Abstract
Background and objectives: Some patients are not optimally treated by conventional in-center hemodialysis (HD) and are unable to perform home HD. We examined the effect of in-center thrice-weekly nocturnal HD (INHD) on patient outcomes.
Design, setting, participants, & measurements: Patients who were not optimally treated on conventional HD were offered INHD. Thirty-nine patients’ laboratory data and medication use were analyzed for the 12 mo before and after conversion to INHD until September 1, 2007. Quality of life on conventional HD and INHD was compared.
Results: After conversion to INHD, median values for phosphorus decreased from 5.9 to 3.7 mg/dl (P < 0.01), alkaline phosphatase level increased from 84 to 161 U/L (P < 0.01), and percentage reduction in urea increased from 74 to 89% (P < 0.01). The mean number of antihypertensive drugs prescribed declined from 2.0 to 1.5 (P < 0.05) during the course of INHD, and the mean daily dosage of phosphate binders declined from 6.2 to 4.9 at study end (P < 0.05). There was a significant reduction in erythropoietin-stimulating agent use of 1992 U/wk (P < 0.01). There was no significant change in median hemoglobin, iron saturation, corrected calcium, or parathyroid hormone levels. Overall, quality of life, sleep, intradialytic cramps, appetite, and energy level all improved significantly on INHD.
Conclusions: INHD offers an effective form of HD for long-term dialysis patients who are unable to perform home HD.
The significant morbidity and mortality and poor quality of life among long-term hemodialysis (HD) patients (1–4) has increased interest in more intensive dialysis regimens. Long intermittent HD, performed for 8 h during the day, three times per week, has been associated with better BP control and a lower standardized mortality rate (5). Short daily HD, performed for at least 2 h, five to six times per week, may improve phosphate and BP control (6,7). Home nocturnal HD (HNHD), performed for 7 to 8 h, five to seven nights per week, can normalize phosphate levels without need for binders (8), improve BP control with few or no antihypertensive medications (9), restore impaired left ventricular systolic function (10) and vascular function (11,12), improve left ventricular mass (13), and stabilize coronary artery calcification (14). Better quality of life has been observed with HNHD (4,13,15–17); however, few patients are candidates for HNHD because of physical, psychological, medical, or social barriers, and some patients believe that the potential benefits do not merit the perceived lifestyle intrusion (18).
An in-center nocturnal HD (INHD) program was instituted at our center for patients who might benefit from intensive HD but are not candidates for HNHD. Here we report the 3.5-yr experience of this program with an emphasis on mineral metabolism and quality of life. An informal qualitative description of some of the operational challenges that this program faced is also presented.
Materials and Methods
Study Design
This is a retrospective study of patients who participated in the INHD program at St. Michael's Hospital (an academic acute care hospital affiliated with the University of Toronto, Toronto, Ontario, Canada) from its inception in March 2004 until September 1, 2007, and was approved by our research ethics board.
INHD Program
Nocturnal dialysis treatments were delivered in the conventional HD unit after the last of the three daytime shifts. Treatments began between 9:00 and 10:00 p.m. and lasted 7 to 8 h on Monday, Wednesday, and Friday or on Tuesday, Thursday, and Sunday nights. Nephrology ward nurses were cross-trained to dialyze the nocturnal patients at a patient-to-nurse ratio of 4:1. Patients visited their primary nephrologist at least every 3 mo in an outpatient clinic, which operated during the evening before dialysis or during the morning after dialysis. Ward housestaff were available for medical emergencies. Initially, patients underwent dialysis in hospital beds that were stored in the adjacent nephrology ward during the day. In August 2007, these were replaced with bed chairs (Total Care, Model 1900; Hill Rom, Batesville, IN), which served the needs of both the conventional and the nocturnal patients. For facilitation of sleep, lorazepam 0.5 to 1.0 mg and dimenhydrinate 25 to 50 mg were offered to the patients as needed.
