Abstract
In this study, we propose and evaluate a technique known as common average referencing (CAR) to generate a more ideal reference electrode for microelectrode recordings. CAR is a computationally simple technique, and therefore amenable to both on-chip and real-time applications. CAR is commonly used in EEG, where it is necessary to identify small signal sources in very noisy recordings. To study the efficacy of common average referencing, we compared CAR to both referencing with a stainless steel bone-screw and a single microelectrode site. Data consisted of in vivo chronic recordings in anesthetized Sprague-Dawley rats drawn from prior studies, as well as previously unpublished data. By combining the data from multiple studies, we generated and analyzed one of the more comprehensive chronic neural recording datasets to date. Reference types were compared in terms of noise level, signal-to-noise ratio, and number of neurons recorded across days. Common average referencing was found to drastically outperform standard types of electrical referencing, reducing noise by >30%. As a result of the reduced noise floor, arrays referenced to a CAR yielded almost 60% more discernible neural units than traditional methods of electrical referencing. CAR should impart similar benefits to other microelectrode recording technologies—for example, chemical sensing—where similar differential recording concepts apply. In addition, we provide a mathematical justification for CAR using Gauss-Markov theorem and therefore help place the application of CAR into a theoretical context.
INTRODUCTION
Individual unit recordings from cortical neurons are dependent on separating the recorded extracellular action potential of a neuron from ambient sources of noise. These sources of noise can be correlated or uncorrelated across the electrode array and include motion artifact, 60-Hz noise, instrumentation noise, thermal noise, and biological sources of noise (Horsch and Dhillon 2004; Kovacs 1994; Schmidt and Humphrey 1990; Shoham and Nagarajan 2003; Webster 1998). As cortical recording experiments move away from closed environments designed to reduce noise (e.g., Faraday cages) to more real-world situations (e.g., neuroprosthetic devices), these sources of noise become increasingly problematic (Horsch and Dhillon 2004; Kovacs 1994; Schmidt and Humphrey 1990; Shoham and Nagarajan 2003; Webster 1998).
Recording useful signal is dependent on minimizing sources of noise through the use of an appropriate reference electrode (Horsch and Dhillon 2004; Kovacs 1994; Schmidt and Humphrey 1990; Shoham and Nagarajan 2003; Webster 1998). Typically either an additional microelectrode, or a large electrode such as a stainless-steel bone screw, or stripped wire, is placed in a location with minimal cortical activity and used as a reference to subtract out correlated sources of noise (Blanche et al. 2005; Henze et al. 2000; Ludwig et al. 2006; Nelson et al. 2008; Vetter et al. 2004; Webster 1998; Williams et al. 1999). Both microelectrode and large electrode references have their own specific advantages and disadvantages.
A large electrode is often preferred as a reference to minimize impedance (Horsch and Dhillon 2004; Kovacs 1994; Schmidt and Humphrey 1990; Shoham and Nagarajan 2003; Webster 1998). Thermal noise is proportional to impedance; therefore less thermal noise is “subtracted into” the recordings when using a low-impedance reference electrode (Horsch and Dhillon 2004; Kovacs 1994; Schmidt and Humphrey 1990; Shoham and Nagarajan 2003; Webster 1998). Unfortunately, there is a significant size and impedance mismatch between the reference and the recording sites on the microelectrode array when using a large reference electrode (Horsch and Dhillon 2004; Kovacs 1994; Schmidt and Humphrey 1990; Shoham and Nagarajan 2003; Webster 1998). Consequently, the representation of correlated sources of noise (such as motion artifact and 60-Hz noise) is different between the reference and the microelectrode sites and therefore is not fully removed by reference subtraction (Horsch and Dhillon 2004; Webster 1998).
Because of the sheer size of the large electrode, the reference is typically placed on top of the dural surface, as opposed to implantation in cortical tissue (Ludwig et al. 2006; Vetter et al. 2004). By placing the reference in a location distal from the microelectrode sites, the difference between the voltage representation of correlated sources of noise at the reference and the microelectrode sites is increased (Horsch and Dhillon 2004; Webster 1998). In addition, there remains the possibility of the reference adding an ECoG (electrocorticogram) signal at the dural surface into the recordings.
As opposed to a large reference electrode, the use of an additional microelectrode implanted in cortical tissue as a reference presents alternative problems. Although microelectrode references match recording sites on the array in terms of geometry and impedance, they have greater impedance than their large electrode counterparts and therefore “subtract in” more thermal noise to the recordings (Horsch and Dhillon 2004; Kovacs 1994; Ludwig et al. 2006; Schmidt and Humphrey 1990; Shoham and Nagarajan 2003). This problem is exacerbated in chronic applications, where the microelectrode reference becomes encapsulated in fibrous tissue, further increasing its impedance (Hochberg et al. 2006; Ludwig et al. 2006; Nicolelis et al. 2003; Otto et al. 2006; Polikov et al. 2005; Rennaker et al. 2007; Seymour and Kipke 2007; Spataro et al. 2005; Suner et al. 2005; Szarowski et al. 2003; Turner et al. 1999; Vetter et al. 2004).
When placed on the electrode array itself, a microelectrode reference may actually record neural signal or uncorrelated biological noise caused by the activity of distal neural sources; this uncorrelated activity is subtracted into the recordings (Horsch and Dhillon 2004; Webster 1998). If placed in a location to minimize the probability of recording neural activity (e.g., corpus callosum), the increased physical separation of the reference electrode from the microelectrode array decreases the correlation between noise at these two locations and therefore decreases the utility of the reference (Horsch and Dhillon 2004; Webster 1998).
In this study, we introduce a technique known as common average referencing (CAR) to generate a more ideal electrode reference for single-unit neural recordings. CAR is commonly used in EEG, where it is necessary to identify small signal sources in very noisy recordings (Cooper et al. 2003; Offner 1950; Osselton 1965). Unlike more complex methods of denoising recorded signals post hoc (Aminghafari et al. 2006; Bierer and Andersen 1999; Oweiss and Anderson 2001), CAR is a computationally simple technique and therefore amenable to both on-chip and real-time applications. As the name implies, an average of all the recordings on every electrode site is taken and used as a reference (Cooper et al. 2003; Offner 1950; Osselton 1965). Through the averaging process, only signal/noise that is common to all sites (correlated) remains on the CAR.1 Signal that is isolated on one site (single-unit activity) does not appear on the CAR, unless the signal is so large as to dominate the average2 (Cooper et al. 2003; Offner 1950; Osselton 1965). Uncorrelated random noise with a zero mean is minimized through the averaging process.3 Because the CAR provides an accurate representation of correlated noise at the location of the microelectrode array, but minimizes the contribution of uncorrelated noise sources, we hypothesize that common average referencing will improve neural recording quality with respect to both large and microelectrode references.
