral inactivation only). Small black dots indicate the infusion sites. Calibration: 2 mm.
Inactivation between weeks 5 and 7, when tested using a maximal current of 60 μA, resulted in fewer effective sites when mapped at weeks 11–16 (Fig. 3A2) than either age-matched controls (unilateral inactivation: 36 ± 12.9%; age-matched controls: 73.7 ± 3.32%; unpaired t-test; t = 3.176; P = 0.009; df = 11; Chakrabarty and Martin 2000) or the untreated M1 (untreated side: 82 ± 11.4%; Fig. 3C2; unpaired t-test; t = 3.744; P = 0.0057; df = 8). There was also a shift to a higher proportion of sites evoking only proximal movements. For comparison, the map of the untreated M1 (Fig. 3C), like controls (Chakrabarty and Martin 2000), had a balance of sites from which proximal and distal movement were evoked. Note that few sites were found from which digit movements were evoked (see following text for further quantification of these effects). Mapping of M1 that was inactivated between weeks 5 and 7, but after alternate inactivation of the unaffected side between weeks 7 and 11 (Fig. 3B2), resulted in a significant increase in the number of effective sites compared with M1 subjected to unilateral inactivation only (alternate inactivation: 72 ± 8%; unpaired t-test; t = 3.477; P = 0.008;df = 8). The percentage of effective sites after alternate inactivation was not different from that of either age-matched controls (unpaired t-test; t = 0.5; P = 0.627; df = 11) or the untreated M1 (t = 0.809; P = 0.442; df = 8). Mean thresholds paralleled these differences in percentage of effective sites. Using the 60-μA cutoff, on average 44.8 ± 5.3 μA was needed to evoke responses in unilateral inactivation animals, 39.8 μA ±1.4 μA for alternate inactivation animals and 37.4 ± 3.3 μA for the untreated side after unilateral inactivation.
We verified that our data did not reflect the number of sites sampled in the different animal groups. We randomly sampled 10 electrode penetrations in each animal. From those penetrations we computed the percentage of effective sites and the mean thresholds. Using an unpaired t-test, we found that there were no significant differences between the values obtained in the randomly reduced sample of penetrations and the full data set. Our findings suggest that alternate inactivation resulted in a balanced and high-density representation of proximal and distal joints. There was a surprising increase in the number of sites from which digit movements were evoked.
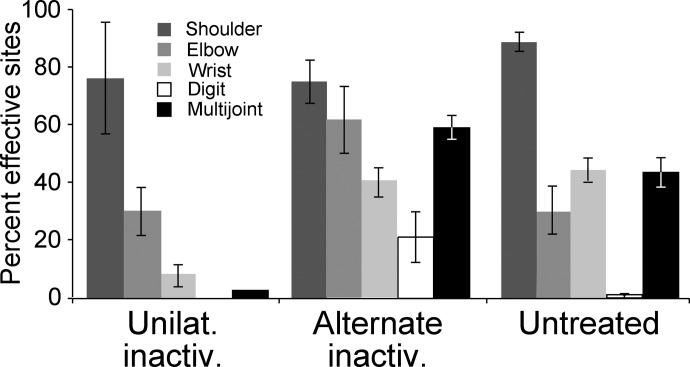
We next quantified the changes we observed in joints represented after inactivation, based on the 60-μA cutoff. We tallied the number of sites evoking particular joint responses for each animal and expressed this as the percentage of the total number of effective sites for the particular animal (Fig. 4). Note that the total of individual joint values for each group exceeds 100% because there were sites from which multijoint movements were evoked. Unilateral inactivation significantly reduced wrist (t = 6.186; P = 0.0003; df = 8) and multijoint (t = 5.904; P = 0.0004; df = 8) responses; digit responses were eliminated. Subsequent alternate inactivation restored the representation of wrist (t = 2.515; P = 0.036; df = 8) and multijoint sites (t = 8.15; P < 0.0001; df = 8), with a possible overshoot to more distal bias because a large number of digit sites were identified. Although proximal joints did not show major differences between groups, there was a significant increase in elbow responses after unilateral inactivation (t = 5.389; P = 0.0007; df = 8) as a result of the increase in multijoint responses. Our findings show that alternate inactivation converts the sparse proximal joint representation after unilateral inactivation to a higher-density proximal-to-distal representation.
FIG. 4.
Proximal sites are represented predominantly after unilateral inactivation, whereas both proximal and distal sites are represented after alternate inactivation. Data from the M1 that received unilateral inactivation (between weeks 5 and 7 only; A), M1 that received the initial inactivation between weeks 5 and 7, but examined after inactivation of the other M1 from weeks 7 to 11 (alternate inactivation; B) and the untreated M1 (C) are shown.
