Abstract
Saccadic eye movements are made both to explore the visual world and to react to sudden sensory events. We studied the ability for humans to execute a voluntary (i.e., nonstimulus-driven) saccade command in the face of a suddenly appearing visual stimulus. Subjects were required to make a saccade to a memorized location when a central fixation point disappeared. At varying times relative to fixation point disappearance a visual distractor appeared at a random location. When the distractor appeared at locations distant from the target virtually no saccades were initiated in a 30- to 40-ms interval beginning 70–80 ms after appearance of the distractor. If the distractor was presented slightly earlier relative to saccade initiation then saccades tended to have smaller amplitudes, with velocity profiles suggesting that the distractor terminated them prematurely. In contrast, distractors appearing close to the saccade target elicited express saccade-like movements 70–100 ms after their appearance, although the saccade endpoint was generally scarcely affected by the distractor. An additional experiment showed that these effects were weaker when the saccade was made to a visible target in a delayed task and still weaker when the saccade itself was made in response to the abrupt appearance of a visual stimulus. A final experiment revealed that the effect is smaller, but quite evident, for very small stimuli. These results suggest that the transient component of a visual response can briefly but almost completely suppress a voluntary saccade command, but only when the stimulus evoking that response is distant from the saccade goal.
INTRODUCTION
Saccadic eye movements can be classified as reflexive, made in response to a sudden sensory event, or voluntary, made at will to a sensory element, generally a visual object, that is part of a visual scene. An exploration of the visual scene will occasionally be interrupted by the sudden appearance of a visual stimulus, which may reflexively elicit a saccadic eye movement toward it. Studying how sudden events interfere with ongoing behaviors is difficult because it requires quantification of the behavioral importance of maintaining an ongoing action relative to the assessed importance of sensory events. Nonetheless, various studies have examined the competition of these voluntary and reflexive processes. The ability for abrupt visual onsets to capture attention has been studied for close to 25 yr (e.g., Egeth and Yantis 1997; Yantis and Jonides 1984, 1990). Such work has examined both “covert” capture of attention, studying how peripheral visual attention is manipulated by suddenly appearing visual targets, as well as “overt” capture of attention or “oculomotor capture,” studying how suddenly appearing visual stimuli can elicit inappropriate saccades when subjects are instructed to direct saccades as part of an ongoing behavioral process, such as visual search (Theeuwes et al. 1999, 2003).
However, the sudden appearance of distracting stimuli can interfere with or delay goal-directed saccades without itself attracting gaze. Walker and colleagues showed that a distracting stimulus can increase the mean latency of a saccade triggered by the appearance of a target whose location is known in advance (Walker et al. 1997; see also Corneil and Munoz 1996; Lévy-Schoen 1969). The appearance of distractors delayed the initiation of saccades when presented far from the target. If presented near the target, however, these distractors influenced the amplitude of the saccade, leaving latency unaffected. The effect of the distractor on overall saccade latency is somewhat weaker if the distractor is presented shortly before or after the target (Walker et al. 1995). Subsequent studies on this “remote distractor effect” have examined how the presence of the distractor can influence saccadic trajectory (e.g., Doyle and Walker 2001).
Left unclear from these studies was the precise temporal nature of these distractor effects, particularly whether these effects were due to a long-lasting inhibition of saccade-related activity—thus reducing the probability of saccade execution for some time—or whether instead the effect was dramatic, but short-lived. Taking a somewhat different experimental approach, Reingold and Stampe have complemented these studies of the spatial specificity of remote distraction by examining the temporal specificity of visual distraction (Reingold and Stampe 1999, 2002, 2003, 2004; Stampe and Reingold 2002). In the experiment most closely resembling the present experiments, consisting of discrete trials, they showed that a visual stimulus consisting of two large, bright bars, extending the entire length of the screen, can cause a transient inhibition in saccade execution occurring around 70–100 ms after the appearance of the distractor. They have referred to this phenomenon as “saccadic inhibition” and speculated that it could be useful to vision by temporarily pausing ongoing natural saccade behavior in the face of sudden sensory events. Additional work has shown that large display-spanning distractors are not necessary for this transient saccade inhibition to occur. Reingold and Stampe (2004) showed that 10 × 10° distractors appearing on one side or the other of the fixation point can inhibit saccades. Smaller distractors have also been shown to have this effect when presented at or near central fixation (Graupner et al. 2007; Pannasch et al. 2001; Sheliga et al. 2002). They can also inhibit saccades made during reading when presented away from the saccade goal (Reingold and Stampe 2003). However, although these studies helped elucidate the temporal nature of saccade distraction, they did not examine the spatial specificity of these effects in the manner of Walker et al. (1997). Specifically, they left unclear whether saccade inhibition occurs irrespective of the distance between the distractor and the saccade goal or whether, like the remote distractor effect, it causes inhibition only when distant from the saccade goal.
The principal aim of the present study was to explore, in conjunction, the spatial and temporal aspects of interference of suddenly appearing distractors on saccade generation (experiment 1). We also investigated how interference with saccade commands depends on whether the planned saccade is visually guided and on whether it is reflexive (experiments 2a and 2b). Finally, we examined how the effect of the distractor depends on its size (experiment 3). Our findings support the idea that the phenomenon of saccade inhibition results from interactions between transient and sustained loci of activity present on a spatial neural map located in an area involved in saccadic programming, such as the superior colliculus or frontal eye fields. We then attempt to integrate the implications of these behavioral findings with those of recent neurophysiological data obtained in recordings of saccade-related areas in the monkey.
METHODS
Subjects, eye movement recording, and visual stimulus display
Four subjects (ages 20–43 yr; two naïve: N1, N2; two authors: A1, A2) participated in these experiments. Part of the motivation for having naïve subjects participate in the task was that the authors, both of whom participated as subjects, had considerable experience in saccade tasks and reliably made express saccades (latencies <110 ms) in a gap task (Fischer and Ramsperger 1984; Saslow 1967). We speculated that such a tendency to make saccades of such short latency reflected sensitivity to the sudden appearance of visual stimuli and that the effects we found here could reflect that sensitivity. To confirm that our naïve subjects did not make express saccades, we ran them in a gap saccade task before and after their participation in this study. In neither session did either subject make an appreciable number of express saccades.
Eye movements were recorded using an Eyelink II video-based eyetracker (SR Research), sampling at 500 Hz. Stimuli were presented on a cathode ray tube monitor (Compaq P1220). Stimulus presentation and data acquisition were controlled by a Macintosh G4 using C programs that were written using the Vision Shell library (Raynald Comtois). The luminance of all stimuli was 100 cd/m2, the maximum brightness setting of the monitor.
Subject setup and calibration
Subjects’ heads were stabilized with a bite bar. The Eyelink II apparatus was placed so that no part of it blocked the view of the screen. Calibration of eye position was performed using nine locations formed in a 3 × 3 grid. An eye position drift offset operation was performed at the start of each block of trials.
Experiment 1: inhibiting learned saccades
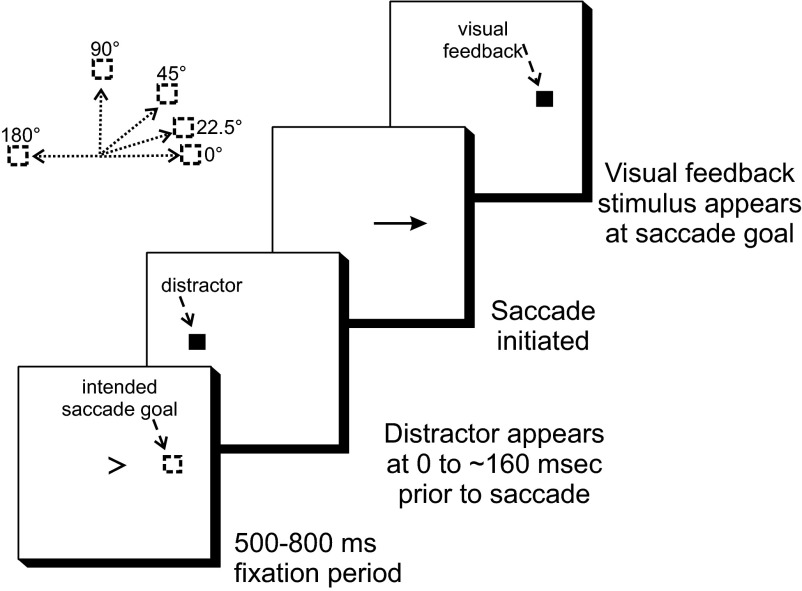
In this experiment subjects made saccades to the left or right as indicated by a central arrow. The task was similar to the “learned saccade task” of Bruce and Goldberg (1985), in that saccades were instructed to be made to a learned or memorized location at which no visible target was present (Fig. 1). The saccade goals were 10° to the left or right of the center of the display. Trials began with the presentation of a small central fixation arrow (“>” or “<” 0.5° from tail to head) pointed either left or right. Subjects were required to fixate this arrow within 500 ms of its appearance. After central fixation had been achieved, the subjects were required to maintain fixation for 500–800 ms, at which point the arrow disappeared, which served as the temporal cue for the subjects to make a saccade to the desired goal. The saccade had to be initiated within 500 ms of target disappearance, or else a loud beep would sound, and the trial was terminated. Data from such trials were not analyzed. Immediately after the saccade had landed, a small “feedback” stimulus, a spot of light (1 × 1°), appeared at the saccade goal for 300 ms, during which subjects had to maintain fixation on it. This provided information to subjects on their saccade accuracy.
FIG. 1.
Schematic of experiment 1. Trials began with the appearance of a central fixation point in the form of an arrow indicating the desired direction of the upcoming saccade. The desired amplitude of the saccade was 10°. The disappearance of the arrow was the cue to make a saccade. At a time between this cue and the initiation of a saccade, a distractor could appear. To provide feedback on the accuracy of the saccade, a visual stimulus appeared at the saccade goal after the saccade landed. For more details see text.