To study the efficacy of common average referencing, we compared CAR to both referencing with a stainless-steel bone screw and a single microelectrode site, using in vivo chronic recordings from a prior study (Ludwig et al. 2006), as well as previously unpublished data. By combining the data from multiple studies, we generated and analyzed one of the largest chronic neural recording datasets to date. Reference types were compared in terms of peak-to-peak noise, signal-to-noise ratio, and number of units recorded across days. Moreover, we provide a mathematical justification for common average referencing based on Gauss-Markov theorem and therefore build a theoretical context for future CAR applications.
METHODS
Microelectrodes
Twenty-one male Sprague-Dawley rats were implanted with 26 16-channel chronic silicon “Michigan” microelectrode arrays, using experimental procedures outlined previously (Ludwig et al. 2006; Vetter et al. 2004). Arrays consisted of four shanks, each with four evenly spaced iridium electrodes. Site and shank spacings were sufficient (100 μm or greater) to limit the probability of an individual neuron being recorded from multiple sites (Henze et al. 2000). All of the electrodes on a specific array were the same site size; electrode site sizes on an individual array were either 703 or 1,250 μm2.
The data for this study was drawn from previous (Ludwig et al. 2006) and ongoing studies aimed at evaluating the efficacy of the conductive polymer poly(3,4-ethylenedioxythiophene) (PEDOT) for improving neural recording quality. Toward that end, eight of the sites on each array were coated with PEDOT using various deposition methods and counter-ions, the details of which are beyond the scope of this study (Ludwig et al. 2006). The remaining eight sites were left uncoated as controls. Sites were stagger coated to prevent bias caused by cortical depth or location (Ludwig et al. 2006).
Overall, PEDOT sites perform similarly to control sites in recording neural activity, with one exception. As noted in other studies, sites coated with PEDOT recorded activity from a slightly larger number of neurons, primarily as a result of reduced thermal noise (Cui and Martin 2003; Ludwig et al. 2006; Yang et al. 2005). These slight differences in recording performance did not affect the results in this paper (see results and discussion for details).
Surgical techniques
All of the arrays in this study were implanted in motor cortex, targeting cortical layer V. Initial anesthesia was administered via intraperitoneal injections of a mixture of 50 mg/ml ketamine, 5 mg/ml xylazine, and 1 mg/ml acepromazine at an injection volume of 0.125 ml/100 g body weight. Updates of 0.1 ml ketamine (50 mg/ml) were delivered as needed to maintain anesthesia during the surgery. Animals were secured to a standard stereotaxic frame, and three stainless-steel bone screws were inserted into the skull. The electrode connector was grounded to a bone screw over parietal cortex using a stainless-steel wire.
A craniotomy ∼3 × 2 mm was made over the target area (target location: 3.0 mm anterior to bregma, 2.5 mm lateral from bregma, and 1.4 mm deep from the surface of the brain). Two incisions were made in the dura mater to create four flaps, which were subsequently folded back over the edge of the craniotomy. The electrodes were hand inserted using a microforceps into the approximate target cortical area. Cortical depth was estimated using the known location of the electrode sites on the individual shanks in conjunction with the known length of the individual shanks. Next, the surface of the brain was covered with GelFoam (Henry Schein, Miami, FL) for protection. The silicon cable connector was covered with either remaining Gelfoam or Kwik-Sil silicone polymer (World Precision Instruments). The entire assembly excluding the connector was enclosed using dental acrylic (Co-Oral-Ite, Dental Mfg., Santa Monica, CA). Finally, sutures were used to close the skin around the acrylic, and triple-antibiotic ointment was applied. All procedures complied with the U.S. Department of Agriculture guidelines for the care and use of laboratory animals and were approved by the University of Michigan Animal Care and Use Committee.
Neural recordings and data analysis
For eight of the animals in this study, recorded neural signals were acquired using a Plexon Multi-channel Neural Acquisition Processor (MNAP; Plexon, Dallas, TX). For the remaining animals, signals were acquired using a TDT multi-channel acquisition system (Tucker-Davis Technologies, Gainesville, FL). Neural electrophysiological recordings for all 16 channels were amplified and band-pass filtered; single- and multi-unit recordings were sampled at either 40 kHz (Plexon) or 24,414 Hz (TDT), and band-pass filtered from 450 to 5,000 Hz. During recording sessions, animals were placed in an electrically shielded recording booth, and multiple 30-s segments of continuous neural recordings were taken.
Neural recording segments were analyzed off-line using custom automated MatLab (Mathworks) software, as described in detail elsewhere (Ludwig et al. 2006). In summary, an amplitude threshold window was set 3.5 SD above and below the mean of the sample distribution. For each peak exceeding the threshold window, a 2.4-ms candidate waveform snippet centered on the absolute minimum of the waveform was removed from the recorded segment and stored. The amplitude of the noise voltage for every recording site in each recorded segment was calculated after all candidate waveforms had been removed.
After initial principal component analysis, individual clusters were identified using Fuzzy C-Means clustering (Bezdek 1981; Dunn 1974; Ludwig et al. 2006). When compared with hard clustering, fuzzy clustering reduces classification errors resulting from the synchronous firing of multiple neurons (Zouridakis and Tam 2000). To determine the optimum number of clusters, the number of clusters was iteratively increased until the value for the objective function calculated for k + 1 number of clusters was ≥55% of the value for the objective function calculated for k number of clusters (Karkkainen and Franti 2002).
After clustering, waveforms with a cluster membership index of >0.8 were used to determine a mean waveform for a cluster. Contributions of white noise and waveforms created by the simultaneous firing of multiple neurons generally do not have a membership index of >0.8 for a particular cluster and therefore were limited using this procedure (Zouridakis and Tam 2000). An interspike interval histogram for each cluster was generated and visually inspected for an obvious absolute refractory period as an additional measure of noise rejection. Signal amplitude for a cluster was defined as the peak-to-peak amplitude of the mean waveform for each cluster.
The signal-to-noise ratio (SNR) for a given cluster was defined as follows
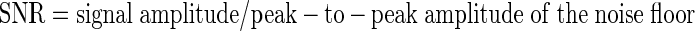 |
The peak-to-peak amplitude of the noise on a given site was calculated as six times the SD of the recording after thresholded waveforms were removed, spanning ∼99.7% of normally distributed noise data (Blanche et al. 2005). By using this method, the calculated SNR and peak-to-peak noise amplitude on a given site was more consistent with a visual inspection of the recorded voltage traces (see Figs. 2 and 3). For example, an SNR of 2 would indicate that the mean peak-to-peak amplitude of the signal was twice as large as the peak-to-peak amplitude of the noise floor. Because the peak-to-peak amplitude of the noise floor for neural recordings is typically between 6 and 10 times larger than the root mean square (RMS) value of the noise floor (Blanche et al. 2005), SNR calculations for neural recordings based on the RMS of the noise floor raise the SNR with respect to peak-to-peak values.
