Abstract
The mechanism(s) of olfactory transduction in invertebrates remains to be fully understood. In lobster olfactory receptor neurons (ORNs), a nonselective sodium-gated cation (SGC) channel, a presumptive transient receptor potential (TRP)C channel homolog, plays a crucial role in olfactory transduction, at least in part by amplifying the primary transduction current. To better determine the functional role of the channel, it is important to selectively block the channel independently of other elements of the transduction cascade, causing us to search for specific pharmacological blockers of the SGC channel. Given evidence that the Na+/Ca2+ exchange inhibitor, KB-R7943, blocks mammalian TRPC channels, we studied this probe as a potential blocker of the lobster SGC channel. KB-R7943 reversibly blocked the SGC current in both inside- and outside-out patch recordings in a dose- and voltage-dependent manner. KB-R7943 decreased the channel open probability without changing single channel amplitude. KB-R7943 also reversibly and in a dose-dependent manner inhibited both the odorant-evoked discharge of lobster ORNs and the odorant-evoked whole cell current. Our findings strongly imply that KB-R7943 potently blocks the lobster SGC channel and likely does so directly and not through its ability to block the Na+/Ca2+ exchanger.
INTRODUCTION
The transient receptor potential (TRP) superfamily of ion channels includes a growing number of nonselective cation channels in organisms from yeast to mammals (Nilius 2007; Venkatachalam and Montell 2007). TRP channels play a major role in sensory signaling, including light, taste, sound, temperature, and touch stimuli, i.e., most major sensory modalities. A notable exception has been the potential involvement of TRP channels in sensory signaling in olfaction. Recently, however, TRPM5 has been suggested to play a functional role in mammalian olfactory receptor neurons (ORNs) (Lin et al. 2007, 2008). Based on its biophysical and pharmacological properties, the lobster olfactory nonselective sodium-gated cation (SGC) channel (McClintock and Ache 1990; Zhainazarov and Ache 1997; Bobkov and Ache 2005) also seems to be a TRP channel involved in olfactory transduction, with potential homology to the mammalian canonical TRP (TRPC) family (Urban et al. 2005). The lobster SGC channel has the interesting property of being both activated by and permeant to Na+ and Ca2+, allowing it to contribute to the generation of a substantial part of the depolarizing receptor potential through regenerative activation (Bobkov and Ache 2003; Zhainazarov et al. 1998). As is true for many TRP channels (Hardie 2003), the lobster SGC channel is the downstream target of phosphoinositide signaling (Zhainazarov et al. 2001). To better determine the role of this and potentially other TRP channels in olfactory transduction, and to understand the mechanism(s) through which phosphoinositides target TRP channels involved in sensory transduction, we sought to specifically block the channel independently of other elements of the transduction cascade (Bobkov and Ache 2005).
Since its introduction as the first specific Na+/Ca2+ exchanger (NCX) inhibitor (Iwamoto et al. 1996), and its emergence as a leading member of a class of synthetic NCX oriented drugs, KB-R7943 has been widely used to study the physiological and/or pathophysiological roles of the NCX exchanger at both the cellular and organismal levels of organization (IC50 = 1–30 μM for NCX; Amran et al. 2003). As research on KB-R7943 expanded, so did the understanding of its action, showing that the drug also blocks transporters (Pintado et al. 2000; Santo-Domingo et al. 2007) as well as voltage- and ligand-gated cation channels (Birinyi et al. 2005; Ouardouz et al. 2005; Sobolevsky and Khodorov 1999), including canonical TRPC channels (Kraft 2007). Given that the functional properties of the lobster SGC channel are consistent with TRPC subfamily assignment (Urban et al. 2005), together with the channel's Na+ sensitivity, we tested the possibility that KB-R7943 could specifically block the lobster SGC channel. Given that Na+ entry and modulation of Na+/Ca2+ exchange has been proposed to be a key mechanism in TRPC signaling (Eder et al. 2005), we also considered whether action of KB-R7943 on the lobster SGC channel would be direct or mediated via an NCX.
We show that KB-R7943 is a potent inhibitor of the lobster SGC channel, that the drug inhibits the odorant-evoked response of individual ORNs in situ in a manner consistent with the blocking action of the drug on the channel itself, and that the drug affects the channel directly and not via action on an NCX. Our findings extend the pharmacological profile of the lobster SGC channel and more further implicate the channel in lobster olfactory transduction.
METHODS
Preparations
The olfactory organ, the lateral filament of the antennule, was excised from the spiny lobster, Panulirus argus, and used for two different preparations. Single-channel inside-out and outside-out recordings were performed on excised patch from cell bodies of cultured ORNs. Clusters of ORN somata were scraped from the lumen of the organ, bathed in Ca2+- and Mg2+-free Panulirus saline (PS; see Solutions) with trypsin (1 mg/ml; Sigma) for 15 min, and mechanically dissociated by trituration in PS. Dispersed cells were plated on 35-mm glass bottom petri dishes filled with PS supplemented with gentamicin solution (4 μl/ml; Sigma). The cultured ORNs were kept at 21°C and used for cell-free patch-clamp recording ≥6 h after dissociation.
In situ recordings were performed on individual sections (annuli) of the olfactory organ prepared for patch-clamp recording. The somata of the ORNs were cleaned by bathing the annuli in Ca2+- and Mg2+-free PS containing trypsin (1 mg/ml; Sigma) and then rinsing with PS. Most of the cuticle not supporting the olfactory sensilla (aesthetascs) was removed to provide access to the ORNs, and the resulting hemi-annulus was mounted in the bottom of a 35-mm petri dish filled with PS. The hair-like aesthetascs containing the outer dendrites of the ORNs were superfused with either PS, PS + drug of interest, or PS + odorant. The somata of the ORNs were continuously superfused with PS to restrict the application of the drug and/or the odorant to the hairs.