Percentage of map representing particular joints
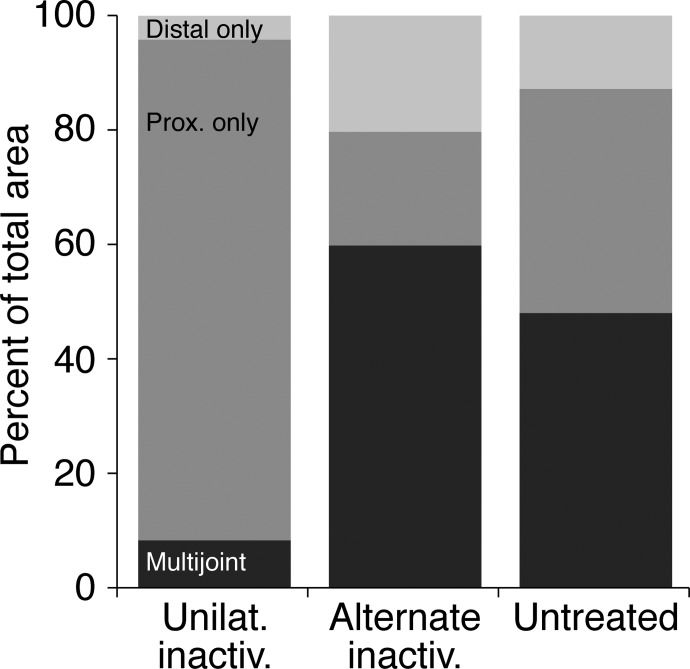
We next determined whether there were any systematic differences in the size of the motor representation in the different animal groups and how space within the motor representation was allocated between the different forelimb joints. For this analysis we determined the area of the map devoted to representing particular joints, using the 60-μA cutoff. When different joints were represented at adjacent sites, we divided the distance between sites in half and apportioned the area between the two joints. There were no significant overall map areal differences between groups (ANOVA; P = 0.25; df = 2), although there was a trend toward the largest maps in the untreated and the smallest maps in the unilateral inactivation group; the alternate inactivation maps were in between (untreated: 18.3 ± 5.9 mm2; unilateral inactivation: 12.3 ± 5.9 mm2; alternate inactivation: 15.1 ± 4.1 mm2). To assess the percentage of the total map area represented by different forelimb joints for each group we combined sites from which shoulder and elbow movements were evoked into a single proximal joint category. Wrist and digit sites were combined into a single distal joint category. Finally, sites evoking any combination of proximal and distal joints were combined into a multijoint category. Figure 5 plots the three joint categories. There was a substantial loss of the multijoint representation in the unilateral inactivation M1; the proximal joint representation dominated the map. By contrast, most of the area in the alternate inactivation M1 and M1 on the untreated side was devoted to multijoint responses, with a mixture of distal and proximal only regions. These findings show that alternate inactivation restored a balance between distal, proximal, and multijoint regions. The Kruskal–Wallis rank test showed a significant difference in the multijoint region only (H = 9.104; P = 0.0104; df = 9). Using a pairwise Mann–Whitney test, the differences between the multijoint regions for the unilateral inactivation and alternate inactivation were significant (Z = 2.611; P = 0.009; df = 9) as were differences between unilateral inactivation and the untreated M1 (Z = 2.402; P = 0.016; df = 9). The multijoint regions were not different between the alternate inactivation and untreated M1 (Z = 0.94; P = 0.347; df = 9). These findings show that alternate inactivation restored M1 territories capable of evoking multijoint responses. M1 sites encoding multijoint movements may be particularly important in motor skills (Nudo et al. 1996).
FIG. 5.
Percentage of total map area devoted to the representation of proximal joints (shoulder and elbow), distal joints (wrist and digits), or proximal–distal multijoint. Data from the M1 that received unilateral inactivation (between weeks 5 and 7 only), M1 that received the initial inactivation between weeks 5 and 7, but examined after inactivation of the other M1 from weeks 7 to 11 (alternate inactivation) and the untreated M1, are shown.