Clusters with a mean SNR of 1.1 or greater were considered discriminable units, because the signal amplitude of these clusters was sufficient to be reliably differentiated from the noise floor. Conversely, clusters generated by random outlying perturbations from sources of noise had mean SNR values of 0.9 or less. Although normally distributed noise sources will occasionally exceed the 3.5 SD threshold by random chance, the average waveform generated by these noise sources returns to zero after crossing threshold (instead of exhibiting an immediate opposing peak). Consequently, the mean waveform of a noise cluster spans <6 SD of the noise floor, resulting in a calculated SNR of <1. When adjusted for the difference between calculating SNR using peak-to-peak amplitude of the noise floor instead of RMS, an SNR of 1.1 or greater corresponded well with observations of “moderate or better” unit quality based on SNR values from similar recording studies (Henze et al. 2000; Ludwig et al. 2006; Suner et al. 2005).
Isolating action potentials from an individual neuron using an individual recording site is inherently prone to classification errors (Harris et al. 2000; Lewicki 1998). The methodology used in this study was intended to minimize these errors and should accurately parallel the true number of underlying neural sources (Ludwig et al. 2006). The sorting routine produces similar results to manual sorting performed by experienced researchers over the same datasets, but with the advantage of being objective and automated (Ludwig et al. 2006).
Referencing techniques
As noted previously, all recordings in this study were initially referenced to a stainless-steel bone screw (screw) located over parietal cortex. Both the microelectrode reference and the CAR were implemented digitally after this initial reference subtraction.
CAR.
The most intuitive implementation of a CAR would be to reference a specific site to the sample by sample average of all of the remaining sites on the array. Unfortunately, this approach presents two problems with respect to real-time cortical recordings. First, each of the 16 sites would have a unique reference, instead of a global reference shared by all sites. This presents an additional layer of complication in translating a CAR from a digital reference to an on-chip analog reference. Second, individual sites on a microelectrode array occasionally fail to function properly. Consequently, these bad sites must be identified and removed from the dataset before generating a CAR.
To address these problems, the common average reference for each array was generated by taking the sample by sample average of all “good” recording sites, creating one global reference for all sites (CAR-16). For a site to be considered good, the RMS of the noise floor on the site was required to be between 0.3 and 2 times the average RMS of the noise floor across all 16 sites on the array. Sites identified as “bad” through impedance spectroscopy (Ludwig et al. 2006) typically exhibited noise floors with an RMS value 3–6 times larger than the average RMS of the noise floor on good sites. This simple methodology for eliminating bad channels proved sufficient for removing the occasional bad site from further analysis in an automated fashion.
Because each good recording site contributes to the average calculated for the global CAR, the amplitude of all samples on a good site is slightly decreased when referenced to the CAR. More specifically, given n good sites, the amplitude of each sample will be (n − 1)/n times the original value (Cooper et al. 2003; Offner 1950; Osselton 1965). As n becomes larger, this scale factor approaches a value of 1. Because this scale factor affects both signal and noise equally, it does not affect the calculated SNR on each site.4 For comparison with other types of referencing, all CAR peak-to-peak noise values denoted in this study have been appropriately adjusted to compensate for this scale factor.
To assess the contribution of recording sites electrochemically deposited with a conductive polymer to the overall data, two additional common average references were created and applied to all data sets. One CAR was generated from only the conductive polymer sites on a given array, and the second CAR from only the control iridium sites. The CAR generated from conductive polymer sites was only applied as a reference to the conductive polymer sites; the CAR generated from control sites was only applied as a reference to the control sites. In this paper, data obtained using this secondary method are referred to as CAR-8.
SINGLE-BEST MICROELECTRODE REFERENCE.
Cortical recordings are often taken in reference to an individual microelectrode site; consequently, we implemented an algorithm to identify the single-best microelectrode reference on an array to compare with common average referencing. Because a single microelectrode may pick up single-unit activity, we first used our automated sorting algorithm in conjunction with CAR to identify candidate sites with no discernible unit activity.5 Each of the candidate sites were used as a reference for the entire array on the original data set. The candidate microelectrode reference that created the lowest average noise floor across the array on a given day was selected as the single-best microelectrode reference. By identifying the single-best microelectrode reference for each array on a daily basis, we created a conservative comparison for CAR.
Theoretical justification of CAR using Gauss-Markov theorem
Multi-channel neural recordings can be described as an observed noisy signal, Y, which is composed of an unknown true signal, X, and an unknown noise, N. This general situation can be described by the following equation (Albert 1972; Stark and Woods 2002)
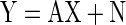 |
(1) |
where Y is the n × 1 vector of observed neural recordings across n channels, X is a k × 1 vector representing the true signal (with k representing the number of underlying neural sources), A is an n × k matrix (n > k) that maps X onto Y, and N is an n × 1 normally distributed random noise vector with zero mean and covariance matrix K.
One useful approach for obtaining a good estimate, X̂, of X from the observed values of Y is to restrict X̂ to be a linear function of Y
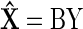 |
(2) |
Gauss-Markov theorem states that, in a linear model in which the errors have expectation zero and equal variances, a best linear unbiased estimate of the coefficients is given by a least squares estimator (Albert 1972; Stark and Woods 2002). In this formulation, we want to determine the matrix B, a spatial filter that provides the best linear unbiased estimate (BLUE) of X based on Y.
The filter, Bi, for each channel, i, can be calculated as follows (Albert 1972; Stark and Woods 2002)
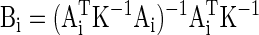 |
(3) |
There are two simplifications that align this general solution with this study. 1) Site spacing is sufficiently large to ensure that neurons are expressed on only one site. Consequently, Ai for each channel has the form [1, 0, 0,… , 0]. 2) The noise model for each channel is identical, producing a covariance matrix K with equal diagonal values (1) and equal off-diagonal components (c).
Consequently, B for each channel has the form
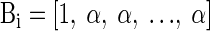 |
(4) |
where α = −c/[1 + (n − 2)c].
As n increases, α approaches −1/(n − 1), leaving Bi as
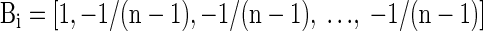 |
(5) |
Placing this result into Eq. 2, obtaining the best linear unbiased estimate of X is equivalent to subtracting the average of all other channels from the current channel—in other words, use a CAR. This formulation is generally true even if the diagonal and off-diagonal values vary across channels. Note that when c is large (near 1), c approaches −1/(n − 1) more quickly. This result is intuitive; when there is high-amplitude correlated noise across channels, referencing is particularly effective. When there is very little or no correlated noise across channels, using a reference only subtracts in uncorrelated noise from the reference to the other channels. Although common average referencing minimizes uncorrelated noise as a function of n, it does not remove it entirely. Consequently, referencing becomes counterproductive when there is no correlated noise between the reference and recordings sites.