Electrophysiological recording and analysis
Patch electrodes for single-channel, extracellular (spikes) and whole cell recordings were pulled from borosilicate capillary glass (BF150-86-10, Sutter Instruments) using a Flaming-Brown micropipette puller (P-87, Sutter Instruments). All currents were recorded using an Axopatch 200A or 200B amplifiers controlled by a Digidata 1322A and pClamp 9.2 and data sampled at 5 kHz. Unitary currents were recorded in inside-out or outside-out configuration. To obtain current-voltage characteristics, in inside-out experiments, series of 50-ms steps at −100 mV followed by a 150- to 1,000-ms voltage ramp from −100 to +100 mV were applied from a holding potential of −60 to −70 mV. In outside-out experiments, series of a 15-ms step at −120 mV followed by a 150-ms voltage ramp from −120 to +100 mV were applied from a holding potential of −60 to −70 mV. Action potentials (APs, spikes) were recorded in track mode using cell-attached loose-patch configuration as described previously (Bobkov and Ache 2005). Whole cell currents were recorded in voltage clamp mode at a holding potential of −70 mV. Bath solution change for single-channel recordings or odor delivery for in situ recordings were performed with a stepper motor (Haydon Switch and Instruments, Waterbury, CT) controlled by a fast-step SF-77B perfusion system (Warner Instruments, Hamden, CT) and pClamp 9.2 software (Molecular Devices) or rapid solution changer, RSC-160 (Bio-Logic–Science Instruments, Claix, France). Experiments were carried out at ∼21°C.
Data were analyzed with Clampfit 9.0 (Molecular Devices) in combination with SigmaPlot 10.0 (SPSS, Chicago, IL). All-points current amplitude histograms were generated to estimate the SGC current amplitude in different conditions. The amplitude of AP discharge frequency was taken as the mean spike frequency within the first 2 s after the beginning of the stimulation and normalized to the maximal response frequency in control conditions. After low-pass filtering, amplitudes of the odorant-evoked whole cell currents were measured and normalized to that before application of KB-R7943. Two modifications of the Hill equation were used to fit the experimental data: 1) F(x) = Fmax × xh/(x1/2h + xh) for activation and 2) F(x) = 1 − Fmax × xh/(x1/2h + xh) for inhibition, where F is the normalized current or frequency of APs, x is the odor or KB-R7943 concentration, x1/2 is the half-maximum odor/KB-R7943 concentration, and h is the Hill coefficient. An additional parameter reflecting the basal level of F (Fb) was incorporated when necessary. All results are expressed as means ± SE of n observations.
Solutions and chemicals
For single-channel experiments, three solutions were used: the Na+-based solution (in mM; 210 NaCl, 1 EGTA, 0.1 CaCl2, 696 glucose, and 10 HEPES); the high-Ca2+/Na+-based solution (in mM; 210 NaCl, 0.1 CaCl2, 696 glucose, and 10 HEPES); and the Li+-based solution (in mM; 210 LiCl, 1 EGTA, 0.1 CaCl2, 696 glucose, and 10 HEPES). The pH of solutions was adjusted with NaOH or Trizma base (Sigma) to 7.9. The free Ca2+ concentration was <10 nM for the Li+-based and Na+-based solutions as calculated with WebmaxC v.2.20 (http://www.stanford.edu/∼cpatton/webmaxcS.htm). To characterize the effects of KB-R7943 on the SGC channel, the pipette was filled with Na+-based solution corresponding to the extracellular medium for inside-out experiments and cytoplasmic medium for outside-out experiments, and the bath consisted of high-Ca2+/Na+-based solution corresponding to the cytoplasmic medium for inside-out experiments and extracellular medium for outside-out experiments. Solutions contained different calcium concentrations are specified in the text.
For APs and whole cell in situ recordings, PS containing (in mM) 486 NaCl, 5 KCl, 13.6 CaCl2, 9.8 MgCl2, and 10 HEPES was used to bath the ORNs. PS also fill the pipette electrode for APs recordings. The whole cell pipette solution contained (in mM) 210 KCl, 0.1 CaCl2, 0–1 EGTA, 570 glucose, and 10 HEPES. The free Ca2+ concentration of the pipette solution was from <10 nM to 100 μM as calculated with WebmaxC v.2.20. At these concentrations of Ca2+ and in the absence of Na+ ions, no current is generated in absence of odorant stimulation. The pH of solutions was adjusted with NaOH or Trizma base (Sigma) to 7.7–7.9.
The odorant consisted of an aqueous extract of a commercial marine aquarium food, TetraMarine (TET; Tetra Werke, Melle, Germany). Before each experiment, aliquots of stock solution were diluted in PS to a final concentration of 0.5 mg/ml.
KB-R7943, 2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea mesylate, and SN-6 (2-[[4-[(4-nitrophenyl)methoxy]phenyl]methyl]-4-thiazolidinecarboxylic acid ethyl ester (Tocris Biosciences) were prepared as stock solution of 100 and 50 mM, respectively, in dimethylsulfoxide (DMSO) and diluted either in PS or solutions used for single-channel recordings. The final DMSO concentration was always at or <0.1%, a concentration without any effect on the lobster SGC channel. The acidic dissociation constant (pKa) value for the isothiourea group of KB-R7943 is ∼10 (Iwamoto et al. 1996). Thus under our experimental conditions (pH 7.9), KB-R7943 was almost completely protonated and carried the charge of +1, which could potentially explain the utility of the drug in our system. Verapamil was prepared as stock solution of 100 mM in water before its dissolution in PS.
RESULTS
KB-R7943 blocks the lobster SGC channel from the intracellular side
The SGC channel is expressed in lobster ORNs in both a Ca2+-sensitive and a Ca2+-insensitive form (Bobkov and Ache 2003). Because of the predominance of the Ca2+-sensitive form in the outer dendrites (transduction zone), we focused on the Ca2+-sensitive form of the channel. The first series of experiments were performed in inside-out patch recording on excised patches taken from the soma of cultured ORNs. In these experiments, a solution rich in Na+ filled the pipette (extracellular medium). In absence of Na+ and Ca2+ ions (Li+-based solution) on the cytoplasmic side of the membrane, the SGC channel was almost totally inactive. To activate the channel, the bath was switched from Li+-based solution to Na+-based solution followed by high-Ca2+/Na+-based bath solution to determine the Ca2+-sensitive form of the SGC channel. In this last condition referred also to control in the text, the concentration of Na+ was symmetrical across the membrane. The only difference between the extracellular and intracellular mediums was the Ca2+ concentration. Figure 1 A shows a representative recording of the Ca2+-sensitive form of the SGC channel. In this example, five SGC channels could be seen in predominantly open state when the solution was switched to the control high-Ca2+/Na+-based bath condition at −60 mV. KB-R7943 applied to the intracellular side of the membrane patch produced a reversible blockage of the SGC current in a voltage-dependent manner (Fig. 1, B). At −60 mV, even a high concentration of the drug (50–100 μM) applied for 15 s failed to block the SGC current (Fig. 1B, top). In contrast, holding the same patch at +60 mV application of 50 μM of KB-R7943 rapidly and totally blocked the SGC current (Fig. 1B, middle). The drug decreased the channel open probability but not the single-channel amplitude as seen at higher time resolution and according to parameters obtained from Gaussian distribution approximation of all-points current amplitude histograms in Fig. 1B (bottom). The value of the unitary amplitude estimated at +75 mV was 12.20 ± 0.21 pA (n = 30) in control high-Ca2+/Na+ solution and 12.02 ± 0.14 pA in the presence of 50 μM of KB-R7943 (n = 43). The channel activity typically recovered within <20 s (Fig. 1B, middle).