Patient Population
In March 2004, there were approximately 200 patients in the in-center HD program at St. Michael's Hospital, 14 patients on home HD (including home nocturnal HD), and 42 patients on peritoneal dialysis. At the study end, the in-center HD population was 180, with 28 patients on home HD and 58 on peritoneal dialysis. Initial recruitment criteria for INHD were uncontrolled hyperphosphatemia, hypotension as a result of ultrafiltration requirements, or poor quality of life on conventional HD. With time, we accommodated patients who requested to be transferred to INHD for personal reasons. This report includes all patients who participated in the INHD program but one who became confused during the night and returned to conventional HD within 1 wk.
Dialysis Treatments
Both the conventional and INHD programs performed HD using the Gambro Phoenix (Gambro Dasco SPA, Mendolla, Italy) HD machine and Baxter CA 210 dialysis membranes (Baxter Healthcare Corp., McGaw Park IL). Patients who were on conventional HD received 4-h treatments thrice weekly with a target blood pump speed of 400 ml/min and dialysate flow rate of 500 to 800 ml/min. INHD was performed for 7 to 8 h thrice weekly with a dialysate flow rate of 600 ml/min and a target blood pump speed of 300 ml/min. Anticoagulation was achieved with heparin (minimum 1000-U bolus, minimum 1000 U/h). A total of 1000 to 5000 U/ml heparin or 4% citrate solution was used as an interdialytic catheter-locking solution. A standard calcium bath of 5.0 mg/dl (1.25 mmol/L) was used and adjusted as clinically indicated. Targets for BP, glycemia, lipids, hemoglobin, and mineral metabolism parameters were the same as for conventional in-center HD. When appropriate, the patients’ diet was liberalized and phosphate binders tapered. Dry weights, hourly rate of ultrafiltration, and antihypertensive agents were adjusted as clinically indicated. The protocol for administration of intravenous erythropoietin-stimulating agent (ESA) and iron supplementation did not change upon conversion to INHD.
We could not accurately determine the medication list at the time of initiation of INHD for some patients; hence, the number of antihypertensives and total daily dosages of phosphate binders prescribed were collected from the point on INHD at which complete data were available, using a 3-mo window, and were compared with the values at study end using Wilcoxon Signed Ranks test. The phosphate binder usage was presented as the total phosphate binder pill number. Dosages of ESA were assessed for the 12 mo before conversion to INHD and compared with those during the course of INHD.
Outcome Assessments
Routine laboratory data were available every 6 wk, except for parathyroid hormone, ferritin, and iron saturation, which were measured every 3 mo. Laboratory data were analyzed for the 12 mo before conversion to INHD and after conversion up to September 1, 2007.
For ensuring that INHD was not having a negative effect on quality of life, a self-developed questionnaire specifically targeted to the INHD patient was administered to the study patients during a structured interview after they had been on INHD for a variable period of time. A single investigator (A.B.) conducted all interviews, except for one that was conducted by a nurse who spoke that patient's primary language. Patients assessed the following quality-of-life indicators on a 10-point Likert scale: Overall energy level; intradialytic cramps and dizziness; postdialysis fatigue; overall quality of life; quality of sleep, which encompassed both the period during dialysis and on nondialysis days; appetite; and level of intrusion of dialysis on daily life. The patients selected a score from 0 to 10 with a score of 0 indicating “poor” and a score of 10 indicating “excellent.” Quality-of-life data from the 23 prevalent patients at the time of the quality-of-life survey are reported here.
Staff Satisfaction
In an effort to improve nursing staff satisfaction and performance, the nurses were asked to complete an anonymous questionnaire indicating their preference as to whether they preferred to continue in the dual role of ward and INHD nurse or to function only as a ward nurse. In a separate nursing project, all INHD nurses were interviewed by a student to evaluate the INHD nursing experience.
