Abstract
Background and objectives: Extended hemodialysis using a high cut-off dialyzer (HCO-HD) removes large quantities of free light chains in patients with multiple myeloma. However, the clinical utility of this method is uncertain. This study assessed the combination of chemotherapy and HCO-HD on serum free light chain concentrations and renal recovery in patients with myeloma kidney (cast nephropathy) and dialysis-dependent acute renal failure.
Design, setting, participants, & measurements: An open-label study of the relationship between free light chain levels and clinical outcomes in 19 patients treated with standard chemotherapy regimens and HCO-HD.
Results: There were sustained early reductions in serum free light chain concentrations (median 85% [range 50 to 97]) in 13 patients. These 13 patients became dialysis independent at a median of 27 d (range 13 to 120). Six patients had chemotherapy interrupted because of early infections and did not achieve sustained early free light chain reductions; one of these patients recovered renal function (at 105 d) the remaining 5 patients did not recover renal function. Patients who recovered renal function had a significantly improved survival (P < 0.012).
Conclusion: In dialysis-dependent acute renal failure secondary to myeloma kidney, patients who received uninterrupted chemotherapy and extended HCO-HD had sustained reductions in serum free light chain concentrations and recovered independent renal function.
Free light chains (FLCs) are by-products of intact Ig synthesis and, under physiologic circumstances, are rapidly removed from the circulation by renal clearance. In patients with multiple myeloma, however, the clonal proliferation of plasma cells may result in serum concentrations of monoclonal FLCs several thousand times higher than normal (1). These high concentrations may result in cast nephropathy (myeloma kidney) which accounts for around 70% of dialysis-dependent renal failure in multiple myeloma patients (Winearls [14 of 20], 2; Montseny [22 of 43] (3); Innes [11 of 14] (4); Magee [16 of 21] (5). This is associated with high morbidity and mortality (6–9). Patient outcomes improve, however, if the renal function recovers (10). The published studies that report renal outcomes are presented in Table 1. The majority of the patients included in these studies did not require dialysis at presentation and/or had no renal histology reported; of patients with biopsy-proven cast nephropathy and dialysis-dependent renal failure, fewer than 25% became independent of dialysis (3,5,6,19). Only one of these studies also reported serum FLC concentrations (19).
Table 1.
Renal outcomes of patients with acute renal failure secondary to multiple myeloma
| Author | Year Published | No. of Patients | RCT | Intervention | Definition of Renal Failure | Histology Reported | Renal Recovery Rate of Non-Dialysis Dependent | Renal Recovery Rate of Dialysis Dependent | Rate of Independence of Dialysis Where Histology Reported as FLC Related |
|---|---|---|---|---|---|---|---|---|---|
| Zucchelli (11) | 1988 | 29 | Yes | PLEX | SC > 5 mg/dl | Partial | Control 0% (0 of 3) | Control 18% (2 of 11) | — |
| PLEX 100% (2 of 2) | PLEX 85% (11 of 14) | ||||||||
| Johnson (12) | 1990 | 21 | Yes | PLEX | Progressive renal failure | Yes | Control 63% (5 of 8) | Control 0% (0 of 2) | — |
| PLEX 87% (7 of 8) | PLEX 100% (3 of 3) | ||||||||
| Rayner (13) | 1991 | 11 | No | — | Dialysis dependent | No | — | 36% (4 of 11) | — |
| Torra (6) | 1995 | 30 | No | — | Dialysis dependent | No | — | 3% (1 of 30) | 3% (1 of 30) |
| Irish (14) | 1997 | 56 | No | — | Acute | No | — | 15% (7 of 47) | — |
| Bladé (7) | 1998 | 94 | No | Chemotherapy | SC > 2 mg/dl | No | 26% (24 of 91) | — | — |
| Montseny (3) | 1998 | 118 | No | — | Histology | Yes | — | 11% (6 of 52) | 11% (6 of 52) |
| Magee (5) | 1998 | 34 | No | — | Acute | Partial | — | 3% (1 of 28) | — |
| Knusden (8) | 2000 | 225 | No | — | SC > 2.3 mg/dl | No | 58% (130 of 225) | 0% (0 of 8) | — |
| Clark (15) | 2005 | 97 | Yes | PLEX | SC > 2.3 mg/dl+ | No | — | Control 37% (7 of 19) | — |
| PLEX 42% (10 of 24) | |||||||||
| Ludwig (16) | 2007 | 8 | No | Chemotherapy | eGFR < 20 ml/min/1.73 m2 | No | 63% (5 of 8) | — | — |
| Kastritis (17) | 2007 | 41 | No | Chemotherapy | SC > 2 mg/dl | No | 73% (30 of 41) | 80% (8 of 10) | — |
| Chanan-Khan (18) | 2007 | 24 | No | Chemotherapy | Dialysis dependent | No | — | 17% (4 of 24) | — |
| Leung (19) | 2008 | 40 | No | PLEX | 50% above baseline or SC > 2 mg/dl | Yes | 45% (18 of 40) | 22% (2 of 9) | 22% (2 of 9) |
| Current study | 2008 | 19 | No | HCO-HD | Dialysis dependent | Yes | — | 74% (14 of 19) | 74% (14 of 19) |
†with an increase greater than 0.6 mg/dL in the preceding 2 weeks despite correction of reversible factors.
