Abstract
Background and objectives: Proteinuria is associated with chronic kidney disease (CKD), and heavy proteinuria predicts a rapid decline in kidney function. However, the epidemiologic distribution of this important biomarker study is not well described in the pediatric CKD population.
Design, setting, participants & measurements: This cross-sectional study of North American children with CKD examined the association of proteinuria among the baseline clinical variables in the cohort. Urinary protein-to-creatinine ratios (Up/c) were used to measure level of proteinuria.
Results: Of the 419 subjects studied, the median GFR as measured by iohexol disappearance (iGFR) was 42 ml/min per 1.73 m2, median duration of CKD was six yr, and glomerular diseases accounted for 22% of the CKD diagnoses. Twenty-four percent of children had normal range (Up/c <0.2), 62% had significant, and 14% had nephrotic-range proteinuria (Up/c >2.0). A decrease in iGFR was associated with an increase in Up/c. At any level of GFR, a higher Up/c was associated with a glomerular cause of CKD and non-Caucasian race. Among subjects with a glomerular cause of CKD, Up/c was lower in subjects reporting utilization of renin-angiotensin system (RAS) antagonists (median Up/c = 0.93) compared with those who did not (median Up/c = 3.78).
Conclusions: Proteinuria is associated with level of iGFR, cause of CKD, and race. The longitudinal study design of Chronic Kidney Disease in Children (CKiD) cohort study and the large number of subjects being studied has created an opportunity to better define the association between proteinuria and CKD progression.
Proteinuria is an important biomarker strongly associated with chronic kidney disease (CKD) and is an indicator of underlying glomerular disease or renal tubular dysfunction. Recently, the National Kidney Foundation has recommended the evaluation of individuals for CKD by either blood testing to estimate GFR or by urinary screening for the detection of proteinuria. Tests for total urine protein are preferred in children, except for those with diabetes who should be screened for albuminuria (1). Not only is proteinuria a marker of kidney damage, but it is also responsible for progressive kidney injury. The increase in urinary protein either by damage to the glomerular capillary wall or by decrease in tubular reabsorbtion of protein causes injury to renal tubular cells (2). Exposure of tubules to urinary proteins causes interstitial inflammation and subsequent fibrosis, (3) as well as apoptosis in proximal tubular cells (4). Adult and pediatric studies also demonstrate that elevated urine total protein is an independent risk factor for a progressive decline in kidney function (5–9). Although the baseline level of proteinuria is a known risk factor for CKD progression, the determinants of proteinuria and how proteinuria changes with kidney progression are unclear. A better understanding of these phenomena might yield new insights and treatments for kidney disease progression. To that end, an epidemiologic survey of all patients with CKD would be important to assess potential environmental modifiers and differences in groups among children with CKD (10). The principal aim of the present study is to describe the distribution of proteinuria in a large cohort of children with mild to moderate CKD and to identify clinical characteristics, including kidney function, associated with elevated levels of proteinuria. To achieve this, we draw upon baseline data from the Chronic Kidney Disease in Children (CKiD) prospective cohort study.
Materials and Methods
Study Population
The CKiD study is a prospective observational cohort study of children with mild to moderate CKD throughout 43 pediatric nephrology centers across North America. The study design and conduct for CKiD has been approved by the External Advisory Committee appointed by the National Institutes of Health and by internal review boards for each participating center. A detailed description of the study design and methods for CKiD has been previously published (11). Briefly, children enrolled in CKiD were 1 to 16 yr of age and had a Schwartz-estimated GFR (12,13) between 30 and 90ml/min per 1.73m2. Exclusion criteria included: renal, other solid-organ, bone marrow, or stem cell transplantation; dialysis treatment within the past 3 mo; cancer/leukemia diagnosis or HIV diagnosis/treatment within the past 12 mo; current pregnancy or pregnancy within the past 12 mo; history of structural heart disease; genetic syndromes involving the central nervous system; and history of severe to profound mental retardation.
As of February 2008, 528 children had undergone their baseline visit, at which time the iohexol-based GFR (iGFR) was measured and the urine protein/creatinine (Up/c) ratio was determined. In the current study, analysis was restricted to the 419 children with measured iGFR and Up/c at baseline as well as known sex, age, CKD diagnosis, and current medication use.
Measurements and Data Collection
The central laboratory (University of Rochester) processed all laboratory specimens for testing of blood and urine. Serum creatinine was determined enzymatically (creatinine deiminase reaction) using the Bayer Advia 2400 analyzer. Serum electrolytes, BUN, and glucose were also determined by the Bayer Advia 2400 analyzer. Serum albumin was determined by the bromocresol green binding method.
The GFR measurements were determined by plasma iohexol disappearance curves iohexol-based GFR (iGFR) at four time points - 10, 30, 120, and 300 min - after infusion of 5 ml of iohexol (Ominipaque, GE Healthcare, Princeton, NJ). Details of the iGFR assessment methods have been published previously (14).