Statistical analysis
Because the four types of referencing in this study were performed digitally on the same data sets, these data sets are highly correlated. To accommodate the correlations between comparison groups, a repeated-measures ANOVA was performed; a Bonferroni correction was applied to the tests for each group pair to control for experimentwise error rate. The Bonferroni correction is regarded as a very strict/conservative test of significance when comparing multiple groups. At an α value of 0.01, the Bonferroni correction required P < 0.0017 for significance. Equality of variance was verified by the Levene statistic.
RESULTS
Noise
Over the course of this study, the average peak-to-peak noise floor across all sites using CAR was 32.5 μV (Fig. 1), a significant improvement over referencing to either a stainless steel ground screw (45.9 μV; P < 10−15) or to the single-best microelectrode site on the array (40.7 μV; P < 10−15). More specifically, every single recording site in the study (n = 5,010) exhibited a lower noise floor when referenced to the CAR in comparison to the single-best microelectrode reference. In all but three instances, the noise floor using CAR was lower than referencing to a ground screw.
FIG. 1.
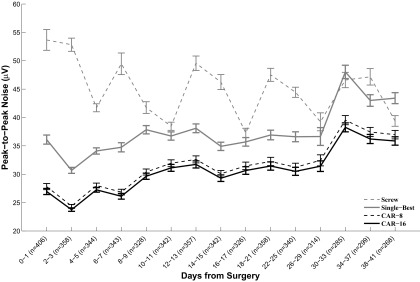
Noise across days. Bars denote SE of the dataset on a given block of days. Number of sites on a given block of days, n, is listed on the x-axis. Over the course of the study, sites referenced to a common average reference exhibited 30% less noise than standard methods of referencing (P < 10−15). Sites referenced to a ground screw placed over parietal cortex exhibited increased noise floor variability in comparison to referencing with common average referencing (CAR) or the single-best microelectrode site. Noise floor amplitude calculated using CAR or single-best microelectrode site references decreased immediately following surgery and increased afterward, consistent with the trend in noise level found in prior studies (Ludwig et al. 2006; Schwartz 2004; Vetter et al. 2004.; Williams et al. 1999).
Using either CAR or single-best references, the trend in the average noise floor across sites following surgery paralleled the results of other microelectrode recording studies. The noise floor typically decreased over the days immediately following surgery and gradually increased over time. An increase in noise over time is expected, as the impedances of the electrodes are known to increase over time due to fibrous encapsulation, increasing the thermal noise on a site (Ludwig et al. 2006; Vetter et al. 2004; Williams et al. 1999).
In contrast to CAR or single-best references, the noise floor using the ground screw as a sole reference was considerably more variable. This variability can be explained by separating the recordings into two distinct cases. In the first case, the recordings made using the ground screw as a reference were contaminated by one or more large sources of correlated noise (Fig. 2). Both the CAR and single-best reference minimize the contribution of correlated noise sources and therefore exhibit lower noise floors and more distinct unit activity than ground screw referencing when large sources of correlated noise are evident. Because the ground screw is a poor match for the implanted microelectrode in terms of location, impedance, and geometry, it does not lower the contribution of correlated noise sources as effectively. The noise floor on electrode sites was larger when using the single-best electrode reference as opposed to the CAR, because the single-best electrode subtracts in the uncorrelated noise from the single electrode site. Conversely, uncorrelated sources of noise with a zero mean tend toward zero when averaged over all the sites on an array using CAR.
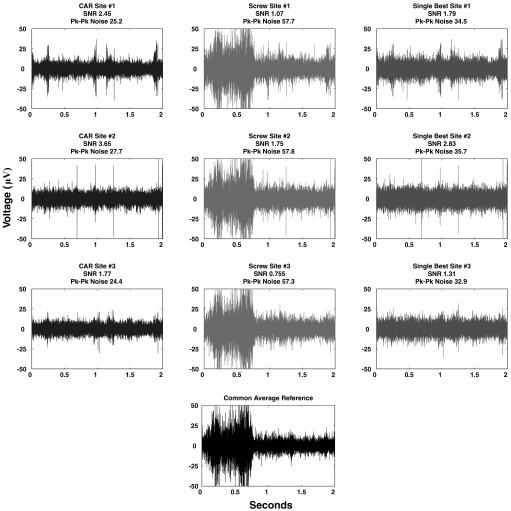
FIG. 2.
Representative example of recordings contaminated by correlated noise. All columns depict the same 2 s of high-speed recordings taken simultaneously across 3 sites on the same array. Column 1 data are referenced to a CAR. Column 2 data are referenced to a stainless-steel screw placed above parietal cortex. Column 3 data are referenced to the single-best microelectrode reference on the array. Sites referenced to CAR exhibit a lower noise floor and higher signal-to-noise ratio than standard electrical references. When sites are referenced to the ground screw, an intermittent correlated noise source is evident that does not appear when using either the CAR or single-best microelectrode reference. This noise source is sufficient to completely obscure unit activity. Also note that, despite the presence of large action potentials on site 2, traces of this signal are not evident on sites 1 and 3 when referenced to CAR (see sites 1 and 3 screw and single-best reference over the same data set for comparison).
In the second case, the recordings were only minimally contaminated by correlated noise (Fig. 3). In this case, the ground screw reference actually lowered the noise floor with respect to the single-best microelectrode reference. Ground screws used in this study had impedances that were multiple orders of magnitude lower than implanted microelectrodes and consequently subtracted less thermal noise into the recordings.6 Because only recordings referenced to the ground screw were significantly altered by the absence or presence of correlated noise, recordings referenced to the ground screw were considerably more variable.7 The CAR, which minimized both correlated and uncorrelated sources of noise, dramatically outperformed the single-best and ground screw references in both cases.
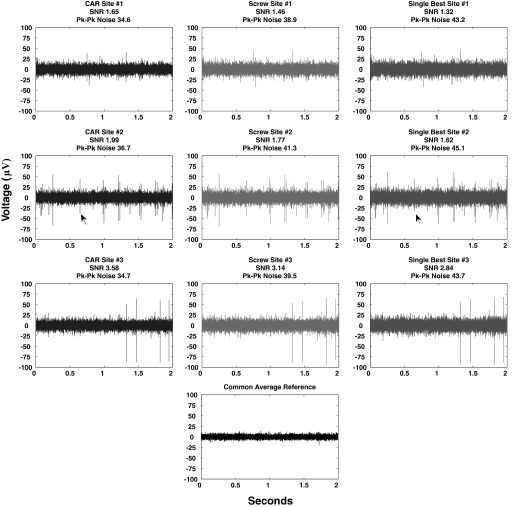
FIG. 3.