Statistical Analysis
Laboratory data were collected 12 mo before INHD conversion and after conversion to September 1, 2007. Continuous variables were reported as medians with the interquartile range (25th and 75th percentiles) or absolute ranges. Nominal variables were reported by proportion. The mean number of antihypertensive medications and phosphate binder pill count in the first documented 3-mo interval were compared with those of the last using the Wilcoxon signed ranks test. The weekly dosage of ESA was examined 12 mo before conversion to INHD and up to September 1, 2007. Dosages of epoetin alfa (Eprex; Ortho Biotech, Toronto, ON, Canada) and darbepoetin alfa (Aranesp, Amgen, Mississauga, ON, Canada) were standardized using two conversion ratios for Aranesp: By mass (200:1) (19) and as per Centers for Medicare and Medicaid Services (330:1) (20).
Laboratory parameters and ESA weekly dosages for each patient were grouped by monthly intervals. The mean was used for multiple measurements occurring in the same interval. Repeated measure analyses were performed on this data using linear mixed modeling with a first order autoregressive covariance structure and 95% confidence interval (CI) in order to examine the effect of conversion to INHD on laboratory parameters and ESA dosage over time. In addition, a subset analysis for patients who were converted to a high-calcium bath (6.2 mg/dl, 1.55 mmol/L) was performed using linear mixed modeling for the effects on alkaline phosphatase (AP) over time, controlling for parathyroid hormone (PTH) and corrected calcium levels as random effects. P < 0.05 was used to define statistical significance in all comparisons. Statistical analysis was performed with SPSS 11.0.1 (SPSS Inc., Chicago, IL).
Results
The demographics of the 39 patients recruited into the INHD program are presented in Table 1. The indications to start INHD were hyperphosphatemia (n = 23), employment and/or lifestyle issues (n = 7), and overall quality of life (n = 3). One patient enrolled to facilitate gentler ultrafiltration in the setting of severe chronic congestive heart failure. Four patients enrolled for other medical reasons. The final patient switched from peritoneal dialysis to HD and sought enrollment into INHD to facilitate employment. Seventeen patients left the program: Five for renal transplantation, three for spousal concerns, two for lifestyle concerns, one for sleeping difficulties, one for transfer to another hospital, one for transfer to peritoneal dialysis, and one for discontinuation of dialysis, and three patients died. The median duration of follow-up on INHD was 1.9 yr.
Table 1.
Patient demographicsa
| Demographic | Value |
|---|---|
| N | 39 |
| Male gender (n [%]) | 26 (67) |
| Age starting INHD (yr; median [25th, 75th percentiles]) | 49 (39, 63) |
| Age (yr; range) | 20 to 84 |
| INHD Treatment time (yr; median [25th, 75th percentiles]) | 1.9 (0.4, 2.8) |
| INHD treatment time (d; range) | 22 to 1246 |
| Time receiving dialysis before INHD (yr; median [25th, 75th percentiles]) | 3.1 (1.3, 6.8) |
| Baseline dialysis modality (n [%]) | |
| in-center hemodialysis | 38 (97) |
| PDb | 1 (3) |
| ever on PD before conversion to INHD | 8 (21) |
| Vascular access (n [%]) | |
| arteriovenous fistula | 17 (44) |
| arteriovenous graft | 2 (5) |
| tunneled dialysis catheter | 20 (51) |
| Cause of kidney disease (n [%]) | |
| glomerulonephritis | 15 (38) |
| diabetes | 7 (18) |
| hypertension | 2 (5) |
| polycystic kidney disease | 3 (8) |
| other | 12 (31) |
INHD, in-center nocturnal hemodialysis; PD, peritoneal dialysis
Treatment time on PD was 5.9 yr.
The laboratory data are presented in Table 2 for the year before conversion to INHD, at the time of conversion, and at 3-mo intervals up to 12 mo. Serum phosphorus and calcium-phosphorus product improved significantly (P < 0.01). The AP increased from 84 to 161 U/L (P < 0.01). Ten patients who had their calcium-based phosphate binders discontinued experienced a fall in their serum calcium and an increase in AP and were placed on a high-calcium bath. Among these 10 patients, two had a parathyroidectomy during the study period. For the remaining eight patients, switching to a high-calcium bath predicted a fall in AP of 66.9 U/L (95% CI 22.6 to 111.3; P < 0.01) independent of corrected calcium and PTH.