SC, serum creatinine; PLEX, plasma exchange; HCO-HD, high cut-off hemodialysis.
In an attempt to improve outcomes, direct removal of FLCs by plasma exchange (PE) has been studied (11,12). A recent randomized controlled trial of 97 patients with multiple myeloma and acute renal failure failed, however, to demonstrate any clinical benefit (15). In this study, renal biopsies were not reported, serum FLC concentrations were not quantified, and the majority of patients were not dialysis dependent at presentation (20). Furthermore, PE does not result in sustained reductions in serum FLC concentrations, as demonstrated by both clinical observations (21) and mathematical modeling (22). Leung et al. recently demonstrated that patients with cast nephropathy are more likely to recover renal function if a 50% decrease in serum FLC concentrations is achieved (19). However, they failed to demonstrate any relationship between the amount of plasma exchange received and the degree of reduction in FLC concentrations (P = 0.9) or the renal response (P = 0.28).
To provide an alternative approach to plasma exchange for direct FLC removal, we recently assessed the utility of extended hemodialysis (HD), using a high cut-off (HCO) dialyzer (1). Detailed mathematical modeling showed HCO-HD to be far more effective for FLC removal than PE. In a pilot study, induction chemotherapy in combination with extended treatment by HCO-HD resulted in sustained reductions in serum FLC concentrations in three of five patients. These three patients subsequently became independent of dialysis.
The aims of the current study were, first, to determine whether chemotherapy in combination with FLC removal by HCO-HD resulted in sustained reductions in serum FLC concentrations in a cohort of patients with cast nephropathy; second, to relate changes in serum FLC levels to renal recovery.
Materials and Methods
This single-center, prospective, pilot study was undertaken between April 2006 and May 2008. The study was approved by the Solihull and South Birmingham Research Ethics Committees and the Research and Development Department of University Hospital Birmingham National Health Service Foundation Trust. The study fully adheres to the Declaration of Helsinki, and all patients gave informed oral and written consent. Patient data were coded and stored anonymously.
Study Populations
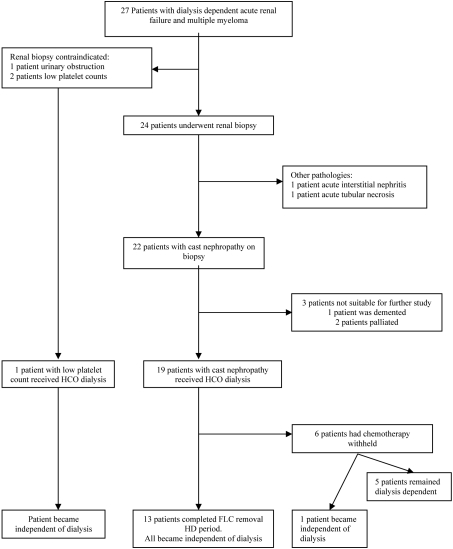
During the study period, 27 patients with multiple myeloma and dialysis-dependent renal failure presented or were referred to our unit (from regional centers). Dialysis-dependent renal failure was defined as an estimated GFR of < 15 mls/min/1.73 m2, as calculated by the abbreviated MDRD equation (23). Twenty-four of the patients were assessed for the pathogenesis of their acute renal failure by renal biopsy. In three patients, renal biopsy was contraindicated: in two because of low platelet counts (< 60 × 109/L), and in one because of urinary tract obstruction. Renal histology showed cast nephropathy in 22 cases, acute interstitial nephritis in one case, and acute tubular necrosis in one case. Patients with biopsy-proven cast nephropathy were then considered for FLC removal HD as an adjunct to chemotherapy. Three patients were excluded: two because of relapsing disease at presentation which was not suitable for new therapies and one because of dementia. Nineteen patients with cast nephropathy were approached and agreed to inclusion in the FLC removal HD study. Sixteen patients had de novo multiple myeloma, and the remaining three had relapsing disease. One additional patient with a heavy serum FLC load (26 g/L), who did not undergo a renal biopsy (because of a low platelet count), received FLC removal HD and induction chemotherapy. The pathway for the patient cohort from presentation with renal failure is shown in Figure 1.
Figure 1.
Schematic of patients assessed for and treated with free light chain (FLC) removal hemodialysis (HD).
