Abstract
Learning and memory depend on neuronal alterations induced by electrical activity. Most examples of activity-dependent plasticity, as well as adaptive responses to neuronal injury, have been linked explicitly or implicitly to induction by Ca2+ signals produced by depolarization. Indeed, transient Ca2+ signals are commonly assumed to be the only effective transducers of depolarization into adaptive neuronal responses. Nevertheless, Ca2+-independent depolarization-induced signals might also trigger plastic changes. Establishing the existence of such signals is a challenge because procedures that eliminate Ca2+ transients also impair neuronal viability and tolerance to cellular stress. We have taken advantage of nociceptive sensory neurons in the marine snail Aplysia, which exhibit unusual tolerance to extreme reduction of extracellular and intracellular free Ca2+ levels. The axons of these neurons exhibit a depolarization-induced memory-like hyperexcitability that lasts a day or longer and depends on local protein synthesis for induction. Here we show that transient localized depolarization of these axons in an excised nerve–ganglion preparation or in dissociated cell culture can induce short- and intermediate-term axonal hyperexcitability as well as long-term protein synthesis–dependent hyperexcitability under conditions in which Ca2+ entry is prevented (by bathing in nominally Ca2+ -free solutions containing EGTA) and detectable Ca2+ transients are eliminated (by adding BAPTA-AM). Disruption of Ca2+ release from intracellular stores by pretreatment with thapsigargin also failed to affect induction of axonal hyperexcitability. These findings suggest that unrecognized Ca2+-independent signals exist that can transduce intense depolarization into adaptive cellular responses during neuronal injury, prolonged high-frequency activity, or other sustained depolarizing events.
INTRODUCTION
A universal assumption about the activity-dependent mechanisms that underlie learning and many other types of adaptive plasticity is that intracellular Ca2+ transients are critical signals. Ca2+ has been considered uniquely suited for transducing electrical activity into cellular responses because the concentration of free intracellular Ca2+ is kept extremely low, its entry into the cytoplasm is enhanced by depolarization, and it can directly activate various enzymes and protein effectors (Burgoyne 2007; Case et al. 2007; Hille 2001), thereby eliciting functional changes within depolarized neurons. Ca2+ transients can also trigger exocytosis from depolarized cells, thereby inducing plastic changes in additional cells. Although the importance of Ca2+ as a trigger for activity-dependent plasticity is indisputable (e.g., Abrams et al. 1991; Malenka 1991; Rao and Finkbeiner 2007; Xu and Kang 2005; Zucker 1999), an unanswered question is whether Ca2+ signaling in neurons is universally required for the transduction of depolarization into adaptive alterations.
Although it might seem unlikely that depolarization-induced plasticity—a biologically important and widespread set of adaptive phenomena—could have evolved without using multiple electrical-to-chemical transduction mechanisms (i.e., transduction signals in addition to Ca2+), conclusive demonstration of Ca2+-independent, depolarization-induced plasticity is quite challenging because it requires the nearly complete removal of an ion that not only is required for chemical synaptic transmission (the subject of most studies of neural plasticity) but also is important for cellular stress tolerance and perhaps viability (Mattson 2007; McNeil and Terasaki 2001; Williams 2006). We have searched for Ca2+-independent, depolarization-induced plasticity using neurons that display surprising tolerance to extreme reduction of extracellular and intracellular free Ca2+ levels. Nociceptive sensory neurons of the marine snail, Aplysia californica, exhibit a depolarization-induced memory-like hyperexcitability of their axons that lasts a day or longer and depends on local protein synthesis (Weragoda et al. 2004). To avoid possible artifacts caused by depolarization-induced contraction of the nerve sheath, Weragoda et al. (2004) used extracellular solutions containing only 1% of the normal Ca2+ concentration, which also blocked synaptic transmission. Robust induction of long-term hyperexcitability (LTH, lasting a day) when extracellular calcium concentration ([Ca2+]o) was thus reduced raised the possibility that depolarization-induced alterations of excitability might be triggered by signals that are independent of Ca2+. Here we show that short-term hyperexcitability (STH, lasting minutes), intermediate-term hyperexcitability (ITH, lasting an hour), and protein synthesis–dependent LTH of these sensory axons can be induced by depolarization in the absence of both a driving force for Ca2+ entry and detectable intracellular Ca2+ transients. These findings indicate that signals in addition to Ca2+ exist for transducing depolarization into memory-like plastic changes in neurons.
METHODS
Nerve–ganglion preparation
Aplysia californica (100–250 g; Alacrity Marine, Redondo Beach, CA) were anesthetized by injection of isotonic MgCl2 solution (337 mM). The pedal–pleural ganglia were excised and placed in chambers with the attached posterior pedal nerve, p9, threaded through a series of connected wells (Fig. 1A). In most experiments the nerve wells and ganglion well were filled, ≥60 min prior to treatment, with a “0Ca/EGTA” solution containing no added Ca2+, and which we have shown to have <100 nM free Ca2+ by fura 2 imaging. The 0Ca/EGTA solution contained (in mM): 460 NaCl, 10.4 KCl, 1 EGTA, 66 MgCl2, and 10 HEPES. In some experiments the concentration of EGTA was increased to 5 or 10 mM. In LTH experiments excised ganglia and nerves were stored overnight at 16°C in artificial seawater (ASW) containing (in mM) 460 NaCl, 11 CaCl2, 10 KCl, 55 MgCl2, 10 HEPES supplemented with 7 glucose, MEM essential amino acids and MEM nonessential amino acids (each at 0.2× normal concentration and obtained from GIBCO), and MEM vitamin solution (0.7× normal concentration, GIBCO). Electrical test stimuli were applied to segments of nerve p9 while monitoring evoked action potentials intracellularly in the soma of a tail sensory neuron in the pleural ganglion (Walters et al. 1983, 2004; Weragoda et al. 2004). Tests were conducted at room temperature (20–22°C) with test currents passed though a narrow 2-mm-long slot between contiguous wells. Axon spike thresholds were tested with ascending series of 50-ms pulses. Repetitive firing was tested with 1-s pulses using a test current twice the magnitude of the 50-ms threshold current. Unless otherwise indicated, pretests and 24-h tests were conducted with the nerve and ganglia bathed in 0Ca/EGTA solution (introduced ≥30 min before the test). Conditioning depolarization (high potassium [“HiK”] treatment) of nerve segments was performed by rapidly replacing all of the 0Ca/EGTA solution in the two silicone-grease-sealed wells with Ca2+-free (and Na+-free) HiK/0Ca/EGTA solution containing (in mM) 470 KCl, 1 EGTA, 66 MgCl2, and 10 HEPES. After 2 min the HiK/0Ca/EGTA solution was completely replaced with 0/EGTA solution. Intracellular measurements from sensory axons were not feasible, but when applied centrally to the soma this solution depolarized the soma to almost 0 mV within 2–3 s. Sham controls received identical treatment, except that 0Ca/EGTA solution was reintroduced rather than HiK/0Ca/EGTA solution.