Example of recordings with low correlated noise. All columns depict the same 2 s of high-speed recordings taken simultaneously across 3 sites on the same array. Column 1 data are referenced to a CAR. Column 2 data are referenced to a stainless-steel screw placed above parietal cortex. Column 3 data are referenced to the single-best microelectrode reference on the array. Sites referenced to CAR exhibit a lower noise floor and higher signal-to-noise ratio than standard electrical references. In cases where large sources of correlated noise are not evident, the ground screw reference routinely outperformed the single-best reference in terms of noise level, signal-to-noise, and number of discriminable units. In these cases, common average referencing still outperformed referencing to either a ground screw or the single-best microelectrode site on the array. Arrows denote a neural signal evident on site 2 when referenced to either CAR or single-best microelectrode reference. Note that the waveform has been distorted on the voltage axis, presumably a result of the increased noise floor when using the single-best microelectrode reference. Even if a neural unit is discernible from the noise, an increased noise floor means more waveform variability, limiting the efficacy of common sorting algorithms (Lewicki 1998).
Signal-to-noise and unit activity
Calculating a meaningful average SNR of unit recordings for comparison between reference types was complicated by the fact that sites registered an overall greater number of discernible units with CAR. More specifically, the reduction in noise on CAR sites was sufficient to separate previously unapparent signals from the noise floor—instead of just increasing the SNR of already discriminable units. Therefore comparing the average SNR of all discriminable units was not representative of the true difference in SNR between reference types.
To calculate the true difference in SNR between CAR and other types of referencing, we first used CAR in conjunction with our automated sorting algorithm to identify the location in time of all unit activity. The known location in time of unit activity was used to calculate the mean signal amplitude for all reference types. Through this methodology, we were able to identify the location of signal that was previously obscured by the noise floor when not using CAR.
Over the course of this study, sites referenced to CAR exhibited a higher SNR than sites referenced to either a ground screw or single-best microelectrode reference (P < 10−10; Fig. 4). The average SNR on CAR sites was 1.59, in comparison to 1.31 for sites referenced to the single-best microelectrode reference and 1.24 for sites referenced to a ground screw. Because CAR does not alter the relative size of signal on a given site, this improvement in performance is attributable to the decreased CAR noise floor.
FIG. 4.
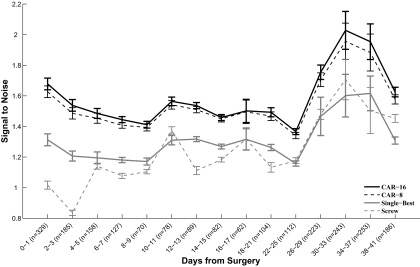
Signal to noise across days. Bars denote SE of the data set on a given block of days. Number of units recorded on a given block of days, n, is listed on the x-axis. Over the course of the study, sites referenced to CAR exhibited a signal-to-noise ratio of 1.59, a significant improvement over referencing to either the single-best microelectrode site (1.31, P < 10−10) or ground screw (1.24, P < 10−10). Variability in signal-to-noise ratio across all types of reference increased toward the end of the study, concurrent with an increase in number of units recorded across all arrays. An increase in number of units recorded starting at 3 wk after implantation has been noted in prior studies (Ludwig et al. 2006; Santhanam et al. 2007; Schwartz 2004).
Referencing with CAR both 1) increased the SNR of units already evident when referencing to the single-best microelectrode reference or the ground screw and 2) decreased the noise floor sufficiently to show units that were previously obscured when referencing to the single-best microelectrode or the ground screw. Consequently, there was a greater number of discernible units when referencing to CAR in comparison to a ground screw or single-best microelectrode reference (P < 10−10; Fig. 5). Over the course of the study, an average of 46.5% of CAR sites exhibited discriminable unit activity, in comparison to 29.6% of the single-best sites and 27.46% of the ground screw sites. CAR sites recorded an average of 0.842 units per day, whereas single-best sites recorded an average of 0.447 units per day and ground screw sites recorded an average of 0.415 units per day. These daily numbers correspond to 4,045 total units registered on CAR sites during this study, in comparison to 2,152 units registered on single-best sites and 1,998 units registered on ground screw sites. As with the improvement in SNR, this improvement is attributable to the markedly decreased CAR noise floor. Consistent with previous studies (Ludwig et al. 2006; Santhanam et al. 2007; Schwartz 2004; Vetter et al. 2004; Williams et al. 1999), there was a notable decrease in unit activity in the days immediately following surgery, followed by an increase in unit activity after day 15. Although the exact reason for this increase in unit activity at the 2- to 3-wk point is unknown, this result has been previously reported in other studies (Ludwig et al. 2006; Santhanam et al. 2007; Schwartz 2004). At 2–3 wk after implantation, the fibrous encapsulation around an implant becomes more defined, and the immune response begins a transition into the chronic phase (Turner et al. 1999).
FIG. 5.

Percentage of sites with units across days. Bars denote SE of the data set on a given block of days. Number of arrays included in analysis, n, is listed on the x-axis. Over the course of the study, sites referenced to a common average reference yielded almost 60% more discernible units than when reference to standard electrical references. This increase in performance is attributable to a reduced noise floor, enhancing signal-to-noise ratio, and therefore increasing the number of discernible units. Unit recordings were strong initially, dipped dramatically in the days following surgeries, and returned to initial levels after the 4-wk point. This trend in recording performance has been noted in previous recording studies (Ludwig et al. 2006; Santhanam et al. 2007; Schwartz 2004).
DISCUSSION
Conductive polymer CAR
As noted in the methods, two additional CARs were created and applied to all data sets to assess the contribution of recording sites electrochemically deposited with a conductive polymer to the overall data. One CAR was generated from only the conductive polymer sites on a given array, and the second CAR from only the control sites (CAR-8). The CAR generated from conductive polymer sites was only applied as a reference to the conductive polymer sites; the CAR generated from control sites was only applied as a reference to the control sites.
If the noise between conductive polymer sites was more correlated than the noise between conductive polymer and control sites, one would expect the noise floor to decrease by generating separate CARs for conductive polymer and control sites. Instead, creating separate CARs marginally increased the noise floor (Fig. 1). This increase in noise is a result of using only 8 sites to calculate the CAR, instead of 16. As shown previously, using CAR more closely approximates the best linear unbiased estimate of the signal as the number of channels increases. The average of uncorrelated sources of noise with a zero mean tends toward zero; the contribution of uncorrelated sources of noise becomes closer to zero when more sites are included to generate the average. Consequently, decreasing the number of sites used to calculate the CAR increases the contribution of uncorrelated noise sources.
