Abstract
Although the trichomes (spicules) of a pod of cowhage (Mucuna pruriens) are known to evoke a histamine-independent itch that is mediated by a cysteine protease, little is known of the itch and accompanying nociceptive sensations evoked by a single spicule and the enhanced itch and pain that can occur in the surrounding skin. The tip of a single spicule applied to the forearm of 45 subjects typically evoked 1) itch accompanied by nociceptive sensations (NS) of pricking/stinging and, to a lesser extent, burning, and 2) one or more areas of cutaneous dysesthesia characterized by hyperknesis (enhanced itch to pricking) with or without alloknesis (itch to stroking) and/or hyperalgesia (enhanced pricking pain). Itch could occur in the absence of NS or one or more dysesthesias but very rarely the reverse. The peak magnitude of sensation was positively correlated for itch and NS and increased (exhibited spatial summation) as the number of spicules was increased within a spatial extent of 6 cm but not 1 cm. The areas of dysesthesia did not exhibit spatial summation. We conclude that itch evoked by a punctate chemical stimulus can co-exist with NS and cutaneous dysesthesias as may occur in clinical pruritus. However, cowhage itch was not always accompanied by NS or dysesthesia nor was a momentary change in itch necessarily accompanied by a similar change in NS or vice versa. Thus there may be separate neural coding mechanisms for itch, nociceptive sensations, and each type of dysesthesia.
INTRODUCTION
Itch is recognized as a fundamental quality of somatic sensation, separate from others such as touch, temperature, and pain. Itch can be a significant clinical problem that sometimes accompanies not only pathologies of the skin but also many diseases and disorders that do not originate in the skin (Binder et al. 2008). For example, chronic neuropathic itch can occur after injuries or other dysfunctions of somatic afferent pathways (Brewer et al. 2008; Oaklander et al. 2003). Sometimes the itch is accompanied by nociceptive sensations such as burning or stinging, as in cases of notalgia paresthetica or postherpetic neuralgia (Yosipovitch and Samuel 2008).
To further our knowledge of the neurobiology of itch and to identify targets for pharmacological treatment of itch, it is essential to identify the primary and central neurons mediating the sensation of itch and to discover how they code and transmit pruritic as opposed to other types of sensory information. For this to occur, there are two objectives. The first is to find and validate an animal model of itch. Certain neural mechanisms cannot be explored at the cellular level in humans and thus must be carried out in animals. For example, we have found that both the monkey and the mouse (CD1 strain) exhibit site-specific scratching to one or more stimuli that elicit itch in humans but not to those that evoke pain (Johanek et al. 2007; Shimada and LaMotte 2008). Thus the monkey and mouse may be valid animal models for studies of neural coding mechanisms in humans.
The second objective, addressed in this study, is to determine psychophysical relationships between pruritic stimuli—ultimately including those of clinical relevance—and the sensory phenomena they elicit in humans. Our purpose is to define the sensory events and sensory states that must be encoded in the responses of sensory neurons. Thus when the same or similar stimuli are presented to the receptive fields of sensory neurons in the peripheral nervous system and CNS, it may be possible to discriminate between neuronal responses that encode itch and pruritic sensory states from those associated with nociceptive and other nonpruritic sensory events.
We focus on the sensations elicited by a spicule (trichome) of cowhage (Mucuna pruriens), a tropical legume. When the tips of multiple spicules lodge in the skin, they deliver a cysteine protease that elicits an itch (Reddy et al. 2008) that is histamine independent (Johanek et al. 2007). However, psychophysical studies are lacking of the incidence, relative magnitude, and time course of itch and accompanying nociceptive sensations evoked by a single spicule of cowhage and of the enhanced pain and itch (Graham et al. 1951) that can develop in the skin surrounding the spicule application site. By asking subjects to report the quality of evoked sensations in addition to their magnitudes, we discovered that itch was typically accompanied by nociceptive sensations of pricking/stinging and burning to an extent that sometimes differed between subjects or repeated tests of the same subject. Also reflecting individual differences was the occurrence of cutaneous dysesthesias—defined by itch to innocuous stroking or an enhanced pricking-evoked itch and pain surrounding the spicule application site. These and other findings indicate the need to find neural mechanisms that can distinguish the pruriceptive from nociceptive sensory qualities of pruritc stimuli.
METHODS
Psychophysical scaling procedure
A total of 47 healthy volunteers, 20 males and 27 females, most of whom were students or employees at Yale University, gave informed consent to protocols approved by the Human Investigation Committee at the Yale School of Medicine.
In most experiments, the subject judged the magnitude of each of three qualities of sensation: itch, pricking/stinging, and burning. Itch was defined as a sensation that evokes a desire to scratch. Pricking/stinging was described as sharp sensations that are qualitatively similar to those produced by an insect bite or a pin prick and can be either intermittent (pricking) or constant (stinging). Burning was defined as the kind of sensation evoked by extreme temperatures or, alternatively, by stimuli such as chemical irritants or mechanical rubbing (like a rug burn) that are not necessarily associated with a thermal stimulation. Subjects were reminded that while pricking/stinging and burning are qualities that are often associated with painful stimuli, for a given stimulus, they may or may not be strong enough to “hurt.” However, the subjects were not asked to judge occurrence or intensity of pain.
Subjects judged the magnitude of each sensory quality using the general version of the Labeled Magnitude Scale (gLMS) (Bartoshuk et al. 2004; Green et al. 1996), which was presented on a computer screen and consisted of a vertical line along which semantic labels of “no sensation,” “barely detectable,” “weak,” “moderate,” “strong,” “very strong,” and “strongest imaginable sensation of any kind,” were spaced in quasi-logarithmic fashion. Use of the gLMS enabled comparison of the perceived intensities of itch and nociceptive sensations on a common scale (Green and Schoen 2007; Green et al. 2008). The quality to be rated was indicated on the screen alongside the scale, and subjects made their ratings by moving an indicator along the scale by means of a computer mouse. The mouse was used to click a box on the screen to record the rating. The subject was instructed that each rating was to indicate the maximum magnitude of a given sensory quality that occurred during the previous 30-s interval. At the start of each interval, the subject was first prompted to provide a rating of itch. After rating itch, the pointer automatically returned to zero and the subject was prompted to rate pricking/stinging, after which the pointer again returned to zero and the subject was prompted to rate burning. The separate ratings of each quality typically took <10 s to complete. The screen went blank until the end of the 30-s interval, after which the scale again appeared and the next interval began. Thus each new judgment was made independently of the previous ratings. This process was continued until all three qualities were either rated as having zero magnitude for three successive intervals or 20 min elapsed since the first rating.
The position of the cursor was saved for each rating and converted to a numerical value between 0 (no sensation) and 100 (strongest imaginable sensation of any kind). The numbers corresponding to the position of each semantic label on the gLMS was 0 for no sensation, 1 for barely detectable, 6 for weak, 17 for moderate, 35 for strong, 53 for very strong, and 100 for the strongest imaginable sensation of any kind. The numbers were not visible to the subject.
Before the first experiment, the subject was given a list of definitions and examples of each sensory quality and asked to rate, using the scale, the magnitude of sensory events that might occur in everyday life, such as the bite of a mosquito, getting your finger caught in a car door, and so on. The purpose was to give subjects practice judging the intensity of qualitatively different sensations in relation to the scale descriptors and in the context of the strongest imaginable sensation of any kind.
Sensations elicited by a single spicule of cowhage
Before this study, we compared the sensory effects of cowhage obtained from India and Honduras. We could find no differences in the sensations or the dysesthesias produced by Indian and Honduran cowhage nor did we find consistent differences between spicules obtained from different pods regardless of the source. We therefore arbitrarily decided to use spicules from different pods of cowhage from India.
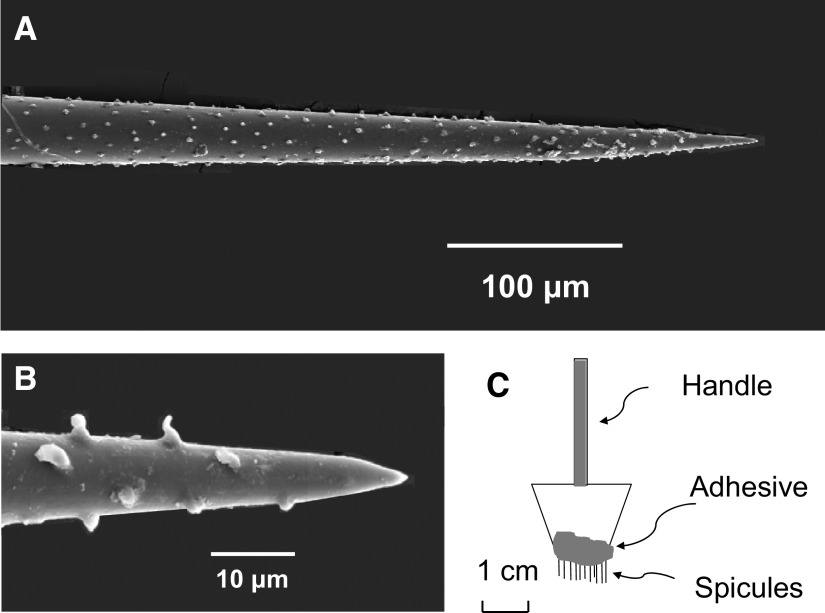
A single trichome or spicule of cowhage used in the study had a length of 2–4 mm, with diameters of 1–3 μm at the tip (Fig. 1, A and B) and ∼50 μm at the other end, The tip of a single spicule was inserted into the skin laterally at an angle of ∼30° from the surface of the skin. Approximately 0.2 mm of the spicule entered the skin. The radius of a spicule ∼0.2 mm from the tip is ∼0.012 mm (Fig. 1A). The volume of skin occupied by the cone-shaped spicule is therefore v = 1/3π r2h = 0.00003 mm3, where the height (h) or length is 0.2 mm, and the radius (r) is 0.012 mm. The protuberances on the outside of the spicule (Fig. 1B) may serve to hold the spicule in place once it is lodged in the skin.
FIG. 1.
The cowhage spicule and a method of applying multiple spicules to the skin. In some experiments, the tip of a single cowhage spicule was inserted ∼200 μm into the skin at an approximate angle of 30° by means of forceps. A: scanning electron micrograph (SEM) of the tip of a cowhage spicule. The total length of a spicule was typically 2–4 mm. B: SEM at a higher magnification of the end of the tip and of the protuberances formed on the outside of the spicule. C: Because a single spicule did not always evoke a sensation, in some experiments, multiple spicules were inserted into the skin by means of a “spicule inserter.” This consisted of 9 or 10 spicules fixed in a row along the cut end of a surgical sponge by means of nail polish. Application of the inserter typically resulted in the insertion of the tips of 7 spicules (±1). A sensation was always evoked.
Once the spicule was inserted into the skin, if no sensation arose within 2 min of insertion, the spicule was removed, and either the same one or a different one was inserted 1–2 mm from the first site. This process was repeated until the subject reported an evoked sensation.