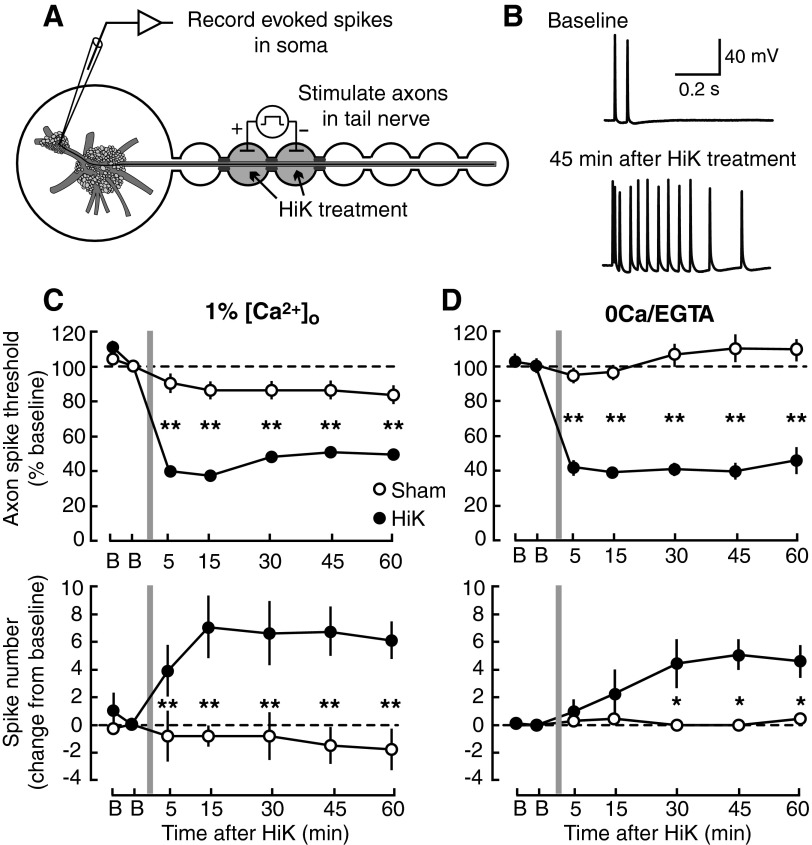
FIG. 1.
Axonal short-term/intermediate-term hyperexcitability (STH/ITH) induced by nerve depolarization does not require Ca2+ entry. A: in situ nerve–ganglia preparation consisting of excised pedal and pleural ganglia with the attached tail nerve (p9). Intracellular recordings were made from central somata of tail sensory neurons having axons in the nerve. Test stimuli were applied to an approximately 2-mm segment of nerve by passing current between 2 wells sealed with silicone grease. High potassium (HiK) solution was applied for 2 min to the two 6-mm wells. B: examples of repetitive firing evoked by a 1-s test pulse to the nerve in 0Ca/EGTA (1 mM) solution before and after HiK treatment. C: STH/ITH induced and expressed in 1% extracellular calcium concentration ([Ca2+]o, n = 5 per group). Top: changes in threshold current required to evoke a spike with 50-ms test pulses. Bottom: changes in repetitive firing (number of spikes evoked by 1-s pulse). D: STH/ITH induced and expressed in 0Ca/EGTA solution (n = 6 per group). Data in this and the other figures are expressed as means ± SE. Differences between sham and HiK treatment outcomes were assessed with 2-way, repeated-measures ANOVA followed by Bonferroni posttests at each time indicated. *P < 0.05; **P < 0.01.
Dissociated sensory neurons
Sensory neurons were isolated using conventional methods (Schacher and Proshansky 1983). Pleural–pedal ganglia were excised from juvenile Aplysia (10–20 g) and suspended in L-15 containing 1% protease (Protease IX, Sigma) for about 105–120 min at 34°C. Ganglia were washed and transferred to a Sylgard-coated dish containing L-15 and 25% hemolymph. Individual sensory neurons with axons >500 μm were pulled from the ganglion using a fine capillary tube and moved to a glass dish coated with poly-l-lysine (Sigma), which contained L-15 and 50% hemolymph. Dishes were left at 20–22°C in the dark 3–7 days before use. Axons in vitro received extracellular stimulation, in 0Ca/EGTA solution or ASW, ≥300 μm from the soma, through a fire-polished glass pipette (1–1.5 MΩ) pressed gently against the surface of the axon. Axon spike thresholds were tested with ascending-amplitude series of 2-ms pulses. Repetitive firing was tested with ten 2-ms pulses (25-ms interpulse interval) using the threshold current. To depolarize the axons of dissociated sensory neurons, a stream of HiK solution with Fast Green dye was delivered for 2 min through a glass micropipette (5- to 10-μm tip diameter) by gravity feed (Fig. 2A) or, in most cases, by pressure ejection, with the visible plume restricted to the distal part of the axon. Because of dilution of the stream of HiK solution under these conditions, we doubled the K+ concentration to 940 mM to produce intense depolarization. To match the increased osmolarity of this solution, we added 470 mM sucrose to the 0Ca/EGTA solution used for sham treatment. No obvious shrinkage of the axon occurred during delivery of either solution to the axon and, when the hyperosmotic sham solution was delivered to the soma, no effect was observed on resting membrane potential, spike threshold, or repetitive firing. Immediate and delayed effects of HiK treatment on the properties of the sensory neuron soma will be described elsewhere (K. K. Kunjilwar, Q. Yang, F. D. Reyes, and E. T. Walters, unpublished observations).
FIG. 2.
Axonal STH/ITH induced by local depolarization in isolated sensory neurons does not require entry of Ca2+ into the axon. A: dissociated sensory neuron showing the relative positions of the soma (which contained the intracellular recording electrode) and the axonal region contacted by the stream of HiK solution (indicated by plume of Fast Green dye). Extracellular test currents were delivered from the test pipette. B: examples of repetitive firing during test sequence of ten 2-ms pulses (40 Hz). The stimulus current was the threshold level for generating a spike with a single pulse. C: axonal STH/ITH induced and expressed in normal [Ca2+]o (n = 7 per group). Top: changes in threshold current required to evoke a spike with 2-ms test pulses. Bottom: changes in repetitive firing during train of 10 pulses. D: axonal STH/ITH induced and expressed in 0Ca/EGTA solution (n = 5 per group). Differences between sham and HiK treatment outcomes were assessed with 2-way, repeated-measures ANOVA followed by Bonferroni posttests. **P < 0.01.
Ca2+ imaging
Dissociated sensory neurons were incubated in 5 μM fura 2-AM in ASW at 20–22°C for 1 h and then washed four times with ASW (Wertz et al. 2006). Neurons were left in ASW or 0Ca/EGTA solution for ≥30 min and in BAPTA-AM (10 μM) for ≥1 h before imaging began. Fluorescence images were acquired with a Hamamatsu C2400 iCCD camera every 6–9 s. Quantitative measurements of intracellular calcium concentration ([Ca2+]i) in single axons were made with an InCyt Im2 Fluorescence Imaging System (Intracellular Imaging, Cincinnati, OH) equipped with a PixelFly CCD camera (Cooke, Romulus, MI) as previously described (Wu et al. 2007). A series of 5–11 regions of interest (ROIs, each 5–10 μm long) were selected along the axon (diameter 2–5 μm in each ROI) for calcium imaging. R, the ratio of the fluorescence intensity (511 nm), was obtained on excitation at 340 and 380 nm for each ROI and all the ROI values were averaged for each time point. We report R values because changes in R provide a direct index of changes in [Ca2+] while avoiding the limitations inherent in estimating cytosolic [Ca2+] from intracellular calibration curves (Grynkiewicz et al. 1985). To compare our results to those from other studies we also present summary data in terms of estimated cytosolic [Ca2+] based on our calibration curves.
Drug treatments
Thapsigargin, BAPTA-AM, and A23187 were obtained from Invitrogen and all other drugs from Sigma. In the acute studies each was applied for the entire period of the experiment, beginning ≥60 min prior to HiK or sham treatment of the nerve or axon. In a long-term study emetine was applied from 80 min before HiK or sham treatment until 2 h afterward.