Urinary protein and creatinine measurements were obtained from urine samples collected for the study visit either at home by the participant or upon arrival at the clinical center. All patients were requested to collect and submit a first-morning urine sample, which had been defined as before 9:00 a.m. The collection times for urine samples were known for 89% (n = 374) of subjects. Of these, 79% (n = 294) were collected before 9:00 a.m. Proteinuria was defined by the Up/c level. Participants were identified as having normal ratios (Up/c <0.2), significant proteinuria (0.2 – 2 Up/c), or nephrotic-range proteinuria (Up/c >2).
Urine total protein was measured by binding to pyrogallol red in an acid environment containing molybdate ions. The resulting blue-colored complex absorbs maximally at 600 nm and is proportional to the protein concentration. The method is linear up to 125 mg/L and has an overall coefficient of variation of 3.7 to 4.9%. Urine creatinine was measured using the kinetic reaction of picric acid with creatinine in an alkaline medium (Jaffe). The rate of complex formation is measured at 505 nm and is proportional to the creatinine concentration. The method is linear from 1.5 to 300 mg/dl and the overall coefficient of variation is 1.4 to 2.1%. Both the urine total protein and urine creatinine concentrations were measured by the Bayer Advia 2400 analyzer with the coefficient of variations given by the technical specifications of the manufacturer.
Nonlaboratory data were obtained at the baseline visit using standardized forms. Data collected and analyzed in the current analysis includes age, sex, race, ethnicity, height, weight, clinical BP, reported intake of renin-angiotensin system (RAS) antagonists during the past 30 d, primary CKD diagnosis, and age at which the parent or child was first aware of the child's CKD. RAS antagonists were classified either as angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARBs). Height and weight were determined as the mean of three independent measurements, from which body mass index (BMI) was determined. Height, weight, and BMI percentiles were calculated (15) using NHANES III age- and sex- specific norms (16); obesity was defined as a BMI >95th percentile. Casual BP were determined as the average of three measurements obtained by auscultation using an aneroid sphygmomanometer (Mabis MedicKit 5, Mabis Healthcare, Waukegan, IL). Participants with BP ≥95th percentile for age, sex, and height were defined as hypertensive (HTN); the percentiles cutoffs were determined using the National High Blood Pressure Education Program 4th Report on the diagnosis, evaluation, and treatment of high BP in children and adolescents (17). The subject's severity of CKD was classified by strata defined by the National Kidney Foundation (NKF) Kidney Disease Outcomes Quality Initiative (K/DOQI) CKD staging system, (18) as defined by the subject's iGFR (and not estimated GFR) at baseline.
The diagnoses of CKD were reviewed by the members of the CKiD Steering Committee and categorized as either glomerular or nonglomerular. Glomerular diagnoses include chronic glomerulonephritis, congenital nephrotic syndrome, diffuse mesangial sclerosis (Denys-Drash syndrome), diabetic nephropathy, familial nephritis, focal segmental glomerulosclerosis, hemolytic uremic syndrome, Henoch Schonlein nephritis, idiopathic crescentic glomerulonephritis, IgA nephropathy, membranoproliferative glomerulonephritis types I and II, membranous nephropathy, sickle cell nephropathy, and systemic immunological disease including systemic lupus erythematosis. Nonglomerular diagnoses included aplastic-, hypoplastic, and dysplastic kidneys; cystinosis; medullary cystic disease/juvenile nephronophthisis, obstructive uropathy; oxalosis; autosomal dominant and recessive polycystic kidney disease; pyelonephritis/interstitial nephritis; reflux nephropathy; renal infarct; syndrome of agenesis of abdominal musculature; and Wilm's Tumor. CKD diagnoses not subscribing to one of the groups listed above were reviewed by the Steering Committee and, if necessary, discussed with the clinical site, so as to be properly categorized. The duration of a participants’ CKD was determined as the time between their baseline study visit and when they first became aware of their CKD diagnosis.
Statistical Analysis
The demographic and clinical characteristics for the overall study population were summarized using median and interquartile range (IQR) for continuous variables and percentages and frequencies for categorical variables. To assess the relationship of these clinical and demographic characteristics with measured proteinuria, median and IQR for continuous variables, as well as percentages and frequencies for categorical variables, were calculated for each level of proteinuria – normal, significant, and nephrotic (see above definitions). Trends of continuous and nonbinary ordinal variables across the three levels of proteinuria were determined nonparametrically using Spearman Rank Correlations; the Cochran-Armitage Test for trends was used for dichotomous variables. P values were reported to summarize the strength of the trend. The threshold for statistical significance for these investigations and for other analyses was set to 0.05.