FIG. 1.
KB-R7943 blocks the lobster sodium-gated cation (SGC) channel recorded in inside-out configuration. A: representative inside-out single-channel recordings from a patch taken from the soma of a cultured lobster olfactory receptor neuron (ORN) showing the activation of Ca2+-sensitive SGC channels. The patch contained 5 SGC channels. The pipette was filled with Na+-based solution. In Li+-based bath solution, the SGC channel was almost totally inactive (Po ∼0.03). To activate the channel, the bath was switched to Na+-based solution. B: inside-out recording from a different patch than A containing 7 SGC channels simultaneously opened in high Ca2+/Na+ bath condition (referred as control in the figures) at −60 or +60 mV. At +60 mV, the SGC current activity was inhibited by KB-R7943 but not at −60 mV. Current traces in higher time resolution show single-channel currents in presence of 50 μM of KB-R7943. The amplitude histogram corresponding to currents recorded in control and in the presence of KB-R7943 (50 μM) provided on the bottom of the panel indicates a decrease in channel activity but not in single channel current amplitude (12.20 ± 0.21 pA, n = 30 in control solution and 12.02 ± 0.14 pA, n = 46 in presence of KB-R7943). The inhibition was reversible with a time constant close to 5 s for this patch.
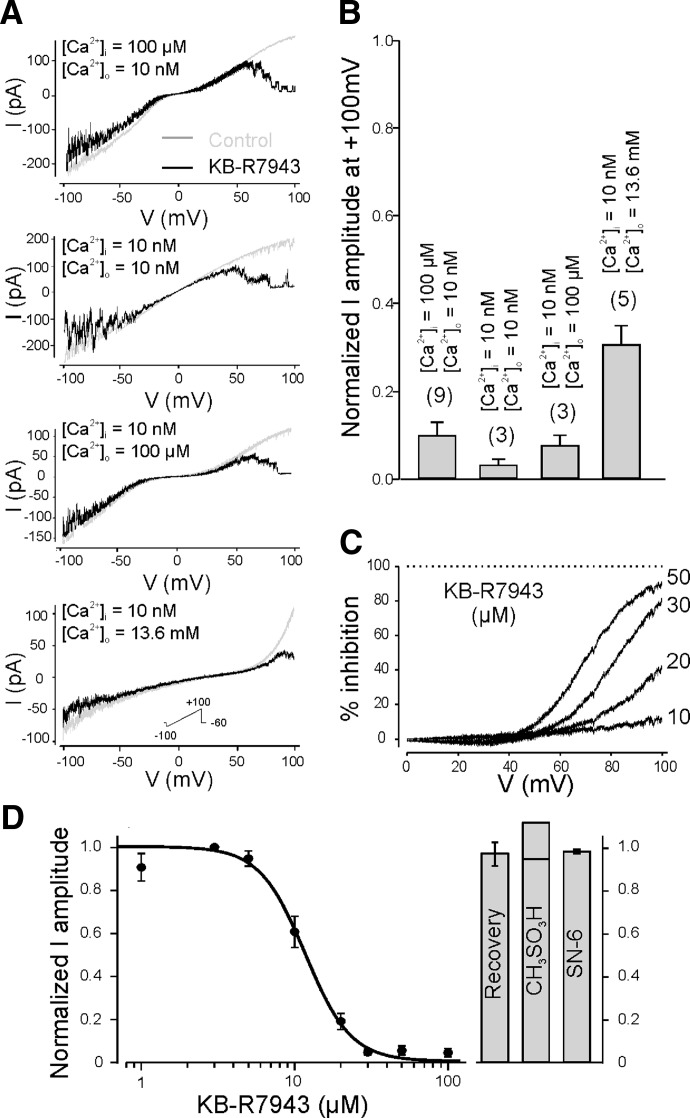
To better characterize the voltage sensitivity of the drug blockage of the SGC current, we applied ramp protocols (linear change in membrane voltage) to membrane patches from a holding potential of −60 mV (Fig. 2 A). In control high-Ca2+/Na+ bath solution, the current-voltage (I-V) relationship of the SGC current generated by ramp protocols had a characteristic double rectification in the presence of divalent cations (in given case 100 μM intracellular Ca2+) with a reversal potential close to 0 mV (Fig. 2A, gray trace, top graph). Application of 50 μM KB-R7943 on the intracellular side of the patch mainly blocked the outward current component at voltages higher than +40 mV confirming that the channel blockage was voltage dependent (Fig. 2A, black trace, top graph).
FIG. 2.
KB-R7943 blocks the lobster SGC channel in a dose- and voltage-dependent manner. A: current-voltage (I-V) relationships determined from different patches recorded in control condition (gray trace) and in presence of 50 μM KB-R7943 (black trace) in different conditions of Ca2+ concentrations across the membrane. To generate I-V relationships, series of ramp protocols from −100 to +100 mV were applied. Whatever the internal and external Ca2+ concentrations, KB-R7943 appeared to strongly block the SGC current only at positive voltages. Slight reduction of the current seen at negative voltages in the presence of the drug seems to result from a short interval between the ramps and incomplete recovery of the channel activity after preceding inhibition. Note, when pipette was filled with PS the reversal potential of SGC current shifted by approximately +35 mV; corresponding corrections need to be done to directly compare voltage dependence of SGC channel conductance in different ion conditions. B: histogram of the averaged amplitude of the SGC current in the presence of KB-R7943 normalized to the SGC current in control at +100 mV from several patches in the same internal and external Ca2+ concentrations as in A. The blockage of the SGC current at positive voltages by KB-R7943 was strongly present in all Ca2+ conditions across the membrane tested. C: concentration dependence and voltage dependence of the blocking effect of KB-R7943 on the SGC channel. The percent inhibition of the averaged I-V curves for different concentrations of KB-R7943 was plotted as a function of the positive voltages. The percent inhibition was calculated as (mean current in control − mean current in presence of KB-R7943)/(current at +100 mV) × 100. D: concentration-inhibition curve for KB-R7943 on the mean amplitude of the SGC current at +100 mV. Averaged currents were normalized to the averaged current in absence of KB-R7943. Data points represent means ± SE of n = 3–11 patches and were fit to a Hill equation providing an IC50 of 11.74 μM and a Hill coefficient 2.92. KB-R7943 blockage was reversible (n = 13, histogram). Mesylate (CH3SO3H, 200 μM) alone did not block the SGC channel (n = 2 both shown on the histogram). SN-6, a potent Na+/Ca2+ exchanger inhibitor, did not block the SGC current.
