Abstract
γ-Aminobutyric acid type A (GABAA) receptor α5 subunits, which are heavily expressed in the hippocampus, are potential drug targets for improving cognitive function. They are found at synaptic and extrasynaptic sites and have been shown to mediate tonic inhibition in pyramidal neurons. We tested the hypothesis that α5 subunits also contribute to synaptic inhibition by measuring the effect of diazepam (DZ) on spontaneous and stimulus-evoked inhibitory postsynaptic currents (IPSCs) in genetically modified mice carrying a point mutation in the α5 subunit (α5-H105R) that renders those receptors insensitive to benzodiazepines. In wild type mice, DZ (1 μM) increased the amplitude of spontaneous IPSCs (sIPSCs) and stimulus-evoked GABAA,slow IPSCs (eIPSCs) and prolonged the decay of GABAA,fast sIPSCs. In α5-mutant mice, DZ increased the amplitude of a small-amplitude subset of sIPSCs (<50 pA) and eIPSCs (<300 pA) GABAA,slow and prolonged the decay of GABAA,fast sIPSCs, but failed to increase the amplitude of larger sIPSCs and eIPSCs GABAA,slow. These results indicate that α5 subunits contribute to a large-amplitude subset of GABAA,slow synapses and implicate these synapses in modulation of cognitive function by drugs that target α5 subunits.
INTRODUCTION
γ-Aminobutyric acid type A receptors (GABAARs) are anion-selective ion channels that underlie inhibitory neurotransmission in the brain. These receptors are assembled as pentamers from several closely related classes of subunits (α1–6, β1–3, γ1–3, δ, π, θ, and ɛ) (McKernan and Whiting 1996). Individual subunits differ in their regional and subcellular patterns of distribution, with different subunits conferring distinct biophysical and pharmacological properties to receptors that incorporate them (Fritschy and Mohler 1995; Pirker et al. 2000). Pharmacological modulation of specific GABAARs assemblies has been linked to a number of behavioral effects, including sedation, anxiolysis, amnesia, and reduced seizure susceptibility (Low et al. 2000; McKernan et al. 2000; Rudolph et al. 1999).
Receptors that incorporate α5 subunits show a unique distribution in the brain. They are expressed primarily in the hippocampus, where they comprise close to 25% of all GABAA receptors (Pirker et al. 2000; Sur et al. 1998). Studies using pharmacological agents and genetic manipulations have demonstrated that α5 subunits play a role in hippocampus-dependent learning (Chambers et al. 2004; Collinson et al. 2002; Crestani et al. 2002; Dawson et al. 2006; Gerdjikov et al. 2008; Yee et al. 2004), in generating gamma oscillations (Towers et al. 2004), and in controlling hippocampal network excitability (Glykys and Mody 2006; Houser and Esclapez 2003; Scimemi et al. 2005).
Because they do play important roles in hippocampal function, the physiological nature of inhibition mediated by α5-GABAARs is of considerable interest. Until recently it was thought that the majority of receptors containing α5 subunits are located at extrasynaptic sites (Brunig et al. 2002). This suggested that α5-GABAARs underlie a nonsynaptic form of “tonic inhibition” found in pyramidal cells under conditions of elevated extracellular GABA concentration (Caraiscos et al. 2004; Prenosil et al. 2006; Scimemi et al. 2005). More recent electron microscopic studies have revealed that although many α5-subunits are located at extrasynaptic sites, they are also found at synapses on the dendrites of hippocampal pyramidal cells (Serwanski et al. 2006). Furthermore, it was recently shown that inhibitory postsynaptic potentials (IPSPs) produced by dendrite-targeting interneurons in neocortex use receptors that incorporate α5 subunits (Ali and Thomson 2008). These findings raise the possibility that receptors incorporating α5 subunits may also contribute to some forms of synaptic inhibition in hippocampal CA1 pyramidal neurons. In particular, α5 subunits may contribute to a form of synaptic inhibition located in the dendrites of CA1 neurons that has been termed “GABAA,slow” to reflect its distinctive activation and deactivation kinetics (Pearce 1993). However, studies addressing this issue have yielded conflicting results. Some have supported a contribution of α5 subunits to fast spontaneous and evoked inhibitory postsynaptic currents (sIPSCs and eIPSCs) (Collinson et al. 2002), others have reported no contribution to fast sIPSCs (Caraiscos et al. 2004; Glykys and Mody 2006) but to a fraction of slow sIPSCs (Caraiscos et al. 2004; Glykys and Mody 2006), and yet others have demonstrated a partial contribution of α5 subunits to spontaneous GABAA,slow IPSCs but no contribution to slow eIPSCs (Prenosil et al. 2006).
If α5 subunits do indeed contribute to phasic inhibition, this would have important implications for understanding the mechanisms by which these receptors and the synapses that incorporate them influence cognitive function. Therefore we assessed the contribution of α5-GABAARs to fast somatic and slow dendritic synaptic inhibition by studying genetically altered mice that carry a mutation in the α5 subunit (α5-H105R) rendering α5-containing GABAARs insensitive to diazepam (Crestani et al. 2002). We found that α5 subunits do contribute to a subset of GABAA,slow IPSCs characterized by large-amplitude spontaneous and evoked responses.
METHODS
Mouse breeding
The generation and breeding of α5-H105R mice has been described previously (Crestani et al. 2002). In short, a mutation in the gene of the α5-subunit of the GABAA receptor was generated in RW-4 embryonic stem cells, derived from strain 129X1/SvJ, and resulting mice were bred with 129X1/SvJ mice originally purchased from Research and Consulting Company (RCC, Füllinsdorf, Switzerland). The mutation was maintained on this “Swiss 129X1/SvJ” inbred background. For electrophysiology experiments, male offspring from homozygote or heterozygote breeding pairs were studied. Mice from heterozygote pairs were genotyped using the primers 5′-TTA AAC CGC AGC CTT TCA TCT TTC and 5′-GAG GCC ACC TAA TGC TTC CAG CTT. Because of the inclusion of loxP in an intron adjacent to the exon harboring the H105R point mutation, the amplified fragments are about 50 base pairs larger in the mutant mice than in wild type (WT) mice.
Slice preparation
All animal procedures were performed according to a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee. Male mice, aged 24–54 days (mostly between 30 and 37 days), were decapitated under 3% isoflurane anesthesia. The heads were immediately immersed in cold (∼0°C) “cutting solution” containing (in mM): NaCl 127, KH2PO4 1.2, KCl 1.9, NaHCO3 26, CaCl2·2H2O 0.9, MgSO4·7H2O 2.7, and glucose 10 (pH 7.3 when saturated with 95% O2-5% CO2; 290–300 mOsm). The brain was glued to the tray of a vibrating microtome (Leica Model VT1000S; Leica Microsystems, Nussloch, Germany) using cyanoacrylate glue. Slices 350 or 400 μm thick were cut in a plane approximately 15° off the frontal plane and transferred to an incubation chamber filled with artificial cerebrospinal solution (ACSF) containing (in mM): NaCl 127, KH2PO4 1.2, KCl 1.9, NaHCO3 26, CaCl2·2H2O 2.2, MgSO4·7H2O 1.4, and glucose 10 (pH 7.3 when saturated with 95% O2-5% CO2; 290 mOsm). Slices kept at room temperature remained in the incubation chamber for ≥1 h before being transferred to a recording chamber perfused at 2.5–3.0 ml/min with ACSF.
Electrophysiology
Voltage-clamp recordings were performed at room temperature using standard whole cell patch-clamp techniques (Hamill et al. 1981). Signals were amplified using a MultiClamp 700A amplifier (Molecular Devices, Sunnyvale, CA), filtered at 4 kHz, digitized at 10 kHz using a Digidata 1322A A/D converter (Molecular Devices), and acquired using Clampex (version 9.0.1.25; Molecular Devices).
Inhibitory currents were investigated in isolation. To block glutamatergic transmission, d-2-amino-5-phosphonovaleric acid (AP-5, 20 μM) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μM) were applied in the bath solution. Bicuculline methiodide (20 μM) or picrotoxin (100 μM) were used in a subset of experiments to confirm that responses were mediated by GABAA receptors. Pipettes were filled with an intracellular solution containing (in mM): CsCl 110, KCl 30, MgCl2 2, NaHEPES 10, BAPTA 10, Na2ATP 2, Na3GTP 0.3, and N-(2,6-dimethylphenyl carbamoylmethyl)triethylammonium bromide (QX-314) 4 (pH 7.3; 290–300 mOsm, 2.5–3 MΩ). For some experiments with evoked responses, the internal solution contained (in mM): CsCl 30, K-gluconate 90, KCl 3.5, NaCl 5, NaHEPES 10, EGTA 5, MgATP 4, Na3GTP 0.4, Na2phosphocreatine 10, and QX-314 5 (pH 7.2–7.3; 290–300 mOsm, 3–5 MΩ).
Electrically evoked IPSCs (eIPSCs) and spontaneous IPSCs (sIPSCs) were recorded at −60 mV from pyramidal-shaped neurons (presumed pyramidal neurons) located in the stratum pyramidale of the CA1 region of the hippocampus. Slow eIPSCs were elicited by electrical stimuli (30–200 μA at 0.033 Hz) using a glass monopolar stimulating electrode filled with 1 M NaCl (tip resistance ∼3 MΩ), placed at the border of stratum radiatum and stratum lacunosum-moleculare, at a site displaced laterally by about 100–250 μm from the site directly opposite the recording electrode. Current pulses (0.1 ms duration) were delivered via a constant-current stimulus isolator (Model A365D; WPI, Sarasota, FL). Stimulus intensity was adjusted to evoke responses with a monophasic rise and with amplitudes close to 50% of the maximal response. The access resistance was typically 5–15 MΩ and was compensated by 50–80% using amplifier circuitry. Cells that exhibited >20% change in access resistance were excluded from analysis.