Data analysis
Data are reported as means ± SE, with the n values indicating the numbers of neurons tested in each condition. Comparisons between treatments of unpaired nerve or axon segments were made with unpaired t-tests. Comparisons of a single group before and after treatment were made with paired t-tests. Comparisons of two groups across multiple tests were made using one-way ANOVA with repeated measures followed by Bonferroni post hoc tests. Statistically significant differences (P < 0.05) are indicated by asterisks in each figure.
RESULTS
Induction of axonal STH and ITH by local depolarization does not require entry of Ca2+ into the axon
We first asked whether early phases of depolarization-induced hyperexcitability could be triggered in the absence of Ca2+ entry, using both an in situ nerve–ganglion preparation and in vitro dissociated sensory neurons from Aplysia. In the in situ preparation (Fig. 1A) (Weragoda et al. 2004), axonal action potentials evoked by shocking a short segment of the tail nerve were recorded intracellularly in the sensory neuron soma within the pleural ganglion. Previously we showed that K+-induced depolarization (HiK treatment) of a nerve segment for 2 min produced axonal LTH that persisted for ≥24 h (Weragoda et al. 2004). To avoid possible artifacts from nerve contraction, this LTH study had been performed after reducing Ca2+ levels outside the cells ([Ca2+]o) to 1% of normal (0.1 instead of 11 mM). This concentration eliminates obvious chemical synaptic transmission and contractile responses in Aplysia (Weragoda et al. 2004), but still leaves a nearly 1000-fold concentration gradient to drive Ca2+ entry. In the present study we found that axonal hyperexcitability was also expressed immediately (STH) and for ≥60 min (ITH) after HiK treatment in 1% [Ca2+]o. The hyperexcitability was expressed as both a significant decrease in the current needed to reach axonal action potential (spike) threshold during a 50-ms test pulse (Fig. 1C, top) and an increase in the number of spikes evoked by a 1-s test pulse (Fig. 1C, bottom). Significant decreases in spike threshold and increases in repetitive firing were also found (Fig. 1, B and D) when the nerve segment was depolarized and repeatedly tested in 0Ca/EGTA solution. This solution contained <100 nM free Ca2+ and thus eliminated or drastically reduced the driving force for Ca2+ entry (see discussion). The effects on repetitive firing, but not spike threshold, appeared somewhat larger in 1% [Ca2+]o than in 0Ca/EGTA solution (although this difference was not statistically significant), raising the possibility that a small amount of extracellular Ca2+ may promote the enhancement of repetitive firing by depolarization of the nerve. Importantly, the significant hyperexcitability remaining in 0Ca/EGTA solution demonstrates that axonal STH and ITH can be induced in situ by depolarization in the probable absence of Ca2+ entry and that Ca2+ entry following the depolarization is not needed to maintain axonal STH and ITH.
To determine whether depolarization-induced hyperexcitability that is independent of Ca2+ entry involves signals intrinsic to the sensory axon, we dissociated sensory neurons from the ganglion along with a 0.5- to 1.5-mm length of the primary axon. After 3–7 days in culture, extracellular test stimuli were applied to a point on the distal half of the primary axon before and after delivery of a 2-min stream (100–200 μm wide) of a modified HiK solution (see methods) to the tested region (Fig. 2A). When the depolarization and testing of single axons were conducted in ASW containing normal [Ca2+ ]o (11 mM), axonal STH and ITH were produced, as expressed by decreased spike threshold (Fig. 2C, top) and increased repetitive firing (Fig. 2C, bottom). ITH remained significant for both measures through 90 min of testing, although for comparison only 60 min are shown on the graph. The same transient depolarization in 0Ca/EGTA solution also produced significant reduction of spike threshold and enhancement of repetitive firing (Fig. 2, B and D). The lack of significant effects observed following sham treatment of dissociated neurons with hypertonic 0Ca/EGTA/sucrose demonstrates that the axonal hyperexcitability produced by HiK treatment was not caused by possible osmotic effects of the hypertonic HiK solution ejected near the axon (see methods). One significant difference was found in axons of dissociated neurons compared with those tested in the nerve; the reduction of threshold in 0Ca/EGTA solution, albeit significant, was less in vitro (Fig. 1D) than in situ (Fig. 2D) (minimum thresholds during all posttests relative to baseline, 69 ± 6 vs. 34 ± 4%, respectively; means ± SE, P < 0.001, unpaired t-test). Interestingly, in vitro the increase in repetitive firing was induced to a greater degree in 0Ca/EGTA solution than in ASW (maximum change in number of evoked spikes during all posttests relative to baseline, 6.2 ± 1.0 vs. 2.8 ± 0.9 spikes, respectively; means ± SE for groups shown in Fig. 2, C and D, P < 0.05, unpaired t-test). This difference is consistent with recent data suggesting that, in addition to Ca2+-independent mechanisms that induce hyperexcitability, sensory neurons may possess Ca2+-dependent mechanisms that oppose the induction of hyperexcitability by depolarization (K. K. Kunjilwar, Q. Yang, R. Crook, and E. T. Walters, unpublished observations). Most important, the findings in axons of dissociated sensory neurons show once again that significant axonal STH and ITH induced by depolarization can be triggered and maintained by signals that do not depend on diffusion of Ca2+ from extracellular compartments into the axon and that this signaling is intrinsic to the depolarized axon.
Depolarization in the absence of extracellular Ca2+ evokes a small increase in intraaxonal [Ca2+] that can be prevented by intracellular Ca2+ chelation
The occurrence of depolarization-induced axonal STH and ITH in the primary axon of dissociated sensory neurons allowed us to measure relative changes in [Ca2+]i during and after depolarization under different conditions. Many Ca2+-imaging studies of Aplysia neurons have used the indicator dye, fura 2, which must be injected to load the cell. To avoid possible alterations of the neuron caused by depolarization or Ca2+ entry during injection, we bath-applied the membrane-permeant analog, fura 2-AM. Acetoxymethyl ester (AM) forms of dyes or chelators have rarely been used in studies of Aplysia neurons (Wertz et al. 2006), but we had observed that two AM dyes, calcein-AM and fluo 4-AM (each 5 μM), were readily visualized within axons both in cell culture and in intact peripheral nerves 1 h after bath application and washout. This indicated that AM forms of impermeant dyes and chelators can cross the membrane in Aplysia axons, whereupon intracellular esterases remove the AM moiety, yielding active, membrane-impermeant forms (in these cases, fluorogenic calcein or fluo 4), as shown previously in other neurons (e.g., Bozyczko-Coyne et al. 1993; Tymianski et al. 1993). When [Ca2+]o was normal (11 mM in ASW), axons that had been loaded with fura 2-AM rapidly responded to 2-min HiK/ASW superfusion with a large and prolonged increase in [Ca2+]i, as indicated by an increase in the ratio R of imaged fluorescence intensities at excitation wavelengths of 340 and 380 nm (340/380) (Fig. 3A, top and middle). The approximate [Ca2+]i before and after HiK treatment is shown in the bottom panels of Fig. 3A. Recovery of [Ca2+]i close to baseline levels took >10 min. Sham treatment (superfusing ASW instead of HiK/ASW) produced no significant change in [Ca2+]i. When testing and HiK treatment were performed in 0Ca/EGTA solution, a small but significant rise in [Ca2+]i occurred after HiK/0Ca/EGTA but not sham treatment (Fig. 3B), suggesting that depolarization may trigger the release of a small amount of Ca2+ from intracellular stores. After preexposure of the preparation to ASW containing the membrane-permeant Ca2+ chelator BAPTA-AM (10 μM), HiK/ASW caused a large increase in [Ca2+]i (Fig. 3C). However, when the large increase in [Ca2+]i evoked by HiK treatment was eliminated by EGTA, the 0Ca/EGTA/BAPTA-AM prevented any significant change in axonal [Ca2+]i caused by HiK treatment (Fig. 3D). This condition also prevented detectable Ca2+ transients during depolarization of the sensory neuron soma (data not shown). These results indicate that the amount of active, intracellular BAPTA resulting from incubation in 10 μM BAPTA-AM is sufficient to prevent the small HiK-evoked Ca2+ transient (perhaps originating from intracellular stores) that occurs when extracellular Ca2+ is effectively absent, even though the same exposure to BAPTA-AM is insufficient to effectively chelate the enormous amounts of Ca2+ that enter the axon during 2-min depolarization in normal [Ca2+]o.