Chemotherapy Regimes
Cyclophosphamide (seven patients), thalidomide (14 patients), vincristine/doxorubicin (one patient), and high dose dexamethasone (all patients) were the principal chemotherapies used for patients with de novo multiple myeloma in the study group. Patients with relapsing multiple myeloma received bortezomib in combination with doxorubicin and high-dose dexamethasone.
FLC Removal by HCO-HD
FLC removal HD was undertaken using extended HD with the Gambro HCO 1100 dialyzer (Gambro Dialysatoren GmbH, Hechingen, Germany). Two HCO 1100 dialyzers were used in series as described previously (1). Blood and dialysate flow rates were 250 ml/min and 500 ml/min, respectively. Fluid removal was determined on clinical grounds by the supervising clinician, with the aim not to dehydrate the patient. Hemodiafiltration was not used. A dialysis schedule of eight hours daily for five days, eight hours on alternate days for the next 12 d, then six hours three times per week was followed. Extended dialysis periods were supported by the regular replacement of human albumin solution, magnesium, and calcium by protocol. Previous experience has demonstrated that all patients receiving extended HCO-HD will require human albumin solution to replace that lost, and many will also require magnesium and calcium replacement (24). Therefore, 40 g of salt-poor human albumin solution was given at the end of every 8-h dialysis session. If predialysis serum concentrations of magnesium or calcium were low, intravenous magnesium was given at the end of dialysis and oral calcium was supplemented, respectively. FLC removal HD was continued until the patients became independent of dialysis. At 6 wk, patients who remained dialysis dependent were reassessed for continuation of HCO-HD. Renal recovery was defined as being clinically independent of dialysis 2 wk after the last dialysis session, as determined by the supervising nephrologist, with a minimum estimated GFR of > 10 ml/min/1.73 m2.
Serum FLC Concentrations After Combined Chemotherapy and HCO-HD
Serum κ and λ FLC concentrations were measured by nephelometry, on a Dade-Behring BNII Analyzer, using a particle-enhanced, high specificity, homogeneous immunoassay (FREELITE, The Binding Site, Birmingham, UK) (25). Normal serum reference ranges used were κ: 7.3 mg/L (range 3.3 to 19.4) and λ: 12.7 mg/L (range 5.7 to 26.3), with an assay sensitivity of < 1 mg/L (26). The percentage reductions in the monoclonal serum FLC concentrations achieved by extended HCO-HD were assessed at 5, 12, and 21 d after the commencement of treatment.
A two-compartment mathematical model, previously described, was used to calculate the FLC production rates at the above-referenced time points (1,22). Briefly, this consisted off intra- and extravascular compartments. FLCs are produced by the bone marrow into the intravascular compartment and removed from both compartments by the reticuloendothelium at previously calculated rate constants. For the calculations, clearance of FLCs from the intravascular compartment by the kidneys was 0 ml/min, and direct removal by HCO-HD was based on previously calculated rate constants.
Statistical Analysis
The statistical package SPSS (version 14.0) was used to analyze results. Nonparametric results were expressed as medians with ranges. Comparisons between clinical outcome groups were analyzed by the Mann-Whitney U test. Reductions in serum FLC concentrations were compared using the Kruskal-Wallis test. Rates of renal recovery and survival were assessed using Kaplan-Meier estimates.
Results
During the study period, 27 patients with multiple myeloma and dialysis-dependent acute renal failure presented or were referred to our center. Nineteen of these patients met study criteria (Figure 1). Table 2 presents the demographic and baseline biochemical and immunological characteristics of the study population. Table 3 provides a detailed description of the 19 study patients’ demographics, myeloma type (including FLC concentrations), renal presentation, and outcomes.
Table 2.
Summary of the demographics and presenting features of study population
| Feature | Study group (n = 19) |
|---|---|
| Age, years | 60 (38 to 81) |
| Male gender, % | 74% |
| Presenting creatinine, μmol/L | 714 (427 to 1508) |
| Presenting eGFR | 7 (3 to 13) |
| Patients with κ FLCs, % | 50% |
| Presenting serum FLC concentration, g/L | 2.6 (0.8 to 69) |
Data are shown as median (range). eGFR, estimated glomerular filtration rate (ml/min/1.73 m2, calculated by the four-variable MDRD equation) (15).
Table 3.