To compare IPSCs under control and drug conditions, analyses were restricted to the times exhibiting steady-state effects. For diazepam (DZ, 1 μM), full effects were observed about 8 to 10 min following the initiation of drug application (Fig. 5, E–H). Flumazenil (FLUM, 5 μM), a wide-spectrum benzodiazepine site antagonist (Hunkeler et al. 1981) or weak inverse agonist (De Vry and Slangen 1985; King et al. 1985), reversed the effect of DZ in about 5–8 min. For analyses we used the last 3 min of recordings obtained in each condition. Because the α5-H105R mutation has been reported to cause FLUM to act as a partial agonist (Kelly et al. 2002), we did not use measurements of IPSC amplitude in the presence of FLUM to calculate effects of DZ on IPSCs. Pharmacological effects of DZ on evoked and spontaneous IPSCs were investigated in different groups of cells.
Data analysis
Spontaneous inhibitory postsynaptic currents (sIPSCs) were analyzed using computer software (Mini Analysis 6.0.3; Synaptosoft, Decatur, GA). To detect sIPSCs, the search protocol threshold was set at threefold the root-mean-square (RMS) noise level, which typically was 3–5 pA. To assess effects of DZ on fast sIPSC kinetics, for each cell (10 cells in CTRL and DZ, 4 cells in DZ + FLUM), ≥40 fast sIPSCs were averaged, normalized, and characterized by their 10–90% rise and decay times. Events were chosen based on the presence of a stable baseline level and the lack of spontaneous events during the deactivation phase and aligned at the time of half-maximal amplitude of slow the rising phase. The decay phases of averaged fast sIPSCs were fitted to a biexponential function using a Simplex fitting algorithm. Individual slow sIPSCs were characterized by rise time (time to peak) and decay time (time from peak to 0.3-fold the peak). Spontaneous IPSCs were considered as GABAA,fast if rise time was <4 ms and decay time was <40 ms and GABAA,slow if rise time was >4 ms and decay time was >40 ms (Banks et al. 1998, 2002).
Slow eIPSCs were analyzed using Clampfit (version 9.0.1.25; Molecular Devices, Sunnyvale, CA). Responses were characterized by 10–90% rise time, and by the time constant of decay (τdecay), which was obtained by fitting the decay phase to a mono- or biexponential function using the Levenberg–Marquardt algorithm. The accuracy of the fit was judged by eye. Evoked IPSCs were usually best fit by a single-exponential function, as described previously (Banks et al. 1998). For those cases in which the current was better fit by a biexponential function, τdecay was taken as the weighted time constant. Only IPSCs with a monophasic rise were used for amplitude and rise time measurements and only IPSCs that lacked large-amplitude spontaneous currents during the decay phase were used for decay measurements.
Statistics
Statistical analyses were performed using GraphPad PRISM (version 5.00 and 8.00; GraphPad Software, San Diego, CA; http://www.graphpad.com) or Origin (version 7.5; OriginLab, Northampton, MA).
For slow eIPSCs, amplitude distributions were fitted with a single Gaussian function or the sum of two Gaussian functions. For slow sIPSCs, amplitude distributions were fitted with one-component and two-component log-normal functions using Origin. To achieve fit convergence, the three parameters characterizing each individual component (A, amplitude; xc, center; w, width) were evaluated iteratively, whereas the three parameters of the other component were fixed. This procedure was repeated for each component alternately until the sum of errors was minimized. Comparisons between one-component and two-component models were performed using the F-test, with values of P < 0.001 required to favor a sum of two over one component. The Kolmogorov–Smirnov (K-S) test (Kirkman 1996) was used to compare the amplitude distributions of each component in the absence versus the presence of DZ by separating the cumulative distributions according to the ratios of their areas from the two-component log-normal fits.
The critical value for statistical significance was set at P < 0.05, unless indicated otherwise. Data are presented as means ± SD.
Chemicals
All salts and drugs were obtained from Sigma (St. Louis, MO). Stocks of diazepam (10 mM) and flumazenil (30 mM) were made in DMSO and stored in a freezer at −20°C. The final concentration of DMSO was <0.1%, which was found to have no effect on GABAAR-mediated currents (Harney et al. 2003).
RESULTS
GABAA,fast sIPSCs
More than 99% of spontaneous IPSCs (sIPSCs) in CA1 pyramidal neurons have kinetic and pharmacological properties classifying them as GABAA,fast IPSCs. They are generated by somatic and perisomatic inhibitory synapses made by several different classes of inhibitory neurons including basket cells, axo-axonic cells, and trilaminar cells (Buhl et al. 1994) and they arise from receptors containing α1 and α2 subunits (Nusser et al. 1996). However, a recent publication reporting a heavy expression of the α5 subunit in stratum pyramidale of the CA1 region (Prenosil et al. 2006) prompted us to test whether GABAA,fast IPSCs may be mediated in part by receptors containing α5 subunits. Thus we examined the characteristics of GABAA,fast sIPSCs under control conditions and the impact of a saturating concentration of DZ (1 μM) on GABAA,fast sIPSCs in α5-H105R and in WT mice.
Under control conditions, characteristics of fast sIPSCs were similar in the two genotypes (Table 1, Fig. 1). In both cases, DZ increased the relative proportion of the slow component and increased the weighted τdecay (Table 1, Fig. 2, A and B). The similarity of fast IPSC properties under control conditions and the similarity in effects of DZ in the two genotypes indicate that α5 subunits do not contribute substantially to GABAA,fast sIPSCs.
TABLE 1.
Properties of fast spontaneous IPSCs (sIPSCs) recorded under various conditions in WT and α5-H105R mice
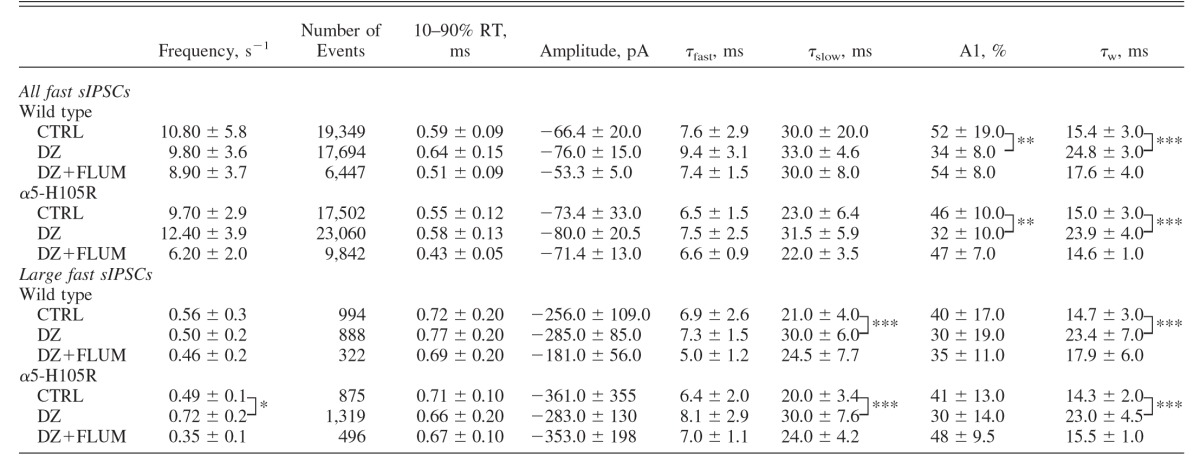 |
Values are means ± SD. Conditions: control (CTRL, 10 cells); in the presence of 1 μM diazepam (DZ, 10 cells); and in the presence of DZ and 5 μM flumazenil (DZ+ FLUM, 4 cells). Data were compared using unpaired t-test. Significance:
*P < 0.05,
**P < 0.01,
***P < 0.0001. The P values (statistically insignificant) for within-genotype effects of DZ versus CTRL on frequency of sIPSCs varied between 0.1 and 0.7.
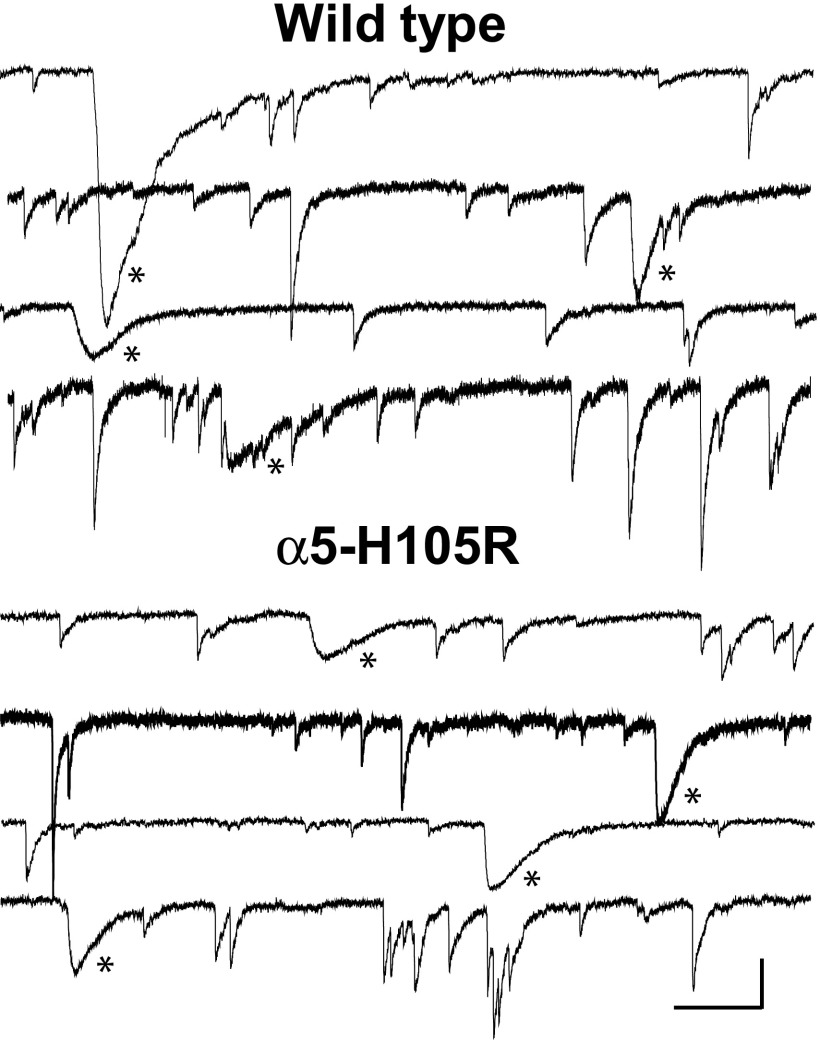
FIG. 1.