Effects of multiple cowhage spicules in eliciting spatial summation of itch and nociceptive sensations
A group of 32 subjects, each of whom had been tested with a single spicule, were subsequently asked to rate the sensations elicited by a group of cowhage spicules inserted into the forearm by means of a specially designed inserter (Si) prepared from a cut-off Weck-Cel surgical spear (Xomed Surgical Products, Jacksonville, FL) to which spicules were attached by nail polish (Fig. 1C). The Si applied a row of 9 or 10 spicules to the skin, in a mediolateral orientation and ∼1-mm spacing, at an angle of ∼30° with respect to the surface of the skin. This typically resulted in the insertion of seven spicules (±1). Allowing for the occasional insertion of 9 or 10 spicules and a cutaneous width of ∼1 mm diam about the tip of each spicule within which a micro-skin reaction of slight edema or redness was sometimes observed, the maximal area of skin locally influenced by a row of spicules was estimated as having a length of 1 cm and a width of 1 mm (area of 10 mm2). The subjects judged the magnitude of itch, pricking/stinging, and burning every 30 s as described for the insertion of a single spicule.
Fifteen subjects, previously tested with one cowhage spicule, were each asked to rate sensations elicited by four groups of cowhage spicules, each inserted into the forearm in rapid sequence by means of an Si. The four groups, each of approximately seven spicules and mediolaterally oriented, were spaced at distances of 2 cm along the longitudinal axis of the forearm. There were two experimental conditions. In one condition, each group contained active, native, cowhage spicules. In another condition, administered during a different test, the first and most proximal Si contained active spicules. However, unknown to the subject, the three additional Si's each contained spicules that had been autoclaved at 250°C for 30 min to render them chemically inert. Under each condition, the subjects judged the magnitude of itch, pricking/stinging, and burning every 30 s as described.
Temporal relationship between momentary changes in the magnitude of itch and pricking/stinging evoked by cowhage
We studied whether momentary changes in the magnitude of itch that subjects reported as occurring at various times during each 30-s period were accompanied by similar changes in nociceptive sensations. We determined that the subject's ratings of two sensory qualities could be obtained within successive periods of 10 s but that three qualities required a sufficiently greater amount of time. Because pricking/stinging was felt more often than burning, we chose the former quality to compare with itch on a moment to moment basis. Nine subjects made separate ratings of itch and pricking/stinging every 10 s throughout the test The data were analyzed to determine the extent to which a relative increase or decrease in itch from one interval to the next was accompanied by a similar change in pricking/stinging. Only successive pairs of ratings greater than barely detectable, and an increase/decrease greater/lesser than 10% than the previous rating was used in the analyses.
Another group of 11 subjects made continuous ratings of itch alone or, in separate tests in the same subjects, pricking/stinging alone, at a sampling interval of 0.5 s. The purpose was to test whether there were common oscillatory patterns in each quality of sensation when sampling at a higher temporal resolution than in the previous tests.
Effects of cowhage in eliciting cutaneous dysesthesias (alloknesis, hyperknesis, and hyperalgesia)
After the sensations evoked by one or more spicules had disappeared or the 20 min allotted for such ratings expired, the presence and borders of the following three abnormal sensory states (dysesthesias) were mapped on the skin surrounding the cowhage application site. 1) Alloknesis, defined as the area within which itch was produced by a normally nonitchy stimulus consisting of a light stroke with a cotton swab. The itch is initiated during the stroke. 2) Hyperknesis, defined as an enhanced itch from a normally itchy stimulus consisting of a von Frey filament that had a tip diameter of 50 μm and exerted a bending force of 20 mN when applied for 1 s. When applied to the volar forearm, this stimulus typically evoked a faint pricking sensation followed by an itch; 3) Hyperalgesia, defined as the enhanced pain from a normally painful indentation with a von Frey filament that had a tip diameter of 200 μm and a bending force of 80 mN, applied for 1 s. Each von Frey stimulus “typically” but not always evoked the sensations described. That is there was the occasional application that evoked a sensation of touch without even a faint pricking pain or, in the case of the filament with the smaller tip, the absence of an itch even in the presence of a faint pricking pain. Before the application of a cowhage spicule, the subject was asked to judge the presence and magnitude of pricking evoked pain and itch in response to a series of applications of each von Frey filament.
The border of each type of dysesthesia was mapped by applying a series of short-duration stimuli (∼1 s for each stroke or punctuate indentation) proceeding from outside the anticipated area toward the cowhage application site along eight radially organized trajectories (Atanassoff et al. 1999). Occasionally, the same stimulus was applied to an homologous region of skin on the other arm to provide the subject with intermittent information about the range of perceived intensities evoked in the absence of the application of a pruritic chemical. During the tests for hyperalgesia using the von Frey filament with the larger tip, subjects were instructed to attend only to the presence or absence of an increase in pricking pain (beyond that which typically occurred in normal skin) as the stimulus was advanced toward the spicule application site. Conversely, during tests for hyperknesis, using the filament with the smaller tip, subjects were told to ignore pricking-evoked pain and to attend to the presence or absence of an increase in the magnitude of any pricking-evoked itch. Such enhanced itch should exceed the perceived intensities of itch evoked on “normal skin” not exposed to a pruritic chemical. The subject was not allowed to view the skin during the mapping procedure.
The point along a trajectory beyond which pricking evoked an enhanced pain or itch, or a light stroke elicited an itch, was marked in ink on the skin. These points were connected to form the outline of an area of a given type of dysesthesia. The areas were photographed and measured using ImageJ, an image processing software developed at the National Institutes of Health.
Dysesthesias were not always present, or, if one type was present, one or both of the others might or might not be. A dysesthetic area was considered as present only if it extended beyond 0.5 cm in every direction away from a spicule (or group of spicules). It did not seem practical to deliver stimuli closer to one or more spicules without possibly mechanically disturbing the spicule. Thus for a single spicule, the dysesthesia had to extend beyond an area of 1 cm2. For a row of spicules delivered by an Si, the dysesthesia had to extend beyond an area of 2 cm2 (2 × 1 cm). The same criterion was applied for the existence of a neurogenic reddening of the skin or “flare” reaction. The area of flare had to exceed 2 cm2.
In most experiments, the borders of dysesthetic areas were mapped only after cowhage evoked sensations had disappeared (typically 5–20 min after spicule application). The reason for this is that the application of mechanical stimuli to the skin transiently altered the ongoing sensations evoked by cowhage. In one experiment, the time course of changes in the areas of each type of dysesthesia was mapped during the ratings. Typically, ongoing cowhage-evoked sensations were transiently suppressed for several seconds by the application of mechanical stimuli (even when the stimulus itself evoked an itch that was referred to the locus of application). Thus, although subjects continued to rate each quality of sensation (in addition to assessing enhanced sensations evoked by the mechanical stimuli), the sensory ratings were not included in our data base.
Data analyses
In each experiment and for each subject and sensory quality, the following measurements were obtained: 1) the latency of the onset of sensation, defined as the time between the application of the spicule(s) and the first nonzero rating, 2) the peak magnitude of sensation (the greatest magnitude of rating obtained during a single experimental test), 3) the latency to the peak (time between the 1st rating and the peak rating), 4) the duration of sensation (the total elapsed time between the onset of ratings and the 1st 0 rating after the sensation disappeared), and 5) the area under the rating curve over time (AUC) over the course of the experiment. For each of these parameters, a mean of the values from each subject was obtained. A subject with a peak rating of zero for a given quality was included only in calculations of the group mean for the peak magnitude but not for the calculations of the mean latency, duration, or area. The calculations of the peak magnitude and AUC were based on logarithmic transformations after a value of 1.0 was added to each sensory rating (to eliminate any values of 0).
The distribution of values of a particular response parameter was tested with the D'Agostino and Pearson omnibus normality test. Sensory magnitude ratings were logarithmically transformed to achieve normality after first adding a value of 1.0 to each score (to avoid the problem of calculating the log of 0). Student's t-test, paired t-test, or ANOVAs (with Bonferroni tests for post hoc multiple comparisons) were used to compare the means of normally distributed values. For data not normally distributed, the means, or more typically, medians, were compared with the Kruskal-Wallis nonparametric analyses of variance followed by postcomparisons with Dunn's multiple comparison test. Correlations between sets of data were calculated either with the Pearson correlation coefficient or with the nonparametric Spearman rank correlation coefficient. Calculations were performed using GraphPad Prism 4.03 software for Windows (GraphPad Software, San Diego, CA). Data are shown as means ± SE.
RESULTS
Incidence of sensations evoked by a single spicule of cowhage
The superficial insertion of the tip of a single, heat-inactivated cowhage spicule into the skin of the forearm evoked no sensation other than an occasional faint prickle at the moment of insertion (data not shown). A native, active cowhage spicule did not always elicit a sensation beyond the moment of its insertion. In tests with 45 subjects, 25 felt a sensation on the first insertion of a spicule, i.e., the probability of a sensation in response to the first insertion was 0.56. If after 2 min no sensation was reported, the spicule was removed, and either the same or another spicule was inserted 1–2 mm away. Ten more subjects reported sensations after a second insertion, for a combined probability of 0.78 (35/45 subjects). When sensations where not elicited and the same spicule was used again in one or more subsequent insertions, sensations were typically elicited. This indicated that the absence of sensation was not merely caused by the lack of chemical potency of the spicule. In addition, the sensations evoked by the first insertion of a spicule did not seem consistently different in magnitude or time course from those that occurred after one or more failed attempts.
The probability of 0.56 for eliciting a sensation on the first insertion of a single spicule is probably an underestimate, possibly because of statistical fluctuations in sampling. In a subsequent experiment in which 10 of the 45 subjects were each given four subsequent tests, 38 of the total number of 50 tests yielded a sensation on the first spicule insertion (a probability of 0.76). A similar proportion of 0.77 was obtained in a new sample of subjects used in a recent experiment (Sikand et al. 2008).
The fact that the same spicule, having evoked no sensation on first insertion, could do so on a second or a third suggests that the active pruritic ingredient in the spicule, a cysteine protease (Reddy et al. 2008), is effective over a very limited area that does not always contain one or more responsive nerve endings.
Quality, magnitude, and time course of sensations evoked by a single cowhage spicule
The typical sensations evoked by a single spicule were itch accompanied by one or both types of nociceptive sensations. Only one subject, on one test, reported nociceptive sensations in the absence of any itch. The mean rating (perceived intensity) of each sensory quality was initially obtained at the time of spicule insertion and subsequently for consecutive intervals of 30 s (Fig. 2A). There was a similarity in the time course of change in the magnitude of each sensory quality with each reaching a peak rapidly and then decreasing more slowly over time.