Study populations demographics, presenting features and outcomes
| Patient | Age | Gender | Ethnicity | Myeloma
|
Previous GFRa | GFR at Presentation | Oligo-anuricb | Hyper-calcaemia | NSAIDc | Presenting FLC Concentration (mg/L) | Interruption to Chemotherapyd | Renal Recovery | Percentage FLC Reduction at Outcomee | Duration of HCO-HD (days) | GFR at 3 monthsf | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Presentation | Type | |||||||||||||||
| 1 | 51 | Male | Ca | New | IgA K | n/a | 11 | No | Yes | No | 42,000 | Yes | No | n/a | 27 | n/a |
| 2 | 68 | Female | Ca | New | IgG L | n/a | 5 | No | No | No | 1120 | Yes | No | n/a | 39 | n/a |
| 3 | 58 | Male | Ca | New | IgG L | 58 | 13 | No | No | No | 855 | Yes | No | n/a | 32 | n/a |
| 4 | 64 | Female | Ca | New | IgA L | n/a | 5 | Yes | Yes | Yes | 1740 | Yes | No | n/a | 5 | n/a |
| 5 | 52 | Male | Ca | Relapsing | Free L | 23 | 9 | No | No | No | 69,430 | Yes | No | n/a | 29 | n/a |
| 6g | 59 | Male | Ca | New | Free L | n/a | 4 | No | No | No | 26,382 | No | Yes | 96 | 14 | 83 |
| 7 | 68 | Female | Ca | New | Free K | 30 | 8 | No | No | No | 18,500 | No | Yes | 81 | 8 | 45 |
| 8 | 62 | Male | AC | MGUS | IgG K | n/a | 6 | No | No | Yes | 1030 | No | Yes | 83 | 18 | 45 |
| 9 | 53 | Male | IA | New | IgA K | >60 | 13 | No | No | No | 13,500 | No | Yes | 50 | 34 | 48 |
| 10 | 81 | Male | Ca | New | IgG L | n/a | 7 | Yes | No | No | 2110 | No | Yes | 90 | 29 | 34 |
| 11 | 61 | Male | IA | Relapsing | IgG K | >60 | 3 | No | No | No | 2520 | No | Yes | 60 | 18 | 76 |
| 12 | 71 | Male | Ca | New | IgA L | 49 | 3 | No | No | No | 4200 | No | Yes | 65 | 22 | 34 |
| 13 | 46 | Male | Ca | New | IgG K | n/a | 9 | No | No | No | 1780 | No | Yes | 86 | 26 | 29 |
| 14 | 64 | Male | IA | Relapsing | IgG L | 31 | 9 | No | No | No | 2530 | No | Yes | 93 | 19 | 37 |
| 15 | 61 | Female | Ca | New | Free K | n/a | 5 | No | No | No | 3000 | No | Yes | 87 | 45 | 11h |
| 16 | 38 | Male | Ca | New | Free K | n/a | 7 | No | No | No | 27,000 | No | Yes | 87 | 28 | 27 |
| 17 | 60 | Male | Ca | New | IgG K | n/a | 12 | No | No | No | 2254 | No | Yes | 79 | 12 | 67 |
| 18 | 67 | Male | Ca | CLL | IgM L | >60 | 4 | No | No | No | 2585 | No | Yes | 82 | 39 | 43 |
| 19 | 55 | Female | Ca | New | Free K | n/a | 8 | No | No | No | 8076 | Yes | Yes | 97 | 105 | 17h |
| 20 | 56 | Male | Ca | New | IgA L | n/a | 6 | No | No | No | 9918 | No | Yes | 97 | 56 | 27 |
n/a, not available; Ca, Caucasian; AC, Afro-Caribbean; IA, Indo-Asian; MGUS, monoclonal gammopathy of undetermined significance.
GFR if known 3 to 12 mo before presentation.
Defined as a urine output of < 500 ml/24 h.
Nonsteroidal anti-inflammatory drug (NSAID) use preceding presentation with renal failure.
Interruption to chemotherapy during the first 6 wk of treatment.
Free light chain (FLC) concentration at dialysis independence.
GFR at 3 mo from enrollment.
Patient 6 did not have a renal biopsy because of low platelets but was treated to protocol; the 19 remaining patients all had cast nephropathy on renal biopsy.
These patients became independent of dialysis after 3 months therefore the GFRs presented were those at 2 wk after the last dialysis session.
Safety and Tolerability of Induction Chemotherapy and FLC Removal HD
In the first 6 wk of treatment, six patients had chemotherapy interrupted because of complications, which were predominantly infections; these are documented in Table 4. Two of these patients had chemotherapy restarted within the first 6 wk, two died, and two had chemotherapy restarted after the first 6 wk.
Table 4.
Complications within the study population, necessitating early withdrawal of chemotherapy
| Patient Number | Myeloma | Complications (day) | Chemotherapy Withheld (day) | Chemotherapy Restarted Within the 6-wk Treatment Perioda | Chemotherapy Restarted After the First 6 wk | Cause of Death (day) |
|---|---|---|---|---|---|---|
| 1 | De novo | Line sepsis (19), LRTI (39) | Thal/Dex (27) | No | Yes | Progressive disease (331) |
| 2 | De novo | Line sepsis (14), ACS (30) | Dex (14), Thal (30) | No | Yes | Progressive disease (220) |
| 3 | De novo | ACS (8), line sepsis (9) | Thal (8), Dex (9) | Dex (27) | n/a | Fungal pneumonia (35) |
| 4 | De novo | Pneumonia (3) | Thal/Dex (3) | No | n/a | Intracranial hemorrhage (21) |
| 5 | Relapsing | Neutropenic sepsis (14), LRTI + AF (20) | Dex (14)b | Yes (30) | — | Progressive disease (53)c |
| 19 | De novo | Dermatitis (35) | Thal (36) | Yesd | — | n/a |
Complications that resulted in early withdrawal of chemotherapy were predominantly infections. LRTI, lower respiratory tract infection; ACS, acute coronary syndrome; AF, atrial fibrillation; Thal, thalidomide; Dex, dexamethasone; n/a–not applicable.