FIG. 3.
Depolarization triggers axonal Ca2+ transients that can be prevented by chelating [Ca2+]o and intracellular calcium concentration ([Ca2+]i). Dissociated sensory neurons were loaded with fura 2-AM. Top panels: examples of Ca2+ transients. Middle panels: mean differences in maximal R (treatment period − pretreatment period) in sham-treated and HiK-treated axons. Bottom panels: mean values of peak [Ca2+]o (determined from calibration curves) during the 2-min period preceding (Pre) and the 2-min period during HiK treatment. A: Ca2+ transients produced by HiK treatment in normal [Ca2+]o (11 mM in artificial seawater [ASW]). B: Ca2+ transients produced by HiK treatment in 0Ca/EGTA solution. C: Ca2+ transients produced by HiK treatment in ASW after pretreatment with BAPTA-AM (10 μM). D: lack of significant Ca2+ transients produced by HiK treatment in 0Ca/EGTA/BAPTA-AM solution. Traces in top panels of A and B are from the same cell and C and D are from another cell. Middle and bottom panels: means ± SE for each condition. *P < 0.05; **P < 0.01; ***P < 0.001, paired t-test.
Conditions that prevent depolarization-evoked Ca2+ transients fail to reduce depolarization-induced STH and ITH
Pretreatment of sensory neurons in dissociated cell culture with BAPTA-AM (10 μM) for ≥1 h failed to block axonal STH and ITH (expressed as alterations in spike threshold and repetitive firing) induced and maintained in 0Ca/EGTA solution (Fig. 4A). Similarly, pretreatment of ganglia–nerve preparations with BAPTA-AM (10 μM) failed to block STH and ITH induced and maintained in 0Ca/EGTA (Fig. 4B). Significant differences were observed for 90 min after depolarization. These results suggest that neither Ca2+ entry nor release of Ca2+ from intracellular stores is required for the induction or maintenance of axonal STH and ITH.
FIG. 4.
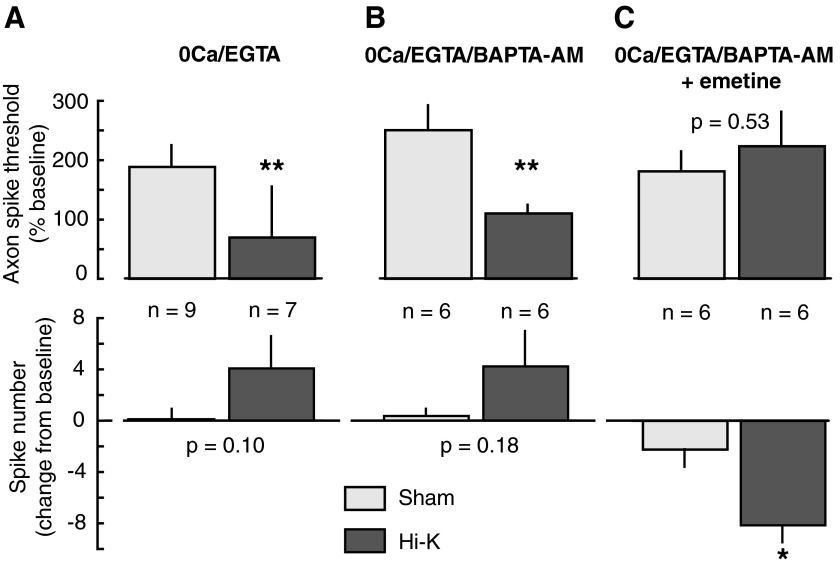
Axonal STH/LTH can be induced under conditions in which detectable Ca2+ signals are absent. A: axonal STH/ITH induced and expressed in dissociated, isolated sensory neurons in 0Ca/EGTA(1 mM)/BAPTA-AM(10 μM) solution (n = 5 per group). Hyperexcitability was expressed as decreased spike threshold (top) and increased repetitive firing (bottom). B: axonal STH/ITH induced and expressed in excised nerves in 0Ca/EGTA/BAPTA-AM solution (n = 5 per group). C: axonal STH/ITH induced and expressed in excised nerves under conditions of maximal practical Ca2+ chelation, with 0Ca solution containing 5 mM EGTA and 20 μM BAPTA-AM (n = 4 per group). D: axonal STH/ITH induced and expressed in excised nerves in 0Ca/EGTA solution containing thapsigargin (10 μM) and A23187 (100 μM) (n = 5 per group). Differences between sham and HiK treatment outcomes were assessed with 2-way, repeated-measures ANOVA followed by Bonferroni posttests. *P < 0.05; **P < 0.01.
Although imaging of dissociated axons revealed no detectable Ca2+ transients under these conditions (Fig. 3), the possibility remained that some Ca2+ signals might still be present in situ or in vitro. Therefore we increased the concentrations of EGTA and BAPTA-AM (from 1 mM and 10 μM, respectively) to as high as compatible with normal baseline responsiveness of the axons. We found that 10 mM EGTA plus 20 μM BAPTA-AM caused dramatic instability of axonal spike threshold in the ganglia–nerve preparation (not shown), whereas 5 mM EGTA plus 20 μM BAPTA-AM permitted stable baseline responses. Thus we used 5 mM EGTA plus 20 μM BAPTA-AM to produce “maximal” practical chelation of extracellular and intracellular free Ca2+ and again examined the effect of HiK treatment. This increase in the chelator concentrations failed to reduce either the STH or ITH produced by HiK treatment (Fig. 4C).
Other manipulations expected to block or reduce intracellular Ca2+ transients also failed to affect the magnitude of STH and ITH, including pretreatment of nerve–ganglia preparations with thapsigargin (10 μM) in a nominally Ca2+-free solution containing both EGTA (1 mM) and the Ca2+ ionophore A23187 (100 μM) (Fig. 4D). Thapsigargin inhibits Ca2+ uptake into endoplasmic reticulum, greatly reducing the stores that can be subsequently released (e.g., Kachoei et al. 2006), whereas the Ca2+ ionophore was added to help clamp [Ca2+]i to [Ca2+]o, which was close to zero. Pretreatment with dantrolene (50 μM), an inhibitor of intracellular Ca2+ release that occurs via ryanodine receptors in mammalian cells, also failed to prevent STH or ITH (n = 5 HiK-treated and 5 sham-treated axons, P < 0.01 at each time point from 5 to 60 min after treatment), although the effectiveness of this agent in Aplysia neurons is not yet known. These results indicate that axonal STH and ITH can be induced and maintained in the absence of significant intracellular Ca2+ transients caused by either Ca2+ influx or release from intracellular stores.