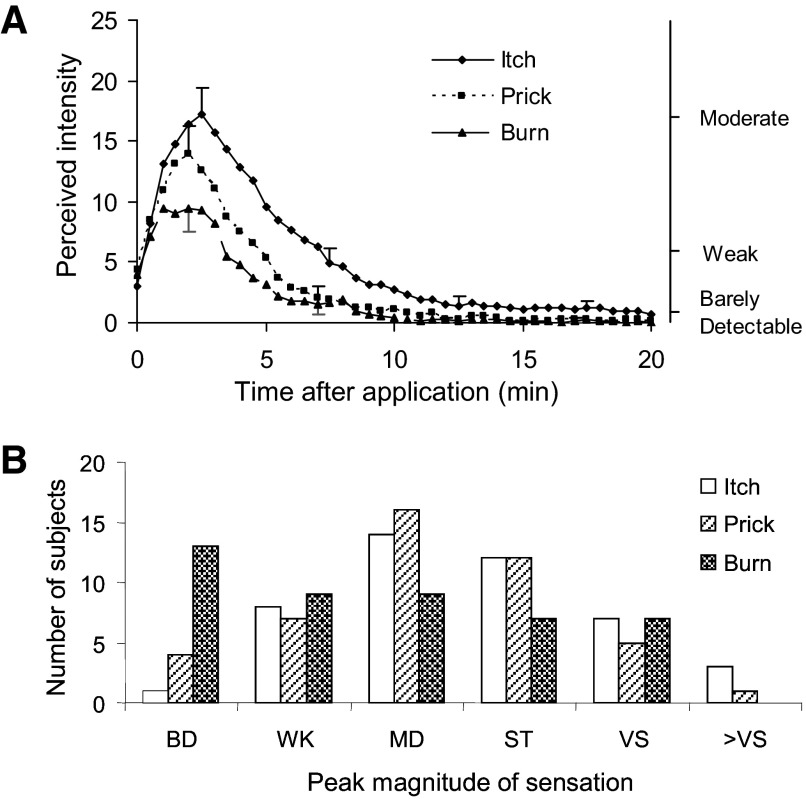
FIG. 2.
The magnitude of itch, pricking/stinging, and burning evoked by a single spicule of cowhage. A: the mean rating of perceived intensity from 45 subjects obtained at successive intervals of 30 s after application (insertion) of the spicule. The SE is plotted every 5 min starting with the peak magnitude for the particular quality of sensation. Pricking/stinging is abbreviated as “prick” and burning as “burn.” The locations of 3 of the verbal descriptors are shown on the right vertical axis in correspondence with the numerical ratings of perceived intensity indicated on the left vertical axis. B: distributions of the categories chosen by the 45 subjects to indicate the peak magnitude of itch, pricking/stinging, and burning (from the same database used for calculating the means in A). The label corresponds to the upper limit of a numerical range defined as barely detectable (BD, ≤1), weak (WK, >1 to ≤6), moderate (MD, >6 to ≤17), strong (ST, >17 to ≤35), very strong (VS, >35 to ≤53), and greater than very strong (>VS, >53).
There were no significant differences among the three sensory qualities in the latency of the onset of sensation or the time from the onset to the peak sensation. In comparison with pricking/stinging, itch had a similar peak magnitude but a significantly greater duration and the area under the rating curve over time (AUC). In comparison with pricking/stinging, burn had a similar duration but a significantly lesser peak magnitude and AUC. Thus itch was more sustained than either of the two nociceptive sensations and, when persisting longer, existed as a pure or single quality of sensation.
There was a significant correlation in peak magnitude between itch and pricking/stinging and between itch and burning (Pearson r values of 0.62 and 0.34, respectively). The same was true between the AUCs for itch and pricking/stinging (r = 0.61) and for itch and burning (0.47). Thus itch co-existed with nociceptive sensations and was positively correlated in overall magnitude with pricking/stinging and burning.
Distributions of labels assigned to the peak magnitude of sensations evoked by a single cowhage spicule
The numerical values for the peak magnitude of sensation for each of the three sensory qualities were grouped by corresponding adjective labels or categories of perceived intensity. Each category included numbers equal or below the numerical position of the label but greater than the position of the label below on the gLMS (e.g., “weak” included numbers >1 to ≤ 6). The distributions of categories chosen for peak magnitude were very similar for itch and pricking/stinging (Fig. 2B). Both were approximately normal and centered on moderate. In contrast, of the categories chosen for burning, the most frequent was barely detectable (which included not detected) and the others were evenly distributed from weak to very strong. Thus burning seeemed to be more or less randomly distributed between subjects and between labels. That is, subjects could agree on itch and pricking/stinging as appropriate labels for the qualities of sensation evoked by cowhage but not on burning.
Differences between subjects and tests of the same subject in sensations evoked by a single spicule
The mean responses of the 45 subjects tested with a single spicule of cowhage are representative of the individual responses of a majority of the subjects tested. However, the differences in results obtained in each test with a different subject were suggestive of separate neural mechanisms mediating itch and each nociceptive sensory quality. Throughout the test, nociceptive sensations were rated as lesser in peak magnitude than itch for about one half of the subjects, greater for 17 others, and absent altogether for 2. These findings indicate that, although all three qualities can be elicited by cowhage, each quality exhibited some degree of independence in magnitude and time course.
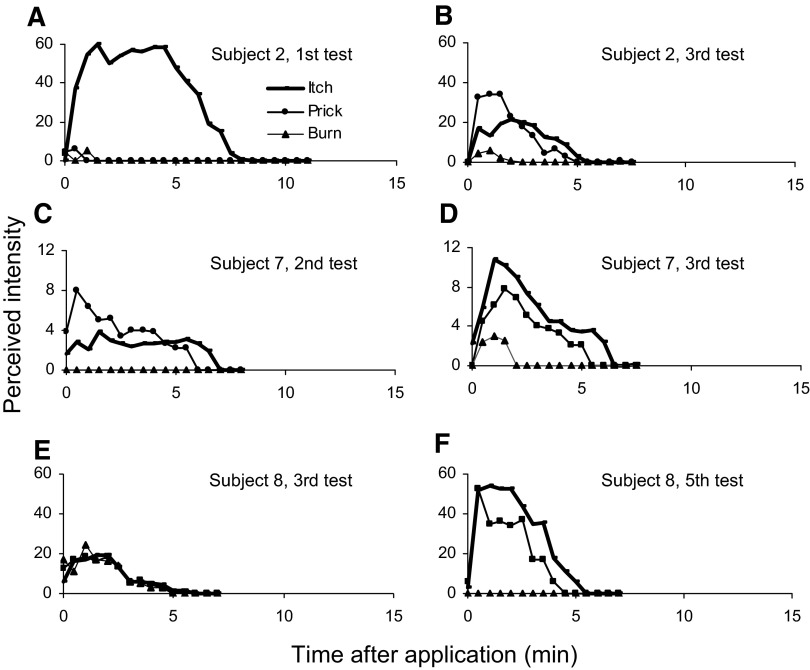
To explore whether such differences in findings between tests were caused solely by consistent individual differences between subjects or regional variations in cutaneous sensitivity and/or other factors, the single-spicule test was repeated in each of nine subjects on five different occasions and at slightly different loci on the midportion of the volar forearm. For most subjects, there was a variability between tests in which itch was greater than nociceptive sensations for most but not all judgments during one or two tests (Fig. 3, A, D, and F) but not on other tests (Fig. 3, B, C, and E). The nociceptive sensations were a mixture of pricking/stinging and burning except in the case of one subject who never reported burn. One subject consistently felt only itch without any nociceptive sensations during these tests on the arm. However, this same subject did report significant pricking/stinging sensations accompanying cowhage-evoked itch on the back of the hand (data not shown). Thus there was evidence both for individual differences between subjects and for regional variations in cutaneous sensitivity between tests of the same subject. In future studies, a large population of subjects might be screened to determine whether there is a genetic basis for differences between subjects in susceptibility to cowhage evoked sensations, dysesthesias, or skin reaction.
FIG. 3.
The sensory ratings of 3 different subjects each given 2 different tests with a single spicule. These findings indicate the diversity in sensory response profiles evoked by a single spicule.
Effects of the number of spicules on the incidence of each sensory quality
In the second series of experiments, we tested whether increasing the number of inserted spicules would increase the likelihood of an evoked sensation. Thirty-two of the subjects tested with a single spicule were given a subsequent test on the arm in which approximately seven (±1) spicules were simultaneously inserted along a row by means of a special inserter (Fig. 1C). Itch was evoked in all subjects and rated, for most, as the strongest sensation (20/32) during the test. However, although the increase in the number of spicules from one to seven increased the likelihood of a sensation (from 0.56 to 1.0), there were no consistent or overall changes in the incidence of each sensory quality or of one quality dominating over the other. In addition, few subjects rated the same qualities in exactly the same order of peak magnitude (e.g., itch > pricking/stinging > burning). These findings, in addition to those obtained with repeated tests on the arm using a single spicule, suggest that separate neural mechanisms mediating each sensory quality may be differentially engaged, possibly because of differences in neuronal activation or sensitivity from one local region of skin to another.
Effects of the number of spicules on the magnitude and time course of itch and nociceptive sensations
We compared the magnitude ratings of each sensory quality obtained from the 32 subjects tested with one versus seven spicules inserted into the volar forearm to determine whether the increased incidence of sensation in response to seven spicules was accompanied by an increased magnitude or duration (spatial summation) of sensations within a local region occupied by a row of spicules. For each of the three qualities of sensation, separate Student's t-tests were performed to test the significance of differences, caused by experimental condition (1 vs. 7 spicules), in the latency of the onset of sensation, in the peak magnitude of sensation, the latency to the peak, the duration of sensation, and the AUC. No significant differences for any of these parameters were found between the two conditions. Thus the time course and magnitude of itch and each nociceptive sensation were similar in response to one or seven spicules. In addition, the distributions of the categories used by the subjects to indicate the peak magnitude of each quality of sensation were virtually the same for one and seven spicules (data not shown). In comparison with one spicule, seven spicules did not evoke an increase in the relative number of barely detectable to weak ratings or in the use of higher categories as one might expect if there were a spatial summation of subliminal or suprathreshold sensory events (no statistical difference in the distributions of numbers 1–6 assigned to the categories). Thus there was no evidence of spatial summation of itch or nociceptive sensations within a region of ∼1 × 0.1 cm.
However, a different result was found when four groups of spicules (7 spicules per group or a total of 28 ± 4) were inserted via the spicule applicator along a proximal-to-distal line on the forearm (2 cm between groups of spicules). For each sensory quality, the mean perceived intensity of sensation was plotted as a function of time after spicule application for one or four Si insertions (Fig. 4). For each sensory quality, four Si insertions evoked a significantly greater peak magnitude of sensation and AUC than one Si insertion (paired t-test). However, these differences were not accompanied by any significant differences in any of the other parameters, such as the duration of sensation. The peak mean magnitude of sensation for each quality was reached within the same period of time, i.e., the first few minutes after the onset of sensation. Thus within a spatial extent of 6 cm, the magnitude but not the time course of itch, pricking/stinging and burning exhibited spatial summation.
FIG. 4.