Although the correlation in noise between pairs of sites across a single array was similar on a given day, this correlation value could vary dramatically from day to day. The average cross-channel correlation on an array was 0.66 over the course of this study, but this average value ranged from 0.2 to 0.95 depending on the day. These values are similar to results reported by Rebrik et al. (1999), who found that cross-channel correlation coefficients within the same tetrode implanted in cat visual cortex ranged between 0.8 and 0.92, whereas the correlation coefficients across channels of different tetrodes ranged between 0.47 and 0.51.
Application of CAR to tetrodes
Under certain circumstances, there is a possibility of registering neural signal on a single site so large that it dominates the average of all sites, and therefore appears on the CAR (Cooper et al. 2003; Offner 1950; Osselton 1965). Accordingly, this neural signal would appear as a small false unit on all sites referenced to the CAR. For this to occur, given n recording sites, the average amplitude of the signal on a given site must be n times larger than the peak-to-peak amplitude of the noise floor (Cooper et al. 2003; Offner 1950; Osselton 1965). In this study, the signal amplitude on one site was never sufficient to dominate the average (Fig. 4); given 16 recordings sites on every array, the signal on a single site would have to regularly exceed 16 times the peak-to-peak amplitude of the noise floor (Figs. 2 and 3). Based on the largest peak-to-peak amplitudes for action potentials observed in this study in comparison to the peak-to-peak amplitude of the noise floor, a minimum of five electrodes must be used to calculate the CAR to limit the possibility of an action potential dominating the common average reference. As sites were separated by >100 μm in this study, the probability of recording signal from an individual neuron on multiple sites simultaneously was low (Henze et al. 2000).
For some neural recording applications, microelectrodes are manufactured with groups of four recording sites closely spaced to deliberately detect neural activity from an individual neuron simultaneously, known commonly as a tetrode configuration (Gray et al. 1995). As a result, the probability of signal from an individual neuron dominating the CAR average becomes much greater. If m sites of n total electrodes record similar signal from an individual neuron, the average signal across m electrodes needs only to exceed n/m times the peak-to-peak amplitude of the noise floor to dominate the CAR average. One solution to this problem would be to increase n, the number of electrode sites used to calculate the CAR. In addition, a site or sites recording aberrantly large signal could simply be removed when calculating the common average reference (Cooper et al. 2003; Offner 1950; Osselton 1965).
When using a CAR, the potential contribution from a neural source on one electrode in a tetrode could diminish the measured potential from the neural source on the three remaining electrodes in the tetrode. This distortion should be constant across all four electrodes and therefore should not adversely affect standard amplitude-based tetrode clustering techniques (Gray et al. 1995). The distortion could be problematic, however, when using mathematical techniques to locate the neural source in space.
Comparing CAR to alternative correlation-based denoising methods
A number of post hoc methods have been previously suggested that identify and use the correlated noise across channels to reduce overall noise and improve spike sorting (Bierer 1999; Musial et al. 2002; Rebrik et al. 1999). These post hoc analysis techniques require initial segments of data to define the correlation in noise between multiple channels. Consequently, these strategies are computationally intensive in comparison to CAR and are not easily performed in real time. Moreover, these methods require additional recalibration steps as the correlation in noise across the array changes over time. As a result, these techniques are not easily implemented on-chip. CAR is not a post hoc analysis technique but instead an alternative electrical reference, which can be implemented in real time without the need for calibrations to account for transient changes in noise content. In addition, CAR can be implemented with a simple circuit either on-chip or at the recording headstage of the microelectrode array, thereby minimizing the contribution of noise before subsequent amplification and digitization.
To implement CAR on-chip, it is necessary to ensure that the amplitude of the incoming signal is within the dynamic range of the operational amplifier (op-amp). In some cases, noise transients are large enough to exceed the dynamic range of the op-amps due to the large amplification that is traditionally required for front-stage neural acquisition hardware, causing “saturation” or “clipping” of the output signal. To avoid this problem, a large electrode such as a ground screw is normally used as an electrical reference to minimize incoming noise transients. To successfully implement real-time hardware CAR, it would be necessary to either 1) increase the dynamic range of the op-amps to exceed maximum values of expected noise transients given the gains necessary for signal reconstruction or 2) first use a ground screw as an electrical reference to prevent amplifier saturation.
Additional benefits of reducing noise
In some cases in this study, CAR provided a significant improvement in recording performance, in other cases CAR salvaged recordings that would have otherwise been unusable. Decreasing the noise floor not only increases the total number of units recorded across an array but also decreases the variability of those units. Additional noise can alter the instantaneous shape of an action potential, potentially distorting the recorded waveform in principal component analysis (PCA) space, deleteriously affecting common sorting techniques (Lewicki 1998) (Fig. 3).
Perhaps even more significant, CAR mitigates the contribution of intermittent sources of correlated noise (Fig. 2), such as motion artifact. These noise sources distort the relationship between neural firing rates and underlying physiological processes. This is especially problematic in real-time applications such as brain–machine interfaces, where intermittent artifact cannot be removed through post hoc analysis. Reducing variability in the noise floor can also be critical for comparison studies of electrode performance. Significant differences in electrode performance as a function of electrode design or a drug treatment could potentially be masked by the large variance in noise floor from day to day.
Conclusions
According to standard differential recording theory, the reference electrode and the recording electrode must be matched as closely as possible in terms of geometry, electrode material, location, and electrical characteristics (Kovacs 1994; Schmidt and Humphrey 1990; Shoham and Nagarajan 2003; Webster 1998). These requirements would seem to suggest the use of a single microelectrode as a reference, instead of an unmatched larger electrode. Unfortunately, because of the small size of a microelectrode, coupled with fibrous encapsulation endemic to long-term cortical implants (Edell et al. 1992; Johnson et al. 2005; Ludwig et al. 2006; Polikov et al. 2005; Schmidt et al. 1993; Szarowski et al. 2003; Turner et al. 1999; Vetter et al. 2004; Williams et al. 1999; Xindong et al. 1999), the impedance of a microelectrode reference adds significant uncorrelated thermal noise to cortical recordings (Kovacs 1994; Schmidt and Humphrey 1990; Shoham and Nagarajan 2003; Webster 1998). Moreover, the microelectrode reference could potentially register neural signal as well as uncorrelated biological noise—adding both to neural recordings. In contrast, a common average reference minimizes uncorrelated sources of signal and noise through averaging, while eliminating sources of noise common to all sites. Therefore a common average reference more closely approximates the theoretical differential recording ideal.