There were two types of such trials in this block, both using the temporal schematics described earlier. In “Invisible Distractor” trials no distractor appeared (the reason why these trials were referred to as “invisible” is described in the following text). In “Distractor” trials a bright square (2 × 2°) appeared at varying times relative to the disappearance of the fixation point, at varying locations on the screen. The temporal separation of fixation point disappearance and distractor appearance was fixed at one of four intervals, each separated from the next in duration by 30 ms. To determine the range of timings for a subject, practice trials were run and the subject's mean reaction time (i.e., interval between fixation arrow disappearance and saccade initiation) was calculated. Using this mean, intervals were set for each subject such that the average time between distractor appearance and saccade initiation was 85 ms, a typical time of maximum inhibition in the experiments of Reingold and Stampe (2002). For example, if a subject's mean reaction time was 150 ms, then the intervals between fixation arrow disappearance and distractor appearance were set at 110, 80, 50, and 20 ms, such that the expected intervals between distractor and saccade initiation were 40, 70, 100, and 130 ms (the average of which is 85 ms). The distractor would disappear as soon as the saccade was initiated. The possible distractor locations lay on a circular locus centered on the fixation point, with a directional difference of 0, 22.5, 45, 90, or 180° from the position of the saccade goal, at an eccentricity equivalent to that of the saccade goal. The distractors appeared only in the upper hemifield.
Trials, varying in the location of the saccade goal and the presence, location, and timing of the distractor, were run randomly within a session. Within a session, 288 trials were run, 144 each with the left or right saccade goal. For each side's set of 144 trials, 120 were Distractor trials and 24 were Invisible Distractor trials. Of the 120 Distractor trials, 24 had distractors at each of the five positions relative to the saccade goal. For each of the 24 trials with a particular distractor position, there were six of each of the four possible fixation arrow disappearance/distractor appearance intervals, as specified earlier. Each subject participated in five identical sessions for a total of 1,440 trials. Thus across the entire data set, there were a total of 30 trials for each combination of saccade direction, distractor location, and distractor timing, along with a total of 240 Invisible Distractor trials.
We also collected data for a follow-up to this experiment in which the distractors appeared in the lower instead of the upper hemifield (experiment 1b); otherwise, this follow-up was identical to experiment 1.
Experiment 2a: inhibiting visually guided delayed saccades
Experiment 2a was identical to experiment 1 except that subjects made visually guided delayed saccades to a 2 × 2° square that appeared at the saccade goal 100 ms after the fixation arrow appeared. Subjects were still required to wait for the fixation arrow to disappear before making a saccade, making this a visually guided delayed saccade task (Fischer and Boch 1981). The square disappeared 300 ms after the end of the saccade made to it. Also, because there was a visible target at the saccade goal, in contrast to experiment 1a, there were no trials with a 0° saccade goal/distractor angle difference. Thus there were a total of 240 trials in each session. Again, five sessions were run for a total of 1,200 trials. All four subjects participated in this experiment.
Experiment 2b: inhibiting reflexive visually guided saccades
Experiment 2b was identical to experiment 2a, except that the square at the saccade goal appeared coincident with the disappearance of the fixation arrow and saccades were instructed to be made when this peripheral stimulus appeared, rather than when the central fixation stimulus disappeared, as was the case in experiments 1 and 2a. This is thus similar to the remote distractor experiments of Walker et al. (1997), except that distractors were generally presented after the saccade target rather than coincident with it. The same four subjects of experiments 1 and 2a participated here.
Experiment 3: inhibiting learned saccades with distractors of various sizes
This experiment was identical to experiment 1, except the only distractor location was opposite the saccade goal and five different distractor sizes (all squares) were used (4, 2, 1, 0.5, and 0.25°). Within a session, 50% of trials had invisible distracters, whereas the other 50% of trials had distractors of one of the five sizes. Six sessions were run. The two authors participated in this experiment.
Data analysis
Data analysis was performed using routines from Matlab (The MathWorks) and SigmaStat (SYSTAT). All stated statistically significant differences were significant at α = 0.05.
CALCULATION OF SACCADE METRICS.
To compute the start and end of each saccade, a saccade velocity trace was obtained by differentiating the horizontal and vertical components of the eye position trace by a central difference algorithm implemented in Matlab and then using the Pythagorean theorem to calculate radial velocity as a function of time. The following algorithm for determining saccade latency was used in Matlab: the eye position trace just after the time of the cue to make a saccade (either target appearance or fixation point disappearance; see following text) was examined to determine the first point at which velocity exceeded 35°/s. Next, the trace was evaluated backward in time until the first point <15 deg/s was found. The end of the saccade was determined in an analogous manner, but with time reversed.
ASSESSMENT OF INFLUENCE OF DISTRACTORS ON SACCADE INITIATION.
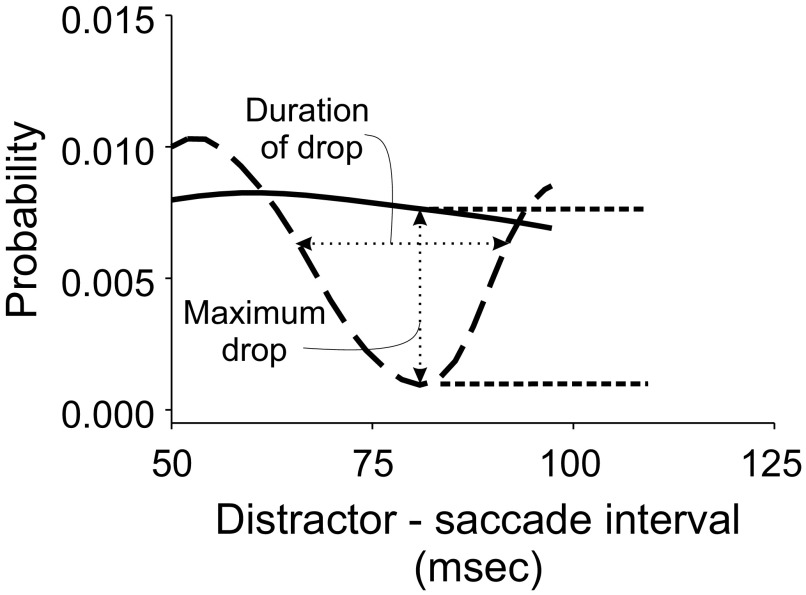
To determine the influence of distractor timing on saccade initiation, we constructed histograms of saccade initiation as a function of time after the appearance of the distractor. Thus if distractors at a particular location inhibited saccade initiation with a delay of, say, 80–120 ms, such histograms should show a “notch” at that delay where the probability of saccade occurrence dropped. To quantify this drop we calculated a continuous estimate of the probability of saccade occurrence, which replaced each saccade event in the histogram with a Gaussian kernel of SD of 3 ms (analogous to how spike density functions are calculated for use in analyzing single-unit neurophysiological data; Richmond and Optican 1987). We then compared these continuous estimates for each condition in which a distractor appeared with the condition in which no distractor appeared.
The Invisible Distractor condition was run using a temporal framework identical to that of the other trial types, only with the distractors appearing black (i.e., matching the appearance of the background), so that they were invisible (thus the name). This is equivalent to randomly assigning each trial of the Invisible Distractor condition to one of the four possible temporal intervals between distractor appearance and the cue to make a saccade (the disappearance of the fixation point). This puts the histogram of saccade initiation on an equal footing for the different trial types, such that if the visible distractors had no influence on saccade initiation, their histograms of saccade initiation would look the same as that of the Invisible Distractor trials. This approach has been used previously (e.g., Reingold and Stampe 2002). Next, we calculated the maximum probability drop as the maximal difference between the curve for the Distractor trials and the curve for the Invisible Distractor trials (Fig. 2) and noted the time relative to distractor appearance at which this maximum drop occurred. We also assessed the duration of this notch by calculating the half-width of this notch. This was done by noting the separation in the times when the probability function for the distractor condition crossed a level corresponding to 25% of the total drop.
FIG. 2.
Schematic of analyses of saccadic inhibition effect. For each subject's data for a particular target/distractor separation, a density function estimating saccade probability was calculated. This curve was then compared with an analogous one calculated for trials in which the distractor was invisible. We estimated the maximum decrease in saccade probability over time for each set of distractor trials relative to the Invisible Distractor trials, as well as the duration of this reduction in probability. We also calculated the time of peak saccade occurrence. For more details see text.
SACCADE CURVATURE.
If the effect of the distractor did not become manifest until late into the saccade, a curved saccade might result. To assess this possibility, we defined saccade curvature as the maximum perpendicular distance of any point on the saccade trajectory from a straight line connecting the start and endpoints divided by the distance between the two points (Smit and Van Gisbergen 1990). We then assigned a sign to this value depending on whether the curvature toward the end of the saccade was toward the right (+) or the left (−).
RESULTS
Experiment 1: no visible target at saccade goal
We found that the presence of a visual distractor far away from the intended goal of the saccade caused a transient inhibition in saccade generation that occurred 70 to 100 ms after the appearance of the distractor. In contrast, when the distractor was presented close to the saccade goal, it tended to elicit a saccade within 80–90 ms of its appearance, although the saccade tended to land much closer to the intended saccade goal than to the distractor.