Of primary interest was the cross-sectional relationship of Up/c and iGFR and how it may be modified by CKD diagnosis. With this in mind, a log-log scatterplot of Up/c against iGFR that distinguished between nonglomerular and glomerular CKD patients was overlaid with nonparametric smoothing splines – one for each diagnosis group. The smoothing spline is a flexible line that can be thought of as smoothly connecting a series of averaged Up/c's; each average is calculated within a narrow range of continuous iGFR values (19). Each smoothing spline suggested a log-log linear relationship between Up/c and iGFR. As such, unadjusted and adjusted regression models quantifying the relationship between Up/c and iGFR were developed. A Shapiro-Wilks Test (P = 0.22) of the residuals from the log(Up/c) on log(iGFR) linear regression model confirmed the appropriateness of the normality assumption for the multivariate analysis. Expected percentage changes in Up/c for defined unit changes in iGFR and other covariates were determined by exponentiating the respective regression coefficients.
Of secondary interest was the relationship between Up/c, utilization of RAS antagonists, and CKD diagnosis. With this in mind, RAS antagonist use and proteinuria levels were compared between glomerular and nonglomerular patients. Multivariate linear regression models were developed to test the effect modification that CKD diagnosis might have on the relationship between ACEI/ARB use and log(Up/c). All data analysis for this study was performed using SAS 9.1 statistical software (SAS Institute, Cary, NC); figures were produced using S-Plus 8.0 (Insightful Corporation, Seattle, WA).
Results
Demographic and clinical characteristics of the 419 children are displayed in Table 1. The median age was 11 yr; 62% were male; 68% were Caucasian, 16% were African-American, and 16% reported multiracial or other racial background. Fourteen percent reported Hispanic ethnicity. Among the subjects included in the study, the median iGFR was 42 ml/min per 1.73 m2, the median duration of CKD was 6 yr, and glomerular diseases accounted for 22% of the CKD diagnoses. The median Up/c for the cohort was 0.53 with an interquartile range of 0.20 to 1.27. Table 1 also summarizes participants’ clinical and demographic characteristics by their level of proteinuria. Twenty-four percent of children had normal range Up/c (<0.2), 62% had significant proteinuria, and 14% had nephrotic-range proteinuria.
Table 1.
Patient characteristicsa
| Overall (n = 419) | Level of Proteinuria
|
P value for trendb | |||
|---|---|---|---|---|---|
| Normal (n = 101) | Significant (n = 258) | Nephrotic (n = 60) | |||
| Age, years | 11 [7,14] | 9 [7,13] | 11 [7,14] | 12 [9,15] | <0.01 |
| Male, % (n) | 62 (258) | 61 (62) | 62 (160) | 60 (36) | 0.90 |
| Race, % (n)f | 0.03c | ||||
| Caucasian | 68 (285) | 75 (76) | 68 (174) | 58 (35) | |
| African-American | 16 (67) | 13 (13) | 16 (41) | 22 (13) | |
| Multi-racial or other | 16 (66) | 12 (12) | 16 (42) | 20 (12) | |
| Hispanic ethnicityf | 14 (59) | 10 (10) | 15 (39) | 17 (10) | 0.17 |
| BMI | 18 [16, 21] | 17 [16, 20] | 18 [16, 21] | 20 [17, 23] | <0.01 |
| BMI standard deviation scoref | 0.36 [-0.38,1.19] | 0.24 [-0.41,0.91] | 0.32 [-0.43,1.25] | 0.66 [0.05,1.25] | 0.07 |
| Obese, BMI > 95th percentile (SDS)f | 16% (66) | 14% (14) | 17% (42) | 18% (10) | 0.46 |
| Serum albumin, g/dlf | 4.3 [4.1,4.5] | 4.4 [4.3,4.6] | 4.3 [4.1,4.5] | 3.9 [3.3,4.2] | <0.01 |
| Hypoalbuminemia (albumin <4 g/dl) | 14% (60) | 4% (4) | 9% (24) | 54% (32) | <0.01 |
| Glomerular CKD diagnosis, % (n) | 22% (91) | 12% (12) | 19% (50) | 48% (29) | <0.01 |
| Iohexol-based GFR, ml/min per 1.73 m2 | 42 [32,52] | 50 [38,59] | 43 [32,51] | 33 [28,40] | <0.01 |
| K-DOQI CKD Stage, % (n) | <0.01d | ||||
| I | <1% (3) | 1% (1) | <1% (2) | 0% (0) | |
| II | 15% (61) | 24% (24) | 13% (33) | 7% (4) | |
| III | 65% (274) | 65% (66) | 66% (171) | 62% (37) | |
| IV | 19% (81) | 10% (10) | 20% (52) | 32% (19) | |
| CKD duration, yearsf | 6 [3,10] | 6 [3,10] | 6 [3,10] | 7 [3,9] | 0.63 |
| Uncontrolled Systolic Hypertension, % (n)e,f | 14% (59) | 13% (13) | 14% (35) | 19% (11) | 0.35 |
| Uncontrolled Diastolic Hypertension, % (n)e,f | 15% (60) | 13% (13) | 13% (33) | 24% (14) | 0.10 |
| ACE-Inhibitor/ARB use, % (n) | 54% (228) | 51% (51) | 56% (144) | 55% (33) | 0.49 |
SDS, standard deviation score.