Given that the inhibitory effect of KB-R7943 on the NCX was shown to be Ca2+ dependent (Iwamoto et al. 1996), we next examined the possible Ca2+ dependence of the blocking effect of KB-R7943 on SGC current. KB-R7943 (50 μM) was applied on the intracellular face after activation of the SGC channel recorded in different combinations of external and internal Ca2+ concentrations chosen among 10 nM, 100 μM, or 13.6 mM. As seen in Fig. 2A, Ca2+ did not seem to be an important factor of KB-R7943–mediated inhibition of the SGC channel. With PS outside, the increase in Ca2+/divalent cations concentration reduced the single SGC channel amplitude, shifted the reversal potential from 0 mV (Fig. 2A, top graphs) to ∼+35 mV (Fig. 2A, bottom graph) and changed the voltage dependence of the SGC currents. However, the pattern of KB-R7943–mediated inhibition is still similar at all Ca2+ concentrations tested (cf. I-Vs in Fig. 2A). Overall, saturating concentration of KB-R7943 inhibited the SGC channel activity by 93.11 ± 1.98% ([Ca2+]i/[Ca2+]o = 100 μM/10 nM, 10 nM/10 nM, and 10 nM/100 μM) and by 69.38 ± 4.37% when PS was an extracellular solution ([Ca2+]i/[Ca2+]o = 10 nM/13.6 mM; Fig. 2B).
KB-R7943 blocked the SGC channel in a dose-dependent manner (Fig. 2, C and D). Figure 2C shows the inhibition of the SGC channel activity recorded from the same patch at increasing concentrations of KB-R7943 within positive voltage range. For this example, 85.8 ± 1.8% of the SGC channel activity was inhibited at +100 mV with 50 μM KB-R7943 in high-Ca2+/Na+ bath solution ([Ca2+]i/[Ca2+]o = 100 μM/10 nM). A concentration-inhibition curve of KB-R7943 (Fig. 2D) was generated with eight concentrations of the drug at +100 mV. Three to 11 patches were tested for each KB-R7943 concentration. Fitting of the curve with the Hill equation yielded a half-maximal inhibitory concentration (IC50) of 11.74 μM and a corresponding Hill coefficient of 2.92. Because KB-R7943 is complexed with mesylate (CH3SO3H), we had to test that the inhibition we observed was caused by KB-R7943 and not mesylate. Mesylate alone, even at high concentration (200 μM, n = 2; Fig. 2D, histogram), did not block the SGC current. Additionally, SN-6, another blocker with a greater selectivity for the NCX (Niu et al. 2007), tested at the maximum concentration permitted by the solubility properties of the compound in water at pH = 8 [5 μM, calculated using Advanced Chemistry Development (ACD)/Labs; software V8.14 for Solaris), had no effect on the SGC current (n = 7; Fig. 2D, histogram).
KB-R7943 blocks the lobster SGC channel from the extracellular side
The effect of the drug was also studied by recording in the outside-out configuration. In these experiments, a solution rich in Na+ filled the pipette (intracellular medium) to activate the SGC channel. In control conditions, the patch was perfused in high-Ca2+/Na+-based bath solution (extracellular medium). KB-R7943 applied to extracellular side of the membrane patch reversibly blocked the SGC channel in a dose-dependent manner (Fig. 3). Application of 50 μM of KB-R7943 potently inhibited the SGC current at a holding potential of −70 mV (Fig. 3A). The drug decreased the channel open probability without changing single-channel amplitude as seen at higher time resolution and according to amplitude histograms of the top panel of Fig. 3A. The value of the unitary amplitude estimated at −70 mV was 11.20 ± 0.18 pA (n = 12) in control high-Ca2+/Na+ solution and 11.32 ± 0.11 pA (n = 16) in the presence of 50 μM of KB-R7943. To test if the inhibition of the channel was sensitive to membrane potential, we applied ramp protocols to membrane patches (Fig. 3B). In control high-Ca2+/Na+ solution, the I-V relationship of the SGC current generated by ramp protocols had a characteristic double rectification with a reversal potential close to 0 mV. Application of 50 μM KB-R7943 equally blocked both the inward and outward current components, suggesting that the channel blockage was not voltage dependent (Fig. 3B).
FIG. 3.
KB-R7943 reversibly blocks the lobster SGC channel recorded in outside-out configuration. A: outside-out patch recording from a cultured lobster ORN showing the inhibition of the lobster SGC channel activity by KB-R7943 in a concentration-dependent manner. The patch contained ≥11 SGC channels. Holding potential was −70 mV. The white dotted lines indicate the averaged current amplitude for each concentration of KB-R7943 tested for this particular example. The percentage of inhibition of the averaged current by KB-R7943 is shown above the dotted line. Current traces in higher time resolution allow observing single-channel currents in control condition and in presence of 50 μM of KB-R7943 (gray plots). Current traces and corresponding amplitude histogram provided on the right of the panel indicate a decrease in channel activity but not in single channel current amplitude (11.20 ± 0.18 pA, n = 12 in control and 11.32 ± 0.11 pA, n = 16 in presence of KB-R7943). B: I-V relationships of the SGC current recorded before, during, and after application of 50 μM KB-R7943. To generate I-V relationships, series of 150-ms ramp protocols from −120 to +100 mV were applied every 2 s. Two ramp currents are presented in the graph for each condition. The I-V relationships show the reversible blockage of the SGC current by KB-R7943. C: concentration dependence of KB-R7943 effects. Averaged steady-state currents were normalized to the averaged current in absence of KB-R7943 (holding potential: −70 mV). Data points represent means ± SE of n = 7–9 patches and were fit to a Hill equation providing an IC50 of 13.82 μM and a Hill coefficient of 1.32. KB-R7943 blockage was reversible (n = 4; histogram). Mesylate (CH3SO3H, 200 μM) alone did not block the SGC channel (n = 5; histogram).