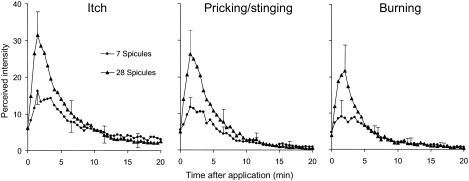
The effects of increasing the number of spicules from 1 group of 7 to 4 groups applied at 2 cm spacing along the forearm. The increase in number of spicules from 7 to 28 resulted in the spatial summation in the magnitude of Itch, pricking/stinging and burning.
For ratings of itch or pricking/stinging, there was a significant shift in the distributions toward higher categories of intensity in response to four Si insertions (statistical comparisons using a t-test of the distributions of numbers 1–6 corresponding respectively to the chosen categories). The most frequently chosen categories were moderate and strong in response to one Si insertion but strong to very strong or greater in response to four insertions. The shifts to higher intensity ratings in response to a fourfold increase in the number of spicules seem unlikely to result from the increased probability of hitting a more sensitive spot. As described, a sevenfold increase from one to seven spicules produced no such shift in intensity ratings for itch or either nociceptive quality. Rather, the increase in ratings of itch and pricking/stinging are more compatible with spatial summation across afferent fibers.
The distribution of categories for the peak magnitude of burning did not shift with the increase in number of insertions. For both distributions, intensity ratings peaked at barely detectable and were approximately evenly distributed across the higher categories. Similar to the responses to a single spicule, responses to multiple insertions support the conclusion that the label of burning is less commonly and less reliably associated with cowhage-evoked sensations than itch and pricking/stinging.
Temporal relationship between momentary changes in the magnitude of itch and pricking/stinging evoked by cowhage
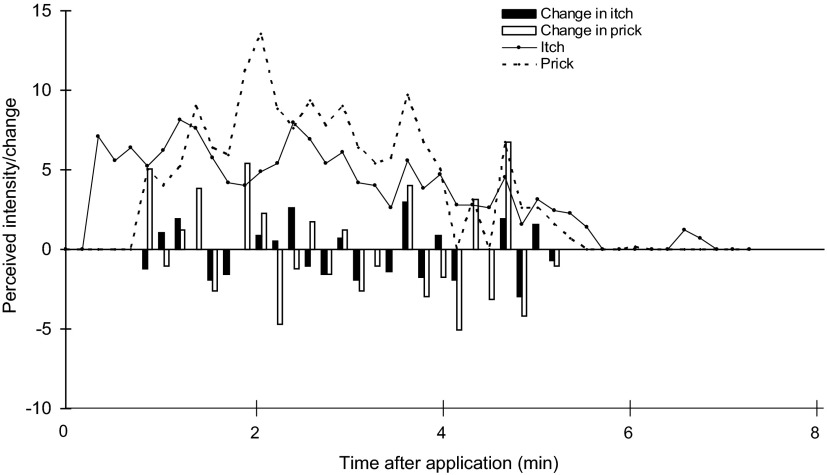
In response to cowhage delivered with the Si, nine subjects were asked to rate the magnitude of itching and pricking/stinging at successive intervals of 10 s. In the analyses for each subject, each rating of a given sensory quality (itch or pricking/stinging) was subtracted from the previous rating, starting with the second rating and continuing until the last rating to form a succession of relative changes in that quality. This analysis is shown for one subject in Fig. 5. This particular subject initially felt itch in the absence of pricking/stinging. Once both qualities of sensation were present, there were about as many occasions when the two qualities changed in the same direction as there were when one changed and the other did not or changed in the opposite direction.
FIG. 5.
The perceived intensity of itch and pricking/stinging rated by a subject every 10 s in response to the application of 7 cowhage spicules. Also shown is the difference (change) in the magnitude rating of each sensory quality between the plotted rating and the rating obtained 10 s earlier. The ratings of itch and pricking/stinging are represented by solid and dashed lines, respectively. Solid bars, change in itch; open bars, change in pricking/stinging. Changes <10% are not shown.
In the analyses to determine whether there was a significant correlation between the changes in each quality for each subject, if a given change in rating was not >10% of the previous rating, it was considered to be zero; if each rating in a pair was not above the number corresponding to barely detectable, the difference between the two was not counted. Two subjects exhibited significant correlation coefficients of 0.43 (the subject whose responses are shown in Fig. 5) and 0.48. In contrast, the absence of comparable changes in each quality was more common for the remaining subjects for whom the correlation coefficients of 0.07, 0.35, −0.10, −0.05, 0.28, 0.36, and 0.28 were not significant. The fact that for most subjects itch and pricking/stinging did not exhibit momentary changes of similar magnitude and in the same direction suggests that the two qualities are served by different neural mechanisms.
Eleven subjects received spicules using the Si and were instructed to make continuous ratings of itch alone or, in separate tests, pricking/stinging alone. The purpose was to count how many times the rating of a sensory quality exhibited an oscillation, defined as an increase in perceived intensity followed by a decrease (each by ≥10%). For two subjects, the sensations increased and decreased only once during the test. That is, each quality increased in stepwise fashion to a peak followed by a stepwise decrease to zero. The other nine subjects exhibited a series of mean oscillations (in cycles/s) of 0.03 ± 0.006 (3 times in 100 s or 9 times in 300 s; i.e., 5 min) for ratings of itch and 0.0294 ± 0.01 for pricking/stinging.
Cutaneous dysesthesias evoked by a single cowhage spicule
A single spicule often elicited one or more cutaneous areas of dysesthesia within which light stroking evoked an itch (alloknesis) or a punctate indentation elicited a greater than normal itch (hyperknesis) or pricking pain (hyperalgesia). The borders of each area, mapped with the appropriate stimuli as described in methods, are shown for a subject who exhibited all three types of dysesthesia in response to a single spicule (Fig. 7A). The same methods of testing for dysesthesia were applied even after those instances where a single spicule evoked no sensation. However, in no case where a spicule failed to elicit a sensation was there evidence for the presence of any type of dysesthesia. A dysesthesia developed only in those tests in which cowhage evoked an itch with or without one or both nociceptive sensations.
FIG. 7.
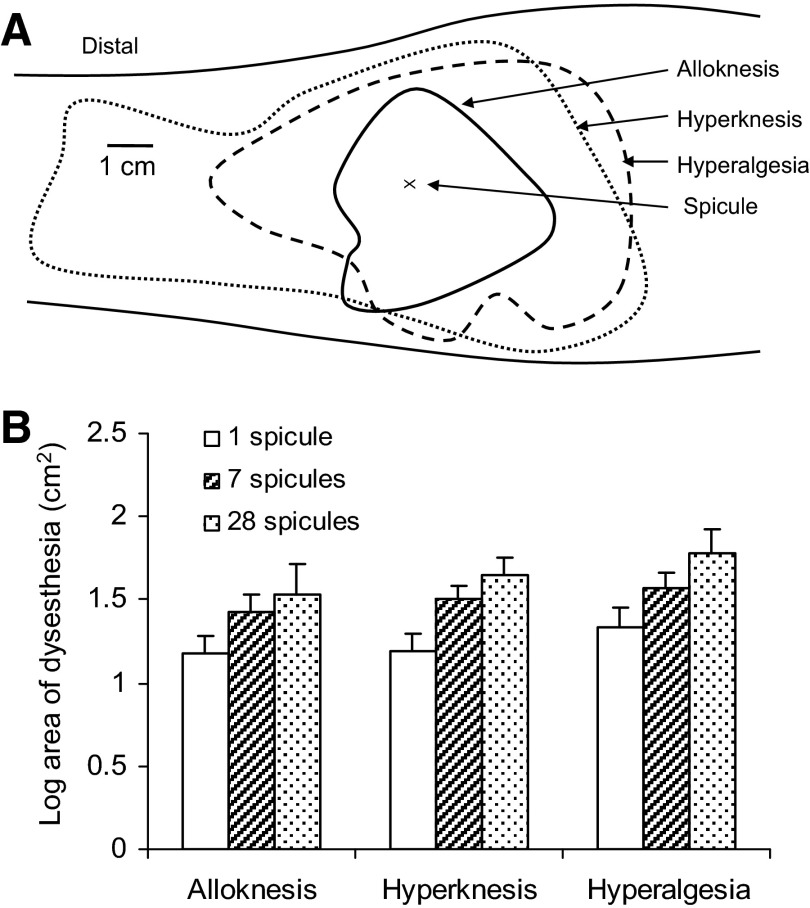
Areas of cutaneous dysesthesia evoked by cowhage. A: borders of alloknesis, hyperknesis, and hyperalgesia mapped on the volar forearm of a subject 20 min after the insertion of a single cowhage spicule. B: the mean log area of alloknesis, hyperknesis, and hyperalgesia that developed in response to a single spicule (open bars) or a group of 7 or 28 spicules (hashed and dotted bars, respectively).
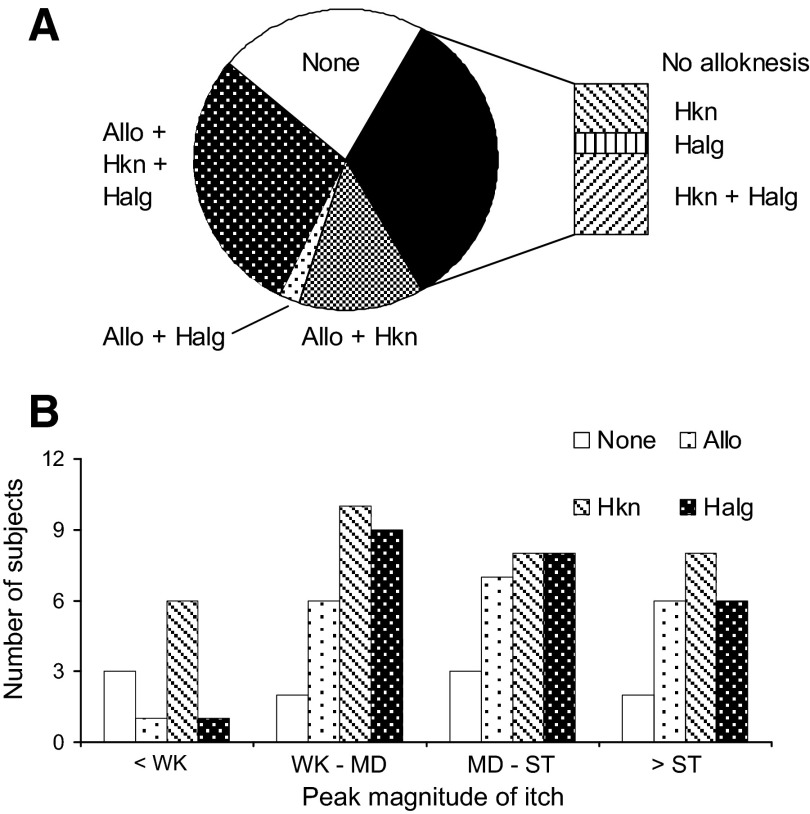
In the experiment in which a single spicule was administered, the presence of dysesthesias was tested only after all cowhage-evoked sensations had disappeared and/or 20 min had elapsed. Most of the 45 subjects tested exhibited hyperknesis (32 subjects) accompanied by hyperalgesia (21) and/or alloknesis (Fig. 6A). Alloknesis was exhibited in 20 of the subjects but always accompanied by hyperknesis alone (6), hyperalgesia alone (1), or both (13).