Median [IQR], unless otherwise noted.
P values for trend determined using: 1) Spearman Rank-Correlation for continuous and non-binary ordinal variables (e.g., CKD Stage), and 2) the Cochran-Armitage test for trends for dichotomous variables.
For Caucasian vs. non-Caucasian race.
Modeled as median iGFR for each K-DOQI stage of CKD.
Defined as BP ≥95th percentile for age, sex, and height.
Missing data: n = 11 missing systolic hypertension; n = 12 missing diastolic hypertension; n = 1 missing race; n = 5 missing ethnicity; n = 16 missing BMI percentile; n = 1 missing serum albumin; n = 1 missing BMI; n = 7 missing CKD duration.
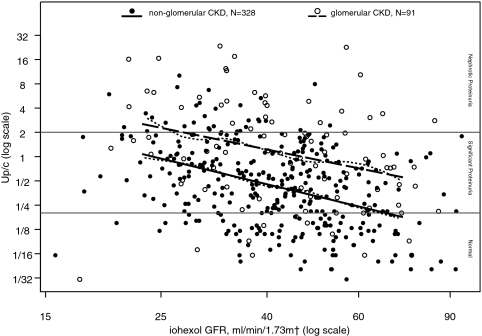
Figure 1 shows the log-log linear relationship between Up/c and iGFR for patients with nonglomerular and glomerular CKD, respectively. In the unadjusted linear regression analysis, we found that level of proteinuria was associated with iGFR, age, race, and glomerular cause of CKD (see Table 2). We estimate that for every 10% decrease in iGFR, Up/c increased on average by 14% (95% CI: 10%, 18%). While the magnitude of the association between Up/c and iGFR did not differ by CKD diagnosis, patients with glomerular CKD had Up/c levels on average 140% greater than those of nonglomerular patients. After adjusting for age, gender, race, BMI, cause of CKD, and RAS antagonist use in the multivariable regression analysis, the relationship between iGFR and Up/c remained statistically significant, whereby a decrease in iGFR was associated with an increase in Up/c. In addition to decreased iGFR and glomerular CKD, the multivariate regression analysis identified non-Caucasian children as having Up/c levels 40% higher (95% CI: 9%, 80%) than Caucasian children.
Figure 1.
Urine Protein:Creatinine ratios (Up/c) by iohexol-measured GFR (iGFR) for 328 nonglomerular CKD children and 91 glomerular CKD children, respectively. Nonparametric smoothing splines for the Up/c versus iGFR relationship for each of the CKD diagnosis groups are represented by the small dashed lines. Linear regression models for the nonglomerular and glomerular CKD patients are represented by the solid and large dashed straight lines, respectively. Both the smoothing splines and linear regression lines are drawn from the 5th to the 95th percentile iGFR values.
Table 2.
Patient characteristics and change in proteinuriaa
| Percent difference in Up/c
|
||||
|---|---|---|---|---|
| Unadjusted, n = 419
|
Adjustedb, n = 402
|
|||
| Estimate [95% CI] | P value | Estimate [95% CI] | P value | |
| iGFR, per 10% Decrease | 14% [10%, 18%] | <0.01 | 17% [13%, 21%] | <0.01 |
| Age, per 2-yr Increase | 8% [1%, 14%] | 0.01 | 6% [0%, 12%] | 0.07 |
| Male versus female Sex | −2% [−24%, 27%] | 0.88 | 12% [−12%, 41%] | 0.36 |
| Non-Caucasian versus Caucasian race * | 39% [6%, 81%] | 0.02 | 40% [9%, 80%] | 0.01 |
| BMI SDS, z-score, per 1 unit increasec | 3% [−8%, 15%] | 0.60 | 3% [−6%, 14%] | 0.52 |
| Glomerular versus non-glomerular CKD diagnosis | 140% [79%, 221%] | <0.01 | 134% [73%, 219%] | <0.01 |
| ACE and/or ARB use | 8% [−16%, 38%] | 0.57 | −16% [−34%, 7%] | 0.16 |
SDS, standard deviation score.
Linear regression of log Up/c as a continuous variable.
Controlling for iGFR, age, gender, race, BMI percentile, cause of CKD, and ACEI or ARB use.
Missing data: race, n = 1; BMI SDS, n = 16.