A concentration-inhibition curve was generated with six concentrations of KB-R7943 using steady-state averaged current at −70 mV (Fig. 3C). Fitting the data with the Hill equation yielded an IC50 of 13.82 μM and a corresponding Hill coefficient of 1.32. The channel activity totally recovered within 5–20 s after drug removal. As a control, mesylate alone even at high concentration (200 μM, n = 5) did not block the SGC channel (Fig. 3C, histogram).
KB-R7943 blocks the odorant-evoked discharge in lobster ORNs in situ
Previous studies identified at least two different patterns of odorant-evoked discharge in lobster ORNs, tonically discharging cells and bursting cells (Bobkov and Ache 2007b). We tested the effect of KB-R7943 on the tonic cells because they represent the predominant ORN subpopulation. Tonically active ORNs gradually increase their ongoing rate of odorant-evoked discharge in response to increasing stimulus intensities (Fig. 4, A and B). KB-R7943 (50 μM) decreased the spontaneous discharge of the cells by 81% in the presence of 50 μM of the drug in 7 of 10 cells, almost completely inhibited the odorant-evoked discharge at low odorant concentrations, and strongly decreased the response to high odorant concentrations, as shown for one cell in Fig. 4, A and B. The inhibition by KB-R7943 could not be reversed for ≥5 min after washout. Importantly, KB-R7943 was not observed to change either shape or spike amplitude (Fig. 4B, insets). This would suggest the drug did not change the activity of ion channels directly involved in AP generation.
FIG. 4.
KB-R7943 reversibly blocks the odorant-evoked discharge of lobster ORNs in a dose-dependent manner. A: representative raster displays of action potentials (APs) from a single lobster ORN recorded in the loose-patch configuration in response to increasing odor stimulus intensity (shortest duration at the top of each block) before (top), during (middle), and after (bottom) application of 50 μM of KB-R7943. B: normalized peak AP discharge frequency of the same ORN as in A plotted as a function of odor stimulus duration before, during, and after application of 50 μM of KB-R7943. Data points were fit to a Hill equation. KB-R7943 reversibly blocked the output of the cell. KB-R7943 did not change either spike shape or the spike peak-to-peak amplitude (212.7 pA in control vs. 215.1 pA in presence of 50 μM KB-R7943, insets). C: representative raster displays of APs from a single lobster ORN in response to identical odor stimulus intensity in control condition (top), during applications of increasing concentrations of KB-R7943 (middle), and wash-out (bottom). KB-R7943 reversibly blocked the output of the cell in a dose-dependent manner. D: concentration dependence of KB-R7943 effects on the odorant-evoked ORN discharge. Data points were fit to a Hill equation providing an IC50 of 16.57 μM and a Hill coefficient of 1.74. Data points represent means ± SE of n = 4–8 cells. SN-6, a potent Na+/Ca2+ exchanger inhibitor, did not block the odorant-evoked AP discharge.
Changing the concentration of the drug altered the response to a fixed odorant intensity in a concentration-dependent manner (Fig. 4, C and D). Increasing the inhibitor concentration decreased the stimulus evoked discharge as shown for one cell in Fig. 4C and collectively for four to eight cells in Fig. 4D. The concentration-dependent inhibition of the discharge by KB-R7943 fit with a Hill equation gave an IC50 of 16.57 μM and a Hill coefficient of 1.74. Inhibition was almost total between 50 and 100 μM KB-R7943. The blockade was relatively slow; KB-R7943 blocked the odorant-evoked discharge in 91% of the cells tested (21 of 23) after 5–10 min. Washout was also relatively slow, with, in some cases, only partial recovery after 5–10 min of washout (Fig. 4A). Additionally, verapamil (100 μM), another NCX blocker (Amran et al. 2003), had no effect on the odorant-evoked discharge tested on four tonically discharging cells (AP discharge frequency in presence of verapamil/AP frequency in control = 1.03 ± 0.05). Finally, SN-6 (5 μM) had no effect on the odorant-evoked discharge tested on three tonically discharging cells (AP discharge frequency in presence of SN-6/AP frequency in control = 1.00 ± 0.07).
KB-R7943 blocks the whole cell current in lobster ORNs in situ
As mentioned earlier, the parameters of spikes (spike positive/negative peak amplitudes and temporal characteristics) did not noticeably change in the presence of KB-R7943. This would suggest that the drug did not affect the activity of ion channels directly involved in AP generation. On the other hand, given the reduction in ORN spontaneous spike discharge frequencies, there is a possibility that KB-R7943 does not actually block the stimulus activated channels but rather affects ion transport systems leading to ORN hyperpolarization significant enough to dramatically decrease odorant evoked ORN activity. To examine the possibility, we studied the effects of the drug on the odorant-evoked ORN responses using the whole cell voltage-clamp recordings (Fig. 5). Typically, the lobster ORNs recorded in voltage-clamp mode generated inward currents in response to stimulus application in a dose-dependent manner (Fig. 5A). As in many other primary chemosensory neurons from a variety of different species, the lobster ORN odorant-evoked currents exhibited rundown when recorded in standard whole cell mode, although the response rundown kinetics was relatively slow. KB-R7943 (50 μM) applied focally to outer dendrites area (transduction zone) of the ORNs did not considerably change resting whole cell currents (Fig. 5B), whereas almost completely and reversibly inhibited the odorant-evoked currents (Fig. 5B). Overall, KB-R7943–dependent inhibition of the normalized peak amplitude of the whole cell current was 69 ± 22% (n = 7; Fig. 5C). These results indicate that the drug-dependent ORN hyperpolarization seems to have little if any effect on odor-evoked ORN discharge and KB-R7943 targeted primarily ion channels involved in the lobster chemosensory transduction cascade.
FIG. 5.