Table 2.
Comparison of laboratory data from the 12 mo before conversion to INHD with the intervals up to 12 mo after conversion to INHDa
| Parameter | 12 Mo before INHD | Baseline at Conversion | 3 Mo after INHD | 6 Mo after INHD | 9 Mo after INHD | 12 Mo after INHD | Pa |
|---|---|---|---|---|---|---|---|
| N | 39 | 39 | 33 | 28 | 26 | 25 | |
| PRU (%)b | 0.74 (0.69 to 0.78) | 0.76 (0.71 to 0.82) | 0.88 (0.71 to 0.91) | 0.89 (0.87 to 0.90) | 0.90 (0.85 to 0.93) | 0.89 (0.79 to 0.93) | <0.01 |
| Corrected calcium (mg/dl) | 9.6 (9.1 to 10.0) | 9.5 (9.1 to 10.2) | 9.4 (9.2 to 9.7) | 9.6 (8.9 to 9.8) | 9.2 (9.0 to 9.6) | 9.3 (9.2 to 9.8) | NS |
| Phosphorus (mg/dl) | 5.9 (4.3 to 7.5) | 5.4 (3.6 to 6.9) | 5.0 (4.4 to 6.3) | 4.7 (4.2 to 5.9) | 4.4 (3.8 to 6.7) | 3.7 (3.3 to 5.0) | <0.01 |
| C × P (mg2/dl2) | 56.6 (39.1 to 75.0) | 51.3 (32.8 to 70.4) | 47 (40.5 to 61.1) | 45.1 (37.4 to 57.8) | 40.5 (34.2 to 64.3) | 34.4 (30.4 to 49.0) | <0.01 |
| PTH (pg/ml) | 220.9 (75.5 to 391.8) | 234.5 (93.6 to 525.5) | 340 (197.3 to 357.3) | 302.7 (102.7 to 547.3) | 311.8 (241.8 to 697.3) | 326.4 (117.3 to 643.6) | NS |
| AP (U/L) | 84 (63 to 129) | 102 (74 to 168) | 122 (74 to 158) | 142 (105 to 245) | 134 (107 to 201) | 161 (105 to 234) | <0.01 |
| Hemoglobin (g/dl) | 11.5 (10.6 to 12.2) | 11.5 (10.2 to 12.7) | 11.7 (10.7 to 12.4) | 11.7 (10.5 to 12.9) | 11.9 (11.2 to 13.0) | 12.6 (11.4 to 12.8) | NS |
| Iron saturation (%) | 0.22 (0.17 to 0.29) | 0.24 (0.16 to 0.34) | 0.22 (0.18 to 0.34) | 0.29 (0.19 to 0.36) | 0.29 (0.18 to 0.30) | 0.27 (0.19 to 0.30) | NS |
Statistical analysis, by repeated measures, included all of the data to study end. To convert phosphorus in mg/dl to mmol/L, multiply by 0.323; calcium in mg/dl to mmol/L, multiply by 0.25; parathyroid hormone in pg/ml to pmol/L, multiply by 0.11; ferritin in ng/ml to pmol/L, multiply by 2.247; hemoglobin in g/dl to g/L, multiply by 10; albumin in g/dl to g/L, multiply by 10. AP, alkaline phosphatase; C × P, calcium-phosphorus product; PTH, parathyroid hormone.
PRU, percent reduction of urea.
The mean number of antihypertensive medications taken daily declined from 2.0 to 1.5 (n = 18; P < 0.05) and the total daily dosages of phosphate binders declined from 6.2 to 4.9 (n = 19; P < 0.05) at study end.
Complete data for ESA use were available for 34 patients. ESA dosage fell significantly from starting INHD to the end of the study. Using a conversion ratio for darbepoetin alfa to epoetin alfa of 200:1 there was a 2089 U/wk decrease (95% CI 999 to 3178; P < 0.01). The results were similar using a 330:1 conversion whereby a 1992-U/wk decrease was observed (95% CI 873 to 3111; P < 0.01).