Over half (54%) of the patients in the cohort reported taking a RAS antagonist with a significantly heavier prevalence being reported by glomerular CKD patients (80%) as compared with nonglomerular CKD patients (47%) (Table 3). Within the strata of glomerular CKD subjects, those who reported use of a RAS antagonist had a lower prevalence of nephrotic- range proteinuria compared with those who were not using these agents (respectively, 23% [17/73] versus 67% [12/18]). Within the strata of nonglomerular subjects, the prevalence of nephrotic-range proteinuria was similar between those who were and were not using an ACEI or ARB (10% [16/155] versus 9% [15/177], respectively). Moreover, among subjects with glomerular causes of CKD, average Up/c levels were significantly lower in subjects reporting ACEI/ARB intake (median Up/c = 0.93) compared with those who did not (median Up/c = 3.78); among nonglomerular patients, average Up/c levels did not differ by RAS antagonist use (median Up/c of 0.45 and 0.42, respectively).
Table 3.
Proteinuria by CKD diagnosis and ACEI/ARB use
| CKD diagnosis | ACEI/ARB use/% (N) | Median Up/c | Level of proteinuria
|
||
|---|---|---|---|---|---|
| Normal (n = 101) | Significant (n = 258) | Nephrotic (n = 60) | |||
| Glomerular | |||||
| n = 91 | No/20% (18) | 3.78 | 11% (2) | 22% (4) | 67% (12) |
| Yes/80% (73) | 0.93 | 14% (10) | 63% (46) | 23% (17) | |
| Non-Glomerular | |||||
| n = 328 | No/53% (173) | 0.45 | 28% (48) | 64% (110) | 9% (15) |
| Yes/47% (155) | 0.42 | 26% (41) | 63% (98) | 10% (16) | |
Table 4 shows the association between current ACEI/ARB use and Up/c level as modified by CKD diagnosis. After adjusting for iGFR, Up/c levels for glomerular CKD patients utilizing a RAS antagonist were 54% lower (95% CI: −75%, −15%) than those not taking a RAS antagonist; nonglomerular CKD patients on ACEI/ARBs showed Up/c levels nonsignificantly lower by 9% (95% CI: −29%, 18%) when compared with those not on ACEI/ARBs. The statistical model presented in Table 4 demonstrates a significant relationship between difference in proteinuria by ACEI/ARB use and cause of CKD. The difference in proteinuria is more pronounced in patients in glomerular disease above and beyond what is expected based on the effect of ACEI/ARB use and the nonglomerular group (P value for the interaction <0.01). The adjusted model demonstrates that the inter-relationships of ACEI/ARB use, cause of CKD, and proteinuria are still present when comparing patients within the same strata of iGFR (P value for the interaction = 0.04).
Table 4.
ACE or ARB Use and Difference in Proteinuria by CKD Diagnosisa
| ACE or ARB yes versus no | Percent difference in Up/c
|
|||||
|---|---|---|---|---|---|---|
| Unadjusted [95% CI] | Exp^β | P valuec | Adjustedb [95% CI] | Exp^β | P valuec | |
| n = 419 | n = 419 | |||||
| Glomerular Dx | −63% [−81%, −30%] | 0.37 | <0.01 | −54% [−75%, −15%] | 0.46 | 0.04 |
| Non-Glomerular Dx | 2% [−22%, 33%] | 1.02 | −9% [−29%, 18%] | 0.91 | ||
| Interaction | 0.36 | 0.51 | ||||
Linear regression of log Up/c as a continuous variable.
Adjusted for iGFR.
P value for the interaction of ACE/ARB and CKD diagnosis.
Discussion
The baseline data from this North American-wide cohort study provides important observations regarding the patterns of proteinuria in children with mild to moderate CKD. We found that the level of proteinuria tended to be higher as the level of iGFR decreased irrespective of the cause of CKD. Other factors that were independently associated with proteinuria were race and glomerular cause of CKD. Furthermore, among patients with glomerular causes of CKD, those who reported ACEI or ARB intake tended to have significantly lower levels of proteinuria compared with those who were not taking these medications.
Other pediatric studies noted that high levels of proteinuria were associated with a more rapid decline in kidney function. In 1997, the European Study Group for Nutritional Treatment of Chronic Renal Failure in Childhood reported that high-grade proteinuria at the baseline of their three-year study was associated with a more rapid decline in renal function (20). Furthermore, in 2006, Wong et al. demonstrated that the prevalence of proteinuria increased with CKD severity from their cross-sectional study of 366 pediatric subjects with CKD (21). Studies limited to children with nonglomerular nephropathies also observed that level of proteinuria at baseline was associated with renal failure progression (6,22). The pediatric observations are consistent with adult CKD studies (8,9) demonstrating that proteinuria is a risk factor for kidney disease progression. With the baseline assessment of subjects in this cohort, we found that higher levels of proteinuria were associated with a lower iGFR. Given that the median duration of CKD in the cohort is 6 yr, the high levels of proteinuria associated with a lower GFR likely reflect prior exposure of these patients to heavy proteinuria. Alternatively, the progressive loss of kidney function may lead to an increase in proteinuria. The continued observation of the CKiD cohort will be important to characterize the changes in proteinuria over time as well as clarify the relationship between proteinuria and iGFR.