Fast and slow spontaneous IPSCs (sIPSCs) are present in both genotypes. Examples of current traces that include both fast and slow sIPCSs are shown for 8 different pyramidal cells (4 wild type mice and 4 genetically modified mice carrying a point mutation in the α5 subunit [α5-H105R]). Asterisks mark slow sIPSCs. Scale bar: 100 ms, 100 pA.
FIG. 2.
Effect of diazepam (DZ, 1 μM) on GABAA,fast sIPSCs in WT and α5-H105R mice. A and B: normalized, averaged sIPSCs, from WT (CTRL τfast 6.4 ms, τslow 23 ms; DZ τfast 7.9 ms, τslow 39 ms) and α5-H105R mice (CTRL τfast 5.5 ms, τslow 26 ms, DZ τfast 6.8 ms, τslow 32 ms). C and D: normalized, averaged sIPSCs derived from the largest 5% of events, from WT (CTRL τfast 6.6 ms, τslow 23 ms; DZ τfast 8.1 ms, τslow 36 ms) and α5-H105R mice (CTRL τfast 6.2 ms, τslow 32 ms, DZ τfast 7.2 ms, τslow 30 ms). Averages were derived from 42–51 events. Deactivation was fitted to biexponential functions (black curves superimposed on gray traces). Scale bar: 5 ms.
Although spontaneous IPSCs result from the activation of predominantly synaptic receptors, synchronous activation of several adjacent synapses may also activate perisynaptic and extrasynaptic receptors (Overstreet and Westbrook 2003; Wei et al. 2003). This mechanism promotes the generation of GABAB IPSPs (Scanziani 2000) and has been proposed to underlie the slow component of fast IPSCs with biexponential decay kinetics (Roepstorff and Lambert 1994). In a previous study of receptors in excised membrane patches exposed to brief pulses of exogenously applied GABA we found that a large proportion of receptors on the somata of CA1 pyramidal neurons have slow kinetics and are likely located at extrasynaptic sites (Banks and Pearce 2000). Reasoning that the largest sIPSCs arise from cells that form several closely spaced synapses on a single pyramidal neuron, such as basket cells (Buhl et al. 1995), and that these would be the most likely to activate perisynaptic or extrasynaptic receptors containing α5 subunits, we compared the effects of DZ on sIPSCs with the greatest amplitudes (the largest “5%” of all sIPSCs under each condition) in WT and α5-H105R mice.
Under control conditions, the characteristics of large fast sIPSCs were indistinguishable in the two genotypes, including rise time, decay times, and relative amplitudes of the individual exponential components, weighted decay rates, and the frequency of spontaneous IPSCs (Table 1). DZ did not alter the mean amplitude of large fast sIPSCs in either genotype (WT: 112 ± 33% of control; α5-H105R: 80 ± 36% of control, P = 0.5 for both). The weighted τdecay was significantly prolonged by DZ, by 50 ± 14% in WT, and by 56 ± 9% in α5-H105R mice (P > 0.01 for both; Table 1, Fig. 2, C and D). These effects did not differ between genotypes (P = 0.5–0.9). These results show that receptors containing the α5 subunit do not contribute substantially to either the early or late component of fast phasic inhibition.
GABAA,slow sIPSCs
Although the great majority of sIPSCs in CA1 cells arise from GABAA,fast synapses, a small proportion of sIPSCs have kinetic and pharmacological characteristics that closely match those of GABAA,slow eIPSCs (Banks et al. 1998; Glykys and Mody 2006; Prenosil et al. 2006). We found that slow sIPSCs were present in both WT and α5-H105R mice (Fig. 1). Under control conditions, they did not differ in rise time, decay, or mean amplitude in the two genotypes (P > 0.05, unpaired t-test, Table 2). However, the frequency of slow sIPSCs, which ranged from 0.03 to 0.18 s−1 (mean 0.09 ± 0.01 s−1) in WT mice and from 0.03 to 0.11 s−1 (mean 0.05 ± 0.01 s−1) for α5-H105R mice, was significantly lower in α5-H105R mice compared with that in WT mice (P < 0.05, unpaired t-test, Table 2).
TABLE 2.
Properties of slow spontaneous IPSCs (sIPSCs) recorded under various conditions in WT and α5-H105R mice
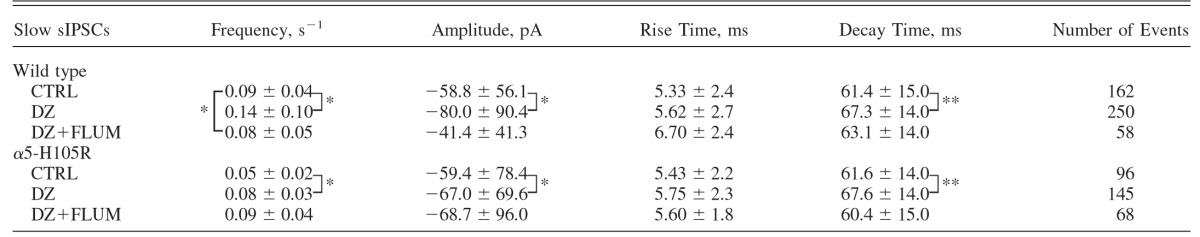 |
Values are means ± SD. Conditions: control (CTRL, 10 cells); in the presence of 1 μM diazepam (DZ, 10 cells); and in the presence of DZ and 5 μM flumazenil (DZ+FLUM, 4 cells). Data were compared using unpaired t-test. Significance:
*P < 0.05,
**P < 0.01. The P values (statistically insignificant) for within-genotype effects of DZ versus CTRL on frequency of sIPSCs varied between 0.1 and 0.2.
To test for the presence of α5 subunits at slow synapses we compared the impact of DZ on slow sIPSCs in the two genotypes. DZ increased sIPSC frequency (WT: +54 ± 13%; α5-H105R: +51 ± 5%), modestly slowed sIPSC decay (WT: +9.6 ± 23%; α5-H105R: +9.7 ± 23%), and increased IPSC mean amplitude (WT: +37 ± 54%; α5-H105R: +13.4 ± 17%). None of these effects differed in the two genotypes. However, there was a trend toward a greater effect of DZ on amplitude in WT mice (0.05 < P < 0.10, Table 2).
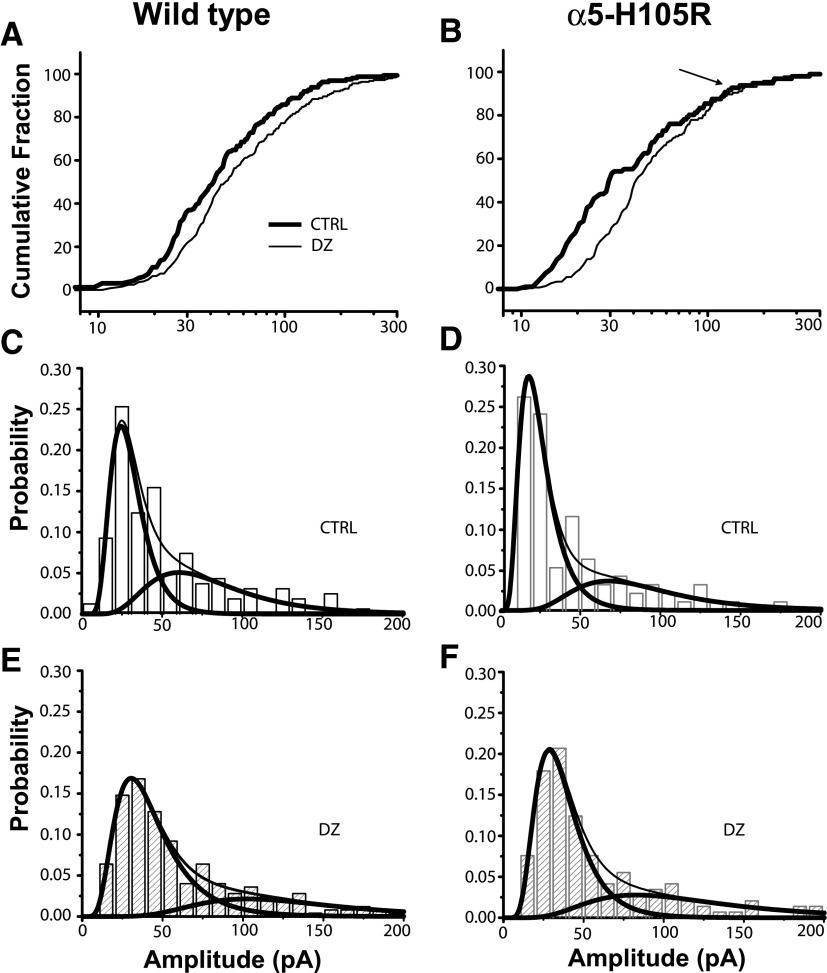
Examination of cumulative amplitude distributions revealed that in WT mice DZ caused a shift to larger values across the entire range of IPSC amplitudes, whereas in α5-H105R mice DZ increased the amplitude of the smaller but not larger IPSCs (Fig. 3, A and B). This amplitude-dependent difference in DZ modulation in cells from α5-H105R mice suggests that multiple forms of slow IPSCs may exist, with only large-amplitude synapses using α5 subunits. To examine this possibility in more detail, we fit the amplitude distributions of slow sIPSCs in WT and α5-H105R mice in the absence and the presence of DZ. Amplitude distributions were skewed toward small-amplitude events (Fig. 3, C–F); in all cases these distributions were better fit by the sum of two log-normal distributions than by a single log-normal distribution (F-test, P < 0.0001). To perform statistical comparisons of the effects of DZ on the two components in the two genotypes, we separated IPSCs into small- and large-amplitude groups according to the ratios of the areas of individual components in the two-component log-normal fits (Table 3) and compared these distributions using the K-S test. In WT mice, DZ shifted the amplitude distribution of both the smaller and larger components to larger values (small: +45%; large: +81%, P < 0.01 for both; Fig. 4, A and C), whereas in α5-H105R mice, DZ shifted the amplitude distribution of the smaller component (+34.5%, P < 0.01; Fig. 4B) but not the larger component (+3%, P = 0.32; Fig. 4D).