FIG. 6.
The incidence of each type of dysesthesia evoked by a single cowhage spicule and the incidence in relation to the peak magnitude of itch. A: the incidence of hyperknesis (Hkn) and hyperalgesia (Halg) with or without alloknesis (Allo). B: the incidence of each type of dysesthesia in relation to the peak magnitude of itch. The peak magnitude ratings were categorized as less than weak (<WK), weak to moderate (WK–MD), moderate to strong (MD–ST), and greater than strong (>ST). In some instances, there was no dysesthesia even in the presence of a strong itch. However, there was rarely any alloknesis or hyperalgesia in the absence of at least a weak itch.
There were no significant correlations between the peak magnitude of sensation and the incidence of any type of dysesthesia (Fig. 6B). For example, of the 10 (of 45) subjects reporting no dysesthesias at 20 min after the spicule insertion, one half reported a peak itch that was moderate to very strong. On the other hand, it was rare for alloknesis or hyperalgesia to occur in the absence of at least a weak itch (only 1 subject for each dysesthesia). Thus while there was a relation between the presence of a minimal sensation and the occurrence of one or more dysesthesias, the occurrence of a strong itch did not necessarily result in a dysesthesia, at least at the time of testing.
In the repeated tests of the same subjects there was evidence for both differences between subjects and between tests of the same subject in the occurrence of dysesthesias. Of the nine subjects tested, each exhibited at least one type of dysesthesia and typically more than one, on at least one of the five tests. Every subject felt enhanced pricking evoked sensations (hyperknesis or hyperalgesia or both) on one, or more commonly, on most of the tests. However, two subjects exhibited no alloknesis on any test despite peak magnitude ratings of itch that varied on different tests from weak to very strong. Conversely, two subjects had alloknesis on four or all five tests. For each of these subjects, the peak ratings of one or both nociceptive qualities were similar in magnitude to the ratings of itch, whereas on other occasions they were considerably lower. Again, there was no consistent relationship between the peak magnitude ratings of any sensory quality and the occurrence of alloknesis or enhanced responses to pricking.
Areas of cutaneous dysesthesias evoked by single and multiple spicules
There was considerable variability in the areas of dysesthesia in response to cowhage. The mean log areas of each dysesthesia was obtained in response to a single spicule (45 subjects tested) and in response to a group of about seven spicules (32 subjects) or four such groups applied at proximal-distal spacings of 2 cm as described in the spatial summation experiment (15 subjects; Fig. 7B). For a given number of spicules, there were no significant differences in mean log areas between the types of dysesthesia. In addition, the mean log areas of a given type of dysesthesia did not differ significantly with differences in the number of spicules applied. Thus there was no evidence for spatial summation of the dysesthesias that accompanied the spatial summation of the peak magnitude of sensation in response to an increase in the number and spatial distribution of spicules applied to the forearm.
There was support for the existence of individual differences between subjects that were tested with different numbers of spicules. Although every subject felt enhanced pricking sensations (hyperalgesia and/or hyperknesis) in response to one or more of the three experimental conditions (1, 7, and 28 spicules), four subjects experienced no alloknesis even in response to 28 spicules. In contrast, six subjects exhibited all three types of dysesthesia under every condition.
Time course of dysesthesias
In a separate experiment, an approximate measure of the time course of development and disappearance of dysesthesias was measured for 12 subjects who experienced a sensation within 2 min of application of a single spicule. The ratings of sensation were interrupted periodically to test the presence and to map the borders of each type of dysesthesia. This was carried out at 3 and 10 min and thereafter at intervals of 10 min until all dysesthesias were gone or 80 min had elapsed (at which time only a single subject reported any area remaining). One subject had no dysesthesias. The others had dysesthesias that reached maximal size within 3–10 min, thereafter gradually decreasing in size and typically disappearing in less than an hour.
Cutaneous reactions to cowhage
None of the subjects in any of the experiments with single or multiple spicules exhibited a wheal, defined as a noticeable edema extending beyond the occasional local vascular reaction produced by a cowhage spicule. The latter consisted of a local red area and an edema of about a millimeter in diameter. However, 6 of the 45 subjects experienced a flare in response to one or more tests with a single spicule. A flare was defined as a clearly visible, well-demarcated area of redness that extended farther than 2 cm from the site of spicule insertion. Subsequent tests of some of these same subjects using multiple spicules again exhibited a flare. For these subjects, the mean areas of flare in response to 1, 7, and 28 spicules, respectively, were 4.9 ± 0.6, 10.2 ± 3, and 20 ± 3 cm2. We could find no indications of any differences in the characteristics of the sensory ratings or dysesthesias that might distinguish subjects that did or did not exhibit a flare.
DISCUSSION
A central finding of this study is that punctate delivery of a minute amount of a pruritic chemical from the tip of a single spicule of cowhage (0.0003 mm3) can evoke itch accompanied by nociceptive sensations and can enhance mechanically evoked itch and pain in surrounding skin. When the enhancement occurred, it was manifested in one or more cutaneous dysesthesias wherein a light, moving, tactile stimulus evoked itch (alloknesis), or, a punctate indentation with a fine von Frey filament elicited a greater than normal itch (hyperknesis) and/or pricking pain (hyperalgesia). The nociceptive sensations evoked by cowhage were characterized more as pricking/stinging than burning and were positively rather than inversely correlated with itch in magnitude and duration. Similarly, hyperalgesia was manifested in the presence of hyperknesis. Thus pruritic and nociceptive sensations could co-exist as could pruritic dysesthesias and hyperalgesia. However, cowhage itch was not always accompanied by one or more dysesthesias, nor was the presence or change in itch accompanied by the presence or similar change in nociceptive sensations and vice versa. That is, there may be separate neural coding mechanisms for each sensory quality and type of dysesthesia. Last, the magnitude of itch and nociceptive sensations exhibited spatial summation with increases in the number of spicules but only when the spicules were distributed over a region of 6 cm. Applications within a region of 1 cm failed to produce summation. Each of these findings has implications for peripheral or central neural coding of itch, pain, and enhanced pruritic and nociceptive sensory states.
The possible mechanistic independence of itch and different types of nociceptive sensation receives support from prior studies of chemesthesis. A single nociceptive quality can dominate over others in the absence of itch. For example, burning is the dominant nociceptive sensation in response to a topical application of methyl salicylate (Green and Flammer 1989b), capsaicin (Green and Bluth 1995), and mustard oil (Handwerker et al. 1991), and stinging in response to lactic acid (Green and Bluth 1995) and, under certain stimulus conditions, innocuous cold (Green and Pope 2003; Green et al. 2008). On the other hand, itch can sometimes dominate over lesser sensations of pricking or burning when capsaicin is topically applied to the forearm (Green 1990; Green and Flammer 1989a).
It is a common belief that “pain blocks itch.” However, we found both a positive correlation between the peak magnitudes of itch and nociceptive qualities and the absence of a correlation between momentary changes in itch and changes in pricking/stinging. These observations indicate that the nociceptive sensations were not acting to suppress itch. Indeed, clinical pruritus can occur in the presence and absence of ongoing nociceptive sensations or pain (Binder et al. 2008). In this study, subjects were not asked to rate the magnitude of pain. However, there is ample evidence that when asked to do so, nociceptive sensations of burning, stinging, or pricking can be evoked in the absence of any reports of pain both in the presence of itch evoked by a topical application of capsaicin (Green and Shaffer 1993) or in the absence of itch in response to innocuous thermal stimuli (Green and Pope 2003). To what extent the modality, painfulness and other aspects of a stimulus can inhibit itch warrants investigation in future studies.
Combined itch and nociceptive sensations were also evoked when various chemical stimuli were applied to the skin in the same manner as native cowhage. Cowhage spicules rendered chemically inert by heating and subsequently soaked in cowhage extract containing the native cysteine protease (mucunain), an identical recombinant mucunain (Reddy et al. 2008), or various concentrations of histamine or capsaicin (Sikand et al. 2008), elicited itch that was typically accompanied by pricking/stinging and, to a lesser degree, burning. Thus it is possible that at least one or more types of primary sensory neurons can be activated by all of these pruritic stimuli.
There is evidence that pruriceptive primary sensory neurons are nociceptive neurons that respond to pruritic stimuli and can be subcategorized as either mechanosensitive or mechano-insensitive afferents (MSAs and MIAs, respectively). Cutaneous MSAs with unmyelinated or myelinated axons responsive to cowhage have been reported in cat (Tuckett and Wei 1987a,b). In monkey, subpopulations of MSAs with C-fibers [C-mechanoheat (CMHs)] and MSAs with A-fibers responded to cowhage applied in a manner similar to that used in this study (Johanek et al. 2008; Schepers et al. 2008). Most of the CMHs also responded to an intradermal injection of histamine. In humans, CMHs also responded to cowhage (Namer et al. 2008; Torebjork and Hallin 1976), but their responses to histamine, while sometimes corresponding to the time course of itch (Handwerker et al. 1991), were weaker than the responses of a subpopulation of MIAs with C fibers (Schmelz et al. 1997, 2003). However, the C-fiber MIAs recorded to date in monkeys and humans and the A-fiber MIAs recorded in monkey have been reported to be unresponsive to cowhage (Johanek et al. 2008; Namer et al. 2008). Thus there are subpopulations of nociceptive A- and C-fibers that can be subclassified as pruriceptive, because they respond to histamine (MIAs more than MSAs) and/or to cowhage (MSAs).
The widespread erythema known as a neurogenic flare is an axon reflex thought to be mediated by MIAs and not MSAs (Schmelz et al. 2000). Cowhage spicules are capable of eliciting itch in the absence of any evidence of a flare (Johanek et al. 2008; Shelley and Arthur 1957). However, there is older, anecdotal evidence that a flare occasionally does develop (Graham et al. 1951). This is born out in this study by the occurrence of a flare in 13% of the subjects tested with a single spicule. Such flares were never accompanied by a wheal and thus not likely to be mediated by a release of histamine. These subjects, but not the others, typically exhibited a flare in subsequent tests in which one or multiple spicules were delivered, suggesting individual differences between subjects in susceptibility to flare from cowhage There were no apparent differences between these subjects and those who did not exhibit a flare. This is indicative that independent mechanisms mediate the flare and both the sensations and dysesthesias evoked by cowhage and probably by other pruritic stimuli (Sikand et al. 2008). A further implication of these findings is that if MIAs mediate the flare, then at least some MIAs are responsive to cowhage. In addition, or alternatively, some MSAs might contribute to the cowhage evoked flare.