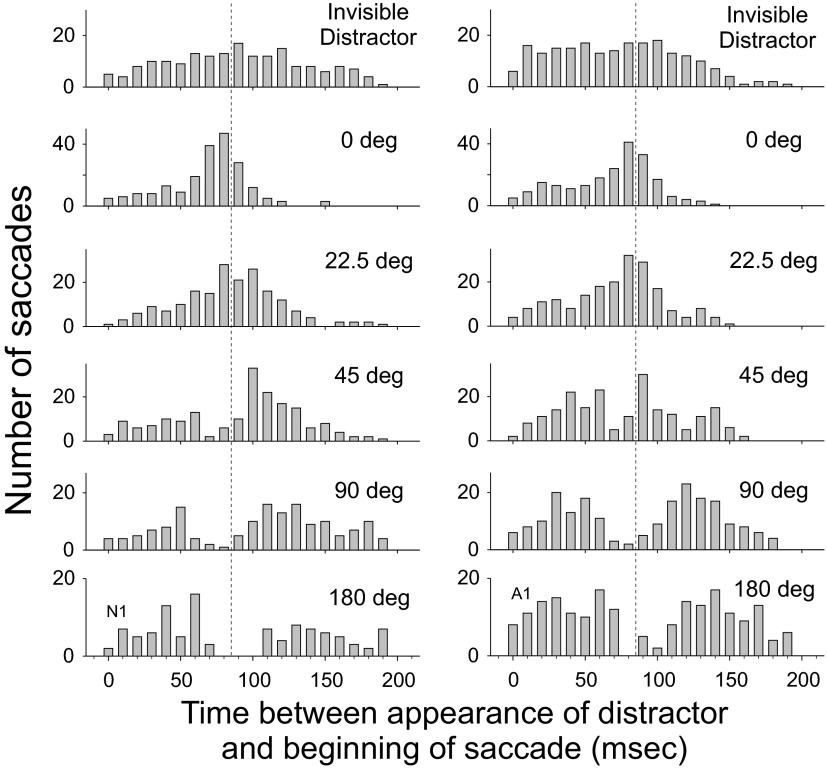
Histograms were constructed that reflected the probability of the time of saccade initiation with respect to the appearance of the distractor. They are shown for two subjects at the five different spatial relationships between distractor and saccade goal, along with the Invisible Distractor condition (Fig. 3).
FIG. 3.
Histograms for experiment 1 showing occurrence of saccades as a function of the interval between target disappearance and saccade initiation for 2 subjects. As a reference, at the top are shown trials in which the distractor was not visible. Below this are histograms of data for each target/distractor separation, with leftward and rightward saccades grouped together.
The most noteworthy finding is the “notch” visible in the histogram showing saccade initiation with respect to the appearance of the distractor. This was present for all four subjects when the directional difference between saccade goal and distractor was 180 or 90° and was present to a more modest extent when the spatial difference was 45°. We performed pairwise comparisons between the Invisible Distractor condition and each of the other conditions using the Kolmogorov–Smirnov test. Distributions were significantly different for all four subjects when the target/distractor separation was 90 or 180°, for two subjects when the separation was 45°, and for three subjects when the separation was 22.5 and 0°.
As described in methods (Fig. 2), to quantify the characteristics of the notch for the larger saccade goal/distractor spatial separations we calculated a continuous probability density estimate of the distribution of saccade latencies and compared this estimate using trials of a particular saccade goal/distractor angle with that of the Invisible Distractor trials (Fig. 4). We also computed the half-width of the density estimate of the notch (see methods). The mean peak reduction in saccade probability was 93% when separation was 180°, 91% at 90°, and 65% at 45°. The mean half-width of the notch was 49 ms for a 180° separation, 44 ms for 90°, and 25 ms for 45°. The time of the minimum of this notch ranged from 82 to 105 ms when separation was 180° and 79 to 92 ms when the separation was 90°. To confirm that the probability of saccade occurrence was lower in this notch than in the same time interval when no distractor appeared, we compared these two probabilities using a z-test for proportions. We found significant reductions in probability of saccade occurrence for all four subjects for each of the 45, 90, and 180° separations. Finally, we confirmed the dependence of the magnitude of inhibition on the goal/distractor separation across the four subjects with a one-way repeated-measures ANOVA (P < 0.001). A multiple-comparisons procedure (Student–Newman–Keuls [SNK]) revealed that the effects across the different saccade goal/distractor separations were all significantly different from each other, except for the difference between the 90 and 180° separation conditions. A similar repeated analysis procedure performed for analyzing the temporal duration of the inhibition (just for 180, 90, and 45° separations) also showed a dependence on angular separation; multiple comparisons showed that the duration of the inhibition was significantly shorter in the 45° condition than that in the other two conditions.
FIG. 4.
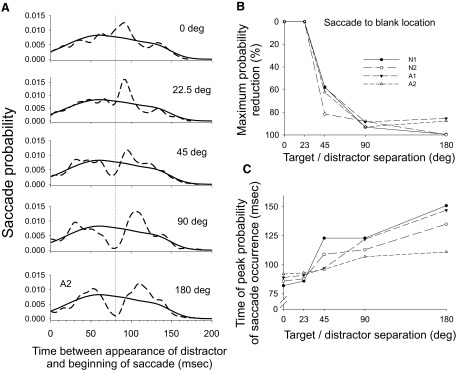
A: probability density functions for different target/distractor separations are shown for one subject. As a comparison, these curves are each superimposed with the corresponding curve from Invisible Distractor trials. B: maximum decreases in saccade probability are shown for the 5 target/separations for each of the 4 subjects. A value of 0% indicates that no distinct notch was found. C: time of peak probability of saccade occurrence is shown for each of the 5 target/distractor separations and for each of the 4 subjects.
When the distractor appeared at the saccade goal (0° separation) or else when the separation was small (22.5°) the distractor tended to elicit a saccade within 80–100 ms rather than suppress it, as depicted by the large peak in the distribution of saccade initiation times relative to distractor appearance in Fig. 3. There was a significant increase in the number of saccades made between 80 and 100 ms after distractor appearance for three of four subjects for the 0° separation condition and for all four subjects in the 22.5° separation condition. Overall, there was a 117% increase in the proportion of saccades in that time window for 0° separation and an 88% increase in the proportion of saccades for trials with a 22.5° separation. The time of this peak averaged 87 ms for 0° separation and 89 ms for 22.5°. These times are well within the range of human express saccade latencies (Edelman et al. 2007; Fischer and Ramsperger 1984) and are virtually identical in timing to the notch described earlier, when distractors appeared far away from the saccade goal.
DISTRACTOR INFLUENCE ON SACCADE DIRECTION.
Despite the intention to make a saccade to a particular location, it would seem possible for the sudden appearance of the distractor to elicit a saccade directly to the distractor itself. We found that when the directional difference between the saccade goal and distractor was at 180°, one of the four subjects (N2) made a large number of saccade errors, with roughly 30% of the saccades landing close to the distractor. The proportions of errors for the other three subjects at the 180° separation and for all four subjects at the 90° separation were <5%.
One could speculate that with smaller distances between targets and distractors, saccade endpoint could be influenced by the distractor, especially since the data on saccade initiation described earlier indicated that saccades were being triggered by the appearance of the distractor. However, even when this distance was small, saccades overall landed much closer to the desired goal than to the distractor. We calculated a “distractor angular percentage gain” by dividing the upward deflection in saccade angle with respect to the horizontal meridian by the angular distance of the distractor from the horizontal meridian. Thus if a saccade in the 45° condition landed directly on the distractor then the distractor angular percentage gain would be 100%, whereas if it landed at the desired goal (along the horizontal meridian), this measure would be zero. For saccades initiated between 80 and 100 ms after the distractor, we found that distractor angular gain tended to be very small, on average 21, 11, and <1% for 22.5, 45, and 90° separations, respectively. These values largely reflect the gains of one subject, N2, which were much larger than that of the others, although even for this subject gains were <50% (22.5°: 44%; 45°: 31%).
Although the spatial effect of the distractor was generally small, we compared these saccades to those initiated within 30 ms of distractor appearance (a time frame in which the distractor presumably has no effect on the saccade). The differences were significant for all four subjects when the distractor distance was 22.5° and for two of four subjects when the distance was 45° (t-test). Given these small gains, one might suggest that saccades were initiated toward the distractor and then bent back toward the saccade goal. An analogous analysis of saccades maximally affected and nonaffected by the distractor, as described earlier, showed that saccade curvatures were small for all trial types (curvature index: unaffected: 0.035; 22.5°: 0.039; 45°: 0.038).
DISTRACTOR INFLUENCE ON SACCADE AMPLITUDE AND KINEMATICS.
Distractor opposite saccade goal. As described earlier, the sudden appearance of a distractor opposite the saccade goal reduced the likelihood of initiating a saccade within about 80 ms of its appearance. The probability of initiating saccades within 40–80 ms of distractor appearance did not generally decrease significantly, although, given the duration of a typical saccade of 10° in amplitude (∼40–50 ms), saccades initiated within 40–80 ms after target appearance could be in progress when the distractor made its presence felt. Thus it is possible that the kinematics or endpoint of such saccades could be different from that of saccades made with no distractor.
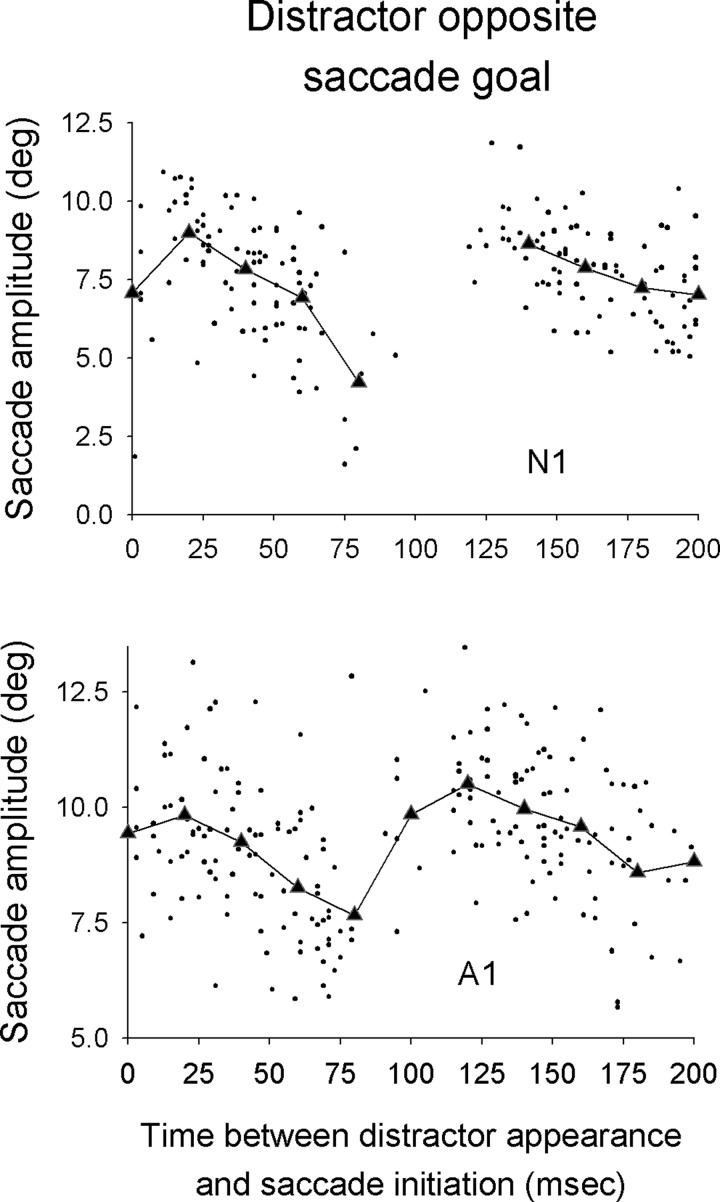
Indeed, when the distractor was opposite the saccade goal (180° separation) there was a tendency for saccades initiated 50–75 ms after distractor appearance to be more hypometric than saccades initiated at other times. We compared the amplitude of saccades initiated within 20 ms of the beginning of the reduction in saccade probability (typically 50–70 ms after distractor appearance) with the amplitude of saccades occurring within 25 ms of the distractor's appearance. Amplitudes were significantly reduced in this later period for all four subjects (P < 0.05, two-tailed t-test), with an average reduction in amplitude of 28% (Fig. 5).