KB-R7943 reversibly blocks the odorant-evoked whole cell current of lobster ORNs. A and B: whole cell recordings from lobster ORNs in situ held at −70 mV and stimulated by odorant pulse. A and B: different ORNs. A: representative odorant-induced whole cell currents in response to increasing stimulus intensity (160- to 800-ms odorant pulse) in control and the corresponding peak current amplitude plotted in function of odorant stimulus. B: representative odorant-induced whole cell currents in response to constant 200-ms odorant pulse recorded before, during, and after application of 50 μM of KB-R7943. C: histograms showing the averaged peaks of whole cell currents measured before, during, and after application of 50 μM of KB-R7943. Averaged peak currents were normalized to the averaged peak current in absence of KB-R7943. Data points represent means ± SE. KB-R7943 greatly reduced the whole cell peak current compared with control (paired t-test, P < 0.001).
DISCUSSION
We showed that KB-R7943 blocks the SGC channel. The lobster SGC channel occurs in both calcium-sensitive and calcium-insensitive forms (Bobkov and Ache 2003). The data we reported were obtained exclusively from the calcium-sensitive form of the channel because that form is predominantly expressed in the outer dendrites (transduction zone) of the ORNs. However, preliminary data suggest that the calcium-insensitive form of SGC channel seems to be equally sensitive to the effects of KB-R7943 (50–100 μM, n = 6). We also showed that KB-R7943 blocks odorant-evoked activity in tonically active ORNs. These cells represent the major subpopulation of ORNs in the lobster olfactory organ (Bobkov and Ache 2007b). However, preliminary data suggest that a subpopulation of rhythmically active ORNs in the olfactory organ are also blocked by KB-R7943 (n = 6), suggesting that the SGC channel occurs and subserves a common transduction and/or amplification mechanism in all ORNs.
Although our results are qualitatively consistent, showing similar voltage dependence and comparable half-maximal concentrations of inhibition across the three electrophysiological approaches (IC50 = 12, 14, and 17 μM in inside-out, outside-out, and in situ recordings, respectively), the Hill coefficients showed considerable variability (h = 2.92, 1.32, and 1.74 in inside-out, outside-out, and in situ recordings, respectively), suggesting different degrees of cooperativity and therefore different numbers of inhibitor molecules (3 and 1, respectively, between inside-out and outside-out recording) are needed to block the channel from the intracellular and or extracellular face. However, this difference could result from the different voltages (−60 vs. +100 mV) and/or the different experimental protocols (gap-free recording vs. ramp) used to collect the data. Adequately interpreting this potentially interesting finding would demand detailed biophysical analysis and be well beyond the scope of this study.
Our finding that KB-R7943 (50 μM) decreased the spontaneous discharge frequency of 7 of 10 tonically active ORNs suggests that a fraction of SGC channels are constitutively active and may contribute to the resting state of the cell, as known to occur for transduction channels in vertebrate ORNs (Kleene 2000; Pun and Kleene 2003). The extent to which the channel contributes to the resting state of the cells, however, presumably would be subject to agonist concentration, e.g., intracellular sodium, intracellular calcium, and phosphoinositides, but that question was not pursued as part of this study.
One of the interesting properties of the lobster SGC channel is its complex interaction with Na+ and Ca2+ ions. Opening of the channel evokes Na+ and Ca2+ influx into the cell that, in turn, potentially can serve as positive or negative (high intracellular Ca2+) feedback for channel gating. It has been proposed that Na+ and Ca2+ transport systems, including nonselective cation channels, must closely communicate with NCXs to integrate Ca2+ signaling in cells and/or control intracellular Na+/Ca2+ homeostasis (Algara-Suarez et al. 2007; Eder et al. 2005, 2007; Rosker et al. 2004). Indeed, using a glutathione S-transferase pull-down assay, Rosker et al. (2004) found that NCX1 associates with the cytosolic C terminus of TRPC3, suggesting such a functional interaction could be mediated by physical interaction between the TRPC channel and NCX proteins. The functional consequences of having an intimate linkage between the TRPC channel and NCX is not completely understood. It is still unclear, for instance, whether the linkage reflects direct interaction or interaction mediated through scaffolding proteins and, related to that, whether pharmacological intervention targeting one element of such a signaling complex could somehow disrupt the structural and/or more importantly the functional integrity of the whole complex. Evidence that KB-R7943 is a potent blocker of TRPC3, TRPC5, and TRPC6 channels (Kraft 2007) suggests that co-assembly with exchangers could be a general property of TRPC channels, possibly including the lobster SGC channel.
However, our data herein and elsewhere strongly suggest the inhibitor acts directly on the SGC channel and not via an NCX. First, of the broad spectrum of drugs known or suspected to modify NCX activity in other systems (Amran et al. 2003; Blaustein and Lederer 1999)—verapamil (IC50 = 0.1–10 μM; Erdreich et al. 1983), amiloride (IC50 = ∼1 mM), benzamil, and 3′,4′-dichloro-benzamil (DCBA) in a variety of animals including crustaceans (IC50 = ∼3–30 μM; Danaceau and Lucero 2000; Niggli and Lederer 1991; Wheatly et al. 2002), and diltiazem in crustaceans per se (Zhuang and Ahearn 1998), none of the ones we tested—verapamil (10–200 μM; see also Zhainazarov et al. 1998), amiloride (2 mM; Bobkov and Ache 2007a), DCBA (400 μM; Bobkov and Ache 2007a), and diltiazem (400 μM, n = 6 in situ and n = 4 in inside-out)—noticeably changed the gating of the SGC channel and/or ORN activity in situ. Second, the inhibitory effect of KB-R7943 on the NCX was shown to be NCX substrate dependent, especially Ca2+ dependent (Iwamoto et al. 1996), suggesting that KB-R7943 may compete with Ca2+ to bind to the exchanger. In our experiments, however, neither changing extracellular (10 nM to 13.6 mM) nor intracellular (10 nM to 100 μM) Ca2+ seemed to change the blocking effect of KB-R7943 (50–100 μM). Third, complete or partial replacement of monovalent ions did not significantly alter the effects of KB-R7943 blockade, excluding the possibility that an NCX is likely involved in mediating SGC channel activity. Finally, preliminary experiments using a newer blocker with greater selectivity for NCX, SN-6 (IC50 = ∼2 μM; Niu et al. 2007), tested at 5 μM had no effect on either the odorant-evoked discharge of the ORNs or on the SGC channel recorded in inside-out patches. We conclude therefore that KB-R7943 acts directly on the channel and not by blocking an odor-induced NCX current.