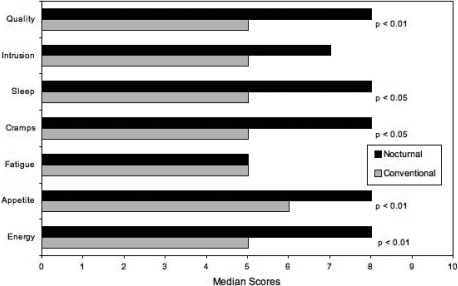
The quality-of-life questionnaire was completed by 23 patients (Figure 1). Overall quality of life, overall sleep on both dialysis and nondialysis nights, intradialytic cramps and dizziness, appetite, and energy level were improved on INHD when compared with conventional HD (P < 0.05 for these parameters). Fatigue and the intrusion of dialysis into daily life did not change significantly.
Figure 1.
Quality-of-life questionnaire (n = 23); see text for details.
The nurse's preference questionnaire revealed that among the 21 ward nurses who had worked both on the ward and in the INHD program, 16 were happy to continue in the dual role, whereas five preferred to work only on the ward. Among the six new recruits, all were interested in being trained for the dual role. During the interviews, some ward nurses who expressed concerns about their dual role later enjoyed the INHD experience and preferred to continue with it.
Discussion
Lower serum phosphate levels and calcium-phosphate products were achieved in patients who previously had poor control while on conventional dialysis despite aggressive dietary and pharmaceutical interventions. These findings are consistent with those reported with other intensive dialysis regimens (21,22). Improved phosphate control should lead to reductions in PTH and AP. We observed a significant rise in AP and a trend toward higher PTH, as seen in patients who converted to HNHD (23). Previous studies of HNHD patients suggested that typical dialysate calcium concentrations led to a net removal of calcium while on dialysis, with rates estimated at approximately 2 mmol/h elemental calcium (80 mg/h) with a standard-calcium bath (5.0 mg/dl, 1.25 mmol/L) and 0.5 mmol/h (20 mg/h) with a high-calcium bath (6.0 mg/dl, 1.5 mmol/L) (24). If the estimates from HNHD are similar to those performing INHD, then a standard calcium bath would result in a weekly loss of nearly 2 g of elemental calcium. This could potentially be replaced with oral calcium; however, patients who received nocturnal HD, in both our study and previous reports (23), typically reduced or discontinued oral calcium as a phosphate binder. We have demonstrated that the unfavorable trends in AP and PTH can be reversed by switching to a high-calcium bath, as has been reported with HNHD (23). The optimal dialysate calcium concentration remains to be defined; however, one might gain guidance from the measurement of postdialysis serum calcium and phosphate levels.
Patients reported improvements in global quality of life and parameters on INHD; however, these results should be viewed as an exploratory assessment. The quality-of-life tool was designed for quality control and has not been validated, and patients were asked to assess their current quality of life on INHD and retrospectively rate it when on conventional dialysis, which is limited by a variety of recall biases. Selection biases also may have affected our results, because patients who were doing well on conventional HD were not recruited for the INHD program.
An additional weakness of this study is the incomplete information on medications at the outset of this study, because phosphate binder and antihypertensive prescriptions before conversion to INHD were available for a limited number of patients. Nevertheless, an analysis of the medication consumption part way through the INHD experience, when an electronic medication list was available, still showed a significant reduction in dosages by study end. Although these data are suggestive, complete data will be required to make definitive statements concerning antihypertensive and phosphate binder consumption after conversion to INHD.