Four of five patients did not have chemotherapy restarted during the 6-wk treatment period.
Patient was also receiving bortezomib, which was not stopped.
The patient subsequently opted for palliative care after no response to chemotherapy.
Patient developed a severe dermatitis that improved on stopping thalidomide. Dexamethasone was never withheld, and cyclophosphamide was started when thalidomide was withdrawn. Subsequently the patient received bortezomib. Because of poor response to treatment, the patient became independent of dialysis at 105 days.
Thirteen patients completed 6 wk of uninterrupted treatment. Three of these patients had infections (two urinary tract and one line related) that did not necessitate suspension of chemotherapy. Two of the three relapsing patients treated with bortezomib developed grade 2 sensory neuropathy, a recognized side-effect, and treatment was not stopped.
The FLC removal HD was well tolerated by all patients, with no unexpected adverse events related to the dialysis. All patients received human albumin solution at the end of each dialysis session, 15 patients required intermittent magnesium supplementation, and 12 required calcium replacement. There were no noninfective complications related to venous access or anticoagulation.
Reductions in Serum FLC Concentrations After Combined Chemotherapy and FLC Removal HCO-HD
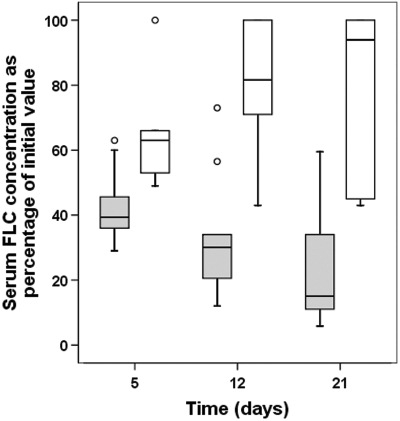
There were sustained early reductions in serum FLC concentrations in 13 of the 19 study patients. The 13 patients who achieved sustained reductions in serum FLC concentrations were those who had uninterrupted courses of chemotherapy. The six patients who did not achieve sustained early reductions in serum FLC concentrations all had chemotherapy interrupted for variable periods, because of complications, principally infective. The results of these six patients were compared with the 13 patients who completed the treatment period without a break in chemotherapy. The details of the FLC removal HCO-HD received are presented in Table 5. There were no significant differences in the amount of dialysis received between the two groups with respect to number of dialysis sessions (P = 0.3), hours per day of HCO-HD during the first 21 treatment days (P = 0.5), and the total duration of FLC removal HCO-HD (P = 0.62). The effectiveness of FLC removal HCO-HD sessions was comparable between the groups. Median percentage reductions in serum FLC concentrations for single dialysis sessions for patients who received uninterrupted chemotherapy and interrupted chemotherapy for elevated κ FLCs, were 69% (range 45 to 84) and 65% (41–70) (P < 0.2) respectively, and for elevated λ FLCs were 71% (60–90) and 72% (60–92) (P < 0.45) respectively. Figure 2 presents the predialysis serum FLC concentrations for the two groups at days 5, 12, and 21 as percentages of the presenting value. Mathematical modeling demonstrated that the failure to reduce serum concentrations in patients who had chemotherapy withheld was due to sustained high production rates of FLCs, compared with patients who had uninterrupted therapy (median 266% [range 98 to 433]) of presenting production rate versus median 1% [range 0 to 16] at day 12, respectively).
Table 5.
Description of the free light chain (FLC) removal hemodialysis (HD) received by the study population
| Group | No. of FLC Removal HD Sessions Received | No. of Days FLC Removal HD Received | No. of Hours of FLC Removal HDa |
|---|---|---|---|
| Patients completing treatment period (n = 13) | 14 (3–46) | 24 (5–105) | 4.3 (3–5.6) |
| Patients not completing treatment period (n = 6) | 22 (3–29) | 30 (5–58) | 4.6 (3.5–5.7) |
| P | 0.3 | 0.62 | 0.5 |
Patients who completed the study period of combined chemotherapy and FLC removal HD are compared with those who had chemotherapy withheld. There were no significant differences in the dialysis received between these populations. Data is reported as median (range).
During the first 21 days of treatment.
Figure 2.