Not surprisingly, CAR was found to drastically outperform standard types of electrical referencing over the course of this study, reducing noise by >30%. As a result of the reduced noise floor, arrays referenced to a CAR yielded almost 60% more discernible units than traditional methods of electrical referencing. However, these results need to be considered in context of the experimental methodology; recordings in this study were taken from anesthetized animals placed in a Faraday cage designed to reduce ambient noise. Recordings taken from awake and behaving animals in nonshielded environments would have much more noise. Consequently, the drawbacks of either single microelectrode or large electrode references would be further exacerbated. Under these circumstances, the CAR would likely exhibit an even more dramatic increase in recording performance. CAR may impart similar benefits to other microelectrode recording technologies—for example, chemical sensing (Burmeister and Gerhardt 2001; Dressman et al. 2002; Johnson et al. 2007)—where similar differential recording concepts apply.
GRANTS
This work was supported by the National Institutes of Health P41 Center for Neural Communications Technology (EB002030), Biotectix, and the Whitaker Foundation.
Acknowledgments
We thank all of the members of the Neural Engineering Laboratory at the University of Michigan for assistance in this study; S. Richardson-Burns for coating some of the probes used in this study; and E. Purcell, J. Seymour, M. Gibson, and H. Parikh for invaluable advice and manuscript review.
D. R. Kipke and D. J. Anderson have significant financial interest in NeuroNexus Technologies.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
For example, 60-Hz noise and motion artifact.
Given n electrodes, this occurs if the signal is n times larger than the peak-to-peak value of the noise floor.
For example, thermal noise and distal neural sources.
Although SNR is not affected by this scale factor, CAR improves SNR by removing noise common to all sites without adding uncorrelated noise.
Using a common average reference was necessary to identify sites without unit activity, because the noise floor using the ground screw as a sole reference was sufficient to mask unit activity on many days.
Many students in our laboratory were surprised to note that digitally referencing an individual electrode site would often increase the noise floor across the array and initially attributed this result to an equipment malfunction.
Within array variances when using all types of referencing in this study were equivalent, as verified by the Levene statistic. Ground screw peak-to-peak noise values varied greatly between arrays, depending on the presence of correlated noise sources. As noted in methods, both the single-best reference and CAR were implemented after initial referencing to a ground screw.
REFERENCES
- Albert 1972.Albert AE Regression and the Moore-Penrose Pseudoinverse. New York: Academic Press, 1972.
- Aminghafari et al. 2006.Aminghafari M, Cheze N, Poggi JM. Multivariate de-noising using wavelets and principal component analysis. Comput Stat Data Analy 50: 2381–2398, 2006. [Google Scholar]
- Bezdek 1981.Bezdek JC Pattern Recognition With Fuzzy Objective Function Algorithms. New York: Plenum Press, 1981.
- Bierer and Andersen 1999.Bierer SB, Andersen DJ. Multi-channel spike detection and sorting using an array processing technique. Neurocomputing 26–27: 947–956, 1999.
- Blanche et al. 2005.Blanche TJ, Spacek MA, Hetke JF, Swindale NV. Polytrodes: high-density silicon electrode arrays for large-scale multiunit recording. J Neurophysiol 93: 2987–3000, 2005. [DOI] [PubMed] [Google Scholar]
- Burmeister and Gerhardt 2001.Burmeister JJ, Gerhardt GA. Self-referencing ceramic-based multisite microelectrodes for the detection and elimination of interferences from the measurement of L-glutamate and other analytes. Analyt Chem 73: 1037–1042, 2001. [DOI] [PubMed] [Google Scholar]
- Cooper et al. 2003.Cooper R, Binnie CD, Osselton JW, Prior PF, Wisman T. EEG, paediatric neurophysiology, special techniques and applications. In: Clinical Neurophysiology, Vol. 2, edited by Cooper R, Mauguiere F, Osselton JW, Prior PF, and Tedman BM. Amsterdam: Elsevier, 2003, p. 8–103.
- Cui and Martin 2003.Cui X, Martin DC. Electrochemical deposition and characterization of poly(3,4-ethylenedioxythiophene) on neural microelectrode arrays. Sensors Actuators B 89: 92–102, 2003. [Google Scholar]
- Dressman et al. 2002.Dressman SF, Peters JL, Michael AC. Carbon fiber microelectrodes with multiple sensing elements for in vivo voltammetry. J Neurosci Methods 119: 75–81, 2002. [DOI] [PubMed] [Google Scholar]
- Dunn 1974.Dunn JC A fuzzy relative of the ISODATA process and its use in detecting compact well compact well-separated clusters. J Cybern 3: 32–57, 1974. [Google Scholar]
- Edell et al. 1992.Edell DJ, Toi VV, McNeil VM, Clark LD. Factors influencing the biocompatibility of insertable silicon microshafts in cerebral cortex. IEEE Trans Biomed Eng 39: 635–643, 1992. [DOI] [PubMed] [Google Scholar]
- Gray et al. 1995.Gray CM, Maldonado PE, Wilson M, McNaughton B. Tetrodes markedly improve the reliability and yield of multiple single-unit isolation from multi-unit recordings in cat striate cortex. J Neurosci Methods 63: 43–54, 1995. [DOI] [PubMed] [Google Scholar]
- Harris et al. 2000.Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsaki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol 84: 401–414, 2000. [DOI] [PubMed] [Google Scholar]
- Henze et al. 2000.Henze DA, Borhegyi Z, Csicsvari J, Mamiya A, Harris KD, Buzsaki G. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J Neurophysiol 84: 390–400, 2000. [DOI] [PubMed] [Google Scholar]
- Hochberg et al. 2006.Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442: 164, 2006. [DOI] [PubMed] [Google Scholar]
- Horsch and Dhillon 2004.Horsch KW, Dhillon GS. Neuroprosthetics Theory and Practice. River Edge, NJ: World Scientific, 2004.
- Johnson et al. 2007.Johnson MD, Kao OE, Kipke DR. Spatiotemporal pH dynamics following insertion of neural microelectrode arrays. J Neurosci Methods 160: 276–287, 2007. [DOI] [PubMed] [Google Scholar]
- Johnson et al. 2005.Johnson MD, Otto KJ, Kipke DR. Repeated voltage biasing improves unit recordings by reducing resistive tissue impedances. IEEE Trans Rehab Engineer 13: 160–165, 2005. [DOI] [PubMed] [Google Scholar]
- Karkkainen and Franti 2002.Karkkainen I, Franti P. Dynamic Local Search for Clustering with Unknown Number of Clusters. International Conference on Pattern Recognition, Quebec, Canada, August 11–15, 2002.
- Kovacs 1994.Kovacs GTA Introduction to the theory, design, and modeling of thin-film microelectrodes for neural interfaces. In: Enabling Technologies for Cultured Neural Networks, edited by Stenger DA and McKenna T. New York: Academic, 1994, p. 121–165.