Ca2+-independent signals can induce protein synthesis–dependent axonal LTH
To see whether depolarization-induced axonal hyperexcitability lasting 24 h can be induced in the absence of Ca2+ entry, we incubated nerve–ganglia preparations in 0Ca/EGTA (1 mM) solution for ≥80 min prior to HiK or sham treatment and for ≥2 h afterward. Baseline excitability tests began 20 min prior to treatment. The preparations were than kept overnight in ASW and tested in 0Ca/EGTA 24 h after treatment. Elimination of Ca2+ entry at the time of depolarization failed to prevent axonal LTH 24 h later, as revealed by significantly lower spike thresholds after HiK treatment than those after sham treatment (Fig. 5A). The increase in threshold in the sham-treated axons at 24 h has been seen in previous studies (Weragoda and Walters 2007; Weragoda et al. 2004) and confirms that maintaining the axons in our ex vivo ganglia–nerve preparation over this period reduces axonal excitability. Similar LTH (compared with the excitability of sham-treated axons) was observed when depolarization occurred in 0Ca/EGTA/BAPTA-AM solution to block intracellular Ca2+ transients (Fig. 5B). Unlike spike threshold, repetitive firing was not significantly different in HiK-treated axons than that in sham-treated axons 24 h after depolarization (Fig. 5, A and B), although a trend to increase was noted, similar to the pattern found previously for axonal LTH induced in 1% [Ca2+]o (Weragoda et al. 2004). Like the previously described LTH, axonal LTH of spike threshold induced in 0Ca/EGTA/BAPTA-AM solution was blocked by pretreatment with a protein synthesis inhibitor, emetine (100 μM; Fig. 5C, top). Interestingly, in the presence of emetine and the apparent absence of Ca2+ signals, HiK treatment caused a significant reduction (rather than enhancement) of repetitive firing compared with that of sham treatment (Fig. 5C, bottom), perhaps because of the combined stress-related effects of transient depolarization, temporary inhibition of protein synthesis, and prolonged maintenance ex vivo. In contrast to the effects on 24-h LTH, inhibition of protein synthesis failed to block the short-term and intermediate-term reductions in spike threshold that were induced and maintained in 0Ca/EGTA solution (HiK vs. sham treatment in the presence of 10 μM anisomycin, P < 0.01 during each test 5–60 min after treatment, n = 5 in each group). Anisomycin also failed to prevent a significant overall effect of HiK treatment on repetitive firing 5–60 min after treatment (two-way ANOVA treatment effect, P = 0.0034), although none of the individual posttests reached statistical significance. Both emetine and anisomycin inhibit protein synthesis by close to 95% in Aplysia sensory neurons (Casadio et al. 1999; Montarolo et al. 1986). These results indicate that protein synthesis–dependent, depolarization-induced axonal LTH can be triggered by signals other than Ca2+ transients.
FIG. 5.
Protein synthesis–dependent long-term hyperexcitability (LTH) of sensory axons lasting ≥24 h does not require Ca2+ signaling for its induction. A: long-term depression (LTD) of axonal spike threshold is not prevented by eliminating [Ca2+]o with 0Ca/EGTA solution during induction. B: LTD of axonal spike threshold is not prevented by combining the 0Ca/EGTA (1 mM) with BAPTA-AM (10 μM). C: LTD of axonal spike threshold is prevented by adding the protein synthesis inhibitor emetine (100 μM) to the 0Ca/EGTA/BAPTA-AM solution. The 0Ca/EGTA/BAPTA-AM and emetine were present from 1 h before to 2 h after HiK treatment; 24-h tests were conducted in 0Ca/EGTA. Differences between sham and HiK treatment outcomes were assessed with unpaired t-test. *P < 0.05; **P < 0.01.
DISCUSSION
Ca2+-independent, depolarization-dependent plasticity is unexpected
The present results show that early and late phases of depolarization-induced hyperexcitability of sensory axons can be induced in the absence of detectable Ca2+ transients. This provides direct evidence for Ca2+-independent, depolarization-induced plasticity, which is important because Ca2+ is often assumed to be the only significant transducer of depolarization (caused by electrical activity or injury) into adaptive responses in neurons (e.g., Hille 2001). Ca2+ signals have long had central roles in cellular hypotheses about learning mechanisms (e.g., Brown et al. 1988; Eccles 1983; Hawkins et al. 1983; Walters and Byrne 1983;) and nearly all reported examples of activity-dependent or depolarization-dependent neuronal plasticity either have been shown or suggested to utilize Ca2+ as a trigger. These include short-term and intermediate-term synaptic plasticity (Abrams et al. 1991; Glanzman 2008; Malenka 1991; Zucker 1999), long-term synaptic plasticity (Kovacs et al. 2007; Malenka and Bear 2004; Rao and Finkbeiner 2007), and short-term and long-term plasticity of intrinsic excitability (Xu and Kang 2005; Zanzouri et al. 2006). In addition to its pervasive role in the induction of activity-dependent plastic alterations (i.e., deviations from a normal state of excitability and synaptic strength), Ca2+ appears to be an important feedback signal in some forms of electrophysiological homeostasis (Ibata et al. 2008; Turrigiano et al. 1994). Examples of activity-dependent homeostatic effects that depend on Ca2+ signals include a compensatory regulation of excitability during both synaptic LTP (Fan et al. 2005) and synaptic LTD (Brager and Johnston 2007). Depolarization-dependent Ca2+ signaling in neurons can alter gene transcription (Kingsbury et al. 2007; West et al. 2001), protein synthesis (Scheetz et al. 2000), and perhaps protein degradation (Deng and Lei 2007; Dong et al. 2008). In most cases, Ca2+ signals are generated by entry of the ion through voltage-sensitive channels in the plasma membrane (including N-methyl-d-aspartate receptor-gated channels), but Ca2+ released from intracellular stores can also contribute to the induction of plastic changes (Bardo et al. 2006; Fitzjohn and Collingridge 2002). In reported examples of plasticity in which Ca2+ does not appear to be a direct trigger (e.g., heterosynaptic facilitation) there is usually assumed to be at least an indirect Ca2+ requirement, involving Ca2+-dependent exocytosis of plasticity-inducing neuromodulators (Kandel 2001).
Very few reports have implicated depolarization-induced, Ca2+-independent triggering of long-lasting plastic changes. Potentiation of hippocampal synapses lasting >30 min has been induced by a 3-min HiK/0Ca treatment following a 4-min infusion of a nominally Ca2+-free solution (no chelators were used) (May et al. 1987). Interestingly, in clonal GH3 pituitary cells, 1- to 5-h treatment with a HiK/0Ca/EGTA solution caused a down-regulation of Kv1.5 mRNA, potentially causing long-term hyperexcitability (Levitan et al. 1995). However, Ca2+ entry could not be ruled out in the former study and neither of these studies provided evidence against depolarization-induced Ca2+ signals coming from intracellular stores. The most convincing evidence for depolarization-induced, Ca2+-independent triggering of plastic changes is long-term facilitation at the crayfish neuromuscular junction evoked by 10 min of 20-Hz stimulation; a phase lasting >1 h can be induced in a nominally Ca2+-free solution containing 25 μM BAPTA-AM (Wojtowicz and Atwood 1988). The present results extend this finding to long-term hyperexcitability and provide strong evidence for depolarization-dependent plasticity occurring in the absence of detectable intracellular Ca2+ transients that might act as either a direct or an indirect trigger of the alterations.
Is undetected Ca2+ signaling involved?