FIG. 3.
Two populations of slow sIPSCs. A and B: cumulative amplitude distributions of slow sIPSCs in WT and α5-H105R mice under control conditions (thick lines) and in the presence of 1 μM DZ (thin lines). DZ failed to increase the amplitude of the largest sIPSCs in α5-H105R mice (arrow). C–F: amplitude distributions of slow sIPSCs in WT and α5-H105R mice under control conditions (C, D) and in the presence of diazepam (E, F), fitted to 2-component log-normal distributions. Thick lines show individual components of fits, thin lines the sum of the 2 components.
TABLE 3.
Parameters of the two-component log-normal fits of amplitude distributions shown in Fig. 3 under control conditions (CTRL) and in the presence of 1 μM diazepam (DZ)
| Slow sIPSCs | Wild Type |
α5-H105R | ||||
|---|---|---|---|---|---|---|
| A | xc | w | A | xc | w | |
| CTRL | ||||||
| Small | 5.7 ± 0.1 | 28.2 ± 2.7 | 0.38 ± 0.04 | 7.0 ± 0.3 | 22.5 ± 1.3 | 0.48 ± 0.06 |
| Large | 3.8 ± 0.6 | 74.2 ± 15.4 | 0.45 ± 0.18 | 2.9 ± 0.5 | 82.2 ± 12.1 | 0.45 ± 0.15 |
| DZ | ||||||
| Small | 7.1 ± 0.2 | 38.8 ± 1.6 | 0.49 ± 0.02 | 7.0 ± 0.5 | 34.4 ± 1.6 | 0.43 ± 0.02 |
| Large | 2.5 ± 0.3 | 121.9 ± 11.0 | 0.42 ± 0.09 | 3.2 ± 0.3 | 106.2 ± 14.5 | 0.50 ± 0.13 |
Values are means ± SD. Amplitude values were used to calculate the number of small versus large events and to create cumulative distribution plots of amplitudes presented in Fig. 4. A, amplitude; xc, center; w, width.
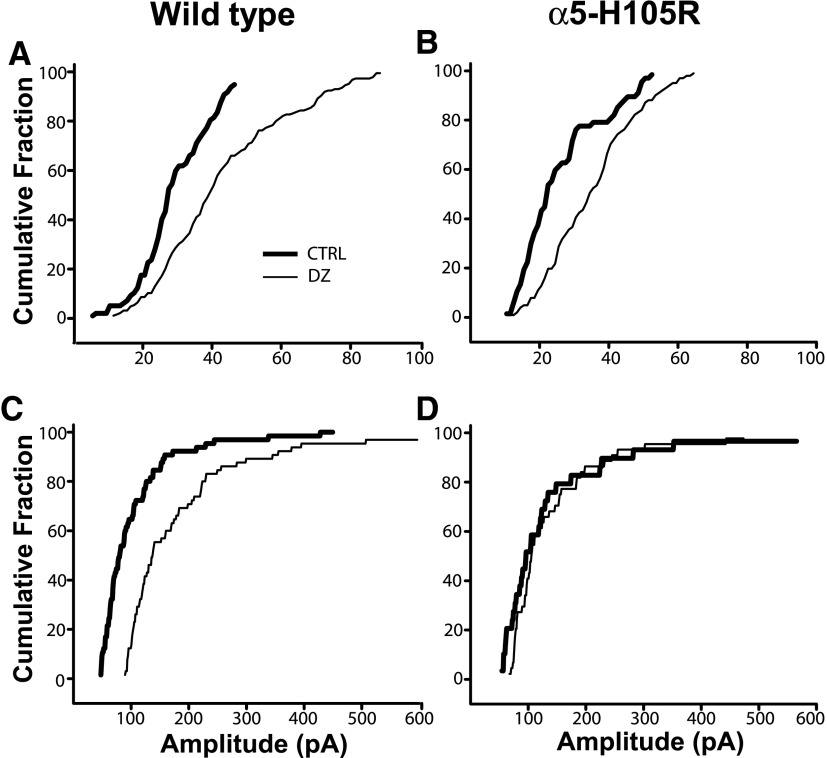
FIG. 4.
Statistical comparison of DZ modulation of small and large sIPSCs in WT and α5-H105R mice. Small (A, C) and large (B, D) slow sIPSCs were separated according to the ratios of their areas from the 2-component log-normal fits (Fig. 3, Table 3) and plotted separately as cumulative probability distributions. DZ significantly increased the amplitudes of small and large sIPSCs in WT mice (A and C, Kolmogorov–Smirnov [K-S] test, P < 0.01) and of small sIPSCs in α5-H105R mice (B, K-S test, P < 0.01), but did not alter characteristics of large sIPSCs in α5-H105R mice (D, K-S test, P = 0.32).
We were concerned that assigning a “cutoff” between large- versus small-amplitude sIPSCs based on the ratio of the areas of the two components (as described earlier) would introduce a systematic bias through misclassification of events. For example, large events would be biased most since these comprise only 40% of events in WT and 29% of events in α5-H105R mice (Table 3). Therefore to minimize this error, we also compared the effect of DZ on only the largest 20% of IPSCs in each group. Again, we found that DZ increased amplitudes of large-amplitude sIPSCs in WT mice (+47%, P < 0.01), but failed in α5-H105R mice (+1%, P = 0.361).
These results indicate that α5 subunits are present at a large-amplitude subset of synapses that give rise to GABAA,slow sIPSCs.
GABAA,slow eIPSCs
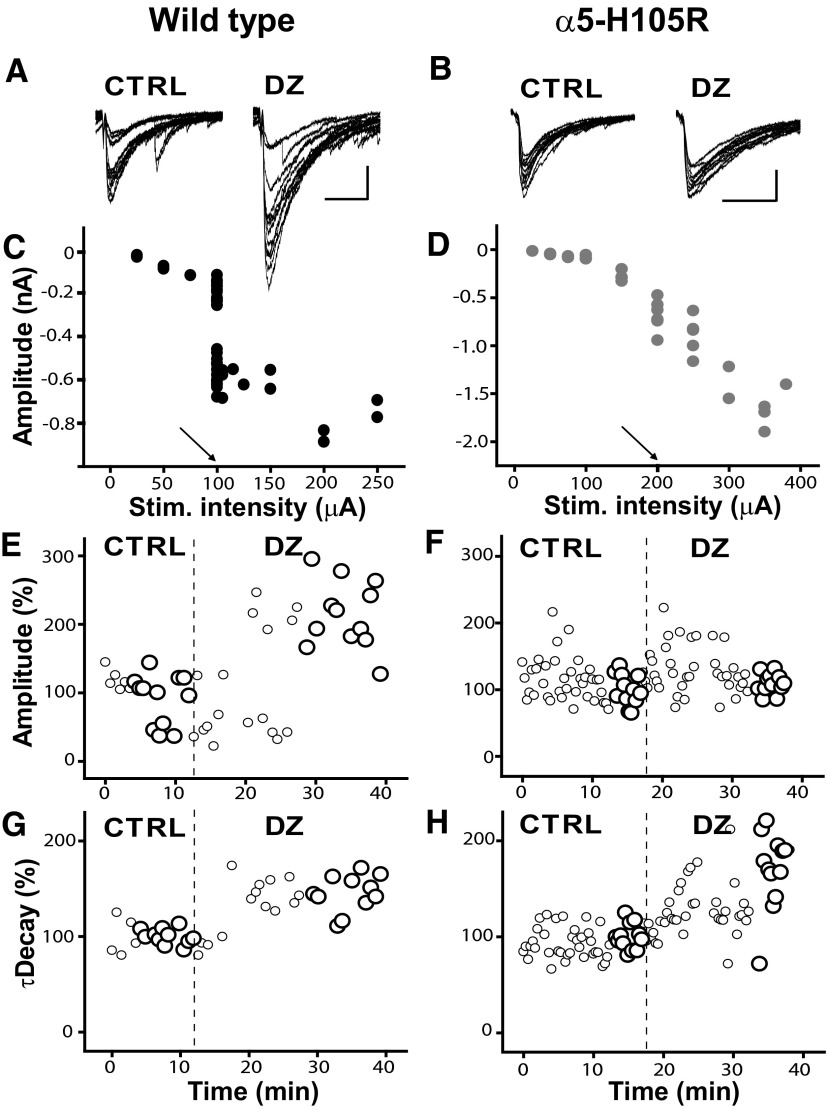
To test for a possible contribution of α5 subunits to evoked GABAA,slow IPSCs, we compared the effects of DZ on responses evoked by electrical stimuli delivered to the border of stratum radiatum and stratum lacunosum-moleculare in WT versus α5-H105R mice (Fig. 5). Under control conditions, with the stimulus intensity adjusted to elicit IPSCs with a monophasic rising phase and an amplitude approximately one half of the maximum (e.g., 100 μA in Fig. 5C and 200 μA in Fig. 5D), there were no differences between genotypes in mean amplitude (WT: −315 ± 160 pA; α5-H105R: −307 ± 130 pA), 10–90% rise time (WT: 6.7 ± 2.1 ms; α5-H105R: 7.4 ± 2.3 ms), or τdecay (WT: 56.1 ± 22 ms; α5-H105R: 56.1 ± 16 ms). These kinetic characteristics are similar to those that we, and others, have described previously under drug-free conditions (Banks et al. 1998; Glykys and Mody 2006). DZ significantly increased the mean amplitude in WT mice, by 46 ± 9% (paired t-test, P < 0.01) and slowed decay by 32 ± 17% (P < 0.05). In α5-mutant mice, DZ also significantly increased the mean amplitude, by 38 ± 11% (P < 0.05), and slowed decay, by 28 ± 18% (P < 0.001). These average effects were not different in the two genotypes (unpaired t-test, P = 0.6).