FIG. 5.
Plot of saccade amplitude as a function of the time between distractor appearance and saccade initiation for 2 subjects for experiment 1. Data are from trials in which the distractor appeared opposite the saccade goal. Each dot corresponds to data for one saccade. The lines denote binned averages where the bins were of 20-ms width. Binned averages are shown only if there were ≥6 saccades in the bin.
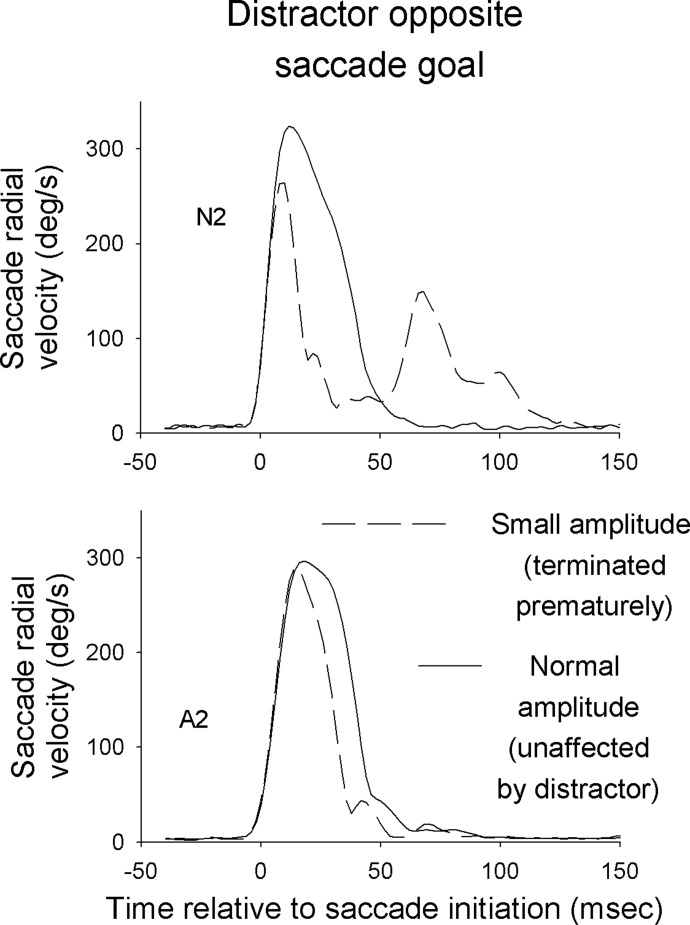
Interestingly, a comparison of peak velocity between these two sets of saccades revealed much smaller differences than one would expect given their relative amplitudes. The overall percentage decrease in velocity (12.5%) was much smaller than that of saccade amplitude (28.5%), suggesting that these saccades were higher in peak velocity than what would be normal for their amplitude. Figure 6 contrasts, for two subjects, the velocity profiles of saccades with relatively small amplitudes (N2: <5°; A2: <7.5°) that were initiated between 50 and 75 ms after distractor appearance with those of saccades not affected by the distractor. That the small-amplitude saccades achieve relatively high velocities before suddenly slowing down is consistent with the idea that the distractor, although not delaying the saccade, has prematurely terminated it. Note also for subject N2 eye velocity picks up around 25 ms after the small-amplitude saccades end, a velocity profile reminiscent of saccades interrupted by electrical stimulation of pontine omnipause neurons or of the rostral superior colliculus (Keller 1977; Keller and Edelman 1994; King and Fuchs 1977; Munoz et al. 1996).
FIG. 6.
Plot of mean radial saccade velocity as a function of time for 2 subjects in experiment 1, showing mean velocity both for saccades that were unaffected by the distractor and those that had relatively small amplitudes (N2: <5°; A2 <7.5°). Data are from trials in which the distractor appeared opposite the saccade goal (180° saccade goal/distractor separation).
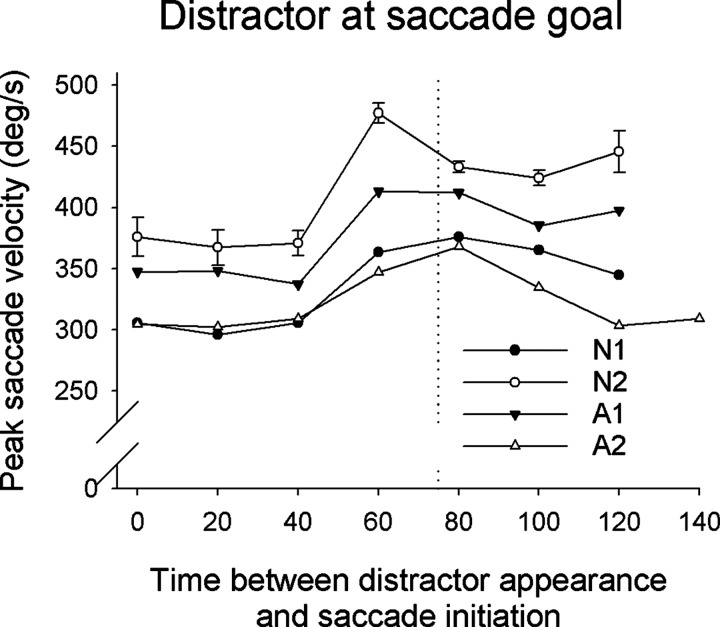
Distractor located at saccade goal. The presence of a distractor at the saccade goal also had an effect on saccade kinematics, in this case increasing saccade velocity. As mentioned earlier, when the distractor appeared at the saccade goal it had a tendency to elicit the saccade very rapidly, with a latency typical of express saccades. Saccades initiated 60–80 after distractor appearance had a velocity higher than that of saccades initiated 0–25 ms after distractor appearance. This increase was highly significant for all four subjects (P < 0.0001, two-tailed t-test) and averaged 23.8% across all four subjects (Fig. 7).
FIG. 7.
Plot of mean peak saccade velocity as a function of time of saccade initiation relative to the appearance of distractor for experiment 1 for trials in which the distractor appeared at the saccade goal (0° saccade goal/distractor separation). The symbols and lines represent 20-ms-width binned averages. Data for a particular bin are shown only if there were ≥6 saccades in the bin.
Surprisingly, for the three subjects who initiated a considerable number of saccades >100 ms after distractor appearance, it was evident that the velocity of saccades made at longer delays decreased substantially. For each of these three subjects we compared the peak velocity of saccades in the bin at which saccades were initiated most frequently (see Fig. 3) with peak velocities of the following bin. The peak velocities in the earlier bin were significantly higher for all three subjects (P < 0.0001, two-tailed t-test), by an average of 13.7% (Fig. 7). In contrast, the amplitude of saccades varied little as a function of the time between distractor appearance and saccade initiation.
EXPERIMENT 1B: CAN DISTRACTORS APPEARING IN THE LOWER VISUAL FIELD ALSO INHIBIT SACCADES?
To reduce the number of possible conditions in experiment 1 distractors were presented only in the upper visual field. We chose the upper visual field because we surmised that, because saccade latencies to the upper visual field are generally smaller than those to the lower visual field (Goldring and Fischer 1997), the effect of suddenly appearing distractors could be stronger. Although this maneuver increased the statistical power of our analyses, it left open the question of whether distractors in the lower visual field could also transiently inhibit saccades. To test this, we repeated experiment 1 but instead flipped visual stimuli about the horizontal meridian so that all distractors appeared either in the lower visual field or along the horizontal meridian. The two authors served as subjects.
We found an effect on saccade likelihood very similar to that found for experiment 1. The reduction in saccade likelihood was again similar to that found for experiment 1, with maximum decreases of saccade initiation of 87, 84, and 63% for distractor/saccade goal separations of 180, 90, and 45°, respectively. These decreases were all significant (P < 0.05, z-test of proportions) for both subjects.
Experiment 2: do visual stimuli at the saccade goal mitigate distraction?
The results described earlier show that saccades made to a blank location are inhibited by the sudden appearance of a visual distractor when it appears at a position with a large angular separation from the location of the saccade goal. On one hand, it is possible that the inhibition is an inevitable consequence of the appearance of the sudden onset. However, it is also possible that the inhibition is a net result of competition between saccade-related processes present at the saccade goal with those present at the distractor location. If so, inhibition occurs because a command to make a saccade to a blank location is simply not robust enough to resist the effect of a transient burst of activity that codes for visual stimuli or saccades to a distant location. If so, it is possible that the effects of the inhibition would be ameliorated by boosting the command signal for the intended saccade.