Restoration of the M1 map occurs during the period of alternate inactivation
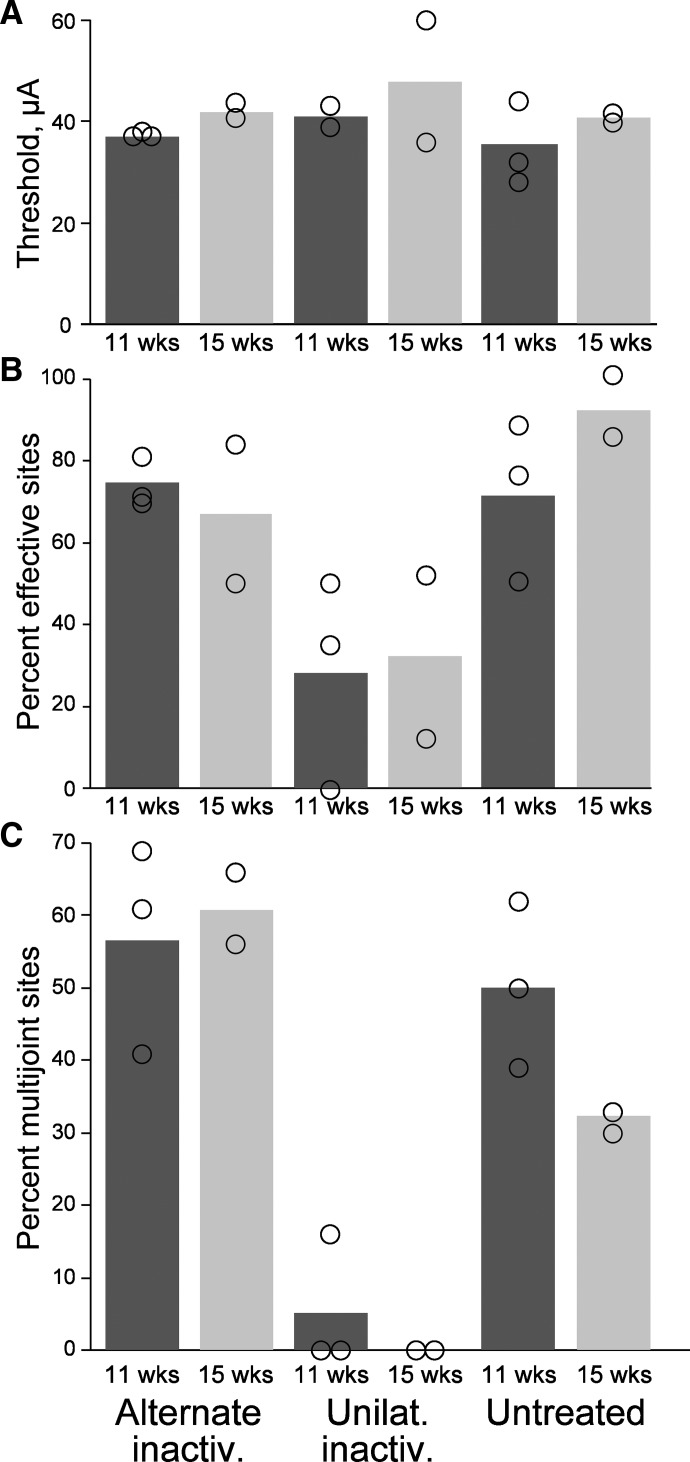
In our previous study we found that alternate inactivation restored CST connections with spinal premotor circuits several weeks before animals recovered normal forepaw accuracy during visually guided locomotion (Friel and Martin 2007). We proposed that further plastic changes were required as the animals' motor systems learned to use the restored connections during this period to achieve recovery of motor function. Plausibly, an important change underlying this recovery of skill is restoration of the M1 motor map during this late period, after the alternate inactivation has restored CST connectivity. We investigated this question in the present study. To determine whether there were systematic changes in the M1 motor map during the period animals regained motor skill, we divided the unilateral and alternate inactivation animals into two groups, depending on when the terminal M1 mapping experiment took place: between weeks 11 and 13 and 15 and 16. Figure 6 plots mean thresholds (A), percentage effective sites (B), and percentage multijoint sites (C) for the two age groups. There were no systematic differences between the two groups, nor within each group. These findings suggest that restoration of the M1 motor map after alternate inactivation occurs by the end of the alternate inactivation period since there is no trend to further improvement after a longer survival period.
FIG. 6.
The M1 motor representations at weeks 11 through 13 and 14 through 16 are not different. Bars plot mean values. Open circles plot data values from individual animals comprising the 2 ages for each group. Column labeled “11 wks” plots data for animals between weeks 11 and 13. Column labeled “15 wks” plots data for animals between weeks 14 and 16.