FIG. 5.
Effect of diazepam on GABAA,slow evoked IPSCs (eIPSCs). A and B: responses evoked under control conditions (CTRL) and in the presence of diazepam (DZ, 1 μM), in cells from WT and α5-H105R mice. Scale bars: 50 ms, 200 pA. C and D: response amplitude as a function of stimulus intensity for cells presented in A and B. Arrows indicate the stimulus intensity used to assess effects of DZ. Note that for the WT cell, a 100-μA stimulus evoked 2 populations of slow IPSCs with mean amplitudes of approximately −250 and −500 pA. E and F: time series plots of amplitudes of slow eIPSCs, normalized to the mean of the final 12 eIPSCs under control conditions, from the cells illustrated in A and B. Each circle represents a single evoked response. The large circles designate the responses under steady-state conditions that were averaged and used for further analysis. Note that for WT mice, amplitudes showed a bimodal distribution. The mean amplitudes of slow eIPSCs under control conditions were −439 and −534 pA for WT and α5-H105R mice, respectively. G and H: time series plots of τdecay, normalized to the mean of the final 12 eIPSCs under control conditions, from the cells illustrated in A and B. The mean values of τdecay of slow eIPSCs in CTRL were 38 and 42 ms for WT and α5-H105R mice, respectively.
The above-cited results, which revealed neither differences between genotypes in mean response characteristics under control conditions nor differences between genotypes in DZ modulation, would thus seem to indicate that α5 subunits do not contribute to evoked GABAA,slow IPSCs. This conclusion would match that made by Prenosil and colleagues (2006) who reported essentially identical effects of DZ on evoked IPSCs in WT mice (46% increase in amplitude, 29% increase in τdecay) and no effect of DZ in “triple-mutant” (α1-H101R/α2-H101R/α3-H126R) mice.
We did note, however, that under control conditions, with stimulus intensity adjusted to elicit half-maximal responses, the mean eIPSC amplitudes from individual cells fell into two groups, distinguished by amplitudes smaller or larger than 300 pA (Fig. 6, A and B). In some cases, even the responses of individual cells to stimuli delivered with a constant intensity fell into two discrete and separate amplitude ranges (e.g., Fig. 5A), although this pattern was seen in a minority of cases (both in WT: 40% and mutant: 14%); more often, responses in individual cells were more uniform (e.g., Fig. 5B). This bimodal distribution was also evident when all responses evoked from all cells were pooled (i.e., 12 individual responses from each of 10 WT and 14 α5-mutant mice, for a total of 120 and 168 responses) and plotted as amplitude histograms (Fig. 6, B and E). Fits of these pooled data to Gaussian distributions were significantly better using two- than one-component models (P < 0.001, F-test).
FIG. 6.
Two populations of slow eIPSCs. A and D: before–after plots of mean peak amplitudes of eIPSCs in individual cells, in brain slices prepared from WT mice (10 cells, A) and α5-H105R mice (14 cells, D) under control conditions (CTRL) and in the presence of diazepam (DZ, 1 μM). Note that mean current amplitudes tend to fall into 2 distinct groups. Gray rectangles mark cells with the mean amplitudes >300 pA. Single data points represent mean amplitudes ± SD of small- and large-amplitude groups. B and E: probability distributions of eIPSC amplitudes of pooled data under CTRL from WT (B) and α5-H105R mice (E), fitted with 2-component Gaussian functions. C and F: cumulative probability distributions of eIPSC amplitudes of pooled data under CTRL (thick lines) and DZ (thin lines), from WT (C) and α5-H105R mice (F), fitted with 2-component Gaussian functions (superimposed gray dotted lines).
To evaluate the effects of DZ on the two populations of IPSCs (i.e., small and large) in the two genotypes, we used two approaches. In the first, we separated cells into two groups based on the mean response amplitudes of eIPSCs under control conditions. In WT mice, DZ increased the mean amplitude of both small and large eIPSCs (P < 0.05 for both, paired t-test; Fig. 6A, Table 4). However, in α5-mutant mice, DZ increased the IPSC amplitude of only the small-amplitude group (P < 0.05, paired t-test; Fig. 6D, Table 4) but not of cells with large-amplitude responses under control conditions (P > 0.05, paired t-test; Fig. 6D, Table 4). In the second approach, we compared the amplitude distributions of the pooled data that had been fitted with the sum of two Gaussian functions (plotted as cumulative amplitude distributions; Fig. 6, C and F) in the absence versus the presence of DZ. The patterns of DZ responsiveness for these evoked responses matched those described earlier for the means of eIPSCs in individual cells (Fig. 6, A and D) as well as sIPCSs (Fig. 3, E and F): both the small- and the large-amplitude responses were increased by DZ in WT mice, whereas in α5-mutant mice, only the small-amplitude responses were increased (Table 4). Nevertheless, DZ did slow IPSC decay of both small- and large-amplitude groups, to comparable degrees in both genotypes (WT: small +32%, large +36%; α5-H105R: small +22%, large +38%; P < 0.05 for all, paired t-test, Table 4). These findings support the conclusion that receptors containing α5 subunits contribute to a subpopulation of large-amplitude GABAA,slow IPSCs.
TABLE 4.
Effects of diazepam on amplitudes and decay times of slow evoked IPSCs (eIPSCs) in WT (10 cells) and α5-H105R (14 cells) mice
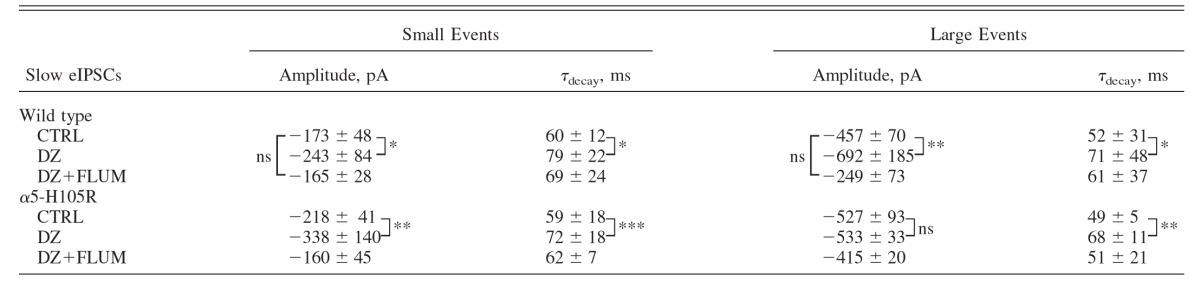 |
Values are means ± SD. The mean amplitudes and time constant of decays of small and large events were measured in groups of cells for which the mean amplitude fell below and above 300 pA, respectively. Statistical comparisons were performed using paired (within genotype) or unpaired (between genotypes) t-tests. Significance:
*P < 0.05,
**P < 0.01,
***P < 0.001.
DISCUSSION
The primary goal of this study was to test whether receptors containing α5 subunits contribute to GABAAR-mediated synaptic inhibition in mouse hippocampus. Our findings indicate that α5 subunits do contribute to a subset of GABAA,slow synaptic currents with large-amplitude evoked responses. We also found that α5 subunits do not contribute to GABAA,fast synaptic currents, even under conditions that favor neurotransmitter spillover. The results hold implications for understanding the impact of synapses that use receptors containing α5 subunits, and their modulation by pharmacological agents, on cognitive function.
α5 subunits contribute to spontaneous and evoked GABAA,slow inhibition
Two lines of evidence support the contribution of α5 subunits to GABAA,slow synaptic inhibition. First, the DZ-induced increase in amplitude of the large-amplitude component of both spontaneous and electrically evoked responses was lost in α5-H105R mice; and, second, the relative frequency of large-amplitude slow spontaneous IPSCs and the proportion of cells with large-amplitude evoked IPSCs were lower in α5-H105R mice than in WT mice. The first effect is directly attributable to the impact of the mutation at a key position within the benzodiazepine-binding site of α5 subunits. The mutation does not prevent receptors from responding to GABA, but it does eliminate benzodiazepine modulation of the responses. Thus the finding that DZ modulation of large-amplitude responses was absent in mutant mice indicates that α5 subunits are present at these synapses. The second, including both the reduced proportion of large-amplitude sIPSCs and the reduced proportion of large-amplitude eIPSCs in mutant mice, may be attributed to a reduced number of α5 subunits in these mice or to reduced affinity of receptors containing the α5-H105R mutation, as documented previously (Crestani et al. 2002).
Our finding that slow IPSCs in the two genotypes had similar kinetics implies that factors other than intrinsic receptor properties (such as neurotransmitter time course) determine the rate of deactivation, since reduced affinity would also be expected to accelerate deactivation if receptors are activated by transmitter transients much briefer than the synaptic responses (Jones and Westbrook 1995; Mozrzymas et al. 2003).