One way to do this is to present a visual stimulus at the goal, since it has been demonstrated that saccades to visual targets are accompanied by more activity than saccades to blank space (Edelman and Goldberg 2001, 2003; Everling et al. 1999). We tested this possibility using two modifications of experiment 1. First, we had subjects make a saccade to a visual stimulus that had been present at the saccade goal for several hundred milliseconds (visually guided delayed saccade task, experiment 2a) and, second, we had subjects make a reflexive saccade to a visual stimulus that suddenly appeared at the saccade goal (experiment 2b). Given that a visual stimulus was now present at the saccade goal, unlike experiment 1, we did not run trials in which a distractor appeared at the saccade goal.
EXPERIMENT 2A: DISTRACTING SACCADES MADE TO A STABLE VISUAL TARGET.
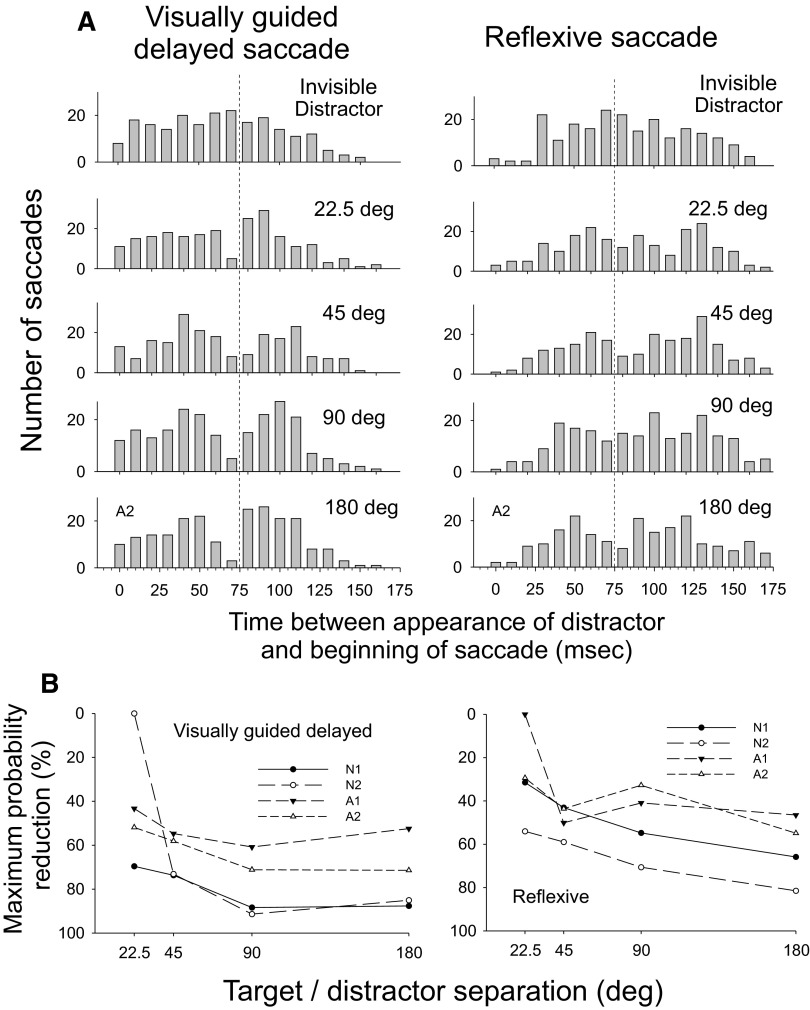
The effect of the distractor was somewhat less strong here than for experiment 1a, although we observed some decreases in saccade probability even for trials with a small distance (22.5°) between the distractor and the saccade goal (Fig. 8). Compared with the Invisible Distractor condition, the mean peak reduction in saccade probability was 74% when separation was 180°, 78% at 90°, 65% at 45°, and 41% at 22.5°. We found significant reductions in probability of saccade occurrence at the time of the notch for three of four subjects at the 180° separation, for all subjects at the 90 and 45° separations, and for two of four subjects at the 22.5° separation. We tested the dependence of the magnitude of inhibition on the angle of separation across the four subjects with a one-way repeated-measures ANOVA on ranks (P = 0.002). Multiple comparisons showed differences between the 22.5° separation and each of the other three separations. Across the four subjects, the mean half-width of the notch in saccade probability was slightly smaller for the 22.5° separation than that of the other three separations grouped together (180°: 35 ms; 90°: 37 ms; 45°: 37 ms; 22.5°: 15 ms, t-test, P = 0.025). For the smallest distractor/saccade goal separation of 22.5°, there was a less distinct peak of saccades at a time 80–100 ms after distractor appearance than was observed in experiment 1. The proportion of saccades made in the 22.5° condition in this time interval compared with the Invisible Distractor condition did not change appreciably because different subjects showed varying amounts of excitation and inhibition during this time window.
FIG. 8.
A: histograms of saccade occurrence for subject A2 for each target/distractor separation for experiment 2a (left) and experiment 2b (right). Other conventions as in Fig. 3A. B: maximum decreases in saccade probability for experiment 2a (left) and experiment 2b (right). Other conventions as in Fig. 4B.
As in experiment 1a, the effect of the distractor on the endpoint of saccades elicited between 80 and 100 ms after distractor appearance was quite small, with an average distractor angular gain (as defined for experiment 1) of 15, 6, and −2% for 22.5, 45, and 90° separations, respectively. Curvatures were also small (curvature index: unaffected: 0.037; 22.5°: 0.035; 45°: 0.045).
EXPERIMENT 2B: SACCADE TRIGGERED BY A SUDDENLY APPEARING VISUAL TARGET.
The effect of the distractor on saccade metrics was substantial, although still smaller than that found in experiments 1 and 2a (Fig. 8). The mean peak reduction in saccade probability was 62% when separation was 180°, 50% at 90°, 49% at 45°, and 29% at 22.5°. We found significant reductions in probability of saccade occurrence at the time of the notch for three of four subjects at 180, 90, and 45° separations and for one of four subjects at 22.5° separation. As in experiment 1a, we confirmed the dependence of the magnitude of inhibition on the goal/distractor separation across the four subjects with a one-way repeated-measures ANOVA (P = 0.003). Multiple comparisons showed differences between the 180° separation and each of the 45 and 22.4° separations. The mean half-width of the notch was similar to those found in experiment 2, although slightly less for 45° (180°: 36 ms; 90°: 24 ms; 45°: 32 ms; 22.5°: 16 ms). A one-way repeated-measures ANOVA of notch duration across the four subjects showed a dependence on separation (P = 0.01), with significant differences found between the 22.5° condition and each of the 45 and 180° conditions. Unlike experiment 1, but similar to experiment 2a, at the smallest distractor/saccade goal separation of 22.5°, there was no distinct peak of saccades at a time 80–100 ms after distractor appearance.
As in experiments 1a and 2a, the effect of the distractor on saccade endpoint in experiment 2b was small. The average distractor angular gain (as defined for experiments 1 and 2a) was 9, −1, and −1% for 22.5, 45, and 90° separations, respectively. Saccade curvatures were similarly low (curvature index: unaffected: 0.037; 22.5°: 0.040; 45°: 0.040).
COMPARISON OF AMOUNT OF INHIBITION ACROSS TRIAL TYPES.
We sought to compare the size of the saccade inhibition effect for the different levels of visual activation (Learned saccade, experiment 1; Visually guided saccade, experiment 2a; Suddenly appearing target, experiment 2b) at the saccade goal. To do this we compared the magnitude and duration of the reduction of saccade probability caused by the distractor as a function of the trial type and target/distractor spatial separation across the four subjects. We conducted a two-factor repeated-measures ANOVA using trial type and target/distractor separation as factors. As in all three experiments the magnitude of the inhibition was largest when the saccade goal/distractor separation was 180 and 90°—we considered trials with only those two separations. Confirming many of the observations described earlier, we found a main effect of trial type (P = 0.002), as well as a significant interaction between task and separation (P = 0.035). A multiple-comparisons procedure (SNK) confirmed that the reduction was largest for the Learned saccade task (experiment 1), intermediate in the Visually guided task (experiment 2a), and lowest in the Reflexive saccade task (experiment 2b). The reduction was also significantly larger for 90 than that for 180° in the Reflexive task, suggesting that this was the source of the interaction.
An analogous analysis of the effect of target condition and target/distractor separation on the duration of the reduction revealed a main effect with separation (P < 0.028), but not task (P > 0.3). Multiple comparisons revealed a significant increase in duration in the Learned saccade task (experiment 1) relative to the Reflexive saccade task (experiment 2b).
Experiment 3: does the magnitude of transient inhibition depend on distractor size?
The results of experiment 1 suggest that the saccade inhibition demonstrated previously during a voluntary saccade task using display-spanning stimuli can also occur using a much smaller stimulus: a bright square of 2° width. This raises the question of whether the effect would exist for even smaller distractors. To test this, we repeated a modified version of experiment 1 using five different sizes of distractors ranging in width from 0.25 to 4°. Distractors, presented on 50% of trials, always appeared opposite the saccade goal. The two authors served as subjects.