Comparison of reductions in serum FLC concentrations at 5, 12, and 21 d. Results presented as patients who completed FLC removal HD with (clear boxes, n = 6) and without (shaded boxes, n = 13) a break in their chemotherapy. Patients with uninterrupted chemotherapy had a more rapid and sustained reduction in their serum concentrations of FLCs, P < 0.0001. The median percentage of presenting FLC concentration at day 5 was 63% (range 49 to 100); day 12, 81% (range 43 to 100); and day 21, 94% (range 43 to 100) in patients with interrupted chemotherapy; compared with 39% (range 29 to 63), 30% (range 12 to 73), and 15% (range 5 to 59) in those patients with uninterrupted chemotherapy.
Renal Recovery Rates, Resolution of Cast Nephropathy, and Patient Survival
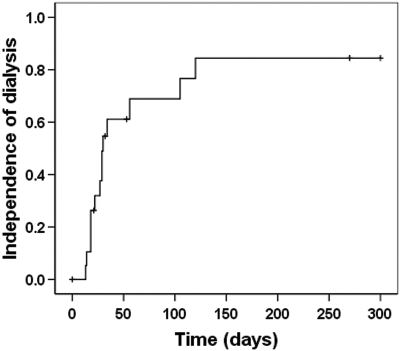
On an intention-to-treat basis, patients receiving chemotherapy and FLC removal HD had a high rate of dialysis independence (14 of 19; Figure 3). This rate was higher than that observed in previous studies in which patients have dialysis-dependent acute renal failure and renal histology is cast nephropathy (Table 1). Twelve of the 14 HCO-HD patients who became independent of dialysis had achieved sustained early reductions in serum concentrations of FLCs. In this group, independence from dialysis occurred at a median of 24 d (range 8 to 58). At 3 mo after following the commencement of treatment, these 13 patients had a median estimated GFR of 40ml/min (range 11 to 83).
Figure 3.
Renal recovery rates of patients who received chemotherapy and FLC removal hemodialysis. Fourteen of the 19 patients who received FLC removal HD became independent of dialysis at a median of 28 d (range 13 to 120).
At 6 wk, three patients were still receiving HCO-HD; one had achieved a sustained reduction in serum FLC concentrations (patient 15) and was converted to standard high-flux dialysis. This patient became independent of dialysis at 120 d. The two patients who did not achieve sustained reductions in serum FLC concentrations were both continued on HCO-HD. One became independent of dialysis at 56 d; the other, 105 d. Two patients had HCO-HD discontinued before 6 wk because it was felt there was a low probability of future recovery in function; one of these patients was converted to a standard high-flux hemodialysis; the other opted for palliative care at this stage.
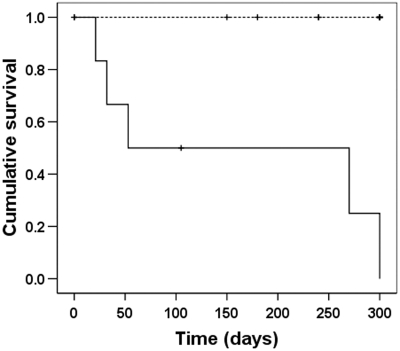
Kaplan-Meier survival analysis demonstrated that early interruption of chemotherapy was associated with significantly worse outcome (P < 0.002) (Figure 4). The median survival for the patients who had chemotherapy stopped was 53 d (range 21 to 331), whereas all patients who did not have chemotherapy stopped were alive at a median follow-up of 360 d (range 150 to 630; P < 0.02). An additional patient who was not included in the analysis because a renal biopsy was not taken, but who received the combined protocol of HCO-HD with uninterrupted chemotherapy, recovered renal function at 21 d
Figure 4.
Kaplan-Meier survival analysis of patients treated with chemotherapy and FLC removal HD. Patients who developed complications requiring early interruption of chemotherapy (solid line, n = 6) had a significantly reduced survival compared with patients who received uninterrupted chemotherapy (broken line, n = 13); P < 0.001.
Discussion
The purpose of this study was to assess the combination of chemotherapy and HCO-HD on serum FLC concentrations in multiple myeloma patients with dialysis-dependent renal failure secondary to cast nephropathy. We also sought to determine the relationship between changes in FLC concentrations and renal recovery.
All of the patients had elevated serum FLC concentrations and cast nephropathy confirmed on biopsy. Thirteen patients (68%) received uninterrupted FLC removal HD and chemotherapy. All 13 achieved sustained reductions in serum FLC concentrations and recovered sufficient renal function to become independent of dialysis. Six patients (29%) had chemotherapy temporarily withheld or discontinued because of early complications, principally infections. These patients did not achieve sustained early reductions in serum FLCs concentrations despite effective removal of FLCs by HCO-HD. Only one of these patients subsequently became independent of dialysis. This occurred after she achieved a late reduction in serum FLC concentration after bortezomib therapy. These results support the finding of previous mathematical modeling which indicated that sustained reductions in serum FLC concentrations require effective chemotherapy. Direct removal of FLCs by HCO-HD in this setting may then lead to more rapid lowering of FLCs (1). However, it is not clear that HCO-HD will provide an additional clinical benefit over standard high-flux HD.