Previous studies that addressed the role of α5 subunits in hippocampal inhibition have consistently reported that they contribute to tonic inhibition (Caraiscos et al. 2004; Glykys and Mody 2006; Prenosil et al. 2006). However, the picture with regard to phasic inhibition has been confusing. In genetically modified mice lacking α5 subunits, spontaneous IPSCs with fast kinetics were reported to be reduced in amplitude, whereas evoked IPSCs, which had slow kinetics, were unchanged in amplitude or decay (Collinson et al. 2002). By contrast, different groups studying these same mice reported no changes in fast sIPSCs or miniature IPSCs, but a modest reduction in the relative frequency of sIPSCs with slow kinetics (Caraiscos et al. 2004; Glykys and Mody 2006). A recent study of mice carrying mutations in α1, α2, and α3 subunits of the GABAA receptor that rendered them benzodiazepine-insensitive indicated that α5 subunits do contribute to slow sIPSCs, but surprisingly, not to evoked responses with similarly slow kinetics (Prenosil et al. 2006). These inconsistencies cannot be explained simply by differences in the mouse models used because opposing conclusions have been reached even based on the same lines of mice. Other possible explanations include the use of different criteria to separate spontaneous events into kinetically distinct groups and the use of techniques for electrical stimulation that elicited mixed GABAA,fast and GABAA,slow responses. Our present results demonstrating that α5 subunits underlie a large-amplitude subset of electrically evoked synaptic currents provide another possible explanation: GABAA,slow IPSCs originate from a heterogeneous population of synapses, only some of which use α5 subunits, and the methods used either to activate or to identify these synapses failed to distinguish between them. Indeed, the small-amplitude evoked responses that we found were unaffected by the α5 mutation (Fig. 4) were similar in amplitude (∼100 pA) to the evoked responses that Prenosil et al. (2006) concluded arose from non-α5-containing receptors.
α5 subunits do not contribute to GABAA,fast inhibition
Our finding that the α5-H105R mutation alters neither the characteristics of fast IPSCs under control conditions nor their response to DZ (Table 1, Fig. 2) indicates that α5 subunits do not contribute to GABAA,fast IPSCs. Other investigators reached this same conclusion by studying α5−/− mice (Caraiscos et al. 2004; Glykys and Mody 2006) and α1-H101R/α2-H101R/α3-H126R mice (Prenosil et al. 2006). Our results are consistent as well with the finding that reduced α5 subunit expression in epileptic rats is associated with reduced levels of tonic GABAAR-mediated current but no change in GABAA,fast synaptic inhibition (Scimemi et al. 2005).
It was suggested previously that the slower biphasic component of fast IPSCs may result from synaptically released transmitter that spills out of synapses onto perisynaptic and extrasynaptic receptors (Overstreet and Westbrook 2003; Roepstorff and Lambert 1994). Indeed, we did find that a large number of extrasynaptic receptors with slow deactivation kinetics are present on the somata of CA1 pyramidal cells (Banks and Pearce 2000) and a recent study reported high expression levels of α5 subunits in stratum pyramidale of the CA1 region (Prenosil et al. 2006). Therefore although the mean properties of GABAA,fast IPSCs were not altered by the α5-H105R mutation, we tested whether α5 subunits contribute to the slower component of some fast IPSCs by examining the properties of the largest fast IPSCs, which would be expected to arise from cells making the largest numbers of synaptic contacts and most likely to allow GABA to reach high levels at extrasynaptic sites. However, again we found no differences between genotypes in GABAA,fast sIPSC characteristics, including the relative amplitudes and time constants of fast and slow components, under control conditions, and no difference in DZ modulation of these parameters. Therefore we conclude that the slower decay component is not due to transmitter spillover from fast synapses onto receptors that incorporate α5 subunits, even at room temperature—a condition that by slowing GABA uptake would be expected to accentuate spillover onto extrasynaptic receptors (Asztely et al. 1997).
The one difference in the effect of DZ on fast IPSCs in the two genotypes that we did observe was an increase in fast sIPSC frequency in α5-H105R but not in WT mice. The effect was evident as a trend for “all events,” although for the largest 5% of events, where the frequency was less variable between cells, it reached statistical significance (Table 1). The result suggests that spontaneous activity in basket cells and/or other interneurons, which generate fast sIPSCs, is under the opposing influence of multiple forms of phasic and tonic inhibition, mediated by α5- versus non-α5-containing receptors. However, without further direct investigation of these various influences, it is difficult to deduce which are instrumental in producing this effect.
Spatiotemporal profile of neurotransmitter at GABAA,slow synapses
Benzodiazepines (BDZs) have been used to estimate the degree of occupancy of postsynaptic receptors at inhibitory synapses (De Koninck and Mody 1994; Nusser et al. 1997; Perrais and Ropert 1999). The finding that the predominant effect of these agents is on the rate of decay rather than amplitude of fast IPSCs has been interpreted as an indication that receptors underlying these synaptic responses are relatively close to saturation (De Koninck and Mody 1994; Hajos et al. 2000). By contrast, receptors at perisynaptic or extrasynaptic sites that might mediate phasic responses by transmitter spillover are likely to be far from saturation. Our finding that DZ substantially increased the amplitude of both spontaneous (Table 2) and evoked GABAA,slow IPSCs (Table 3), together with the previous demonstration that blocking transmitter uptake prolongs GABAA,slow but not GABAA,fast IPSCs (Banks et al. 2000; Prenosil et al. 2006), indicates that the spatiotemporal profile of neurotransmitter at GABAA,slow synapses is quite different from that at GABAA,fast synapses.
What might that spatiotemporal profile be and what factors might contribute? Rather than existing as a cluster of low-affinity receptors that respond to a transmitter transient that is substantially briefer than the relaxation rate of activated channels (Maconochie et al. 1994), dispersed perisynaptic and extrasynaptic receptors with high affinity for GABA (Burgard et al. 1996; Yeung et al. 2003) may respond to a more slowly rising and prolonged transient whose peak concentration is only a fraction of the concentration that leads to full receptor activation. This explanation has been proposed for a slow dendritic IPSC mediated by perisynaptic δ subunit-containing receptors in dentate gyrus (Wei et al. 2003). Alternatively, release of transmitter into a high-volume cleft from which it must be cleared by GABA transporters rather than diffusion might also account for a prolonged, low-concentration transmitter transient, as proposed for slow IPSCs in neocortical pyramidal cells that arise from neurogliaform cells (Szabadics et al. 2007). In either case, the increase in IPSC amplitude caused by DZ may provide an explanation for the increase in frequency that we observed because a larger proportion of spontaneous IPSCs would be brought above the threshold for detection. Alternatively, it is possible that there is a true increase in the frequency of firing of interneurons that generate slow sIPSCs, produced, for example, by a depolarizing GABAAR-mediated response caused by a reversed Cl− gradient (Szabadics et al. 2006; Zhang and Jackson 1993). If this were to occur via presynaptic α5 subunits, this would provide a potential explanation for the reduced frequency of slow sIPSCs in α5-H105R mice.
Heterogeneity of GABAA,slow IPSCs
We focused here on the contribution of α5 subunits to GABAA,slow IPSCs and found that α5 subunits do indeed contribute. However, it is clear that other α subunits also play a role. This is evident in the significant effect of DZ on small-amplitude sIPSCs and eIPSCs (Figs. 4 and 6) and on the decay of slow sIPSCs (Tables 2 and 4) in α5-H105R mice. A likely candidate is the α1 subunit. In contrast to the α2 subunit, which is found primarily at the axon initial segment (Fritschy et al. 1998; Nusser et al. 1996), the α1 subunit is found all along the somatodendritic axis, at both synaptic and extrasynaptic sites. This suggestion is consistent with the results of a recent study of mice carrying mutations in the BDZ-binding sites of α1 and α2 subunits, which showed that DZ modulation of IPSCs evoked by distal stimulation is attenuated in α1-H101R mice and by proximal stimulation in α2-H101R mice (Prenosil et al. 2006).
Our finding that DZ substantially increased the amplitude of the group of large sIPSCs and eIPSCs in WT but not α5-H105R mice, whereas it had similar effects on smaller sIPSCs and eIPSCs (Figs. 4 and 6), suggests that α1- and α5-containing receptors are targeted to subpopulations of GABAA,slow synapses. This arrangement would be analogous to the segregation of α1 and α2 subunits at kinetically similar synapses on the somata of CA1 neurons arising from distinct classes of interneurons (Nyiri et al. 2001). Since α5 subunits preferentially associate with β3 subunits (Sur et al. 1998) and α1 with β2 (Barnard et al. 1998) and γ2 subunits are required for sensitivity to BDZs (Pritchett et al. 1989), the most likely combinations would then be α1β2γ2 and α5β3γ2 receptors.
The finding that DZ slowed decay rate without altering the amplitude of large eIPSCs in α5-H105R mice (Table 4) argues against a strict isolation of α1 and α5 subunits at different classes of slow synapses. Instead, it may reflect a spatial segregation (i.e., synaptic and perisynaptic) at individual synapses that contain both receptor types, with receptors at different locations exposed to different transmitter concentrations. In this scenario, α1-containing receptors that are clustered at subsynaptic sites may be exposed to higher-concentration transmitter transients, so their modulation by DZ would result in a slowing of deactivation without a change in amplitude. By contrast, perisynaptic α1- or α5-containing receptors that are exposed to a lower concentration and slower transmitter transient would be increased in amplitude, but their decay would not necessarily be slowed, if their deactivation primarily reflects the characteristics of the transmitter transient rather than intrinsic receptor kinetics. The net response would then be a combination of these two temporally overlapping events. The higher affinity of perisynaptic α5β3γ2 receptors compared with perisynaptic α1β2γ2 receptors for GABA would then provide an explanation for their unique contribution to the larger subpopulation of GABAA,slow IPSCs: similar transmitter transients experienced by the two receptors types would be expected to lead to stronger activation of the α5-containing population. At some synapses, α1 subunits colocalize with α5 subunits (Hutcheon et al. 2004) and α1 subunits can coassemble with α5 subunits to form functional receptors (Fritschy and Mohler 1995), so it is also possible that some component of slow IPSCs arises from receptors containing both α1 and α5 subunits. Such receptors would display the pharmacological properties of the α5 subunit (Araujo et al. 1999).