A number of operational difficulties were identified. The problem of chair and bed movement and storage was solved with the change to a bed chair that served the needs of both the conventional and nocturnal HD patients. Staffing of an INHD program provided a challenge, because the culture of HD nurses in our institution included an expectation that regular night duty would not be required. Our INHD program was therefore staffed with nephrology ward nurses who had been trained in HD. This added area of responsibility did not meet with universal acceptance. Our questionnaires indicated that some nurses are best excluded from the rotation; however, some who initially disliked the INHD experience (primarily because of insecurity with the new responsibilities) evolved to be committed to the dual role. While staffing an INHD program with experienced HD nurses would be ideal, recruitment may be difficult. Depending on the availability of experienced HD nurses, one could consider making INHD part of the routine HD rotation, but this risks resignations of nurses who may be in short supply. For a hospital-based INHD program, the best option may be our model, with the opportunity for some nurses to opt out, either from the onset or after a 3-mo trial period.
Physician visits also required a new approach. Patients met their staff nephrologist and an advanced practice nurse in a clinic held either before or in the morning after their dialysis. Appointments with other allied health personnel were arranged as needed. There was an occasional need for the ward housestaff to assess urgent issues during the nocturnal treatment, such as chest pain or unexplained hypotension. Although the requirement for physician presence during the nocturnal treatments was rare, it did occur, and dialysis programs who consider INHD will need to allow for this possibility. Certain patient adjustments were also necessary: Accommodating to some less experienced nurses, accepting the absence of physicians during the treatment, and adjusting to sleeping at the hospital. Programs that consider INHD should recognize that the nursing time required per patient is approximately double that required for conventional HD because the treatment time is doubled; however, as treatment and ultrafiltration times are prolonged, alarms are less frequent, and, therefore, an increased patient-to-nurse ratio may be possible.
Conclusions
Our study represents an initial exploration into the effectiveness, safety, and feasibility of INHD. Future larger, controlled, randomized studies are needed to examine prospectively the effectiveness and cost-effectiveness of INHD on long-term outcomes. Notwithstanding cost and the need to train nurses to deliver INHD effectively, our study demonstrates that INHD is safe and effective and offers another option for therapy for patients who require dialysis.
Disclosures
None.
Acknowledgments
This study was supported, in part, by an unrestricted infrastructure support grant to the Division of Nephrology Clinical Research office, from Ortho-Biotech (Toronto, Ontario, Canada). The remainder of the support was from internal funding. Sponsors had no part in the design, execution, analysis, or reporting of this study.
Research was conducted at St. Michael's Hospital, University of Toronto (Toronto, Ontario, Canada).
We acknowledge the commitment and compassion of the nephrology ward nursing staff, who deliver the outstanding care to the INHD patients. The support of the administration in the development and operation of the INHD is gratefully acknowledged.
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “New Options to Improve Hemodialysis Patient Outcomes,” on pages 694–695.
References
- 1.Collins AJ, Foley R, Herzog C, Chavers B, Gilbertson D, Ishani A, Kasiske B, Liu J, Mau LW, McBean M, Murray A, St Peter W, Xue J, Fan Q, Guo H, Li Q, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Zhang R, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L: Excerpts from the United States Renal Data System 2007 annual data report. Am J Kidney Dis 51[Suppl 1]: S1–320, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Gudex CM: Health-related quality of life in endstage renal failure. Qual Life Res 4: 359–366, 1995 [DOI] [PubMed] [Google Scholar]