Furthermore, we identified that non-Caucasian race was an independent determinant of proteinuria. This study indicates that racial/ethnic differences are associated with proteinuria with non-Caucasian patients having a higher level of proteinuria regardless of other factors, as noted above. Thus, the differences in proteinuria by racial/ethnic differences might be related to genetic or environmental factors. This finding in the pediatric CKD population might provide insight for general observations regarding the disproportionate burden of kidney disease in the non-Caucasian populations. Data from the United States Renal Data System show that the 2005 incidence rate for end-stage renal disease in Caucasians was 268 per million population, compared with 991 among African American, 516 among Native Americans, and 355 among Asians (23).
There are studies in both adult and pediatric non-CKD populations that demonstrate racial/ethnic differences in albuminuria and proteinuria. Based on a population-based survey in the United Kingdom of nearly 3000 subjects, Tillin et al. documented differences in the prevalence of microalbuminuria among differing racial/ethnic populations, with albumin excretion rates found to be higher in the African Carribbeans compared with Southeast Asians and Europeans (24). In an adolescent population of 647 subjects, Hanevold et al. demonstrated that healthy, normotensive black adolescents had an approximate 10% higher albumin excretion rate compared with normotensive white adolescents (25). Thus far, the Family Investigation of Nephropathy and Diabetes (FIND) study offers compelling data to support the hypotheses that albuminuria and proteinuria might be influenced by genetic determinants. Preliminary data from the FIND study suggests linkage with albumin-to-creatinine ratios observed on chromosomes 2q14.1 in American Indian families, 7q21.1 in European-American families, and 15q26.3 in African-American families (26). Similar results were obtained in the evaluation of protein to creatinine ratios for 2p and 7q (26).
The analysis of the proteinuria and RAS antagonist intake demonstrated differences in Up/c associated with the reported use of these medications, depending upon the level of proteinuria. Subjects with glomerular causes of CKD had higher levels of proteinuria. In this group of subjects, we demonstrated differences in level of Up/c with patients reporting RAS antagonist use having lower levels of proteinuria compared with patients not using these medications. These findings are consistent with other reports documenting the anti-proteinuric efficacy of these agents in the pediatric CKD population (5,27,28,29). Subjects with nonglomerular disease had significantly lower levels of Up/c compared with subjects with glomerular causes of CKD. Although there were no statistically significant differences in Up/c's among subjects with nonglomerular causes of CKD whether or not ACEI/ARB medications were used, there is insufficient data to conclude that there is no benefit of using such agents among patients with nonglomerular disease. Recently, Ardissino et al. reported that there was no clear evidence of ACEI efficacy on the progression of CKD in children with hypodysplastic nephropathy (30). In their cohort study comparing 41 patients on ACEI therapy and control patients who were not on ACEI therapy, both groups had stable kidney function, with the average decline of −0.31 ml/min/1.73m2/yr with ACEI therapy and −0.33 ml/min/1.73m2 without ACEI therapy. Their study had significant limitations since it was based on registry data and had insufficient time to observe the end point of interest. Given the slow rates of progression in this particular population of CKD patients, longer observation times may be necessary to determine the factors associated with progression in patients with nonglomerular causes of CKD. Patients with hypodysplastic kidneys and other nonglomerular causes of CKD may have to be followed through puberty, during which time subjects with childhood onset CKD tend to have a more rapid decline in kidney function (31).
The main limitation of our study is its cross-sectional design, which prevents the definition of temporal and causal relationships between Up/c and iGFR as well as the other associated factors with Up/c. When the prospective longitudinal data on renal progression become available from the continued follow-up of the CKiD patient cohort, we will able to determine how race, medication use, and cause of CKD affect Up/c, as well as determine their collective effects on the change in iGFR. Given the observational nature of this study, there may be unmeasured factors that could introduce bias into the analysis.
Observational studies pertaining to the effectiveness or side effects of particular drugs or clinical therapies will be affected by a systematic bias termed “confounding by indication” (7,32–34). This bias is likely to be present in the analysis of ACEI/ARB utilization and level of proteinuria. Methods to account for confounding by indication include: stratification of the study population, individual matching of exposed and nonexposed into main prognostic strata, statistical control of confounding factors in multivariable regression models, or subclassification of patients on levels of propensity score (32,35). For the analysis presented in Table 4, we attempted to account for this bias by stratifying the analysis based on cause of CKD and adjusting for iGFR in the multivariable model. However, we acknowledge the possibility of residual bias, especially those with nonglomerular causes of CKD where RAS antagonist use may not be seen as indicated.
Despite these limitations, this study draws tremendous strength from the prospective standardized measurements, the centralized laboratory studies, and the large number of participants across multiple pediatric nephrology centers across North America (11). The standardized measurement of GFR in all participants is a key strength of this study. Previously, large pediatric CKD studies (5,6,31,34) have relied on estimating GFR by the Schwartz formula to evaluate renal progression. Although convenient and practical, the Schwartz formula is imprecise and overestimates the inulin clearance particularly at lower levels of GFR (18).