Do different types of interneurons generate the different populations of slow eIPSCs? If so, do they use different complements of postsynaptic receptors and do they play distinct functional roles in the hippocampal circuit? Although definitive answers to these questions will require paired recordings between presynaptic interneurons and postsynaptic pyramidal cells, as well as more detailed information about those interneurons’ excitatory afferents, existing data do support the possible contribution of several types of interneurons to GABAA,slow IPSCs and of GABAA,slow IPSCs to circuit function. The axonal arbors of all three types of dendrite-targeting interneurons that are most likely to be involved (O-LM, neurogliaform, and ivy cells) are present in the vicinity of our stimulating electrode at the border of SR and SL-M (Elfant et al. 2008; Fuentealba et al. 2008; Price et al. 2005), so any of these may have been activated in our studies of evoked responses. It is certainly possible that the subunit composition at subsynaptic and perisynaptic locations differs for these different types of interneurons. It is also possible that individual neurons engage a range of receptor types, depending on the firing pattern, presynaptic modulation, and other factors that might influence transmitter release.
GABAA,slow IPSCs constitute only a small fraction of spontaneous events, possibly reflecting the low spontaneous firing rates of presynaptic interneurons that generate them. However, they do exert a powerful and long-lasting influence on the ability of pyramidal cells to fire action potentials. This suppression of firing by GABAA,slow is enhanced by a variety of anesthetic agents (Benkwitz et al. 2007; Pearce 1996; Pearce et al. 1989). In vivo recordings from identified neurons have demonstrated that spike timing of cells that generate slow IPSCs (i.e., O-LM, neurogliaform, and ivy cells) is linked to ongoing theta oscillations (Fuentealba et al. 2006, 2008; Klausberger et al. 2003), so GABAA,slow IPSCs are likely to be instrumental in generating or coordinating this plasticity-associated rhythm. Also, evoked and even spontaneously occurring GABAA,slow IPSCs powerfully suppress ongoing activity of interneurons that generate GABAA,fast IPSCs for up to several hundred milliseconds (Banks et al. 2000). This interaction between distinct inhibitory circuits has been proposed as a cellular basis for nested theta-gamma rhythms seen in hippocampus and other cortical structures (White et al. 2000), with inhibitory input from O-LM cells to basket cells serving as one mechanism for synchronizing locally generated gamma oscillations (Tort et al. 2007). Thus accumulating evidence indicates that GABAA,slow synapses have the capacity to play a multitude of important functional roles.
Functional contributions of α5 subunits, GABAA,slow synapses, and tonic inhibition
Several lines of evidence support a role for α5 subunits in learning and memory (Caraiscos et al. 2004; Collinson et al. 2002; Crestani et al. 2002; Gerdjikov et al. 2008; Yee et al. 2004). We suggested previously that by virtue of their position and time course, which overlap anatomically and temporally with dendritic N-methyl-d-aspartate receptors, GABAA,slow synapses are well suited to control synaptic plasticity (Banks et al. 2000; Nicoll et al. 1988; Pearce 1993; White et al. 2000). The present evidence that α5 subunits contribute to GABAA,slow synaptic currents is consistent with this hypothesis. However, α5 subunits also clearly contribute to tonic inhibition and play a role in controlling excitability and epilepsy (Bieda and MacIver 2004; Glykys and Mody 2006; Scimemi et al. 2005). Under which conditions of activation (tonic vs. phasic) α5 subunits are engaged during normal and pathologic processes and how their modulation contributes to the therapeutic actions of benzodiazepines and similar agents remain important unresolved issues.
GRANTS
This research was supported by National Institutes of Health Grants P01-GM-47818 and R01-NS-056411 to R. A. Pearce and Swiss National Science Foundation Grant 3100A0-102113 to U. Rudolph.
Acknowledgments
We thank M. Perkins and A. Bassuener for excellent technical assistance; Drs. Harvey Motulsky, Adin-Cristian Andrei, and Seungbong Han for advice with statistics; and Drs. Cynthia Czajkowski and Mathew Jones for critical reading of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Ali and Thomson 2008.Ali AB, Thomson AM. Synaptic alpha 5 subunit-containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex. Cereb Cortex 18: 1260–1271, 2008. [DOI] [PubMed] [Google Scholar]
- Araujo et al. 1999.Araujo F, Ruano D, Vitorica J. Native gamma-aminobutyric acid type A receptors from rat hippocampus, containing both alpha 1 and alpha 5 subunits, exhibit a single benzodiazepine binding site with alpha 5 pharmacological properties. J Pharmacol Exp Ther 290: 989–997, 1999. [PubMed] [Google Scholar]
- Asztely et al. 1997.Asztely F, Erdemli G, Kullmann DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron 18: 281–293, 1997. [DOI] [PubMed] [Google Scholar]
- Banks et al. 2002.Banks MI, Hardie JB, Pearce RA. Development of GABAA receptor-mediated inhibitory postsynaptic currents in hippocampus. J Neurophysiol 88: 3097–3107, 2002. [DOI] [PubMed] [Google Scholar]
- Banks et al. 1998.Banks MI, Li TB, Pearce RA. The synaptic basis of GABAA,slow. J Neurosci 18: 1305–1317, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks and Pearce 2000.Banks MI, Pearce RA. Kinetic differences between synaptic and extrasynaptic GABAA receptors in CA1 pyramidal cells. J Neurosci 20: 937–948, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks et al. 2000.Banks MI, White JA, Pearce RA. Interactions between distinct GABAA circuits in hippocampus. Neuron 25: 449–457, 2000. [DOI] [PubMed] [Google Scholar]
- Barnard et al. 1998.Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev 50: 291–313, 1998. [PubMed] [Google Scholar]
- Benkwitz et al. 2007.Benkwitz C, Liao M, Laster MJ, Sonner JM, Eger EI, Pearce RA. Determination of the EC50 amnesic concentration of etomidate and its diffusion profile in brain tissue: implications for in vitro studies. Anesthesiol 106: 114–123, 2007. [DOI] [PubMed] [Google Scholar]
- Benson et al. 1998.Benson JA, Low K, Keist R, Mohler H, Rudolph U. Pharmacology of recombinant gamma-aminobutyric acid A receptors rendered diazepam-insensitive by point-mutated alpha-subunits. FEBS Lett 431: 400–404, 1998. [DOI] [PubMed] [Google Scholar]
- Bieda and MacIver 2004.Bieda MC, MacIver MB. Major role for tonic GABAA conductances in anesthetic suppression of intrinsic neuronal excitability. J Neurophysiol 92: 1658–1667, 2004. [DOI] [PubMed] [Google Scholar]
- Brunig et al. 2002.Brunig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol 443: 43–55, 2002. [DOI] [PubMed] [Google Scholar]
- Buhl et al. 1995.Buhl EH, Cobb SR, Halasy K, Somogyi P. Properties of unitary IPSCs evoked by anatomically identified basket cells in the rat hippocampus. Eur J Neurosci 7: 1989–2004, 1995. [DOI] [PubMed] [Google Scholar]
- Buhl et al. 1994.Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature 368: 823–827, 1994. [DOI] [PubMed] [Google Scholar]
- Burgard et al. 1996.Burgard EC, Tietz EI, Neelands TR, Macdonald RL. Properties of recombinant gamma-aminobutyric acid A receptor isoforms containing the alpha 5 subunit subtype. Mol Pharmacol 50: 119–127, 1996. [PubMed] [Google Scholar]
- Caraiscos et al. 2004.Caraiscos VB, Elliott EM, You T, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha 5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA 101: 3662–3667, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers et al. 2004.Chambers MS, Atack JR, Carling RW, Collinson N, Cook SM, Dawson GR, Ferris P, Hobbs SC, O'Connor D, Marshall G, Rycroft W, MacLeod AM. An orally bioavailable, functionally selective inverse agonist at the benzodiazepine site of GABAA alpha 5 receptors with cognition enhancing properties. J Med Chem 47: 5829–5832, 2004. [DOI] [PubMed] [Google Scholar]
- Collinson et al. 2002.Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci 22: 5572–5580, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani et al. 2002.Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha 5 GABAA receptors. Proc Natl Acad Sci USA 99: 8980–8985, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson et al. 2006.Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, Choudhury HI, McDonald LM, Pillai G, Rycroft W, Smith AJ, Sternfeld F, Tattersall FD, Wafford KA, Reynolds DS, Seabrook GR, Atack JR. An inverse agonist selective for alpha5 subunit-containing GABAA receptors enhances cognition. J Pharmacol Exp Ther 316: 1335–1345, 2006. [DOI] [PubMed] [Google Scholar]
- De Koninck and Mody 1994.De Koninck Y, Mody I. Noise analysis of miniature IPSCs in adult rat brain slices: properties and modulation of synaptic GABAA receptor channels. J Neurophysiol 71: 1318–1335, 1994. [DOI] [PubMed] [Google Scholar]
- De Vry and Slangen 1985.De Vry J, Slangen JL. The Ro 15–1788 cue: evidence for benzodiazepine agonist and inverse agonist properties. Eur J Pharmacol 119: 193–197, 1985. [DOI] [PubMed] [Google Scholar]
- Elfant et al. 2008.Elfant D, Pal BZ, Emptage N, Capogna M. Specific inhibitory synapses shift the balance from feedforward to feedback inhibition of hippocampal CA1 pyramidal cells. Eur J Neurosci 27: 104–113, 2008. [DOI] [PubMed] [Google Scholar]
- Fritschy and Mohler 1995.Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol 359: 154–194, 1995. [DOI] [PubMed] [Google Scholar]
- Fritschy et al. 1998.Fritschy JM, Weinmann O, Wenzel A, Benke D. Synapse-specific localization of NMDA and GABAA receptor subunits revealed by antigen-retrieval immunohistochemistry. J Comp Neurol 390: 194–210, 1998. [PubMed] [Google Scholar]
- Fuentealba et al. 2008.Fuentealba P, Begum R, Capogna M, Jinno S, Marton LF, Csicsvari J, Thomson A, Somogyi P, Klausberger T. Ivy cells: a population of nitric-oxide-producing, slow spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron 57: 917–929, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba et al. 2006.Fuentealba P, Klausberger T, Huck JH, Sakatani T, Suen WY, Roberts JD, Studer M, Somogyi P. Firing pattern and synaptic targets for GABAergic neuroglia cells in the rat hippocampus in vivo. Poster 227, FENS Forum, 2006.