- 4.McFarlane PA, Bayoumi AM, Pierratos A, Redelmeier DA: The quality of life and cost utility of home nocturnal and conventional in-center hemodialysis. Kidney Int 64: 1004–1011, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Laurent G, Charra B: The results of an 8 h thrice weekly haemodialysis schedule. Nephrol Dial Transplant 13[Suppl 6]: 125–131, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Yuen D, Richardson RM, Chan CT: Improvements in phosphate control with short daily in-center hemodialysis. Clin Nephrol 64: 364–370, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Ayus JC, Achinger SG, Mizani MR, Chertow GM, Furmaga W, Lee S, Rodriguez F: Phosphorus balance and mineral metabolism with 3h daily hemodialysis. Kidney Int 71: 336–342, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Mucsi I, Hercz G, Uldall R, Ouwendyk M, Francoeur R, Pierratos A: Control of serum phosphate without any phosphate binders in patients treated with nocturnal hemodialysis. Kidney Int 53: 1399–1404, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Chan CT, Floras JS, Miller JA, Richardson RM, Pierratos A: Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney Int 61: 2235–2239, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Chan C, Floras JS, Miller JA, Pierratos A: Improvement in ejection fraction by nocturnal haemodialysis in end-stage renal failure patients with coexisting heart failure. Nephrol Dial Transplant 17: 1518–1521, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Chan CT, Harvey PJ, Picton P, Pierratos A, Miller JA, Floras JS: Short-term blood pressure, noradrenergic and vascular effects of nocturnal home hemodialysis. Hypertension 42: 925–931, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Chan CT, Jain V, Picton P, Pierratos A, Floras JS: Nocturnal hemodialysis increases arterial baroreflex sensitivity and compliance and normalizes blood pressure of hypertensive patients with end-stage renal disease. Kidney Int 68: 338–344, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, Tonelli M, Donnelly S, Friedrich MG, Kumar A, Mahallati H, Hemmelgarn BR, Manns BJ: Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life. JAMA 298: 1291–1299, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Yuen D, Pierratos A, Richardson RM, Chan CT: The natural history of coronary calcification progression in a cohort of nocturnal haemodialysis patients. Nephrol Dial Transplant 21: 1407–1412, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Heidenheim AP, Muirhead N, Moist L, Lindsay RM: Patient quality of life on quotidian hemodialysis. Am J Kidney Dis 42[Suppl]: 36–41, 2003 [DOI] [PubMed] [Google Scholar]
- 16.McPhatter LL, Lockridge RS Jr, Albert J, Anderson H, Craft V, Jennings FM, Spencer M, Swafford A, Barger T, Coffey L: Nightly home hemodialysis: Improvement in nutrition and quality of life. Adv Ren Replace Ther 6: 358–365, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Brissenden JE, Pierratos A, Ouwendyk M, Roscoe JM: Improvements in quality of life with nocturnal hemodialysis [Abstract]. J Am Soc Nephrol 9: 168A, 1998 [Google Scholar]
- 18.Ramkumar N, Beddhu S, Eggers P, Pappas LM, Cheung AK: Patient preference for in-center intense hemodialysis. Hemodial Int 9: 281–295, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Nissenson AR, Swan SK, Lindberg JS, Soroka SD, Beatey R, Wang C, Picarello N, McDermott-Vitak A, Maroni BJ: Randomized, controlled trial of darbopoetin alfa for the treatment of anemia in hemodialysis patients. Am J Kidney Dis 40: 110–118, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Centers for Medicare & Medicaid Services—Medicare Program: Changes to the Hospital Outpatient Prospective Payment System and Calendar Year 2005 Rates; Final Rule. 42 CFR Part 419. Fed Reg 69: 65681–66234, 2004 [PubMed] [Google Scholar]
- 21.Pierratos A, McFarlane P, Chan CT: Quotidian dialysis: Update 2005. Curr Opin Nephrol Hypertens 14: 119–124, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Kurella M, Hung AM, Tichy M, Hsu C, Chertow GM: Intermittent nocturnal in-center hemodialysis: UCSF-Mt Zion experience [Abstract]. J Am Soc Nephrol 13: 410A, 2002 [Google Scholar]
- 23.Lindsay RM, Al-Hejaili F, Nesrallah G, Leitch R, Clement L, Heidenheim AP, Kortas C: Calcium and phosphate balance with quotidian hemodialysis. Am J Kidney Dis 42[Suppl]: 24–29, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Al-Hejaili F, Kortas C, Leitch R, Heidenheim AP, Clement L, Nesrallah G, Lindsay RM: Nocturnal but not short hours quotidian hemodialysis requires an elevated dialysate calcium concentration. J Am Soc Nephrol 14: 2322–2328, 2003 [DOI] [PubMed] [Google Scholar]