DISCUSSION
Alternate inactivation converted the sparse proximal M1 motor representation produced by unilateral inactivation to a high-resolution and complete proximal–distal representation. Importantly, M1 representational plasticity and restoration of CST terminations to more ventral laminae of the dorsal and ventral horns (Friel and Martin 2007) occurred concurrently. We confirmed the timing of CST termination changes in three additional animals in this study (Supplemental Fig. S1).1 After unilateral inactivation, densest labeling is within laminae 3 and 4 of the dorsal horn and the total labeling is shifted dorsal to control levels (contour line). By contrast, after alternate inactivation labeling is densest in laminae 5 and 6 of the dorsal horn and intermediate zone and total labeling overlaps with the territory occupied by CST terminations in controls, both at 11 and 15 wk. Motor map parameters are restored to values similar to those of controls during the alternate inactivation period. Restoration of the M1 motor map after alternate inactivation is coincident with restoration of a normal pattern of CST connections with spinal motor circuits. Unilateral inactivation also impairs motor skills. It produces a consistent endpoint control impairment: the paw overshoots the target during reaching (Martin et al. 2000) and oversteps during visually guided locomotion (Friel et al. 2007). After alternate inactivation, step distance returns to control values but, as we showed previously, this does not occur until 3 (2.9 ± 0.54) wk after the end of the alternate inactivation (Friel and Martin 2007). We also confirmed this in the present study (Supplemental Fig. S2). The motor map is restored before skilled forelimb control, which suggests that the motor map is necessary for motor skill development, but that the motor systems needed additional time after the alternate inactivation, or motor experience, to take advantage of the restored connectivity.
Muscimol infusion inactivates the lateral forelimb areas of rostral and caudal M1
Using the metabolic marker cytochrome oxidase, muscimol infusion between weeks 5 and 7 maximally inhibits a 2.5- to 3-mm radius of cortex surrounding the infusion site and progressively less inhibition for an additional 4–5 mm (Martin et al. 1999). This inactivation also produces a reduction in the level of parvalbumin staining over approximately the same distance as observed for the cytochrome oxidase reduction (Friel et al. 2007). These anatomical correlates of inactivation suggest that the sites mapped were within the area of left M1 inactivated between 5 and 7 wk.
Muscimol infusion does not produce a lesion, as shown by comparing, within the infused and noninfused cortex, cell body density, the distribution of neurofilament-F (SMI-32) immunoreactivity, and parvalbumin cell body staining (Friel et al. 2007). We also found that the cross-sectional areas of the medullary pyramids, where CST axons converge on each side, and SMI-32 staining in the lateral spinal column on each side were not different (Friel and Martin 2005; Martin et al. 1999, 2000). Vehicle infusions at various ages (5–7 wk: Friel and Martin 2005; Martin et al. 1999; >15 wk: I. Salimi, K. M. Friel, and J. H. Martin, Soc Neurosci Abstr 2004) does not produce any noticeable placing/behavioral defect, nor does it change CST terminations (Friel and Martin 2005; Salimi, Friel, and Martin, Soc Neurosci Abstr 2004). Moreover, we showed in this study that saline infusion between weeks 5 and 7 has no effect on the motor map development. These studies show that muscimol infusion produces particular behavioral and CST anatomical changes across all ages studied and that vehicle infusion does not. The effect of alternate inactivation is likely due to the robust actions of muscimol on cortical neuronal excitability.
Stimulation of the untreated side after unilateral inactivation does not produce ipsilateral responses
Unilateral M1 reversible inactivation between weeks 5 and 7 leads to a prominent ipsilateral projection from the untreated (active) side to lamina 7 of the intermediate zone and ventral horn (Friel and Martin 2005; Martin et al. 1999). These connections are likely to be functional. They are located within the laminae containing premotor interneurons (Alstermark and Kümmel 1990) and, when they are electrically stimulated, evoke ipsilateral ventral focal synaptic potentials (Chakrabarty and Martin 2008). Surprisingly, ICMS at no site in any unilaterally inactivated animal produced an ipsilateral forelimb response. Ipsilateral CST projections are thought to be functional in the normal mature nervous system because they are numerous (Brosamle and Schwab 1997; Jankowska and Edgley 2006; Lacroix et al. 2004). There is also a growing literature for ipsilateral CST functions in adult humans (Schaefer et al. 2007; Wang and Sainburg 2006). Moreover, after unilateral reversible inactivation the untreated M1 appears to exert adaptive control of ipsilateral limb movements. We found that inactivation of the untreated M1 in maturity exacerbates ipsilateral motor impairments during reaching, both endpoint errors and grasping impairments (Martin et al. 2000). This suggests that aberrant ipsilateral terminations may be used by the motor systems to help guide the forelimb. A similar adaptive role has been proposed for ipsilateral CST axons after stroke (Ward and Cohen 2004).