- Gerdjikov et al. 2008.Gerdjikov TV, Rudolph U, Keist R, Mohler H, Feldon J, Yee BK. Hippocampal alpha 5 subunit-containing GABAA receptors are involved in the development of the latent inhibition effect. Neurobiol Learn Mem 89: 87–94, 2008. [DOI] [PubMed] [Google Scholar]
- Glykys and Mody 2006.Glykys J, Mody I. Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABAA receptor alpha 5 subunit-deficient mice. J Neurophysiol 95: 2796–2807, 2006. [DOI] [PubMed] [Google Scholar]
- Hajos et al. 2000.Hajos N, Nusser Z, Rancz EA, Freund TF, Mody I. Cell type- and synapse-specific variability in synaptic GABAA receptor occupancy. Eur J Neurosci 12: 810–818, 2000. [DOI] [PubMed] [Google Scholar]
- Hamill et al. 1981.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981. [DOI] [PubMed] [Google Scholar]
- Harney et al. 2003.Harney SC, Frenguelli BG, Lambert JJ. Phosphorylation influences neurosteroid modulation of synaptic GABAA receptors in rat CA1 and dentate gyrus neurones. Neuropharmacology 45: 873–883, 2003. [DOI] [PubMed] [Google Scholar]
- Houser and Esclapez 2003.Houser CR, Esclapez M. Downregulation of the alpha 5 subunit of the GABAA receptor in the pilocarpine model of temporal lobe epilepsy. Hippocampus 13: 633–645, 2003. [DOI] [PubMed] [Google Scholar]
- Hunkeler et al. 1981.Hunkeler W, Mohler H, Pieri L, Polc P, Bonetti EP, Cumin R, Schaffner R, Haefely W. Selective antagonists of benzodiazepines. Nature 290: 514–516, 1981. [DOI] [PubMed] [Google Scholar]
- Hutcheon et al. 2004.Hutcheon B, Fritschy JM, Poulter MO. Organization of GABA receptor alpha-subunit clustering in the developing rat neocortex and hippocampus. Eur J Neurosci 19: 2475–2487, 2004. [DOI] [PubMed] [Google Scholar]
- Jones and Westbrook 1995.Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron 15: 181–191, 1995. [DOI] [PubMed] [Google Scholar]
- Kelly et al. 2002.Kelly MD, Smith A, Banks G, Wingrove P, Whiting PW, Atack J, Seabrook GR, Maubach KA. Role of the histidine residue at position 105 in the human alpha 5 containing GABA(A) receptor on the affinity and efficacy of benzodiazepine site ligands. Br J Pharm 135: 248–256, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King et al. 1985.King GL, Knox JJ, Dingledine R. Reduction of inhibition by a benzodiazepine antagonist, Ro15-1788, in the rat hippocampal slice. Neuroscience 15: 371–378, 1985. [DOI] [PubMed] [Google Scholar]
- Kirkman 1996.Kirkman TW Statistics to use. http://www.physics.csbsju.edu/stats/. 1996.
- Klausberger et al. 2003.Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature 421: 844–848, 2003. [DOI] [PubMed] [Google Scholar]
- Low et al. 2000.Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science 290: 131–134, 2000. [DOI] [PubMed] [Google Scholar]
- Maconochie et al. 1994.Maconochie DJ, Zempel JM, Steinbach JH. How quickly can GABAA receptors open? Neuron 12: 61–71, 1994. [DOI] [PubMed] [Google Scholar]
- McKernan et al. 2000.McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor alpha 1 subtype. Nat Neurosci 3: 587–592, 2000. [DOI] [PubMed] [Google Scholar]
- McKernan and Whiting 1996.McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci 19: 139–143, 1996. [DOI] [PubMed] [Google Scholar]
- Mozrzymas et al. 2003.Mozrzymas JW, Zarnowska ED, Pytel M, Mercik K. Modulation of GABA(A) receptors by hydrogen ions reveals synaptic GABA transient and a crucial role of the desensitization process. J Neurosci 23: 7981–7992, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll et al. 1988.Nicoll RA, Kauer JA, Malenka RC. The current excitement in long-term potentiation. Neuron 1: 97–103, 1988. [DOI] [PubMed] [Google Scholar]
- Nusser et al. 1997.Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABAA receptor number underlie variation in GABA mini amplitude. Neuron 19: 697–709, 1997. [DOI] [PubMed] [Google Scholar]
- Nusser et al. 1996.Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc Natl Acad Sci USA 93: 11939–11944, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyiri et al. 2001.Nyiri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of alpha(2)-subunit-containing GABA(A) receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci 13: 428–442, 2001. [DOI] [PubMed] [Google Scholar]
- Overstreet and Westbrook 2003.Overstreet LS, Westbrook GL. Synapse density regulates independence at unitary inhibitory synapses. J Neurosci 23: 2618–2626, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce 1993.Pearce RA Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron 10: 189–200, 1993. [DOI] [PubMed] [Google Scholar]
- Pearce 1996.Pearce RA Volatile anesthetic enhancement of paired-pulse depression investigated in the rat hippocampus in vitro. J Physiol 492.3: 823–840, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce et al. 1989.Pearce RA, Stringer JL, Lothman EW. Effect of volatile anesthetics on synaptic transmission in the rat hippocampus. Anesthesiol 71: 591–598, 1989. [DOI] [PubMed] [Google Scholar]
- Perrais and Ropert 1999.Perrais D, Ropert N. Effect of zolpidem on miniature IPSCs and occupancy of postsynaptic GABAA receptors in central synapses. J Neurosci 19: 578–588, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker et al. 2000.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101: 815–850, 2000. [DOI] [PubMed] [Google Scholar]
- Prenosil et al. 2006.Prenosil GA, Schneider Gasser EM, Rudolph U, Keist R, Fritschy JM, Vogt KE. Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J Neurophysiol 96: 846–857, 2006. [DOI] [PubMed] [Google Scholar]
- Pritchett et al. 1989.Pritchett DB, Luddens H, Seeburg PH. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science 245: 1389–1392, 1989. [DOI] [PubMed] [Google Scholar]
- Roepstorff and Lambert 1994.Roepstorff A, Lambert JD. Factors contributing to the decay of the stimulus-evoked IPSC in rat hippocampal CA1 neurons. J Neurophysiol 72: 2911–2926, 1994. [DOI] [PubMed] [Google Scholar]
- Rudolph et al. 1999.Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific GABAA receptor subtypes. Nature 401: 796–800, 1999. [DOI] [PubMed] [Google Scholar]
- Scanziani 2000.Scanziani M GABA spillover activates postsynaptic GABAB receptors to control rhythmic hippocampal activity. Neuron 25: 673–681, 2000. [DOI] [PubMed] [Google Scholar]
- Scimemi et al. 2005.Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC. Multiple and plastic receptors mediate tonic GABA(A) receptor currents in the hippocampus. J Neurosci 25: 10016–10024, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwanski et al. 2006.Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li XJ, De Bias AL. Synaptic and nonsynaptic localization of GABA(A) receptors containing the alpha 5 subunit in the rat brain. J Comp Neurol 499: 458–470, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur et al. 1998.Sur C, Quirk K, Dewar D, Atack J, McKernan R. Rat and human hippocampal alpha5 subunit-containing gamma-aminobutyric acid A receptors have alpha5 beta3 gamma2 pharmacological characteristics. Mol Pharmacol 54: 928–933, 1998. [DOI] [PubMed] [Google Scholar]
- Szabadics et al. 2007.Szabadics J, Tamas G, Soltesz I. Different transmitter transients underlie presynaptic cell type specificity of GABAA,slow and GABAA,fast. Proc Natl Acad Sci USA 104: 14831–14836, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadics et al. 2006.Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 311: 233–235, 2006. [DOI] [PubMed] [Google Scholar]
- Tort et al. 2007.Tort AB, Rotstein HG, Dugladze T, Gloveli T, Kopell NJ. On the formation of gammacoherent cell assemblies by oriens lacunosum-moleculare interneurons in the hippocampus. Proc Natl Acad Sci USA 104: 13490–13495, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers et al. 2004.Towers SK, Gloveli T, Traub RD, Driver JE, Engel D, Fradley R, Rosahl TW, Maubach K, Buhl TL, Whittington MA. Alpha 5 subunit-containing GABAA receptors affect the dynamic range of mouse hippocampal kainate-induced gamma frequency oscillations in vitro. J Physiol 559: 721–728, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei et al. 2003.Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci 23: 10650–10661, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White et al. 2000.White JA, Banks MI, Pearce RA, Kopell NJ. Networks of interneurons with fast and slow gamma-aminobutyric acid type A (GABAA) kinetics provide substrate for mixed gamma-theta rhythm. Proc Natl Acad Sci USA 97: 8128–8133, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee et al. 2004.Yee BK, Hauser J, Dolgov VV, Keist R, Mohler H, Rudolph U, Feldon J. GABA receptors containing the alpha5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear. Eur J Neurosci 20: 1928–1936, 2004. [DOI] [PubMed] [Google Scholar]
- Yeung et al. 2003.Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA. Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol 63: 2–8, 2003. [DOI] [PubMed] [Google Scholar]
- Zhang and Jackson 1993.Zhang SJ, Jackson MB. GABA-activated chloride channels in secretory nerve endings. Science 259: 531–534, 1993. [DOI] [PubMed] [Google Scholar]