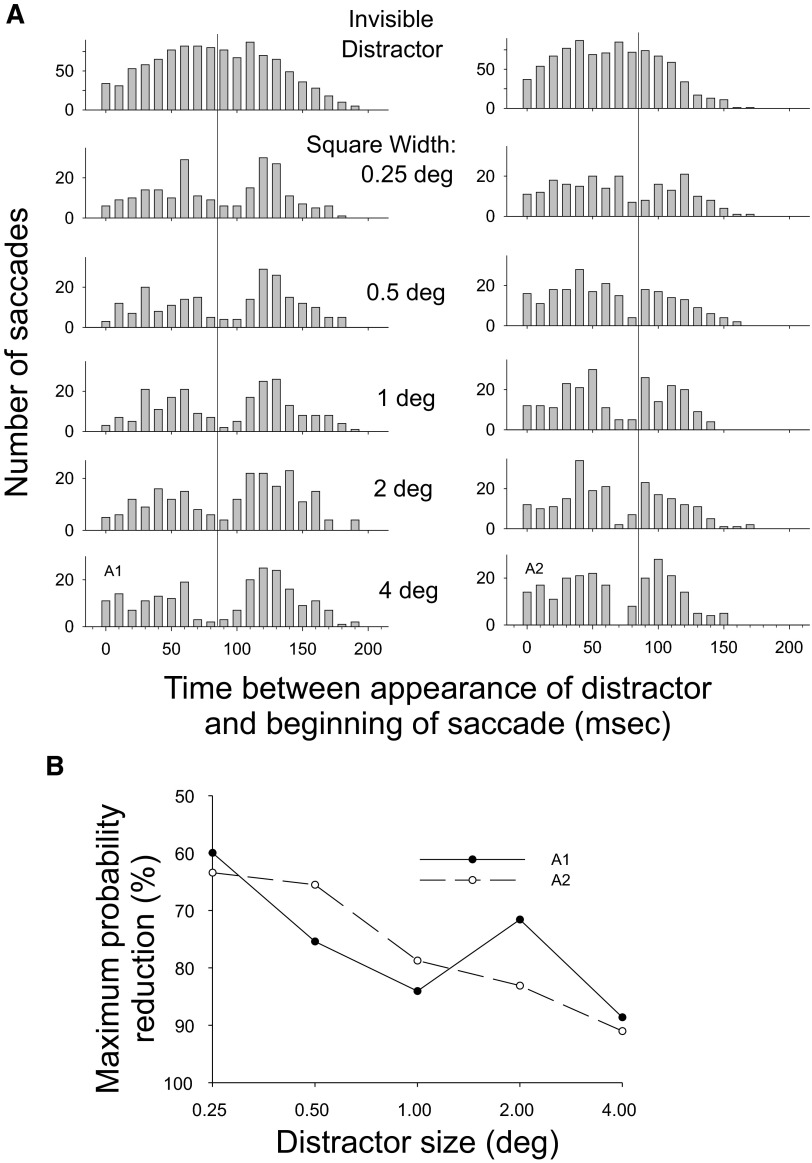
We found that even the smallest distractor (0.25°) inhibited saccade production. Indeed, for both subjects saccade inhibition was statistically significant for all distractor sizes (P < 0.001, z-test on proportions; Fig. 9). However, the inhibitory effect had some tendency to increase as distractor size increased and, in particular, inhibition appeared weakest at the smallest square width. Across the two subjects, the mean peak reduction in saccade probability was 62, 70, 81, 77, and 90% for the square sizes of 0.25, 0.5, 1, 2, and 4°, respectively. A linear regression of the peak magnitude of the probability decrease as a function of the log of the distractor size showed a significant dependence of the effect of distractor size for one subject (A2, P = 0.003) and a similar, though nonsignificant, trend in the other subject (A1, P = 0.13).
FIG. 9.
A: histograms of saccade occurrence for subject A1 for each target/distractor separation for experiment 3. Other conventions as in Fig. 3A. B: maximum decreases in saccade probability as a function of distractor size for experiment 3.
DISCUSSION
These results provide clear evidence that the sudden appearance of a visual stimulus distant from an intended saccade goal can almost completely inhibit the generation of a saccade from 70 to 100 ms after the appearance of the stimulus, regardless of the subject's desire to make a saccade. In contrast, when visual stimuli are presented near the saccade goal, the tendency for short-latency saccades to be elicited with an extremely short latency (express saccades) increases considerably, even for the naïve subjects who had shown little tendency to make express saccades in more conventional saccade tasks. This inhibition is strongest when the saccade is made to blank space, less strong when the saccade is made to a visual stimulus that has been present for several hundred milliseconds, and still less strong when the saccade is elicited by the sudden appearance of a visual object. Inhibition occurs even when the distracting visual stimulus is quite small (0.25 × 0.25°) and occurs regardless of whether the distractor appears in the upper or lower visual field. There were few trials in which no saccade was made, suggesting that this inhibition only delayed saccades, but did not completely abolish the saccade program.
One might expect that the saccades of highly motivated human subjects should not be susceptible to the inhibiting effect of distractors, given that they have a spatially defined movement goal. Indeed, highly focused spatial attention is the one behavioral state that has been shown to mitigate attentional capture by the sudden appearance of visual stimuli (Yantis and Jonides 1990). Yet, saccades do not seem similarly impervious. This was true even for the two authors (subjects A1 and A2) who were both well experienced in eye-movement tasks. Indeed, virtually all the findings of experiments 1, 2a, and 2b held for both the experienced and naïve subjects.
Relation to previous studies of saccade inhibition and distraction
These results extend and clarify implications from work on the “saccadic inhibition” effect of Reingold and Stampe (1999, 2000, 2002, 2003, 2004) and Stampe and Reingold (2002). These previous studies have shown that a flash of the entire display, or else presentation of a large visual stimulus flanking a central fixation point, inhibited saccades for a short period of time. The presence of such a distracting stimulus can inhibit saccades, irrespective of whether they are made during reading (Reingold and Stampe 1999, 2000, 2003, 2004), visual search (Reingold and Stampe 1999, 2000), or more conventional saccade tasks (Reingold and Stampe 2002).
Other studies have shown that the abrupt onset of behaviorally irrelevant visual stimuli can delay saccade commands even when stimuli are small. Sheliga et al. (2002) demonstrated that visual stimuli flashed at the location of fixation (superimposed on a smaller visual stimulus already present at fixation) delayed a volitional eye movement during a centrally cued double-saccade task. However, they found only minor saccadic delays when a visual stimulus was presented opposite the saccade goal, but superimposed on an already visible stimulus. We speculate that the lack of an effect of the peripheral stimulus was attributed not only to an already existing visual stimulus at that location, but also to a somewhat small distractor (0.8 × 0.8°). It has also been shown that small stimuli presented at or near fixation can delay saccades during scanning of a complex visual scene (Graupner et al. 2007; Pannasch et al. 2001). Reingold and Stampe (2003) found that small stimuli could inhibit saccades when superimposed on text and when appearing away from the saccade goal. Such inhibition has also been described in a double-step task in monkey (Sommer and Tehovnik 1999).
However, these experiments did not examine whether small stimuli, distant from the saccade goal, could inhibit saccades in a discrete-trial task in which the saccade goal was predefined. Such a saccade command should have the greatest chance at defying saccadic inhibition. Moreover, these prior studies did not systematically examine the strength of saccade inhibition as a function of distractor size, distractor location, and/or saccade task. Our results demonstrate that saccade inhibition can occur even when there is an explicit motor plan and when the distractor is very small (0.25 × 0.25°) and not present foveally. Our experiments are also the first to show that this inhibition is contingent on the distractor being at some distance from the saccade goal, in that distractors close to the saccade goal tended instead to immediately trigger saccades.
Work on the remote distractor effect has shown that presentation of visual distractors prior to the execution of an impending saccade can, depending on the spatial distance between the saccade goal and the distractor, increase saccade reaction time or influence the endpoint of the saccade (Walker et al. 1997). Of all our experiments, experiment 2b most closely resembles those of Walker et al. (1997), in that the goal-directed saccade was a visually guided reaction-time saccade. Our results are consistent with theirs, since we found saccade inhibition even in this reflexive task. We also found that in this task saccades were almost always eventually made to the saccade goal, which is also consistent with their findings.
Moreover, like previous studies of the remote distractor effect, we found that saccades were much more likely to be delayed when distractors were presented far from the saccade goal than when presented close to the saccade goal. However, unlike the present study, these previous studies did not systematically vary the timing of the distractor and thus could not assess the precise temporal nature of the impact of distractors on saccade initiation. Our results reveal that the remote distractor effect may, in large part, be a result of the short-duration inhibition of the saccade system shown here.
Effect of distractors on saccade vector and kinematics
Another similarity between our results and those from studies of the remote distractor effect is that distractors—even when close to the saccade goal—had little effect on saccade direction. However, the present result is more surprising given that the distractor tended to trigger the saccade very rapidly, such that the saccade had latencies in the express saccade range. This finding suggests that although the sudden visual appearance of the distractor triggers the saccade, the vector of the movement is still governed by the voluntary saccade command.
Our findings contending that the sudden appearance of visual distractors can prematurely terminate a saccade suggests that the influence of the distractor is not confined to simply determining whether a saccade is initiated, but can affect a saccade in progress. This adds further evidence to the idea that saccades, even in midflight, are still susceptible to competing saccade programs and new sensory events (Becker and Jürgens 1979; McPeek 2006; McPeek and Keller 2002; McPeek et al. 2000; Port and Wurtz 2003).
When the distractor was near the saccade goal in experiment 1, we found that saccades that appeared to be visually driven (i.e., made 70–100 ms after distractor appearance) tended to have higher velocities than when the saccades were unaffected by the distractor. This is not surprising, given that visually guided saccades tend to be faster than nonvisually guided saccades (Edelman and Goldberg 2001; Edelman et al. 2006; Gnadt et al. 1991; Ohno et al. 2000; Smit and Van Gisbergen 1989; Smit et al. 1987; Van Gelder et al. 1997). However, we also found that velocities of saccades dropped somewhat as the interval between distractor and saccade initiation further increased. This would suggest that ultrashort-latency saccades like express saccades might be slightly higher in velocity than saccades of slightly longer latency. We know of no study in human subjects that has directly assessed this, although a study in monkeys showed that velocity of express and “regular” latency saccades were similar (Edelman and Keller 1996).
What is the role of saccadic inhibition in natural oculomotor behavior?