The theoretical basis for any clinical benefit of serum FLC removal depends on the principle that a rapid reduction of serum concentrations may facilitate renal recovery in patients with FLC-induced acute kidney injury. In reports of patients with multiple myeloma and dialysis-dependent acute renal failure for whom renal histology is known, cast nephropathy represents from 57% to 78% of the pathologic diagnoses (2–5). These results are consistent with our findings of cast nephropathy in 91% of patients in whom renal biopsies were performed. Cast nephropathy is a direct consequence of elevated concentrations of monoclonal serum FLCs. FLCs are freely filtered by glomeruli and coprecipitate with Tamm-Horsfall protein to form distal tubular casts that cause intranephronal obstruction (27). In addition, monoclonal FLCs also exhibit direct cytotoxicity to tubular epithelial cells (28,29).
Historically, patients with multiple myeloma and renal failure were treated with combinations of alkylating agents and steroids; by the time of the Clark plasma exchange study, the combination of vincrisitine, doxorubicin, and dexamethasone was becoming the standard of care. Recent interest, however, has focused on the use of novel therapeutic agents, particularly bortezomib, in the hope of establishing improved clinical outcomes (16–18,30,31). Early data are encouraging; Ludwig et al. treated eight patients with GFRs of < 20 ml/min, and five improved (16). None of these patients required dialysis at presentation. Kastritis et al. treated 15 patients with good results, including 10 patients who were classified as requiring dialysis; 8 of these recovered renal function (17). However, in the largest study of patients with dialysis-dependent renal failure (n = 25) the use of bortezomib was associated with fewer than 20% of subjects becoming dialysis-independent (18). In these three studies, no renal histology or serum FLC concentrations were reported.
As presented in Table 1 the historical outcomes for patients with renal failure complicating multiple myeloma vary greatly. This makes a direct comparison of the results of this study with historical results difficult. However, key principals can be determined. First, there are many potential causes of acute renal failure in patients with multiple myeloma, and many of these trials did not report renal biopsies. Therefore, direct comparisons of the outcomes of these study populations are difficult. Second, the timing of the intervention is important. Chanan-Khan et al. treated patients who were already dialysis dependent, as were our population, in contrast to the earlier renal injuries of Ludwig et al. and some of the patients reported by Kastritis et al. Patients who present with a less severe renal injury are more likely to have an improvement or stabilization in renal function with appropriate intervention. Third, historically, the combination of dialysis dependence and FLC-induced renal pathology confirmed by renal histology is associated with very poor renal outcomes (Table 1). The indication from uncontrolled studies is that newer chemotherapy drugs may be improving outcomes. If the current chemotherapy standard of care for dialysis-dependent acute renal failure and myeloma kidney becomes bortezomib based, the role of FLC removal by HCO-HD still warrants further investigation as an adjunct to chemotherapy; even with effective chemotherapy, serum FLC concentrations may remain above the level required for cast formation for several weeks. Animal models demonstrate that after 1 mo of obstruction by casts, irreversible damage occurs to a nephron (32). This indicates that there is a short window of opportunity for directed therapy to reduce serum FLC concentrations and facilitate renal recovery. In support of this theory, Leung et al. have recently demonstrated that in this setting the majority of patients who recovered renal function achieve a reduction in serum FLC concentrations of > 50% (19). Similarly, in this study, all patients who became independent of hemodialysis achieved a > 50% reduction in serum FLC concentrations, and 11 of 14 had a > 75% reduction.
The limitations of this current study principally relate to its design as a proof of principal (pilot) study. The study design did not include a control group; therefore, definitive conclusions of possible clinical benefits of HCO-HD cannot be made as yet. The experience reported in this paper and elsewhere (33,34) has led to the development of a multicenter randomized control trial. EuLITE (European Trial of Free Light Chain Removal by Extended Hemodialysis in Cast Nephropathy) (35) aims to recruit 90 patients with cast nephropathy, dialysis-dependent renal failure, and new myeloma. All patients will receive bortezomib-based chemotherapy, and patients will be randomized to FLC removal by HCO-HD or standard dialysis care. The principal outcome is independence from dialysis at 3 mo from recruitment. The trial is powered to detect a difference of 30% between the two study arms, allowing for a 10% drop out. Because plasma exchange remains a treatment option in this setting (19), a head-to-head trial of FLC removal by HD and plasma exchange may be subsequently required if the results of EuLITE demonstrate a benefit.
Conclusion
This study demonstrated that rapid and sustained reductions in serum FLC concentrations in patients with cast nephropathy were achieved by effective chemotherapy, with adjunctive FLC removal HD. On an intention-to-treat basis, 74% of the cohort became independent of dialysis. A multicenter, randomized control trial has now commenced to address the hypothesis that extended HCO-HD increases the percentage of patients with cast nephropathy who become independent of dialysis.