Similar temporal profiles of sensations and MSA discharges evoked by cowhage
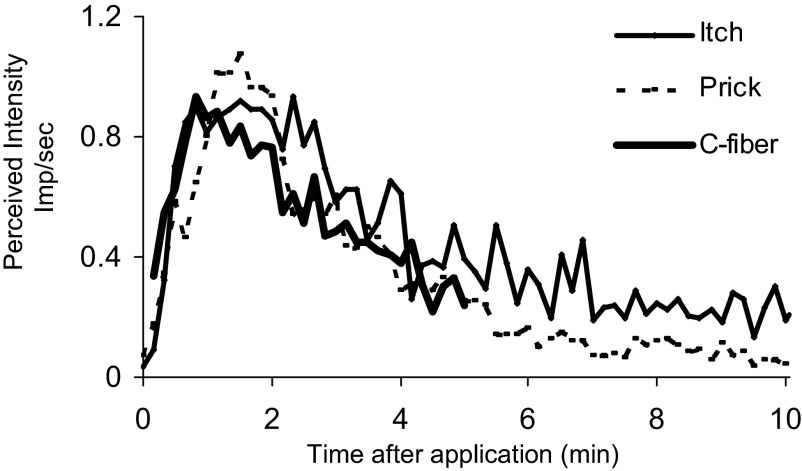
In the following, we compared the mean perceived intensity of simultaneous ratings of itch and pricking/stinging with the mean discharge rates of 39 C-mechanoheat–sensitive nociceptive afferents innervating the hand or arm of the monkey (Johanek et al. 2008). The sensory ratings were obtained from the experiment in which momentary changes in itch and pricking/stinging were compared every 10 s. For each subject, a normalizing factor was obtained that made the peak magnitude rating of itch equal to one. Each rating of itch and pricking/stinging obtained was multipled by this factor and the mean of all individually normalized ratings obtained for successive periods of 10 s. The mean discharge rates were obtained also at successive intervals of 10 s (up to 5 min) each multiplied by a factor that made the peak mean discharge rate equal to the mean peak normalized rating of itch (Fig. 8).
FIG. 8.
Perceived intensity of itch and pricking/stinging in human compared with the mean discharge rates of mechano-heat sensitive nociceptors with C fibers in monkey each obtained in response to a group of cowhage spicules. The mean ratings of the perceived intensity of itch and pricking/stinging simultaneously obtained every 10 s and the mean discharge rates in impulses (imp)/s continuously recorded from 39 mechanosensitive nociceptive C-fibers in monkey were plotted at intervals of 10s after normalizing each to the peak mean perceived intensity of itch. The discharges in monkey were obtained by combining the responses of slowly and quickly adapting C-mechanoheat nociceptors in response to multiple spicules (Fig. 9A of Johanek et al. 2008).
The close relationship between the time course of itch (or pricking/stinging) and the time course of discharge rates of these afferent fibers suggests that the sensations evoked by cowhage may be mediated at least in part by responses in this fiber population, as concluded by Johanek et al. (2008). However, this is not to say that activity in these afferents is necessary or sufficient in mediating these sensations. For example, mechanosensitive nociceptors with A fibers are likely to contribute as well (Schepers et al. 2008) and a conduction block in A fibers in humans significantly reduced the mean magnitude of itch and pricking/stinging evoked by cowhage (Shimada et al. 2007). Possibly the combined responses in both of the C- and A-fiber populations of mechanosensitive nociceptors would provide an even closer relationship with the perceived intensities of itch and pricking/stinging in humans.
How pruriceptive mechanosensitive primary afferents might signal the different qualities of sensation evoked by cowhage
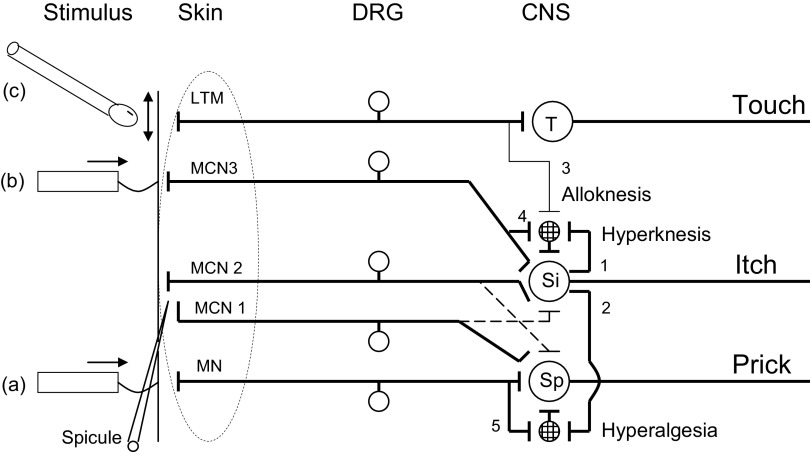
How might three different qualities of sensation (itch, pricking/stinging, and burning) be mediated by the same type of pruriceptive afferent? We hypothesize that the pruriceptive A and C afferents mediating itch and those mediating the nociceptive sensations evoked by cowhage or by capsaicin or histamine (Sikand et al. 2008) may not be distinguishable by their peripheral morphological or functional properties but rather by their central projections. For example, a single cowhage spicule that evokes a strong sensation of itch and a weak magnitude of pricking/stinging might activate one or more nociceptive afferent fibers that have a strong central projection to pruriceptive spinothalamc tract (STT) neurons that, by the nature of the population of higher-order neurons, “mediate itch” (Fig. 10). Conversely, a spicule eliciting a weak sensation of itch and a strong intensity of pricking/stinging might activate afferents of the same type but having strong projections to pruriceptive STT neurons mediating the nociceptive sensations (pricking/stinging) and weak projections to those mediating itch. In addition, there likely exist nociceptive specific STT neurons that would respond to nociceptive but not pruritic stimuli. This would be consistent with findings that intraneural electrical microstimulation that putatively activates a single CMH nociceptive afferent can evoke itch or, on most other occasions, pain (Ochoa and Torebjörk 1989). The central projection hypothesis would also explain why subjects in this study sometimes reported that the pricking of some cutaneous spots with the fine diameter von Frey filament elicited pricking pain or prickle unaccompanied by itch (possibly mediated by nociceptive-specific mechanoreceptive neurons), whereas pricking other spots evoked prickle followed immediately, or after a brief delay, by itch.
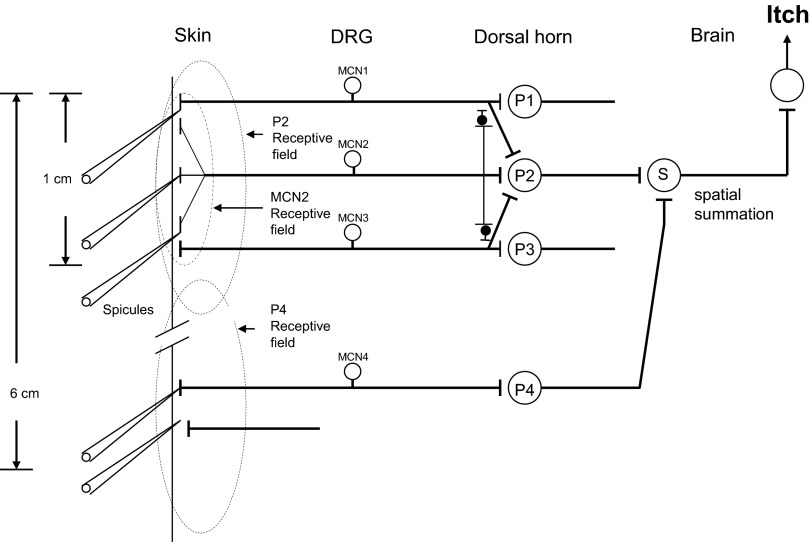
FIG. 10.
Schematic of how pruriceptive neurons might mediate cowhage evoked itch, pricking/stinging, and cutaneous dysesthesias. In this example, a mechanosensitive, nociceptive-specific dorsal root ganglion (DRG) neuron (MN), responsive to a nociceptive punctate stimulus such as a von Frey filament (a), terminates on a central neuron, e.g.,, an STT neuron (Sp), which when activated elicits a sensation of pricking (prick). Three mechano-chemo-sensitive (polymodal) nociceptive neurons (MCN 1, 2, and 3) are responsive to cowhage (spicule). MCN2 and MCN3 each terminate more superficially in the skin than MCN1 and are more responsive to fine, punctuate stimuli (e.g., 50 μm diam) (b) that can normally evoke itch. MCN2 and 3 have strong synaptic projections onto an STT neuron mediating itch (Si), whose cutaneous receptive field is shown on the left (large, dashed oval) and a weak projection (dashed line, shown for simplicity only for MCN2) to an STT neuron (Sp) mediating cowhage-evoked pricking/stinging. In contrast, MCN 1 projects weakly onto Si (dashed line) but strongly onto Sp. MCN2/3 and Si mediate cowhage-evoked itch. MCN 1 and Sp mediate cowhage-evoked pricking/stinging. A low-threshold mechanoreceptive neuron (LTM) terminates on neurons in the dorsal column nuclei (T) that mediate innocuous tactile sensations (touch), e.g., evoked by stroking the skin with a cotton swab (c), and sends collateral projections in the spinal dorsal horn that normally do not contribute to itch. Certain Si neurons, but not all, have the capacity of releasing a neurotransmitter from the terminals of collaterals (1 and 2) that produces a sustained sensitization of 2 types of interneurons (circles with crosshatch pattern). Collateral 1 projects to an interneuron that receives a weak (and normally ineffective) input from an LTM (via collateral 3) and/or an itch mediating, mechano-chemo nociceptive neuron (i.e., MCN2 via collateral 4), each with receptive fields adjacent to or remote from the cowhage application site. When the interneuron is sensitized, its responses to stroking and/or pricking conveyed to Si are enhanced, resulting in alloknesis and/or hyperknesis, respectively. Collateral 2 projects to an interneuron that receives input from MN alone (via collateral 5), and when sensitized, enhances the responses of Sp to pricking adjacent to or remote to the cowhage application site, thereby resulting in punctate hyperalgesia.
Three types of nociceptive STT neurons in monkey have been identified that may be relevant to these findings (Davidson et al. 2007; Simone et al. 2004). One type of nociceptive STT neuron does not respond to either histamine or to cowhage and is therefore considered to be nociceptive specific. For example, one subclass of this type might be of the type known to respond exclusively to noxious mechanical stimuli (Price and Dubner 1976). The other two types are pruriceptive: one of which responds to cowhage but not to histamine and the other to histamine but not to cowhage (Davidson et al. 2007).
The existence of two types of itch, one histamine dependent and the other histamine independent, each possibly mediated by different subpopulations of pruriciceptive neurons, is consistent with recent behavioral pharmacological, c-fos labeling, and electrophysiological findings obtained in the mouse (Carstens et al. 2008a,b; Nakano et al. 2008a,b; Tsujii et al. 2008). In addition, histamine selective STT neurons with properties similar to those of histamine responsive MIAs in humans have been found in the dorsal horn of the cat (Andrew and Craig 2001).