- Lewicki 1998.Lewicki MS A review of methods for spike sorting: the detection and classification of neural action potentials. Network Comput Neural Syst 9: R53–R78, 1998. [PubMed] [Google Scholar]
- Ludwig et al. 2006.Ludwig KA, Uram JD, Yang J, Martin DC, Kipke DR. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J Neural Engineer 3: 59, 2006. [DOI] [PubMed] [Google Scholar]
- Musial et al. 2002.Musial PG, Baker SN, Gerstein GL, King EA, Keating JG. Signal-to-noise ratio improvement in multiple electrode recording. J Neurosci Methods 115: 29–43, 2002. [DOI] [PubMed] [Google Scholar]
- Nelson et al. 2008.Nelson MJ, Pouget P, Nilsen EA, Patten CD, Schall JD. Review of signal distortion through metal microelectrode recording circuits and filters. J Neurosci Methods 169: 141–157, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis et al. 2003.Nicolelis MAL, Dimitrov D, Carmena JM, Crist R, Lehew G, Kralik JD, Wise SP. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc Natl Acad Sci USA 100: 11041–11046, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner 1950.Offner FF The EEG as potential mapping: the value of the average monopolar reference. Electroencephalogr Clin Neurophysiol 2: 213–214, 1950. [DOI] [PubMed] [Google Scholar]
- Osselton 1965.Osselton JW Acquisition of EEG data by bipolar unipolar and average reference methods: a theoretical comparison. Electroencephalogr Clin Neurophysiol 19: 527–528, 1965. [DOI] [PubMed] [Google Scholar]
- Otto et al. 2006.Otto KJ, Johnson MD, Kipke DR. Voltage pulses change neural interface properties and improve unit recordings with chronically implanted microelectrodes. IEEE Trans Biomed Eng 53: 333–340, 2006. [DOI] [PubMed] [Google Scholar]
- Oweiss and Anderson 2001.Oweiss KG, Anderson DJ. Noise reduction in multichannel neural recordings using a new array wavelet denoising algorithm. Neurocomputing 38–40: 1687–1693, 2001.
- Polikov et al. 2005.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods 148: 1–18, 2005. [DOI] [PubMed] [Google Scholar]
- Rebrik et al. 1999.Rebrik SP, Wright BD, Emondi AA, Miller KD. Cross-channel correlations in tetrode recordings: implications for spike-sorting. Neurocomputing 26–27: 1033–1038, 1999.
- Rennaker et al. 2007.Rennaker RL, Miller J, Tang H, Wilson DA. Minocycline increases quality and longevity of chronic neural recordings. J Neural Eng 4: L1–L5, 2007. [DOI] [PMC free article] [PubMed]
- Santhanam et al. 2007.Santhanam G, Linderman MD, Gilja V, Afshar A, Ryu SI, Meng TH, Shenoy KV. HermesB: a continuous neural recording system for freely behaving primates. IEEE Trans Biomed Eng 54: 2037–2050, 2007. [DOI] [PubMed] [Google Scholar]
- Schmidt and Humphrey 1990.Schmidt E, Humphrey DR. Extracellular single-unit recording methods. In: Neurophysiological Techniques, edited by Boulton B, Baker B, and Vanderwolf H. Clifton, NJ: Humana Press, 1990, p. 1–64.
- Schmidt et al. 1993.Schmidt S, Horch K, Normann R. Biocompatibility of silicon-based electrode arrays implanted in feline cortical tissue. J Biomed Mater Res 27: 1393–1399, 1993. [DOI] [PubMed] [Google Scholar]
- Schwartz 2004.Schwartz AB Cortical neural prosthetics. Annu Rev Neurosci 27: 487–507, 2004. [DOI] [PubMed] [Google Scholar]
- Seymour and Kipke 2007.Seymour JP, Kipke DR. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials 28: 3594–3607, 2007. [DOI] [PubMed] [Google Scholar]
- Shoham and Nagarajan 2003.Shoham S, Nagarajan S. The theory of central nervous system recording. In: Neuroprosthetics: Theory and Practice, edited by Horch KW, Dhillon GS. Singapore: World Scientific Publishing, 2003, p. 448–465.
- Spataro et al. 2005.Spataro L, Dilgen J, Retterer S, Spence AJ, Isaacson M, Turner JN, Shain W. Dexamethasone treatment reduces astroglia responses to inserted neuroprosthetic devices in rat neocortex. Exp Neurol 194: 289–300, 2005. [DOI] [PubMed] [Google Scholar]
- Stark and Woods 2002.Stark H, Woods JW. Probability and Random Processes With Applications to Signal Processing. Upper Saddle River, NJ: Prentice Hall, 2002.
- Suner et al. 2005.Suner S, Fellows MR, Vargas-Irwin C, Nakata GK, Donoghue JP. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. IEEE Trans Rehab Eng 13: 524–541, 2005. [DOI] [PubMed] [Google Scholar]
- Szarowski et al. 2003.Szarowski DH, Andersen MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, Turner JN, Shain W. Brain responses to micro-machined silicon devices. Brain Res 983: 23–35, 2003. [DOI] [PubMed] [Google Scholar]
- Turner et al. 1999.Turner JN, Shain W, Szarowski DH, Andersen M, Martins S, Isaacson M, Craighead H. Cerebral astrocyte response to micromachined silicon implants. Exp Neurol 156: 33–49, 1999. [DOI] [PubMed] [Google Scholar]
- Vetter et al. 1998.Vetter RJ, Williams JC, Hetke JF, Nunamaker EA, and Kipke DR. Spike recording performance of implanted chronic silicon-substrate microelectrode arrays in cerebral cortex. IEEE Trans Neural Syst Rehab Eng 52: 896–904, 2004. [DOI] [PubMed]
- Webster 1998.Webster JG Medical Instrumentation: Application and Design. New York: John Wiley, 1998.
- Williams et al. 1999.Williams J, Rennaker R, Kipke D. Long-term neural recording characteristics of wire microelectrode arrays implanted in cerebral cortex. Brain Res Brain Res Protocol 4: 303–313, 1999. [DOI] [PubMed] [Google Scholar]
- Xindong et al. 1999.Xindong L, McCreery DB, Carter RR, Bullara LA, Yuen TGH, Agnew WF. Stability of the interface between neural tissue and chronically implanted intracortical microelectrodes. IEEE Trans Neural Syst Rehab Eng 7: 315–326, 1999. [DOI] [PubMed] [Google Scholar]
- Yang et al. 2005.Yang J, Kim DH, Hendricks JL, Leach M, Northey R, Martin DC. Ordered surfactant-templated poly(3,4-ethylenedioxythiophene) (PEDOT) conducting polymer on microfabricated neural probes. Acta Biomaterialia 1: 125–136, 2005. [DOI] [PubMed] [Google Scholar]
- Zouridakis and Tam 2000.Zouridakis G, Tam DC. Identification of reliable spike templates in multi-unit extracellular recordings using fuzzy clustering. Comput Methods Programs Biomed 61: 91–98, 2000. [DOI] [PubMed] [Google Scholar]