Although saccadic inhibition is now well documented, in the real world our strong sense is that the sudden appearance of a salient visual stimulus will draw the eye toward it. This has been demonstrated in studies of oculomotor capture, where a suddenly appearing visual stimulus can elicit a saccade to itself even though it is irrelevant to the task (Theeuwes et al. 1999, 2003). Why are such saccades not observed in studies of saccadic inhibition? First, the distractors may be too large, even spanning the entire visual display (Reingold and Stampe 1999, 2000, 2002, 2004; Stampe and Reingold 2002), such that there is no distinct visual stimulus that can serve as a target. In the real visual world, suddenly appearing objects are of modest visual extent, such as a car appearing from out of a hidden driveway, or an animal from behind a bush. Second, there may be a predefined saccade goal, as in the present study, as well as in previous studies (Reingold and Stampe 2002; Sheliga et al. 2002; Walker et al. 1997). In contrast, in the real world there is generally no explicit, predefined saccade command. Third, distractors in saccadic inhibition tasks may not be highly salient, such as when they are presented within a cluttered visual field of text (Reingold and Stampe 2003). In such a case, their appearance may be sufficient to inhibit a saccade, but their level of salience may be insufficient to trigger a saccade to themselves. Finally, the distractor may appear too close to fixation to effectively elicit a saccade (Graupner et al. 2007; Pannasch et al. 2001; Sheliga et al. 2002). Given these contrasts between laboratory scenarios and visual ecology, it is not clear whether saccade inhibition is a commonplace occurrence during natural oculomotor behavior.
Neurophysiology of saccade inhibition
Regardless of its natural frequency, the phenomenon of saccadic inhibition can serve as a useful tool for increasing our understanding of the neural mechanics of oculomotor control (Reingold and Stampe 2002). Our results provide a human behavioral counterpart to the recent neurophysiological study of Dorris et al. (2007) in macaque superior colliculus (SC), which showed that the appearance of a remote distractor far away from a saccade goal can cause a transient decrease in low-frequency activity in the deeper layers of the SC, whereas distractors appearing near the saccade goal increased activity, often resulting immediately in a saccade. A similar pause in collicular activity was found during a cued saccade task when a visual stimulus located at fixation changed shape (Li et al. 2006). A simple explanation of our findings and these physiological results is that the transient burst of visual activity temporarily inhibits neurons whose response fields are remote from the distractor. The duration of the high-frequency transient visual burst tends to be on the order of tens of milliseconds (Sparks and Hartwich-Young 1989), similar to the notch seen in our histograms of saccade initiation, and pauses in low-frequency activity observed in neurons in the SC (Dorris et al. 2007; Li et al. 2006).
In contrast, recordings in the monkey lateral interparietal area (LIP) indicate that a behaviorally irrelevant distractor results in a contralateral visual transient, but has no inhibitory effect ipsilaterally (Bisley and Goldberg 2006; Powell and Goldberg 2000). This difference between collicular and LIP activity suggests that inhibition operates at the collicular level, as suggested previously (Reingold and Stampe 2002; Walker et al. 1997). Indeed, one might speculate that areas such as LIP, and possibly the frontal eye fields, maintain a voluntary saccade command signal even in the face of a visual distractor, but can execute this command only after the contralateral visual transient has died down.
It is unclear how these inhibitory influences are mediated across saccade motor maps. Although there has been speculation that intracollicular connections could play such a role (Munoz and Istvan 1998), at least for mediating competitions that existed in just one hemifield (and that thus map out to only one colliculus), recent evidence suggests that the kind of long-range inhibition observed here could not be mediated by intrinsic collicular connections and must thus rely on inputs from other, perhaps cortical, areas (Lee and Hall 2006).
We found that the effect of the remote distractor was weaker, although still quite evident, when a visual stimulus was present at the saccade endpoint (experiment 2a), and still weaker when the goal-directed saccade was itself reflexive (experiment 2b). This suggests that when the distractor is distant from the saccade goal, the voluntary saccade command and the distractor compete for access to the saccadic system. In our experiment 1, a visual transient competed against the low-frequency activity induced by the command to make a voluntary saccade a blank location. Increased activity at a site on the map corresponding to the saccade goal due to the presence of a visual target (experiment 2a) and further increases due to the actual appearance of a visual stimulus at the saccade goal (experiment 2b) would further bias this competition toward the saccade goal, making the goal-directed saccade increasingly less prone to competition from the distractor. This is in accordance with the findings of Reingold and Stampe (2002) who showed that saccade inhibition was stronger when the goal-directed movement was an antisaccade, in which no visual stimulus is present at the saccade endpoint, than when the goal-directed movement was a prosaccade and also stronger in an overlap task than in a gap task.
When a visual target was present at the saccade goal, we found less of a facilitatory effect for distractors appearing at a small (22.5°) angular distance from the saccade goal than when no target was present at the saccade goal. This effect could be the result of inhibition of activity at this location by visually related activity present at the saccade goal. Such flanking inhibition has been demonstrated in the frontal eye fields (Schall and Hanes 1993) and the SC (Li and Basso 2005).
It has been long known that only lesions of both the SC and frontal eye fields can completely eliminate saccades in monkey, whereas lesions of either structure alone do not cause a permanent loss in the ability to make saccades (Schiller et al. 1980). However, lesions of the SC, but not the frontal eye fields, wipe out express saccades—the shortest-latency saccades (Schiller et al. 1987). This finding has led to much speculation that short-latency saccades are generated by the SC, whereas longer-latency saccades are generated by the frontal eye fields and perhaps other cortical structures, although the pathway by which saccade-related signals reach the brain stem saccade generator without passing through the SC remains uncertain (Stanton et al. 1988). If this were true then one could think that a strong motivation to make a voluntary saccade should not be affected by an irrelevant remote distractor. If, as has been suggested, the distractor effects that we and others have found are mediated by the SC (Dorris et al. 2007; Reingold and Stampe 2002; Walker et al. 1997), then our findings suggest that the SC interferes with the production of voluntary saccades and thus, at least in the intact human brain, the SC lies in the final common pathway for the generation of saccadic eye movements, whether reflexive or voluntary.
GRANTS
This work was supported by National Institute of General Medical Sciences Support for Continuous Research Excellence Grant GM-00816-28 and National Institutes of Health/National Center for Research Resources Research Centers in Minority Institutions Grant 5G12-Rr03060.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Becker and Jürgens 1979.Becker W, Jürgens R. An analysis of the saccadic system by means of double step stimuli. Vision Res 19: 967–983, 1979. [DOI] [PubMed] [Google Scholar]
- Bisley and Goldberg 2006.Bisley JW, Goldberg ME. Neural correlates of attention and distractibility in the lateral intraparietal area. J Neurophysiol 95: 1696–1717, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce and Goldberg 1985.Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol 53: 603–635, 1985. [DOI] [PubMed] [Google Scholar]
- Corneil and Munoz 1996.Corneil BD, Munoz DP. The influence of auditory and visual distractors on human orienting gaze shifts. J Neurosci 16: 8193–8207, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris et al. 2007.Dorris MC, Olivier E, Munoz DP. Competitive integration of visual and preparatory signals in the superior colliculus during saccadic programming. J Neurosci 27: 5053–5062, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle and Walker 2001.Doyle M, Walker R. Curved saccade trajectories: voluntary and reflexive saccades curve away from irrelevant distractors. Exp Brain Res 139: 333–344, 2001. [DOI] [PubMed] [Google Scholar]
- Edelman and Goldberg 2001.Edelman JA, Goldberg ME. Dependence of saccade-related activity in the primate superior colliculus on visual target presence. J Neurophysiol 86: 676–691, 2001. [DOI] [PubMed] [Google Scholar]
- Edelman and Goldberg 2003.Edelman JA, Goldberg ME. Saccade-related activity in the primate superior colliculus depends on the presence of local landmarks at the saccade endpoint. J Neurophysiol 90: 1728–1736, 2003. [DOI] [PubMed] [Google Scholar]
- Edelman and Keller 1996.Edelman JA, Keller EL. Activity of visuomotor burst neurons in the superior colliculus accompanying express saccades. J Neurophysiol 76: 908–926, 1996. [DOI] [PubMed] [Google Scholar]
- Edelman et al. 2007.Edelman JA, Kristjánsson Á, Nakayama K. The influence of object-relative visuomotor set on express saccades. J Vis 7: 1–132, 2007. [DOI] [PubMed] [Google Scholar]
- Edelman et al. 2006.Edelman JA, Valenzuela N, Barton JJ. Antisaccade velocity, but not latency, results from a lack of saccade visual guidance. Vision Res 46: 1411–1421, 2006. [DOI] [PubMed] [Google Scholar]
- Egeth and Yantis 1997.Egeth HE, Yantis S. Visual attention: control, representation, and time course. Annu Rev Psychol 48: 269–297, 1997. [DOI] [PubMed] [Google Scholar]
- Everling et al. 1999.Everling S, Dorris MC, Klein RM, Munoz DP. Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. J Neurosci 19: 2740–2754, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer and Boch 1981.Fischer B, Boch R. Enhanced activation of neurons in prelunate cortex before visually guided saccades of trained rhesus monkeys. Exp Brain Res 44: 129–137, 1981. [DOI] [PubMed] [Google Scholar]
- Fischer and Ramsperger 1984.Fischer B, Ramsperger E. Human express-saccades: extremely short reaction times of goal directed eye movements. Exp Brain Res 57: 191–195, 1984. [DOI] [PubMed] [Google Scholar]
- Gnadt et al. 1991.Gnadt JW, Bracewell RM, Andersen RA. Sensorimotor transformation during eye movements to remembered visual targets. Vision Res 31: 693–715, 1991. [DOI] [PubMed] [Google Scholar]
- Goldring and Fischer 1997.Goldring J, Fischer B. Reaction times of vertical prosaccades and antisaccades in gap and overlap tasks. Exp Brain Res 113: 88–103, 1997. [DOI] [PubMed] [Google Scholar]
- Graupner et al. 2007.Graupner ST, Velichkovsky BM, Pannasch S, Marx J. Surprise, surprise: two distinct components in the visually evoked distractor effect. Psychophysiology 44: 251–261, 2007. [DOI] [PubMed] [Google Scholar]
- Keller 1977.Keller EL Control of saccadic eye movements by midline brainstem neurons. In: Control of Gaze by Brain Stem Neurons, edited by Baker R, Berthoz A. Amsterdam: Elsevier, 1977, p. 327–336.