The temporal profile of STT neuronal response to cowhage in the monkey (Davidson et al. 2007, 2008) fits reasonably well with the temporal profile of itch or pricking/stinging or burning documented in the present study. Also, a small subpopulation of histamine- and cowhage responsive neurons became sensitized to stroking and/or to pricking the skin and thus might contribute to the types of cutaneous dysesthesia observed in the present study. However, pruriceptive neurons recorded to date in monkeys and mice respond not only to a pruritic agent but also to thermal, chemical and/or mechanical noxious stimuli (such as a pinch or an intradermal injection of capsaicin) that do not elicit itch. It remains to be determined how information conveyed separately by pruriceptive and nociceptive specific neurons might be decoded by the brain to yield the sensations of itch and different nociceptive sensory qualities.
Neural model of the spatial summation of sensations evoked by pruritic stimuli
There was no spatial summation of itch or nociceptive sensations when the number of spicules was increased from one to seven, within a spatial extent of 1 cm. In contrast, there was a spatial summation of the magnitude of itch and nociceptive sensations when the number of spicules was increased from a group of seven spicules (each separated by 1 mm) to four such groups, each separated by 2 cm. This finding is consistent with the previous observation that itch and pricking/stinging exhibit spatial summation in response to an increase in the number of spatially separated drops of topical capsaicin applied along the forearm (Green 1990).
The number of activated afferents would seem almost certainly greater for seven than for one spicule, although the firing in a single afferent is not greater when multiple spicules as opposed to just one are inserted (Johanek et al. 2008). One possibility is that afferents activated by cowhage may induce a local, lateral inhibitory effect on activity from neighboring afferents as (or before) they converge on second-order neurons, thereby preventing spatial summation within small areas, e.g., <1 cm2 (Fig. 9). Stimulation of more distant fibers may avoid the local inhibition and allow spatial summation via convergence of second-order neurons onto third-order neurons in the dorsal horn or higher up, e.g., in the thalamus. This hypothesis is consistent with the spatial summation of itch when spicules are inserted over an area spanning 6 cm. An identical model could explain the spatial summation of nociceptive sensations in response to cowhage.
FIG. 9.
Neuronal model of spatial summation and segmental inhibition of itch or nociceptive sensations evoked by cowhage. The model provides a candidate mechanism by which spatial summation of itch evoked by multiple spicules is absent within a local extent of 1 cm but present when spicules are spread over a greater extent, such as 6 cm. The same type of model could be used to explain the spatial summation of pricking/stinging or burning in pruriceptive spinothalamic tract (STT) neurons that mediate nociceptive sensations evoked by cowhage. As shown, 1 branch of a “mechano-chemo” nociceptive neuron (e.g., 1 with C-mechanoheat nociceptors), MCN2, responds sooner and more vigorously to a cowhage spicule than other branches of the same neuron or other MCNs (MCN1 and 3 whose branches are not shown). Action potentials generated by the most responsive peripheral branch of MCN2 is hypothesized as traveling not only orthodromically but also antidromically into neighboring branches of the same afferent, thereby resetting or fatiguing the generation of impulses from these fiber terminals (Peng et al. 2003). The central terminals of the same afferent are hypothesized as activating not only a 2nd-order pruriceptive neuron (mediating itch), P2, whose receptive field may be larger than shown, but also inhibitory interneurons (small filled circles). These inhibitory interneurons exert a weak inhibition (e.g., presynaptic) of weaker activity generated by neighboring MCNs whose inputs converge onto P2 (segmental afferent inhibition), accounting for the lack of spatial summation within approximately the size of the receptive field of a MCN. Not shown are the inhibitory interneurons activated by MCN1 and MCN3 similar to MCN2. Spatial summation of input from pruriceptive neurons is hypothesized to occur in 3rd-order neurons to account for the finding that there is spatial summation of itch when 4 groups of cowhage spicules are distributed along an extent of 6 cm on the arm. In this case, each group of spicules is hypothesized to activate some of the same but also different 2nd-order neurons (e.g., P4) whose primary afferent inputs are not subject to inhibitory effects from an adjacent group of spicules. The neuronal input and inhibitory mechanisms for P4 are identical to those of P2 but not shown for simplicity. The convergence of P2 and P4, each mediating itch, onto a spinothalamic tract neuron, S, provides the basis for the spatial summation of itch when the number of spicules is increased over a distance of 6 as opposed to 1 cm.
Dysesthetic sensory states evoked by cowhage
“Itchy skin” was originally described as a cutaneous area within which innocuous stroking evokes or exacerbates an itch (Bickford 1938) and subsequently termed “alloknesis” (Simone et al. 1991). The itchy skin surrounding the site of an intradermal injection of histamine was subsequently found to be characterized not only by alloknesis but also by enhanced pricking evoked sensations in the form of punctate hyperalgesia and/or hyperknesis (Atanassoff et al. 1999). In the single study in which itchy skin was measured in response to cowhage (Graham et al. 1951), an area of hypoalgesia to pin prick developed, within which alloknesis could be elicited. These sensory states were subsequently replaced by a larger area of hyperalgesia to pin prick within which alloknesis could no longer be evoked.
In contrast to these results, we observed that, when alloknesis developed in response to one or more spicules of cowhage, it typically co-existed with an area of hyperalgesia and/or hyperknesis. In addition, each of the three dysesthetic states was occasionally observed in isolation, although rarely so for only alloknesis or hyperalgesia. More commonly, hyperknesis was present alone or accompanied by hyperalgesia with or without alloknesis. The dissociation between each of these enhanced sensory states and the variability in their association with each other in repeated tests with the same subject or between subjects suggests that each may be elicited by a different neural mechanism. Such mechanisms are unlikely to be simply related to activity in afferents mediating sensations of itch and nociceptive sensations. There were several incidences in which a moderate to very strong peak magnitude of itch resulted in no dysesthesias or resulted in hyperknesis in the absence of alloknesis or hyperalgesia. Similar findings were obtained in response to an intradermal injection of histamine (10 μg/10 μl; unpublished observations).
Neural model of dysesthesias evoked by pruritic stimuli
We hypothesize that pruriceptive STT neurons mediating itch have differing capacities to sensitize interneurons that differ, in turn, in the type of primary afferent input they receive and whether they project to an itch-mediating or a nociceptive-specific STT neuron. In this model, the interneuron projecting to the itch mediating STT neuron receives a convergent input either from low-threshold and high-threshold mechanoreceptors or from the high-threshold type alone (Fig. 10). If this type of interneuron is sensitized, its mechanically evoked input to the STT neuron is enhanced, thereby contributing to alloknesis and/or hyperknesis. The other type of interneuron projects to a nociceptive specific STT neuron mediating pricking/stinging. This interneuron receives convergent input solely from high-threshold mechanoreceptors (primarily those with thinly myelinated axons) and, if sensitized, conveys an enhanced response, thereby contributing to enhanced pricking pain (hyperalgesia). Because either type of STT neuron itself is not sensitized, it would not exhibit enhanced responses to all types of stimuli, such as thermal noxious stimuli. If itch-mediating STT neurons differ in their projections to, or capacity to sensitize, one or both types of interneurons, as hypothesized, an itch will not always be accompanied by dysesthesias of one or more types. We hypothesize that such variations in central neuronal properties may account for individual differences between subjects and repeated tests with the same subject in the occurrence of one or more types of dysesthesia.
We observed no hyperknesis or hyperalgesia to thermal stimuli or to blunt noxious mechanical stimuli (e.g., pinch) delivered outside the cowhage application site (unpublished observations). Thus we would hypothesize that such stimuli would elicit sufficient activity in nociceptive specific (and pruriceptive neurons mediating nociceptive sensations) to activate central inhibitory neurons that block the activity in itch-mediating central neurons. Such an inhibitory effect has not yet been observed in candidate pruriceptive STT neurons with the exception of the few neurons tested that exhibited a reduction of ongoing firing to a pruritic chemical stimulus when the skin within or outside the chemical application site was scratched (Davidson et al. 2008).
Why doesn't the activation of polymodal nociceptive afferents by nonpruritic heat or mechanical noxious stimuli elicit itch or pruritic dysesthesias? These stimuli may activate not only pruriceptive neurons but a sufficient number of nociceptive specific afferents (not responsive to pruritic agents) that project to nociceptive specific STT neurons. Candidate nociceptive specific primary sensory neurons include the high-threshold mechanonociceptors that do not respond to cowhage or to histamine (Johanek et al. 2007; Schepers et al. 2008); or to a noxious irritant that elicits burning (Adriaensen et al. 1983). Other types of nociceptive specific afferents might be mechanically insensitive. For example, noxious heat will activate the mechanically insensitive C-heat nociceptors that, unlike polymodal nociceptors, also respond vigorously to a painful intradermal injection of capsaicin (Baumann et al. 1991; Schmelz et al. 1997). An intradermal injection of capsaicin evokes pain and not itch even at low doses (Simone et al. 1989; LaMotte et al., unpublished observations). The inhibitory input to pruriceptive STT neurons from nociceptive specific neurons might be mediated by a descending pathway and/or segmental or suprasegmental inhibitory neurons. Alternatively, perhaps a weaker signal from pruriceptive afferent neurons is merely unable to compete with stronger signals conveyed by nociceptive-specific neurons to higher-order neurons mediating perception.
Conclusions
The punctate delivery of the protease in a single cowhage spicule is an unusually precise and select kind of stimulus, probably activating a very small number of peripheral sensory neurons. However, the sensory consequences, when they occur, can be surprisingly significant—consisting of itch, nociceptive sensations and a radical change in the processing of mechanically evoked sensations in a large area of surrounding skin. Within such an area, a light stroking of the skin may produce itch; a seemingly harmless prickle of the skin may produce an abnormally intense pain or itch. However, there is a local variation within the skin and perhaps between people as to the degree to which nociceptive sensations and each type of dysesthesia accompany the itch evoked by cowhage. Thus the diversity of sensory events evoked by a single spicule provides a microscopic view of the diversity and complexity of the neuronal mechanisms mediating itch, pain, and cutaneous dysesthesias. Our interpretation is that peripheral sensory neurons differ in their capabilities of engaging central neurons mediating itch and pain and that these central neurons, in turn, differ in their capacities to induce a central sensitization to mechanical stimulation in the skin surrounding the site of spicule application.
Because the short lasting activity of a few pruriceptive neurons may have such enormous changes in sensation and sensory processing, a chronic activation in systemic or dermatologic disease would be expected to have significant consequences such as injurious scratching and a decrease in the quality of life.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant 5P01 NS-047399.