An NCX has yet to be identified in lobster ORNs, although NCXs have been identified in ORNs of several phylogenetically diverse animals, including Xenopus (Jung et al. 1994), squid (Lucero et al. 2000), and rodents (Noe et al. 1997; Pyrski et al. 2007; Schulze et al. 2002). Because the precise role of the exchanger in ORNs is unclear, however, they may not be expressed in all ORNs. Nor would they necessarily have the same function if they were. Whereas the Na+/Ca2+ exchange is proposed to be responsible for ∼26% of the odor-induced current in squid (Danaceau and Lucero 2000), for example, it is reportedly involved in termination of the receptor current response in frog ORNs by returning the concentration of intracellular Ca2+ to its basal level after odor stimulation (Reisert and Matthews 1998). Thus while we cannot eliminate the possibility that lobster ORNs express an NCX, and indeed they may well, it does not seem that KB-R7943 acts through or in association with it.
In summary, we showed that KB-R7943 is a potent inhibitor of the lobster SGC channel and that it inhibits the odorant-evoked response of individual ORNs in situ in a manner consistent with its action on blocking the SGC channel. These findings extend the pharmacological profile of the lobster SGC channel and more specifically implicate the channel in lobster olfactory transduction. They also raise the possibility that KB-R7943 can be a useful tool to study chemosensory transduction in other systems where TRP channels, at least TRPC channels, have been implicated in creating or shaping the output of the receptor cells.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grant DC-001655.
Acknowledgments
We thank A. Mistretta-Bradley for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Algara-Suarez et al. 2007.Algara-Suarez P, Romero-Mendez C, Chrones T, Sanchez-Armass S, Meza U, Sims SM, Espinosa-Tanguma R. Functional coupling between the Na+/Ca2+ exchanger and nonselective cation channels during histamine stimulation in guinea pig tracheal smooth muscle. Am J Physiol Lung Cell Mol Physiol 293: L191–L198, 2007. [DOI] [PubMed] [Google Scholar]
- Amran et al. 2003.Amran MS, Homma N, Hashimoto K. Pharmacology of KB-R7943: a Na+-Ca2+ exchange inhibitor. Cardiovasc Drug Rev 21: 255–276, 2003. [DOI] [PubMed] [Google Scholar]
- Birinyi et al. 2005.Birinyi P, Acsai K, Banyasz T, Toth A, Horvath B, Virag L, Szentandrassy N, Magyar J, Varro A, Fulop F, Nanasi PP. Effects of SEA0400 and KB-R7943 on Na+/Ca2+ exchange current and L-type Ca2+ current in canine ventricular cardiomyocytes. Naunyn Schmiedebergs Arch Pharmacol 372: 63–70, 2005. [DOI] [PubMed] [Google Scholar]
- Blaustein and Lederer 1999.Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev 79: 763–854, 1999. [DOI] [PubMed] [Google Scholar]
- Bobkov and Ache 2007a.Bobkov YV, Ache BW. Block by amiloride derivatives of odor-evoked discharge in lobster olfactory receptor neurons through action on a presumptive TRP channel. Chem Senses 32: 149–159, 2007a. [DOI] [PubMed] [Google Scholar]
- Bobkov and Ache 2003.Bobkov YV, Ache BW. Calcium sensitivity of a sodium-activated nonselective cation channel in lobster olfactory receptor neurons. J Neurophysiol 90: 2928–2940, 2003. [DOI] [PubMed] [Google Scholar]
- Bobkov and Ache 2007b.Bobkov YV, Ache BW. Intrinsically bursting olfactory receptor neurons. J Neurophysiol 97: 1052–1057, 2007b. [DOI] [PubMed] [Google Scholar]
- Bobkov and Ache 2005.Bobkov YV, Ache BW. Pharmacological properties and functional role of a TRP-related ion channel in lobster olfactory receptor neurons. J Neurophysiol 93: 1372–1380, 2005. [DOI] [PubMed] [Google Scholar]
- Danaceau and Lucero 2000.Danaceau JP, Lucero MT. Electrogenic Na+/Ca2+ exchange. A novel amplification step in squid olfactory transduction. J Gen Physiol 115: 759–768, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo and Beauge 1994.DiPolo R, Beauge L. Cardiac sarcolemmal Na/Ca-inhibiting peptides XIP and FMRF-amide also inhibit Na/Ca exchange in squid axons. Am J Physiol 267: C307–C311, 1994. [DOI] [PubMed] [Google Scholar]
- Eder et al. 2005.Eder P, Poteser M, Romanin C, Groschner K. Na+ entry and modulation of Na+/Ca2+ exchange as a key mechanism of TRPC signaling. Pfluegers 451: 99–104, 2005. [DOI] [PubMed] [Google Scholar]
- Eder et al. 2007.Eder P, Probst D, Rosker C, Poteser M, Wolinski H, Kohlwein SD, Romanin C, Groschner K. Phospholipase C-dependent control of cardiac calcium homeostasis involves a TRPC3-NCX1 signaling complex. Cardiovasc Res 73: 111–119, 2007. [DOI] [PubMed] [Google Scholar]
- Erdreich et al. 1983.Erdreich A, Spanier R, Rahamimoff H. The inhibition of Na-dependent Ca uptake by verapamil in synaptic plasma membrane vesicles. Eur J Pharmacol 90: 193–202, 1983. [DOI] [PubMed] [Google Scholar]
- Hardie 2003.Hardie RC Regulation of TRP channels via lipid second messengers. Annu Rev Physiol 65: 735–759, 2003. [DOI] [PubMed] [Google Scholar]
- Iwamoto et al. 1996.Iwamoto T, Watano T, Shigekawa M. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J Biol Chem 271: 22391–22397, 1996. [DOI] [PubMed] [Google Scholar]
- Jung et al. 1994.Jung A, Lischka FW, Engel J, Schild D. Sodium/calcium exchanger in olfactory receptor neurones of Xenopus laevis. Neuroreport 5: 1741–1744, 1994. [DOI] [PubMed] [Google Scholar]
- Kleene 2000.Kleene SJ Spontaneous gating of olfactory cyclic-nucleotide-gated channels. J Membr Biol 178: 49–54, 2000. [DOI] [PubMed] [Google Scholar]
- Kraft 2007.Kraft R The Na+/Ca2+ exchange inhibitor KB-R7943 potently blocks TRPC channels. Biochem Biophys Res Commun 361: 230–236, 2007. [DOI] [PubMed] [Google Scholar]