There are two possible explanations for our results. First, despite the lack of a statistically significant Ca2+ transient during repeated axonal depolarizations in 0Ca/EGTA (1 mM) plus 10 μM BAPTA-AM (Fig. 3D), plasticity-inducing Ca2+ transients might have been present that were below the threshold of detection by fura 2 imaging. This would mean that plastic changes were triggered either by 1) Ca2+ transients that were extremely brief (despite the depolarization lasting 2 min), 2) extraordinarily low concentrations of free Ca2+, or 3) high concentrations of free Ca2+ in protected microdomains that were too small and/or too few to be detected. The general possibility of undetected but biologically significant Ca2+ signals cannot be eliminated conclusively, but there is evidence against it. Although we sampled at 6- to 10-s intervals (which might have allowed an early, rapid transient to be missed), the increase in Ca2+ observed in 0Ca/EGTA without BAPTA-AM often did not peak until >2 min after the beginning of depolarization (see Fig. 3B). The sampling was not synchronized to the beginning of the HiK treatment, so the probability of observing a very brief transient (of ≳1 s) in at least one of the nine preparations examined in 0Ca/EGTA/BAPTA-AM (Fig. 3D) would have been high, although no transients were observed in any of the preparations. In addition, in Aplysia neurons both detectable Ca2+ entry (e.g., Gorman et al. 1982; Kachoei et al. 2006) and Ca2+-mediated responses such as Ca2+-activated K+ currents (Kehoe 1985; Walsh and Byrne 1989) and synaptic transmission (Reyes and Walters, unpublished observations; see also Weragoda et al. 2004) are effectively eliminated by 0Ca/EGTA solutions. Indirect evidence that bath-application with 10 μM BAPTA-AM yields sufficient intracellular BAPTA to significantly chelate free Ca2+ during superfusion by 0Ca/EGTA comes from the observation that addition of BAPTA-AM to the 0Ca/EGTA solution significantly decreases the survival of Aplysia sensory and motor neurons during repeated microelectrode impalement (Reyes and Walters, unpublished observations). This increased vulnerability, which can be counteracted partially by the use of sharper microelectrodes, is consistent with a disrupting effect of free intracellular BAPTA on Ca2+-dependent repair of the membrane (Fishman and Bittner 2003; McNeil and Terasaki 2001). Finally, when we used maximal practical Ca2+ chelator concentrations in nominally Ca2+-free solutions, increasing the EGTA fivefold and the BAPTA-AM twofold, we obtained the same robust pattern of STH and ITH (Fig. 4C) as produced with the lower concentrations of chelators that had blocked detectable Ca2+ transients (Fig. 3D).
The effectiveness of the 0Ca/EGTA/BAPTA-AM solution in preventing detectable Ca2+ transients makes any required signaling by free intracellular Ca2+ unlikely. For example, it argues against a consequential elevation of Ca2+ levels caused by a reduction in Na+/Ca2+ exchange (Misler and Hurlbut 1983), which might occur during HiK treatment because of the replacement of extracellular Na+ by K+. Importantly, none of the manipulations we used to reduce Ca2+ transients, including depletion of intracellular Ca2+ stores (Fig. 4D), prevented axonal hyperexcitability. Moreover, conditions that prevent detectable Ca2+ transients failed to block the induction of protein synthesis–dependent LTH by depolarization (Fig. 5). Indeed, in dissociated neurons our efforts to eliminate Ca2+ transients increased axonal hyperexcitability compared with that induced in ASW (Fig. 2). If Ca2+ is a critical transduction signal in our experiments, it must be operating at far lower concentrations or in more restricted domains than would be expected from known examples of Ca2+ signaling in neurons.
These considerations point to a second explanation for our results—that long-lasting hyperexcitability can be induced by Ca2+-independent, depolarization-dependent signals. In particular, the failure of 0Ca/EGTA/BAPTA-AM solution to reduce axonal STH, ITH, or LTH provides strong evidence that signals in addition to Ca2+ must exist for transducing depolarization into plastic changes in neurons. The absence of Na+ in the HiK solution means that Na+ entry cannot be the critical induction signal under our conditions. The near equality of intracellular and extracellular K+ concentrations indicates that very little net flow of K+ out of or into the cell occurs during depolarization to nearly 0 mV by HiK treatment, making K+ an unlikely induction signal. Furthermore, HiK treatment is not the only depolarizing stimulus that can induce LTH; 2 min of electrical stimulation of the nerve in 1% [Ca2+]o can also induce LTH (Weragoda et al. 2004), suggesting that depolarization accompanied by much smaller increases in [K+]o than occurs during HiK treatment is sufficient to induce LTH. Thus our results indicate that long-lasting axonal hyperexcitability, like long-lasting synaptic facilitation reported at the crayfish neuromuscular junction (Wojtowicz and Atwood 1988) and at Aplysia sensorimotor synapses (Reyes and Walters, unpublished observations), may be induced by depolarization-dependent signals that do not depend on fluxes of Ca2+, Na+, or K+.
Additional transducers of depolarization are potentially important for triggering adaptive plasticity under some conditions
In principle, any voltage-sensitive molecule that is associated with the plasma membrane and coupled to cellular signaling pathways could transduce depolarization into neuronal alterations. This might happen indirectly, by Ca2+-independent exocytosis of neurotransmitters (Bernath 1992; Hochner et al. 1989; Zhang and Zhou 2002) that trigger cellular alterations. Although the observed depolarization-induced axonal hyperexcitability resembles serotonin (5-HT)-induced axonal hyperexcitability (Weragoda and Walters 2007), Ca2+-independent exocytosis of 5-HT cannot explain the effects observed in dissociated sensory neurons because these cultures lack any sources of 5-HT. A remaining possibility is that an autocrine factor, such as sensorin (Hu et al. 2004), might be released by depolarization of sensory axons in a Ca2+-independent manner to feed back and induce hyperexcitability.
Coupling of depolarization to cellular signaling pathways might also occur directly in a Ca2+-independent manner. The best-known depolarization-sensitive structures are voltage-gated ion channels. Interestingly, recent studies have demonstrated a number of nonconducting functions of voltage-gated channels (Kaczmarek 2006), including the depolarization-dependent, conduction-independent activation of a protein kinase by a voltage-gated K+ channel (Hegle et al. 2006). Furthermore, voltage sensitivity has been implicated in other membrane-associated proteins, including a G-protein–coupled receptor (Ben-Chaim et al. 2006), a phosphatidylinositol phosphatase (Iwasaki et al. 2008), and a proton permeation protein that is not associated with a channel (Okamura 2007). Depolarization-dependent, Ca2+-independent transcriptional regulation of a hypoxia-inducible factor has been reported in cancer cells (Lan et al. 2007). These emerging observations suggest that the number of possible Ca2+-independent transducers of depolarization may be very large.
Our results suggest that the induction of axonal hyperexcitability during peripheral injury in Aplysia (Weragoda and Walters 2007; Weragoda et al. 2004) involves Ca2+-independent, depolarization-dependent transduction signals that have yet to be identified. During injury in vivo, such signals would be expected to act in parallel with Ca2+-dependent signals and may complement, but also oppose, effects of the Ca2+-dependent signals on the induction of axonal hyperexcitability by depolarization (see Figs. 1, C and D and 2, C and D for potential complementary and opposing effects, respectively). An interesting possibility is that Ca2+-independent, depolarization-dependent signals may be widespread and contribute to forms of activity- or depolarization-dependent plasticity under conditions of intense and prolonged local depolarization. Large, sustained depolarizations might occur in vivo during intense activation in some forms of learning (Destexhe et al. 2003) and are likely during some pathological states, including peripheral nerve injury (Berdan et al. 1993; Ziv and Spira 1993), traumatic brain injury (e.g., Shaw 2002), and epileptic seizures (e.g., de Curtis and Avanzini 2001).
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-35979 to E. T. Walters.
Acknowledgments
We thank R. M. S. Weragoda, F. D. Reyes, and R. Heidelberger for helpful discussions and Q. Yang for expert assistance.