In summary, data from the CKiD study demonstrate that the level of kidney impairment, race, cause of CKD, and reported use of a RAS antagonist are associated with proteinuria (Up/c). Furthermore, the significant differences in levels of proteinuria between glomerular and nonglomerular causes of CKD indicate potential differences in the natural histories and response to therapies to slow or reverse of CKD progression. The longitudinal assessment of the cohort will be able to provide further insights regarding the causal pathways for the factors associated with proteinuria and CKD progression. The study design of CKiD and the large number of subjects being studied has created an opportunity to further define risk factors for CKD progression and to test potentially therapeutic interventions in the years to come.
Disclosures
None.
Acknowledgments
The CKiD prospective cohort study is funded by the National Institute of Diabetes and Digestive and Kidney Disease, with additional funding from the National Institute of Neurologic Disorders and Stroke; National Institute of Child Health and Human Development; and the National Heart, Lung, and Blood Institute (UO1-DK-66143, UO1-DK-66174, and UO1-DK-66116). The CKiD study has been supported by participating institutional General Clinical Research Centers and Clinical Translational Research Centers (University of New Mexico GCRC is supported by grant number M01-RR-00997).
This study was presented as a poster at the annual meeting of the American Society of Nephrology (November 14 through 19, 2006, San Diego, CA) and at the International Pediatric Nephrology Association (August 30 through September 4, 2007, Budapest, Hungary).
The CKiD prospective cohort study has clinical coordinating centers (principal investigators) at Children's Mercy Hospital and the University of Missouri-Kansas City (Bradley Warady, MD) and Johns Hopkins School of Medicine (Susan Furth, MD, PhD), the data coordinating center at the Johns Hopkins Bloomberg School of Public Health (principal investigator Alvaro Muñoz, PhD), and the Central Biochemistry Laboratory at the University of Rochester (principal investigator, George J. Schwartz, MD).
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Proteinuria: Not a Small Problem in the Little Ones,” on pages 696–697.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Vassalotti JA, Stevens LA, Levey AS: Testing for chronic kidney disease: A position statement from the National Kidney Foundation. Am J Kidney Dis 50: 169–180, 2007 [DOI] [PubMed] [Google Scholar]
- 2.D'Amico G, Bazzi C: Pathophysiology of proteinuria. Kidney International 63: 809–825, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Eddy AA: Progression in chronic kidney disease. Adv Chronic Kidney Dis 12: 353–365, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Erkan E, Devarajan P, Schwartz GJ: Mitochondria are the major targets in albumin-induced apoptosis in proximal tubule cells. J Am Soc Nephrol 18: 1199–1208, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Wuhl E, Mehls O, Schaefer F and the ESCAPE trial group: Antihypertensive and antiproteinuric efficacy of ramipril in children with chronic renal failure. Kidney Int 66 (2): 768–776, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Ardissino G, Testa S, Dacco V, Vigano S, Taioli E, Claris-Appiani A, Procaccio M, Avolio L, Ciofani A, Dello Strologo L, Montini G: Proteinuria as a predictor of disease progression in children with hypodysplastic nephropathy. Data from the Ital Kid Project. Pediatr Nephrol 19: 172–177, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez Celedon C, Bitsori M, Tullus K: Progression of chronic renal failure in children with dysplastic kidneys. Pediatr Nephrol 22: 1014–1020, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, King AJ, Klahr S, Massry S, Seifter JL: Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med 123 (10): 754–762, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 349: 1857–1863, 1997 [PubMed] [Google Scholar]
- 10.Toniolo P, Pearce N, Shuker DEG, Boffetta P, Rothman N, Hulka B, International Agency for Research on Cancer.: Application of biomarkers in cancer epidemiology, Lyon, International Agency for Research on Cancer 1997
- 11.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Munoz A, Warady BA: Design and methods of the chronic kidney disease in children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz GJ, Brion LP, Spitzer A: The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34: 571–590, 1987 [DOI] [PubMed] [Google Scholar]
- 13.Schwartz GJ, Haycock GB, Edelmann CM Jr., Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 14.Schwartz GJ, Furth S, Cole SR, Warady B, Munoz A: Glomerular filtration rate via plasma iohexol disappearance: Pilot study for chronic kidney disease in children. Kidney Int 69: 2070–2077, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Pere A: Comparison of two methods for transforming height and weight to normality. Ann Hum Biol 27: 35–45, 2000 [DOI] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics (U.S.): National Health and Nutrition Examination Survey III, 1988–94. NCHS CD-ROM. Series 11; no. 2 a. Hyattsville, Md., U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics, 1998, p. 1 CD-ROM.