With ipsilateral CST projections in place, but no ipsilateral motor representation, we propose that these connections are not optimized for activation of spinal motor circuits at this point in development. Ipsilateral terminations may normally function in coordination with the dense contralateral terminations from the other hemisphere. During unilateral movements, M1 neurons in each hemisphere can modulate their activity (Cisek et al. 2003; Donchin et al. 2002), suggesting some degree of bilateral control of unilateral limb movements. The absence of ipsilateral responses could reflect the absence of the ventral contralateral projection from the CST of M1 in the other hemisphere (Friel and Martin 2005). In the mature rat, ipsilateral responses are commonly evoked by ICMS in M1 (Kartje-Tillotson et al. 1985; Liang et al. 1993). However, ipsilateral responses were rapidly eliminated when M1 in the other hemisphere was reversibly inactivated (M. Brus-Ramer, J. Carmel, and J. Martin, unpublished observations).
M1 map is necessary but not sufficient for expression of accurate visually guided locomotion during development
In maturity, enhanced motor experience leads to an expansion in the motor map and motor skill improvement (Nudo et al. 1996; Sanes and Donoghue 2000). Conversely, rapid elimination of the map correlates with a loss of reaching skills (Kleim et al. 2003). Thus the extent of the M1 map correlates with motor skill. Whereas there are reports of animals recovering skills following CS system damage without concomitant map reorganization (Nudo and Milliken 1996; Plautz et al. 2003), the preponderance of evidence shows strong support for the reemergence of and shifts in M1 representations with recovery after injury (Chen et al. 2002; Mano et al. 1995; Ward and Cohen 2004). The bulk of evidence shows that the M1 motor representation in maturity is necessary for producing many forms of skilled limb movements. Indeed, in the stroke literature, for example, it is implied that a therapeutic strategy that leads to reforming the M1 motor map after injury is tantamount to promoting skill (Brown et al. 2006).
Several studies in healthy animals and humans have noted that reorganization of the M1 motor representation follows the acquisition of new motor skills in maturity, suggesting that the map reflects an established, or consolidated, motor representation (Monfils et al. 2005). Rather, we show that map reorganization precedes restoration of endpoint control. The percentage of effective sites, thresholds, and joints represented are not systematically different at 11 wk, which is before endpoint control is restored, and at 15 wk, when animals position their forepaw normally (Friel and Martin 2007; Supplemental Fig. S2). Similarly, motor map development in normal kittens may lead to motor skill development. The map is expressed around week 7 (Chakrabarty and Martin 2005), following which the kitten's motor repertoire greatly expands (Martin and Bateson 1985). We propose that the motor systems must learn to use the newly developed map, and its associated spinal connections, before skills are expressed. In this way, the map reflects capacity; it is more of a substrate on which motor skills are learned rather than a skilled movement representation, per se.
Alternate inactivation has afforded us an additional way to examine motor development. In both humans and animals, normal development of motor behavior cannot be solely attributed to motor system maturation, much less to M1 map development, because of concurrent maturation of cognitive and visual systems. It is impossible to disentangle the contributions of one from the other. Since M1 inactivation targets a portion of the sensorimotor cortex, based on earlier control experiments (e.g., Friel et al. 2007; Martin et al. 1999), the dissociation between M1 map maturation and recovery of motor skill is likely to reflect changes predominantly in CS system organization, not primarily cognitive or visual, development.
Alternate inactivation may strengthen connections with spinal motor circuits
Competition between the developing CS systems on each side for spinal connections is important for normal development of CST functions (Martin 2005). The significantly increased numbers of sites for evoking motor responses by ICMS after the alternate inactivation (compared with unilateral inactivation) strongly suggest that CST connections with spinal motor circuits were strengthened during this period. This correlates with denser CST terminations in laminae 7 and 9 (Friel and Martin 2007). We propose that this restoration of connectivity is driven by making the initially active CST less competitive in developing synaptic connections, due to reduced activation during the alternate inactivation period.
The activity of two sets of spinal connections could be affected by the alternate inactivation that would reduce competition faced by the initially inactivated CST. First, alternate inactivation leads to a reduction in the aberrant ipsilateral projection from the initially active side (Friel and Martin 2007). Although ipsilateral CST projections normally may be adaptive, as discussed earlier, when their outgrowth is unchecked they may compete with contralateral connections. Second, the alternate inactivation would be expected to reduce activation of the spinal commissural system. The CST can access this system via a cortico-reticulo-spinal pathway, as shown by Jankowska, Edgley, and colleagues (Edgley et al. 2004; Jankowska and Edgley 2006; Jankowska et al. 2006; Stecina et al. 2008).