Acknowledgments
We thank S. Davidson, G. Giesler Jr., R. Meyer, M. Ringkamp, and P. Sikand for valuable comments on the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Adriaensen et al. 1983.Adriaensen H, Gybels J, Handwerker HO, Van Hees J. Response properties of thin myelinated (A-delta) fibers in human skin nerves. J Neurophysiol 49: 111–122, 1983. [DOI] [PubMed] [Google Scholar]
- Andrew and Craig 2001.Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci 4: 72–77, 2001. [DOI] [PubMed] [Google Scholar]
- Atanassoff et al. 1999.Atanassoff PG, Brull SJ, Zhang JM, Greenquist K, Silverman DG, LaMotte RH. Enhancement of experimental pruritus and mechanically evoked dysesthesias with local anesthesia. Somatosens Mot Res 16: 299–303, 1999. [DOI] [PubMed] [Google Scholar]
- Bartoshuk et al. 2004.Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav 82: 109–114, 2004. [DOI] [PubMed] [Google Scholar]
- Baumann et al. 1991.Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol 66: 212–227, 1991. [DOI] [PubMed] [Google Scholar]
- Bickford 1938.Bickford RG Experiments relating to the itch sensation, its peripheral mechanism, and central pathways. Clin Sci 3: 377–386, 1938. [Google Scholar]
- Binder et al. 2008.Binder A, Koroschetz J, Baron R. Disease mechanisms in neuropathic itch. Nat Clin Pract Neurol 4: 329–337, 2008. [DOI] [PubMed] [Google Scholar]
- Brewer et al. 2008.Brewer KL, Lee JW, Downs H, Oaklander AL, Yezierski RP. Dermatomal scratching after intramedullary quisqualate injection: correlation with cutaneous denervation. J Pain 9: 999–1005, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell and LaMotte 1983.Campbell J, LaMotte R. Latency to detection of first pain. Brain Res 266: 203–208, 1983. [DOI] [PubMed] [Google Scholar]
- Carstens et al. 2008a.Carstens E, Merrill AW, Akiyama T, Carstens MI. Intradermal PAR-2 agonist excites superficial dorsal horn neurons in the mouse: potential role in itch. Soc Neurosci Abstr 771.10, 2008a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens et al. 2008b.Carstens MI, Akiyama T, Merrill AW, Anotto KL, Carstens E. Scratching behavior and Fos expression in superficial dorsal horn elicited by protease-activated receptor agonists and other itch mediators in mice. Soc Neurosci Abstr 771.11, 2008b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson et al. 2008.Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ Jr. Responses and modulation of monkey spinothalamic tract neurons to itch producing and itch-inhibiting stimuli. Acta Derm Venereol 87: 475, 2008. [Google Scholar]
- Davidson et al. 2007.Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ Jr. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci 27: 10007–10014, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham et al. 1951.Graham DT, Goodell H, Wolff HG. Neural mechanisms involved in itch, itchy skin, and tickle sensation. J Clin Invest 30: 37–49, 1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green 1990.Green BG Spatial summation of chemical irritation and itch produced by topical application of capsaicin. Percept Psychophysics 48: 12–18, 1990. [DOI] [PubMed] [Google Scholar]
- Green and Bluth 1995.Green BG, Bluth J. Measuring the chemosensory irritability of human skin. J Toxicol Cut Ocular Toxicol 14: 23–48, 1995. [Google Scholar]
- Green et al. 1996.Green BG, Dalton P, Cowart BJ, Shaffer GS, Rankin KM, Higgins J. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses 21: 323–334, 1996. [DOI] [PubMed] [Google Scholar]
- Green and Flammer 1989a.Green BG, Flammer LJ. Localization of chemical stimulation: capsaicin on hairy skin. Somatosens Mot Res 6: 553–566, 1989a. [DOI] [PubMed] [Google Scholar]
- Green and Flammer 1989b.Green BG, Flammer LJ. Methyl salicylate as a cutaneous stimulus: a psychophysical analysis. Somatosens Mot Res 6: 253–274, 1989b. [DOI] [PubMed] [Google Scholar]
- Green and Pope 2003.Green BG, Pope JV. Innocuous cooling can produce nociceptive sensations that are inhibited during dynamic mechanical contact. Exp Brain Res 148: 290–299, 2003. [DOI] [PubMed] [Google Scholar]
- Green et al. 2008.Green BG, Roman C, Schoen K, Collins H. Nociceptive sensations evoked from ‘spots’ in the skin by mild cooling and heating. Pain 135: 196–208, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green and Schoen 2007.Green BG, Schoen KL. Thermal and nociceptive sensations from menthol and their suppression by dynamic contact. Behav Brain Res 176: 284–291, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green and Shaffer 1993.Green BG, Shaffer GS. The sensory response to capsaicin during repeated topical exposures: differential effects on sensations of itching and pungency. Pain 53: 323–334, 1993. [DOI] [PubMed] [Google Scholar]
- Handwerker et al. 1991.Handwerker HO, Forster C, Kirchhoff C. Discharge patterns of human C-fibers induced by itching and burning stimuli. J Neurophysiol 66: 307–315, 1991. [DOI] [PubMed] [Google Scholar]
- Ikoma et al. 2005.Ikoma A, Handwerker H, Miyachi Y, Schmelz M. Electrically evoked itch in humans. Pain 113: 148–154, 2005. [DOI] [PubMed] [Google Scholar]
- Johanek et al. 2008.Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci 28: 7659–7669, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanek et al. 2007.Johanek LM, Meyer RA, Hartke T, Hobelmann G, Maine D, LaMotte RH, Ringkamp M. Psychophysical and physiological evidence for separate neural pathways mediating histaminergic and non-histaminergic itch. J Neurosci 27: 7490–7497, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte et al. 1991.LaMotte RH, Shain CN, Simone DA, Tsai E. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol 66: 190–211, 1991. [DOI] [PubMed] [Google Scholar]
- Nakano et al. 2008a.Nakano T, Andoh T, Lee JB, Kuraishi Y. Different dorsal horn neurons responding to histamine and allergic itch stimuli. Neuroreport 19: 723–726, 2008a. [DOI] [PubMed] [Google Scholar]
- Nakano et al. 2008b.Nakano T, Andoh T, Sasaki A, Nojima H, Kuraishi Y. Different roles of capsaicin-sensitive and H1 histamine receptor-expressing sensory neurones in itch of mosquito allergy in mice. Acta Derm Venereol 88: 449–454, 2008b. [DOI] [PubMed] [Google Scholar]
- Namer et al. 2008.Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol 100: 2062–2069, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaklander et al. 2003.Oaklander AL, Bowsher D, Galer B, Haanpää M, Jensen MP. Herpes zoster itch: preliminary epidemiologic data. J Pain 4: 338–343, 2003. [DOI] [PubMed] [Google Scholar]
- Ochoa et al. 1989.Ochoa J, Torebjörk E. Sensations evoked by intraneural microstimulation of C nociceptor fibres in human skin nerves. J Physiol 415: 583–599, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng et al. 2003.Peng YB, Ringkamp M, Meyer RA, Campbell JN. Fatigue and paradoxical enhancement of heat response in C-fiber nociceptors from cross-modal excitation. J Neurosci 23: 4766–4774, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price et al. 1976.Price DD, Dubner R, Hu JW. Trigeminothalamic neurons in nucleus caudalis responsive to tactile, thermal, and nociceptive stimulation of monkey's face. J Neurophysiol 39: 936–953, 1976. [DOI] [PubMed] [Google Scholar]
- Reddy et al. 2008.Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage evoked itch is mediated by a novel cysteine protease - a ligand of protease activated receptors. J Neurosci 28: 4331–4335, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers et al. 2008.Schepers RJ, Johanek LM, Hartke TV, Shim B, Borzan J, Meyer RA, Ringkamp M. A subpopulation of Aδ nociceptors in monkey is vigorously activated by cowhage spicules. Soc Neurosci Abstr 170.3, 2008. [Google Scholar]
- Schmelz et al. 2000.Schmelz M, Michael K, Weidner C, Schmidt R, Torebjork HE, Handwerker HO. Which nerve fibers mediate the axon reflex flare in human skin? Neuroreport 11: 645–648, 2000. [DOI] [PubMed] [Google Scholar]
- Schmelz et al. 1997.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci 17: 8003–8008, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz et al. 2003.Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol 89: 2441–2448, 2003. [DOI] [PubMed] [Google Scholar]
- Shelley and Arthur 1957.Shelley WB, Arthur RP. The neurohistology and neurophysiology of the itch sensation in man. Arch Dermatol 76: 296–323, 1957. [DOI] [PubMed] [Google Scholar]
- Shimada et al. 2007.Shimada SG, Green BG, LaMotte RH. Effects of a conduction block in myelinated nerve fibers on pruritic and nociceptive sensations evoked by application of cowhage spicules in human. Soc Neurosci Abstr 70.12, 2007. [Google Scholar]
- Shimada and LaMotte 2008.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain 139: 681–687, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikand et al. 2008.Sikand P, Shimada SG, Green BG, LaMotte RH. Capsaicin and histamine produce itch when delivered via inactivated-cowhage spicules. Soc Neurosci Abstr 7.107, 2008. [Google Scholar]
- Simone et al. 1991.Simone DA, Alreja M, LaMotte RH. Psychophysical studies of the itch sensation and itchy skin (“alloknesis”) produced by intracutaneous injection of histamine. Somatosens Mot Res 8: 271–279, 1991. [DOI] [PubMed] [Google Scholar]
- Simone et al. 1989.Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain 38: 99–107, 1989. [DOI] [PubMed] [Google Scholar]
- Simone et al. 2004.Simone DA, Zhang X, Li J, Zhang JM, Honda CN, LaMotte RH, Giesler GJ Jr. Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J Neurophysiol 91: 213–222, 2004. [DOI] [PubMed] [Google Scholar]
- Torebjork and Hallin 1976.Torebjork HE, Hallin RG. C fibre receptors in human skin. In: Sensory Functions of the Skin in Primates, edited by Zotterman Y. Oxford, UK: Pergamon Press, 1976.
- Tsujii et al. 2008.Tsujii K, Andoh T, Lee JB, Kuraishi Y. Activation of proteinase-activated receptors induces itch-associated response through histamine-dependent and -independent pathways in mice. J Pharmacol Sci 108: 385–388, 2008. [DOI] [PubMed] [Google Scholar]
- Tuckett and Wei 1987a.Tuckett RP, Wei JY. Response to an itch-producing substance in cat. I. Cutaneous receptor populations with myelinated axons. Brain Res 413: 87–94, 1987a. [DOI] [PubMed] [Google Scholar]
- Tuckett and Wei 1987b.Tuckett RP, Wei JY. Response to an itch-producing substance in cat. II. Cutaneous receptor populations with unmyelinated axons. Brain Res 413: 95–103, 1987b. [DOI] [PubMed] [Google Scholar]
- Weidner et al. 1999.Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker HO, Torebjörk HE. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. J Neurosci 19: 10184–10190, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosipovitch and Samuel 2008.Yosipovitch G, Samuel LS. Neuropathic and psychogenic itch. Dermatol Ther 21: 32–41, 2008. [DOI] [PubMed] [Google Scholar]