Present address of D. J. Englot: Department of Neurology, Yale University, 111 Park St, New Haven, CT 06511.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Abrams et al. 1991.Abrams TW, Karl KA, Kandel ER. Biochemical studies of stimulus convergence during classical conditioning in Aplysia: dual regulation of adenylate cyclase by Ca2+/calmodulin and transmitter. J Neurosci 11: 2655–2665, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo et al. 2006.Bardo S, Cavazzini MG, Emptage N. The role of the endoplasmic reticulum Ca2+ store in the plasticity of central neurons. Trends Pharmacol Sci 27: 78–84, 2006. [DOI] [PubMed] [Google Scholar]
- Ben-Chaim et al. 2006.Ben-Chaim Y, Chanda B, Dascal N, Bezanilla F, Parnas I, Parnas H. Movement of “gating charge” is coupled to ligand binding in a G-protein-coupled receptor. Nature 444: 106–109, 2006. [DOI] [PubMed] [Google Scholar]
- Berdan et al. 1993.Berdan RC, Easaw JC, Wang R. Alterations in membrane potential after axotomy at different distances from the soma of an identified neuron and the effect of depolarization on neurite outgrowth and calcium channel expression. J Neurophysiol 69: 151–164, 1993. [DOI] [PubMed] [Google Scholar]
- Bernath 1992.Bernath S Calcium-independent release of amino acid neurotransmitters: fact or artifact? Prog Neurobiol 38: 57–91, 1992. [DOI] [PubMed] [Google Scholar]
- Bozyczko-Coyne et al. 1993.Bozyczko-Coyne D, McKenna BW, Connors TJ, Neff NT. A rapid fluorometric assay to measure neuronal survival in vitro. J Neurosci Methods 50: 205–216, 1993. [DOI] [PubMed] [Google Scholar]
- Brager and Johnston 2007.Brager DH, Johnston D. Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in Ih in hippocampal CA1 pyramidal neurons. J Neurosci 27: 13926–13937, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown et al. 1988.Brown TH, Chapman PF, Kairiss EW, Keenan CL. Long-term synaptic potentiation. Science 242: 724–728, 1988. [DOI] [PubMed] [Google Scholar]
- Burgoyne 2007.Burgoyne RD Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat Rev Neurosci 8: 182–193, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadio et al. 1999.Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell 99: 221–237, 1999. [DOI] [PubMed] [Google Scholar]
- Case et al. 2007.Case RM, Eisner D, Gurney A, Jones O, Muallem S, Verkhratsky A. Evolution of calcium homeostasis: from birth of the first cell to an omnipresent signalling system. Cell Calcium 42: 345–350, 2007. [DOI] [PubMed] [Google Scholar]
- de Curtis and Avanzini 2001.de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol 63: 541–567, 2001. [DOI] [PubMed] [Google Scholar]
- Deng and Lei 2007.Deng PY, Lei S. Long-term depression in identified stellate neurons of juvenile rat entorhinal cortex. J Neurophysiol 97: 727–737, 2007. [DOI] [PubMed] [Google Scholar]
- Destexhe et al. 2003.Destexhe A, Rudolph M, Paré D. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci 4: 739–751, 2003. [DOI] [PubMed] [Google Scholar]
- Dong et al. 2008.Dong C, Upadhya SC, Ding L, Smith TK, Hegde AN. Proteasome inhibition enhances the induction and impairs the maintenance of late-phase long-term potentiation. Learn Mem 15: 335–347, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles 1983.Eccles JC Calcium in long-term potentiation as a model for memory. Neuroscience 10: 1071–1081, 1983. [DOI] [PubMed] [Google Scholar]
- Fan et al. 2005.Fan Y, Fricker D, Brager DH, Chen X, Lu HC, Chitwood RA, Johnston D. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in Ih. Nat Neurosci 8: 1542–1551, 2005. [DOI] [PubMed] [Google Scholar]
- Fishman and Bittner 2003.Fishman HM, Bittner GD. Vesicle-mediated restoration of a plasmalemmal barrier in severed axons. News Physiol Sci 18: 115–118, 2003. [DOI] [PubMed] [Google Scholar]
- Fitzjohn and Collingridge 2002.Fitzjohn SM, Collingridge GL. Calcium stores and synaptic plasticity. Cell Calcium 32: 405–411, 2002. [DOI] [PubMed] [Google Scholar]
- Glanzman 2008.Glanzman DL New tricks for an old slug: the critical role of postsynaptic mechanisms in learning and memory in Aplysia. Prog Brain Res 169C: 277–292, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman et al. 1982.Gorman AL, Hermann A, Thomas MV. Ionic requirements for membrane oscillations and their dependence on the calcium concentration in a molluscan pace-maker neurone. J Physiol 327: 185–217, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz et al. 1985.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- Hawkins et al. 1983.Hawkins RD, Abrams TW, Carew TJ, Kandel ER. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science 219: 400–405, 1983. [DOI] [PubMed] [Google Scholar]
- Hegle et al. 2006.Hegle AP, Marble DD, Wilson GF. A voltage-driven switch for ion-independent signaling by ether-a-go-go K+ channels. Proc Natl Acad Sci USA 103: 2886–2891, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille 2001.Hille B Ion Channels of Excitable Membranes (3rd ed.). Sunderland, MA: Sinauer, 2001, p. xviii.
- Hochner et al. 1989.Hochner B, Parnas H, Parnas I. Membrane depolarization evokes neurotransmitter release in the absence of calcium entry. Nature 342: 433–435, 1989. [DOI] [PubMed] [Google Scholar]
- Hu et al. 2004.Hu JY, Glickman L, Wu F, Schacher S. Serotonin regulates the secretion and autocrine action of a neuropeptide to activate MAPK required for long-term facilitation in Aplysia. Neuron 43: 373–385, 2004. [DOI] [PubMed] [Google Scholar]
- Ibata et al. 2008.Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron 57: 819–826, 2008. [DOI] [PubMed] [Google Scholar]
- Iwasaki et al. 2008.Iwasaki H, Murata Y, Kim Y, Hossain MI, Worby CA, Dixon JE, McCormack T, Sasaki T, Okamura Y. A voltage-sensing phosphatase, Ci-VSP, which shares sequence identity with PTEN, dephosphorylates phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA 105: 7970–7975, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachoei et al. 2006.Kachoei BA, Knox RJ, Uthuza D, Levy S, Kaczmarek LK, Magoski NS. A store-operated Ca(2+) influx pathway in the bag cell neurons of Aplysia. J Neurophysiol 96: 2688–2698, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek 2006.Kaczmarek LK Non-conducting functions of voltage-gated ion channels. Nat Rev Neurosci 7: 761–771, 2006. [DOI] [PubMed] [Google Scholar]
- Kandel 2001.Kandel ER The molecular biology of memory storage: a dialogue between genes and synapses. Science 294: 1030–1038, 2001. [DOI] [PubMed] [Google Scholar]
- Kehoe 1985.Kehoe J Synaptic block of a calcium-activated potassium conductance in Aplysia neurones. J Physiol 369: 439–474, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury et al. 2007.Kingsbury TJ, Bambrick LL, Roby CD, Krueger BK. Calcineurin activity is required for depolarization-induced, CREB-dependent gene transcription in cortical neurons. J Neurochem 103: 761–770, 2007. [DOI] [PubMed] [Google Scholar]