- Keller and Edelman 1994.Keller EL, Edelman JA. Use of interrupted saccade paradigm to study spatial and temporal dynamics of saccadic burst cells in superior colliculus in monkey. J Neurophysiol 72: 2754–2770, 1994. [DOI] [PubMed] [Google Scholar]
- King and Fuchs 1977.King WM, Fuchs AF. Neuronal activity in the mesencephalon related to vertical eye movements. In: Control of Gaze by Brain Stem Neurons, edited by Baker R, Berthoz A. Amsterdam: Elsevier, 1977, p. 319–326.
- Lee and Hall 2006.Lee P, Hall WC. An in vitro study of horizontal connections in the intermediate layer of the superior colliculus. J Neurosci 26: 4763–4768, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévy-Schoen 1969.Lévy-Schoen A Détermination et latence de la réponse oculomotrice à deux stimuli. Ann Psychol 69: 373–392, 1969. [Google Scholar]
- Li and Basso 2005.Li X, Basso MA. Competitive stimulus interactions within single response fields of superior colliculus neurons. J Neurosci 25: 11357–11373, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. 2006.Li X, Kim B, Basso MA. Transient pauses in delay-period activity of superior colliculus neurons. J Neurophysiol 95: 2252–2264, 2006. [DOI] [PubMed] [Google Scholar]
- McPeek 2006.McPeek RM Incomplete suppression of distractor-related activity in the frontal eye field results in curved saccades. J Neurophysiol 96: 2699–2711, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPeek and Keller 2002.McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol 88: 2019–2034, 2002. [DOI] [PubMed] [Google Scholar]
- McPeek et al. 2000.McPeek RM, Skavenski AA, Nakayama K. Concurrent processing of saccades in visual search. Vision Res 40: 2499–2516, 2000. [DOI] [PubMed] [Google Scholar]
- Munoz and Istvan 1998.Munoz DP, Istvan PJ. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J Neurophysiol 79: 1193–1209, 1998. [DOI] [PubMed] [Google Scholar]
- Munoz et al. 1996.Munoz DP, Waitzman DM, Wurtz RH. Activity of neurons in monkey superior colliculus during interrupted saccades. J Neurophysiol 75: 2562–2580, 1996. [DOI] [PubMed] [Google Scholar]
- Ohno et al. 2000.Ohno K, Matsuzaki H, Yamada T, Yoshida H, Shimizu K. The difference in saccadic parameters among several visually guided tasks (Abstract). Jpn J Ophthalmol 44: 695, 2000. [DOI] [PubMed] [Google Scholar]
- Pannasch et al. 2001.Pannasch S, Dornhoefer SM, Unema PJ, Velichkovsky BM. The omnipresent prolongation of visual fixations: saccades are inhibited by changes in situation and in subject's activity. Vision Res 41: 3345–3351, 2001. [DOI] [PubMed] [Google Scholar]
- Port and Wurtz 2003.Port NL, Wurtz RH. Sequential activity of simultaneously recorded neurons in the superior colliculus during curved saccades. J Neurophysiol 90: 1887–1903, 2003. [DOI] [PubMed] [Google Scholar]
- Powell and Goldberg 2000.Powell KD, Goldberg ME. Response of neurons in the lateral intraparietal area to a distractor flashed during the delay period of a memory-guided saccade. J Neurophysiol 84: 301–310, 2000. [DOI] [PubMed] [Google Scholar]
- Reingold and Stampe 1999.Reingold EM, Stampe DM. Saccadic inhibition in complex visual tasks. In: Current Oculomotor Research: Physiological and Psychological Aspects, edited by Becker W, Deubel H, Mergner T. New York: Plenum Press, 1999, p. 249–255.
- Reingold and Stampe 2000.Reingold EM, Stampe DM. Saccadic inhibition and gaze contingent research paradigms. In: Reading as a Perceptual Process, edited by Kennedy A, Radach R, Heller D, Pynte J. Amsterdam: Elsevier, 2000, p. 119–145.
- Reingold and Stampe 2002.Reingold EM, Stampe DM. Saccadic inhibition in voluntary and reflexive saccades. J Cogn Neurosci 14: 371–388, 2002. [DOI] [PubMed] [Google Scholar]
- Reingold and Stampe 2003.Reingold EM, Stampe DM. Using the saccadic inhibition paradigm to investigate saccadic control in reading. In: The Mind's Eye: Cognitive and Applied Aspects of Eye Movement Research, edited by Radach R, Hyona J, Deubel H. Amsterdam: Elsevier Science, 2003, p. 347–360.
- Reingold and Stampe 2004.Reingold EM, Stampe DM. Saccadic inhibition in reading. J Exp Psychol Hum Percept Perform 30: 194–211, 2004. [DOI] [PubMed] [Google Scholar]
- Richmond and Optican 1987.Richmond BJ, Optican LM. Temporal encoding of two-dimensional patterns by single units in primate inferior temporal cortex. II. Quantification of response waveform. J Neurophysiol 57: 147–161, 1987. [DOI] [PubMed] [Google Scholar]
- Saslow 1967.Saslow MG Latency for saccadic eye movement. J Opt Soc Am 57: 1030–1033, 1967. [DOI] [PubMed] [Google Scholar]
- Schall and Hanes 1993.Schall JD, Hanes DP. Neural basis of saccade target selection in frontal eye field during visual search. Nature 366: 467–469, 1993. [DOI] [PubMed] [Google Scholar]
- Schiller et al. 1987.Schiller PH, Sandell JH, Maunsell JHR. The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J Neurophysiol 57: 1033–1049, 1987. [DOI] [PubMed] [Google Scholar]
- Schiller et al. 1980.Schiller PH, True SD, Conway JL. Deficits in eye movements following frontal eye field and superior colliculus ablations. J Neurophysiol 44: 1175–1189, 1980. [DOI] [PubMed] [Google Scholar]
- Sheliga et al. 2002.Sheliga BM, Brown VJ, Miles FA. Voluntary saccadic eye movements in humans studied with a double-cue paradigm. Vision Res 42: 1897–1915, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit and Van Gisbergen 1989.Smit AC, Van Gisbergen JA. A short-latency transition in saccade dynamics during square-wave tracking and its significance for the differentiation of visually-guided and predictive saccades. Exp Brain Res 76: 64–74, 1989. [DOI] [PubMed] [Google Scholar]
- Smit et al. 1987.Smit AC, Van Gisbergen JA, Cools AR. A parametric analysis of human saccades in different experimental paradigms. Vision Res 27: 1745–1762, 1987. [DOI] [PubMed] [Google Scholar]
- Smit and Van Gisbergen 1990.Smit AC, Van Gisbergen JAM. An analysis of curvature in fast and slow human saccades. Exp Brain Res 81: 335–345, 1990. [DOI] [PubMed] [Google Scholar]
- Sommer and Tehovnik 1999.Sommer MA, Tehovnik EJ. Reversible inactivation of macaque dorsomedial frontal cortex: effects on saccades and fixations. Exp Brain Res 124: 429–446, 1999. [DOI] [PubMed] [Google Scholar]
- Sparks and Hartwich-Young 1989.Sparks DL, Hartwich-Young R. The deep layers of the superior colliculus. In: The Neurobiology of Saccadic Eye Movements: Reviews of Oculomotor Research, edited by Wurtz RH, Goldberg ME. Amsterdam: Elsevier, 1989, vol. III, p. 213–256. [PubMed] [Google Scholar]
- Stampe and Reingold 2002.Stampe DM, Reingold EM. Influence of stimulus characteristics on the latency of saccadic inhibition. In: Progress in Brain Research, edited by Hyona J, Munoz DP, Heide W, Radach R. Amsterdam: Elsevier Science, 2002, p. 73–87. [DOI] [PubMed]
- Stanton et al. 1988.Stanton GB, Bruce CJ, Goldberg ME. Frontal eye field efferents in the macaque monkey. I. Subcortical pathways and topography of striatal and thalamic terminal fields. J Comp Neurol 271: 473–492, 1988. [DOI] [PubMed] [Google Scholar]
- Theeuwes et al. 2003.Theeuwes J, De Vries GJ, Godijn R. Attentional and oculomotor capture with static singletons. Percept Psychophys 65: 735–746, 2003. [DOI] [PubMed] [Google Scholar]
- Theeuwes et al. 1999.Theeuwes J, Kramer AF, Hahn S, Irwin DE, Zelinsky GJ. Influence of attentional capture on oculomotor control. J Exp Psychol Hum Percept Perform 25: 1595–1608, 1999. [DOI] [PubMed] [Google Scholar]
- Van Gelder et al. 1997.Van Gelder P, Lebedev S, Tsui WH. Peak velocities of visually and nonvisually guided saccades in smooth-pursuit and saccadic tasks. Exp Brain Res 116: 201–215, 1997. [DOI] [PubMed] [Google Scholar]
- Walker et al. 1997.Walker R, Deubel H, Schneider WX, Findlay JM. Effect of remote distractors on saccade programming: evidence for an extended fixation zone. J Neurophysiol 78: 1108–1119, 1997. [DOI] [PubMed] [Google Scholar]
- Walker et al. 1995.Walker R, Kentridge RW, Findlay JM. Independent contributions of the orienting of attention, fixation offset and bilateral stimulation on human saccadic latencies. Exp Brain Res 103: 294–310, 1995. [DOI] [PubMed] [Google Scholar]
- Yantis and Jonides 1984.Yantis S, Jonides J. Abrupt visual onsets and selective attention: evidence from visual search. J Exp Psychol Hum Percept Perform 10: 601–621, 1984. [DOI] [PubMed] [Google Scholar]
- Yantis and Jonides 1990.Yantis S, Jonides J. Abrupt visual onsets and selective attention: voluntary versus automatic allocation. J Exp Psychol Hum Percept Perform 16: 121–134, 1990. [DOI] [PubMed] [Google Scholar]