- Lin et al. 2008.Lin W, Ezekwe EA, Jr, Zhao Z, Liman ER, Restrepo D. TRPM5-expressing microvillous cells in the main olfactory epithelium. BMC Neurosci 9: 114, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al. 2007.Lin W, Margolskee R, Donnert G, Hell SW, Restrepo D. Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proc Natl Acad Sci USA 104: 2471–2476, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero et al. 2000.Lucero MT, Huang W, Dang T. Immunohistochemical evidence for the Na+/Ca2+ exchanger in squid olfactory neurons. Philos Trans R Soc Lond B Biol Sci 355: 1215–1218, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock and Ache 1990.McClintock TS, Ache BW. Nonselective cation channel activated by patch excision from lobster olfactory receptor neurons. J Membr Biol 113: 115–122, 1990. [DOI] [PubMed] [Google Scholar]
- Niggli and Lederer 1991.Niggli E, Lederer WJ. Molecular operations of the sodium-calcium exchanger revealed by conformation currents. Nature 349: 621–624, 1991. [DOI] [PubMed] [Google Scholar]
- Nilius 2007.Nilius B Transient receptor potential (TRP) cation channels: rewarding unique proteins. Bull Mem Acad R Med Belg 162: 244–253, 2007. [PubMed] [Google Scholar]
- Niu et al. 2007.Niu CF, Watanabe Y, Ono K, Iwamoto T, Yamashita K, Satoh H, Urushida T, Hayashi H, Kimura J. Characterization of SN-6, a novel Na+/Ca2+ exchange inhibitor in guinea pig cardiac ventricular myocytes. Eur J Pharmacol 573: 161–169, 2007. [DOI] [PubMed] [Google Scholar]
- Noe et al. 1997.Noe J, Tareilus E, Boekhoff I, Breer H. Sodium/calcium exchanger in rat olfactory neurons. Neurochem Int 30: 523–531, 1997. [DOI] [PubMed] [Google Scholar]
- Ouardouz et al. 2005.Ouardouz M, Zamponi GW, Barr W, Kiedrowski L, Stys PK. Protection of ischemic rat spinal cord white matter: Dual action of KB-R7943 on Na+/Ca2+ exchange and L-type Ca2+ channels. Neuropharmacol 48: 566–575, 2005. [DOI] [PubMed] [Google Scholar]
- Pintado et al. 2000.Pintado AJ, Herrero CJ, Garcia AG, Montiel C. The novel Na+/Ca2+ exchange inhibitor KB-R7943 also blocks native and expressed neuronal nicotinic receptors. Br J Pharmacol 130: 1893–1902, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun and Kleene 2003.Pun RY, Kleene SJ. Contribution of cyclic-nucleotide-gated channels to the resting conductance of olfactory receptor neurons. Biophys J 84: 3425–3435, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrski et al. 2007.Pyrski M, Koo JH, Polumuri SK, Ruknudin AM, Margolis JW, Schulze DH, Margolis FL. Sodium/calcium exchanger expression in the mouse and rat olfactory systems. J Comp Neurol 501: 944–958, 2007. [DOI] [PubMed] [Google Scholar]
- Reisert and Matthews 1998.Reisert J, Matthews HR. Na+-dependent Ca2+ extrusion governs response recovery in frog olfactory receptor cells. J Gen Physiol 112: 529–535, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosker et al. 2004.Rosker C, Graziani A, Lukas M, Eder P, Zhu MX, Romanin C, Groschner K. Ca2+ signaling by TRPC3 involves Na+ entry and local coupling to the Na+/Ca2+ exchanger. J Biol Chem 279: 13696–13704, 2004. [DOI] [PubMed] [Google Scholar]
- Santo-Domingo et al. 2007.Santo-Domingo J, Vay L, Hernandez-Sanmiguel E, Lobaton CD, Moreno A, Montero M, Alvarez J. The plasma membrane Na+/Ca2+ exchange inhibitor KB-R7943 is also a potent inhibitor of the mitochondrial Ca2+ uniporter. Br J Pharmacol 151: 647–654, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze et al. 2002.Schulze DH, Pyrski M, Ruknudin A, Margolis JW, Polumuri SK, Margolis FL. Sodium-calcium exchangers in olfactory tissue. Ann NY Acad Sci 976: 67–72, 2002. [DOI] [PubMed] [Google Scholar]
- Sobolevsky and Khodorov 1999.Sobolevsky AI, Khodorov BI. Blockade of NMDA channels in acutely isolated rat hippocampal neurons by the Na+/Ca2+ exchange inhibitor KB-R7943. Neuropharmacology 38: 1235–1242, 1999. [DOI] [PubMed] [Google Scholar]
- Urban et al. 2005.Urban JM, Bobkov YV, Zhainazarov AB, Ache BW. Molecular identification of a TRPC protein homolog from lobster olfactory receptor neurons. AchemS XXVII Meeting, Sarasota, FL, April 13–17, 2005.
- Venkatachalam and Montell 2007.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem 76: 387–417, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatly et al. 2002.Wheatly MG, Hubbard MG, Corbett AM. Physiological characterization of the Na+/Ca2+ exchanger (NCX) in hepatopancreatic and antennal gland basolateral membrane vesicles isolated from the freshwater crayfish Procambarus clarkii. Comp Biochem Physiol A Mol Integr Physiol 131: 343–361, 2002. [DOI] [PubMed] [Google Scholar]
- Zhainazarov and Ache 1997.Zhainazarov AB, Ache BW. Gating and conduction properties of a sodium-activated cation channel from lobster olfactory receptor neurons. J Membr Biol 156: 173–190, 1997. [DOI] [PubMed] [Google Scholar]
- Zhainazarov et al. 1998.Zhainazarov AB, Doolin RE, Ache BW. Sodium-gated cation channel implicated in the activation of lobster olfactory receptor neurons. J Neurophysiol 79: 1349–1359, 1998. [DOI] [PubMed] [Google Scholar]
- Zhainazarov et al. 2001.Zhainazarov AB, Doolin R, Herlihy JD, Ache BW. Odor-stimulated phosphatidylinositol 3-kinase in lobster olfactory receptor cells. J Neurophysiol 85: 2537–2544, 2001. [DOI] [PubMed] [Google Scholar]
- Zhuang and Ahearn 1998.Zhuang Z, Ahearn GA. Energized Ca2+ transport by hepatopancreatic basolateral plasma membranes of Homarus americanus. J Exp Biol 201: 211–220, 1998. [DOI] [PubMed] [Google Scholar]