M1 motor map reflects CST spinal termination pattern
Prior studies have emphasized the importance of intracortical connectivity shaping the characteristics of the motor map (Sanes and Donoghue 2000). Although we do not deny the importance of intracortical connections, our findings show a strong and consistent association between developmental plasticity of the M1 motor map and plasticity of the topographic features of CST terminations. Even though it is obvious that CST connections in the spinal cord are essential for motor map function, it has been largely overlooked since the early studies of Asanuma, Brooks, and colleagues (for review, see Asanuma 1981). After 7 wk, which is when CST spinal terminations achieve a mature laterality and laminar distribution, maturation of the density (Li and Martin 2001, 2002) and strength (Meng et al. 2004) of CST spinal terminations correlate well with maturation of the M1 motor map. Early postnatal M1 inactivation blocks development of ventrally projecting CST terminations to the deep dorsal horn, intermediate zone, and ventral horn and impairs motor map development. Restoration of connections with alternate inactivation correlates with restoration of the motor map.
Although it may be impossible to dissociate local cortical connections from spinal terminations, we propose that key to restoration of the map and motor control is reestablishing connectivity with particular premotor interneuronal and spinocerebellar circuits. Transneuronally labeled premotor interneurons, following wheat-germ agglutinin–horseradish peroxidase injection into forelimb muscles, are located throughout laminae 5–8 in the cat (Alstermark and Kümmel 1990). Comparison with our findings (Friel and Martin 2007; Supplemental Fig. S1A) shows a virtual elimination of potential connectivity with premotor interneurons after unilateral inactivation. Remarkably, alternate inactivation restores terminations throughout the premotor field. Spinocerebellar neurons are located throughout the spinal gray matter, but are especially dense in the medial portion of laminae 6 and 7 (Matsushita et al. 1979), where CST terminations are consistently dense. Moreover, many spinocerebellar neurons are premotor (Jankowska and Puczynska 2008) and premotor interneurons engage precerebellar circuitry (Alstermark et al. 1981, 1990). The absence of these CST connections could explain both the motor map defects and motor control impairment. Surprisingly, spinal (or brain stem) motor networks are incapable of effectively rerouting information so that CST neurons with aberrant terminations can access spinal motor output circuits controlling diverse forelimb joints. Restoration of CST connections with spinal premotor and spinocerebellar circuits after alternate inactivation could provide an anatomical substrate for reestablishing oligosynaptic access of the M1 motor map to motoneurons.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-33835 to J. H. Martin.
Acknowledgments
We thank X.-L. Wu for assistance with histochemistry; G. Asfaw and Dr. M. Osman for veterinary care; and R. J. Salway, E. Nunnink, and C. Mendoza-Tolentino for help with experiments.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Alstermark et al. 1990.Alstermark B, Isa T, Tantisira B. Projection from excitatory C3–C4 propriospinal neurones to spinocerebellar and spinoreticular neurones in the C6–Th1 segments of the cat. Neurosci Res 8: 124–130, 1990. [DOI] [PubMed] [Google Scholar]
- Alstermark and Kümmel 1990.Alstermark B, Kümmel H. Transneuronal transport of wheat germ agglutinin conjugated horseradish peroxidase into last order spinal interneurones projecting to acromio- and spinodeltoideus motoneurones in the cat. 2. Differential labelling of interneurones depending on movement type. Exp Brain Res 80: 96–103, 1990. [DOI] [PubMed] [Google Scholar]
- Alstermark et al. 1981.Alstermark B, Lundberg A, Norrsell U, Sybirska E. Integration in the descending motor pathways controlling the forelimb in the cat. 9. Differential behavioural defects after spinal cord lesion interrupting defined pathways from higher centers to motoneurons. Exp Brain Res 42: 299–318, 1981. [DOI] [PubMed] [Google Scholar]
- Alstermark and Ohlson 2000.Alstermark B, Ohlson S. Origin of corticospinal neurones evoking monosynaptic excitation in C3–C4 propriospinal neurones in the cat. Neurosci Res 38: 249–256, 2000. [DOI] [PubMed] [Google Scholar]
- Armstrong and Drew 1985.Armstrong DM, Drew T. Electromyographic responses evoked in muscles of the forelimb by intracortical stimulation in the cat. J Physiol 367: 309–326, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma 1981.Asanuma H The pyramidal tract. In: Handbook of Physiology. The Nervous System. Motor Control. Bethesda, MD: Am. Physiol. Soc., 1981, sect. 1, vol. II, pt. 2, p. 703–733.