- 17.Huang G, Niu T, Peng S, Ling D, Liu J, Zhang X, Xu X: Association between the interleukin-1beta C(-511)T polymorphism and blood pressure in a Chinese hypertensive population. Immunol Lett 91: 159–162, 2004 [DOI] [PubMed] [Google Scholar]
- 18.K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–266, 2002 [PubMed] [Google Scholar]
- 19.Steenland K, Deddens JA: A practical guide to dose-response analyses and risk assessment in occupational epidemiology. Epidemiology 15: 63–70, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Wingen AM, Fabian-Bach C, Schaefer F, Mehls O: Randomised multicentre study of a low-protein diet on the progression of chronic renal failure in children. European Study Group of Nutritional Treatment of Chronic Renal Failure in Childhood. Lancet 349: 1117–1123, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Wong H, Mylrea K, Feber J, Drukker A, Filler G: Prevalence of complications in children with chronic kidney disease according to KDOQI. Kidney Int 70: 585–590, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Litwin M: Risk factors for renal failure in children with non-glomerular nephropathies. Pediatr Nephrol 19: 178–186, 2004 [DOI] [PubMed] [Google Scholar]
- 23.USRDS 2008 Annual Data Report: Atlas of End-Stage Renal Disease in the U.S. 2008. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2008
- 24.Tillin T, Forouhi N, McKeigue P, Chaturvedi N: Microalbuminuria and coronary heart disease risk in an ethnically diverse UK population: A prospective cohort study. J Am Soc Nephrol 16: 3702–3710, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Hanevold CD, Pollock JS, Harshfield GA: Racial differences in microalbumin excretion in healthy adolescents. Hypertension 51: 334–338, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Iyengar SK, Abboud HE, Goddard KA, Saad MF, Adler SG, Arar NH, Bowden DW, Duggirala R, Elston RC, Hanson RL, Ipp E, Kao WH, Kimmel PL, Klag MJ, Knowler WC, Meoni LA, Nelson RG, Nicholas SB, Pahl MV, Parekh RS, Quade SR, Rich SS, Rotter JI, Scavini M, Schelling JR, Sedor JR, Sehgal AR, Shah VO, Smith MW, Taylor KD, Winkler CA, Zager PG, Freedman BI: Genome-wide scans for diabetic nephropathy and albuminuria in multiethnic populations: The family investigation of nephropathy and diabetes (FIND). Diabetes 56: 1577–1585, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Ellis D, Vats A, Moritz ML, Reitz S, Grosso MJ, Janosky JE: Long-term antiproteinuric and renoprotective efficacy and safety of losartan in children with proteinuria. J Pediatr 143: 89–97, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Soergel M, Verho M, Wuhl E, Gellermann J, Teichert L, Scharer K: Effect of ramipril on ambulatory blood pressure and albuminuria in renal hypertension. Pediatr Nephrol 15(1–2): 113–118, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Ohta K, Shimizu M, Nakai A, Kasahara Y, Yachie A, Koizumi S: Treatment with low-dose angiotensin-converting enzyme inhibitor (ACEI) plus angiotensin II receptor blocker (ARB) in pediatric patients with IgA nephropathy. Clin Nephrol 64: 35–40, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Ardissino G, Vigano S, Testa S, Dacco V, Paglialonga F, Leoni A, Belingheri M, Avolio L, Ciofani A, Claris-Appiani A, Cusi D, Edefonti A, Ammenti A, Cecconi M, Fede C, Ghio L, La Manna A, Maringhini S, Papalia T, Pela I, Pisanello L, Ratsch IM, on behalf of the ItalKid P: No clear evidence of ACEi efficacy on the progression of chronic kidney disease in children with hypodysplastic nephropathy report from the ItalKid Project database. Nephrol Dial Transplant 22: 2525–2530, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Ardissino G, Dacco V, Testa S, Bonaudo R, Claris-Appiani A, Taioli E, Marra G, Edefonti A, Sereni F: Epidemiology of chronic renal failure in children: Data from the ItalKid project. Pediatrics 111: e382–e387, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Hak E, Verheij TJ, Grobbee DE, Nichol KL, Hoes AW: Confounding by indication in non-experimental evaluation of vaccine effectiveness: The example of prevention of influenza complications. J Epidemiol Community Health 56: 951–955, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Signorello LB, McLaughlin JK, Lipworth L, Friis S, Sorensen HT, Blot WJ: Confounding by indication in epidemiologic studies of commonly used analgesics. Am J Ther 9: 199–205, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Bradbury BD, Wang O, Critchlow CW, Rothman KJ, Heagerty P, Keen M, Acquavella JF: Exploring relative mortality and epoetin alfa dose among hemodialysis patients. Am J Kidney Dis 51: 62–70, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Schmoor C, Caputo A, Schumacher M: Evidence from nonrandomized studies: A case study on the estimation of causal effects. Am J Epidemiol 167: 1120–1129, 2008 [DOI] [PubMed] [Google Scholar]