- Kovacs et al. 2007.Kovacs KA, Steullet P, Steinmann M, Do KQ, Magistretti PJ, Halfon O, Cardinaux JR. TORC1 is a calcium- and cAMP-sensitive coincidence detector involved in hippocampal long-term synaptic plasticity. Proc Natl Acad Sci USA 104: 4700–4705, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan et al. 2007.Lan M, Shi Y, Sun L, Liu L, Guo X, Lu Y, Wang J, Liang J, Fan D. KCl depolarization increases HIF-1 transcriptional activity via the calcium-independent pathway in SGC7901 gastric cancer cells. Tumour Biol 28: 173–180, 2007. [DOI] [PubMed] [Google Scholar]
- Levitan et al. 1995.Levitan ES, Gealy R, Trimmer JS, Takimoto K. Membrane depolarization inhibits Kv1.5 voltage-gated K+ channel gene transcription and protein expression in pituitary cells. J Biol Chem 270: 6036–6041, 1995. [DOI] [PubMed] [Google Scholar]
- Malenka 1991.Malenka RC The role of postsynaptic calcium in the induction of long-term potentiation. Mol Neurobiol 5: 289–295, 1991. [DOI] [PubMed] [Google Scholar]
- Malenka and Bear 2004.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron 44: 5–21, 2004. [DOI] [PubMed] [Google Scholar]
- Mattson 2007.Mattson MP Calcium and neurodegeneration. Aging Cell 6: 337–350, 2007. [DOI] [PubMed] [Google Scholar]
- May et al. 1987.May PB, Goh JW, Sastry BR. Induction of hippocampal long-term potentiation in the absence of extracellular Ca2+. Synapse 1: 273–278, 1987. [DOI] [PubMed] [Google Scholar]
- McNeil and Terasaki 2001.McNeil PL, Terasaki M. Coping with the inevitable: how cells repair a torn surface membrane. Nat Cell Biol 3: E124–E129, 2001. [DOI] [PubMed] [Google Scholar]
- Misler and Hurlbut 1983.Misler S, Hurlbut WP. Post-tetanic potentiation of acetylcholine release at the frog neuromuscular junction develops after stimulation in Ca2+-free solutions. Proc Natl Acad Sci USA 80: 315–319, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarolo et al. 1986.Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science 234: 1249–1254, 1986. [DOI] [PubMed] [Google Scholar]
- Okamura 2007.Okamura Y Biodiversity of voltage sensor domain proteins. Pflügers Arch 454: 361–371, 2007. [DOI] [PubMed] [Google Scholar]
- Rao and Finkbeiner 2007.Rao VR, Finkbeiner S. NMDA and AMPA receptors: old channels, new tricks. Trends Neurosci 30: 284–291, 2007. [DOI] [PubMed] [Google Scholar]
- Schacher and Proshansky 1983.Schacher S, Proshansky E. Neurite regeneration by Aplysia neurons in dissociated cell culture: modulation by Aplysia hemolymph and the presence of the initial axonal segment. J Neurosci 3: 2403–2413, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheetz et al. 2000.Scheetz AJ, Nairn AC, Constantine-Paton M. NMDA receptor-mediated control of protein synthesis at developing synapses. Nat Neurosci 3: 211–216, 2000. [DOI] [PubMed] [Google Scholar]
- Shaw 2002.Shaw NA The neurophysiology of concussion. Prog Neurobiol 67: 281–344, 2002. [DOI] [PubMed] [Google Scholar]
- Turrigiano et al. 1994.Turrigiano G, Abbott LF, Marder E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science 264: 974–977, 1994. [DOI] [PubMed] [Google Scholar]
- Tymianski et al. 1993.Tymianski M, Wallace MC, Spigelman I, Uno M, Carlen PL, Tator CH, Charlton MP. Cell-permeant Ca2+ chelators reduce early excitotoxic and ischemic neuronal injury in vitro and in vivo. Neuron 11: 221–235, 1993. [DOI] [PubMed] [Google Scholar]
- Walsh and Byrne 1989.Walsh JP, Byrne JH. Modulation of a steady-state Ca2+-activated, K+ current in tail sensory neurons of Aplysia: role of serotonin and cAMP. J Neurophysiol 61: 32–44, 1989. [DOI] [PubMed] [Google Scholar]
- Walters et al. 2004.Walters ET, Bodnarova M, Billy AJ, Dulin MF, Diaz-Rios M, Miller MW, Moroz LL. Somatotopic organization and functional properties of mechanosensory neurons expressing sensorin-A mRNA in Aplysia californica. J Comp Neurol 471: 219–240, 2004. [DOI] [PubMed] [Google Scholar]
- Walters and Byrne 1983.Walters ET, Byrne JH. Associative conditioning of single sensory neurons suggests a cellular mechanism for learning. Science 219: 405–408, 1983. [DOI] [PubMed] [Google Scholar]
- Walters et al. 1983.Walters ET, Byrne JH, Carew TJ, Kandel ER. Mechanoafferent neurons innervating tail of Aplysia. I. Response properties and synaptic connections. J Neurophysiol 50: 1522–1542, 1983. [DOI] [PubMed] [Google Scholar]
- Weragoda et al. 2004.Weragoda RM, Ferrer E, Walters ET. Memory-like alterations in Aplysia axons after nerve injury or localized depolarization. J Neurosci 24: 10393–10401, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weragoda and Walters 2007.Weragoda RM, Walters ET. Serotonin induces memory-like, rapamycin-sensitive hyperexcitability in sensory axons of Aplysia that contributes to injury responses. J Neurophysiol 98: 1231–1239, 2007. [DOI] [PubMed] [Google Scholar]
- Wertz et al. 2006.Wertz A, Rossler W, Obermayer M, Bickmeyer U. Functional neuroanatomy of the rhinophore of Aplysia punctata. Front Zool 3: Art. 6, 2006. [DOI] [PMC free article] [PubMed]
- West et al. 2001.West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci USA 98: 11024–11031, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams 2006.Williams RJ The evolution of calcium biochemistry. Biochim Biophys Acta 1763: 1139–1146, 2006. [DOI] [PubMed] [Google Scholar]
- Wojtowicz and Atwood 1988.Wojtowicz JM, Atwood HL. Presynaptic long-term facilitation at the crayfish neuromuscular junction: voltage-dependent and ion-dependent phases. J Neurosci 8: 4667–4674, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. 2007.Wu L, Gao X, Brown RC, Heller S, O'Neil RG. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol 293: F1699–F1713, 2007. [DOI] [PubMed] [Google Scholar]
- Xu and Kang 2005.Xu J, Kang J. The mechanisms and functions of activity-dependent long-term potentiation of intrinsic excitability. Rev Neurosci 16: 311–323, 2005. [DOI] [PubMed] [Google Scholar]
- Zanzouri et al. 2006.Zanzouri M, Lauritzen I, Duprat F, Mazzuca M, Lesage F, Lazdunski M, Patel A. Membrane potential-regulated transcription of the resting K+ conductance TASK-3 via the calcineurin pathway. J Biol Chem 281: 28910–28918, 2006. [DOI] [PubMed] [Google Scholar]
- Zhang and Zhou 2002.Zhang C, Zhou Z. Ca2+-independent but voltage-dependent secretion in mammalian dorsal root ganglion neurons. Nat Neurosci 5: 425–430, 2002. [DOI] [PubMed] [Google Scholar]
- Ziv and Spira 1993.Ziv NE, Spira ME. Spatiotemporal distribution of Ca2+ following axotomy and throughout the recovery process of cultured Aplysia neurons. Eur J Neurosci 5: 657–668, 1993. [DOI] [PubMed] [Google Scholar]
- Zucker 1999.Zucker RS Calcium- and activity-dependent synaptic plasticity. Curr Opin Neurobiol 9: 305–313, 1999. [DOI] [PubMed] [Google Scholar]