- Asanuma and Sakata 1967.Asanuma H, Sakata H. Functional organization of a cortical efferent system examined with focal depth stimulation in cats. J Neurophysiol 30: 35–54, 1967. [Google Scholar]
- Brosamle and Schwab 1997.Brosamle C, Schwab ME. Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. J Comp Neurol 386: 293–303, 1997. [DOI] [PubMed] [Google Scholar]
- Brown et al. 2006.Brown JA, Lutsep HL, Weinand M, Cramer SC. Motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurgery 58: 464–473, 2006. [DOI] [PubMed] [Google Scholar]
- Bruce and Tatton 1980.Bruce IC, Tatton WG. Synchronous development of motor cortical output to different muscles in the kitten. Exp Brain Res 40: 349–353, 1980. [DOI] [PubMed] [Google Scholar]
- Chakrabarty and Martin 2000.Chakrabarty S, Martin JH. Postnatal development of the motor representation in primary motor cortex. J Neurophysiol 84: 2582–2594, 2000. [DOI] [PubMed] [Google Scholar]
- Chakrabarty and Martin 2005.Chakrabarty S, Martin JH. Motor but not sensory representation in motor cortex depends on postsynaptic activity during development and in maturity. J Neurophysiol 94: 3192–3198, 2005. [DOI] [PubMed] [Google Scholar]
- Chakrabarty and Martin 2008.Chakrabarty S, Martin JH. Effects of activity blockade on development of CST monosynaptic connections. Soc Neurosci Abstr 29.13, 2008.
- Chen et al. 2002.Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience 111: 761–773, 2002. [DOI] [PubMed] [Google Scholar]
- Cisek et al. 2003.Cisek P, Crammond DJ, Kalaska JF. Neural activity in primary motor and dorsal premotor cortex in reaching tasks with the contralateral versus ipsilateral arm. J Neurophysiol 89: 922–942, 2003. [DOI] [PubMed] [Google Scholar]
- Donchin et al. 2002.Donchin O, Gribova A, Steinberg O, Mitz AR, Bergman H, Vaadia E. Single-unit activity related to bimanual arm movements in the primary and supplementary motor cortices. J Neurophysiol 88: 3498–3517, 2002. [DOI] [PubMed] [Google Scholar]
- Edgley et al. 2004.Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. J Neurosci 24: 7804–7813, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel and Martin 2005.Friel K, Martin JH. Role of sensory-motor cortex activity in postnatal development of corticospinal axon terminals in the cat. J Comp Neurol 485: 43–56, 2005. [DOI] [PubMed] [Google Scholar]
- Friel and Martin 2007.Friel K, Martin JH. Bilateral activity-dependent interactions in the developing corticospinal system. J Neurosci 27: 11083–11090, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel et al. 2007.Friel KM, Drew T, Martin JH. Differential activity-dependent development of corticospinal control of movement and final limb position during visually guided locomotion. J Neurophysiol 97: 3396–3406, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh 1997.Ghosh S Identification of motor areas of the cat cerebral cortex based on studies of cortical stimulation and corticospinal connections. J Comp Neurol 380: 191–214, 1997. [DOI] [PubMed] [Google Scholar]
- Hassler and Muhs-Clement 1964.Hassler R, Muhs-Clement K. Architektonischer Aufbau des sensorimotorischen und parietalen Cortex der Katze. J Hirnforsch 6: 377–422, 1964. [PubMed] [Google Scholar]
- Jankowska and Edgley 2006.Jankowska E, Edgley SA. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist 12: 67–79, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska and Puczynska 2008.Jankowska E, Puczynska A. Interneuronal activity in reflex pathways from group II muscle afferents is monitored by dorsal spinocerebellar tract neurons in the cat. J Neurosci 28: 3615–3622, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska et al. 2006.Jankowska E, Stecina K, Cabaj A, Pettersson LG, Edgley SA. Neuronal relays in double crossed pathways between feline motor cortex and ipsilateral hindlimb motoneurones. J Physiol 575: 527–541, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartje-Tillotson et al. 1985.Kartje-Tillotson G, Neafsey EJ, Castro AJ. Electrophysiological analysis of motor cortical plasticity after cortical lesions in newborn rats. Brain Res 332: 103–111, 1985. [DOI] [PubMed] [Google Scholar]
- Keller 1993.Keller A Intrinsic connections between representation zones in the cat motor cortex. Neuro Report 4: 515–518, 1993. [DOI] [PubMed] [Google Scholar]
- Kleim et al. 2003.Kleim JA, Bruneau R, Calder K, Pocock D, VandenBerg PM, MacDonald E, Monfils MH, Sutherland RJ, Nader K. Functional organization of adult motor cortex is dependent upon continued protein synthesis. Neuron 40: 167–176, 2003. [DOI] [PubMed] [Google Scholar]
- Lacroix et al. 2004.Lacroix S, Havton LA, McKay H, Yang H, Brant A, Roberts J, Tuszynski MH. Bilateral corticospinal projections arise from each motor cortex in the macaque monkey: a quantitative study. J Comp Neurol 473: 147–161, 2004. [DOI] [PubMed] [Google Scholar]
- Li and Martin 2000.Li Q, Martin JH. Postnatal development of differential projections from the caudal and rostral motor cortex subregions. Exp Brain Res 134: 187–198, 2000. [DOI] [PubMed] [Google Scholar]
- Li and Martin 2001.Li Q, Martin JH. Postnatal development of corticospinal axon terminal morphology in the cat. J Comp Neurol 435: 127–141, 2001. [DOI] [PubMed] [Google Scholar]
- Li and Martin 2002.Li Q, Martin JH. Postnatal development of connectional specificity of corticospinal terminals in the cat. J Comp Neurol 447: 57–71, 2002. [DOI] [PubMed] [Google Scholar]
- Liang et al. 1993.Liang F, Rouiller EM, Wiesendanger M. Modulation of sustained electromyographic activity by single intracortical microstimuli: comparison of two forelimb motor cortical areas of the rat. Somatosens Mot Res 10: 51–61, 1993. [DOI] [PubMed] [Google Scholar]
- Mano et al. 1995.Mano Y, Nakamuro T, Tamura R, Takayanagi T, Kawanishi K, Tamai S, Mayer RF. Central motor reorganization after anastomosis of the musculocutaneous and intercostal nerves following cervical root avulsion. Ann Neurol 38: 15–20, 1995. [DOI] [PubMed] [Google Scholar]
- Martin 1996.Martin JH Differential spinal projections from the forelimb areas of the rostral and caudal subregions of primary motor cortex in the cat. Exp Brain Res 108: 191–205, 1996. [DOI] [PubMed] [Google Scholar]
- Martin 2005.Martin JH The corticospinal system: from development to motor control. Neuroscientist 11: 161–173, 2005. [DOI] [PubMed] [Google Scholar]
- Martin et al. 2005.Martin JH, Engber D, Meng Z. Effect of forelimb use on postnatal development of the forelimb motor representation in primary motor cortex of the cat. J Neurophysol 93: 2822–2831, 2005. [DOI] [PubMed] [Google Scholar]
- Martin et al. 2000.Martin JH, Hacking A, Donarummo L. Impairments in prehension produced by early postnatal sensorimotor cortex activity blockade. J Neurophysiol 83: 895–906, 2000. [DOI] [PubMed] [Google Scholar]
- Martin et al. 1999.Martin JH, Kably B, Hacking A. Activity-dependent development of cortical axon terminations in the spinal cord and brain stem. Exp Brain Res 125: 184–199, 1999. [DOI] [PubMed] [Google Scholar]
- Martin and Bateson 1985.Martin P, Bateson P. The ontogeny of locomotor play behavior in the domestic cat. Anim Behav 15: 59–103, 1985. [Google Scholar]
- Matsushita et al. 1979.Matsushita M, Hosoya Y, Ikeda M. Anatomical organization of the spinocerebellar system in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol 184: 81–106, 1979. [DOI] [PubMed] [Google Scholar]
- Meng et al. 2004.Meng Z, Li Q, Martin JH. The transition from development to motor control function in the corticospinal system. J Neurosci 24: 605–614, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils et al. 2005.Monfils MH, Plautz EJ, Kleim JA. In search of the motor engram: motor map plasticity as a mechanism for encoding motor experience. Neuroscientist 11: 471–483, 2005. [DOI] [PubMed] [Google Scholar]
- Nudo and Milliken 1996.Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol 75: 2144–2149, 1996. [DOI] [PubMed] [Google Scholar]
- Nudo et al. 1996.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci 16: 785–807, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas and Strick 1981.Pappas CL, Strick PL. Physiological demonstration of multiple representation in the forelimb region of the cat motor cortex. J Comp Neurol 200: 481–490, 1981. [DOI] [PubMed] [Google Scholar]
- Plautz et al. 2003.Plautz EJ, Barbay S, Frost SB, Friel KM, Dancause N, Zoubina EV, Stowe AM, Quaney BM, Nudo RJ. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res 25: 801–810, 2003. [DOI] [PubMed] [Google Scholar]
- Sanes and Donoghue 2000.Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci 23: 393–415, 2000. [DOI] [PubMed] [Google Scholar]
- Schaefer et al. 2007.Schaefer SY, Haaland KY, Sainburg RL. Ipsilesional motor deficits following stroke reflect hemispheric specializations for movement control. Brain 130: 2146–2158, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecina et al. 2008.Stecina K, Jankowska E, Cabaj A, Pettersson LG, Bannatyne BA, Maxwell DJ. Premotor interneurones contributing to actions of feline pyramidal tract neurones on ipsilateral hindlimb motoneurones. J Physiol 586: 557–574, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang and Sainburg 2006.Wang J, Sainburg RL. The symmetry of interlimb transfer depends on workspace locations. Exp Brain Res 170: 464–471, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward and Cohen 2004.Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol 61: 1844–1848, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]