Disclosures
S.H. is an employee, and G.P.M. and A.R.B. are directors of The Binding Site Ltd., Birmingham, United Kingdom. The remaining authors have no potential conflicts of interest.
Supplementary Material
Acknowledgments
We thank the patients, nurses and physicians who contributed to this project, in particular, Anne-Marie Phythian and Oliver Foster. We are grateful to the clinicians in the regional centers who referred patients for the study. The study was supported by The British Renal Society (Grant Nos. 05 to 007). We thank The Binding Site Ltd. for financial and technical support, in particular Katie Chandler and Jo Harper. We are grateful to Gambro for supplying the dialyzers with no charge. The early outcomes of the first five patients were included in a preliminary reported previously (1).
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Hutchison CA, Cockwell P, Reid S, Chandler K, Mead GP, Harrison J, Hattersley J, Evans ND, Chappell MJ, Cook M, Goehl H, Storr M, Bradwell AR: Efficient removal of immunoglobulin free light chains by hemodialysis for multiple myeloma: In-vitro and in-vivo studies. J Am Soc Nephrol 18: 886–895, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Winearls CG: Acute myeloma kidney. Kidney Int 48: 1347–1361, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Montseny JJ, Kleinknecht D, Meyrier A, Vanhille P, Simon P, Pruna A, Eladari D: Long-term outcome according to renal histological lesions in 118 patients with monoclonal gammopathies. Nephrol Dial Transplant 13: 1438–1445, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Innes A, Cuthbert RJ, Russell NH, Morgan AG, Burden RP: Intensive treatment of renal failure in patients with myeloma. Clin Lab Haematol 16: 149–156, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Magee C, Vella JP, Tormey W, Walshe JJ: Multiple myeloma and renal failure: One center's experience. Ren Fail 20: 597–606, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Torra R, Blade J, Cases A, Lopez-Pedret J, Montserrat E, Rozman C, Revert L: Patients with multiple myeloma requiring long-term dialysis: Presenting features, response to therapy, and outcome in a series of 20 cases. Br J Haematol 91: 854–859, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Blade J, Fernandez-Llama P, Bosch F, Montoliu J, Lens XM, Montoto S, Cases A, Darnell A, Rozman C, Montserrat E: Renal failure in multiple myeloma: Presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med 158: 1889–1893, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Knudsen LM, Hjorth M, Hippe E: Renal failure in multiple myeloma: Reversibility and impact on the prognosis. Eur J Haematol 65: 175–181, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fronseca R, Rajkumar SV, Offord JR, Larson DR, Plevak ME, Therneau TM, Greipp PR: Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 78: 21–33, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Pozzi C, Pasquali S, Donini U, Casanova S, Banfi G, Tiraboschi G, Furci L, Porri MT, Ravelli M, Lupo A: Prognostic factors and effectiveness of treatment in acute renal failure due to multiple myeloma: A review of 50 cases. Report of the Italian Renal Immunopathology Group. Clin Nephrol 28: 1–9, 1987 [PubMed] [Google Scholar]
- 11.Zucchelli P, Pasquali S, Cagnoli L, Ferrari G: Controlled plasma exchange trial in acute renal failure due to multiple myeloma. Kidney Int 33: 1175–1180, 1988 [DOI] [PubMed] [Google Scholar]
- 12.Johnson WJ, Kyle RA, Pineda AA, O'Brien PC, Holley KE: Treatment of renal failure associated with multiple myeloma. Arch Intern Med 150: 863–869, 1990 [PubMed] [Google Scholar]
- 13.Rayner HC, Haynes AP, Thompson JR, Russell N, Fletcher J: Perspectives in multiple myeloma: Survival, prognostic factors and disease complications in a single centre between 1975 and 1988. Quart J Med 79: 517–525, 1991 [PubMed] [Google Scholar]
- 14.Irish AB, Winearls CG, Littlewood T: Presentation and survival of patients with severe renal failure and myeloma. QJM 90: 773–780, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Clark WF, Stewart AK, Rock GA, Sternbach M, Sutton DM, Barrett BJ, Heidenheim AP, Garg AX, Churchill DN, Canadian Apheresis Group: Plasma exchange when myeloma presents as acute renal failure: A randomized, controlled trial. Ann Intern Med 143): 777–784, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Ludwig H, Drach J, Graf H, Lang A, Meran JG: Reversal of acute renal failure by bortezomib-based chemotherapy in patients with multiple myeloma. Haematologica 92: 1411–1414, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kastritis E, Anagnostopoulos A, Roussou M, Gika D, Matsouka C, Barmparousi D, Grapsa I, Psimenou E, Bamias A, Dimopoulos MA: Reversibility of renal failure in newly diagnosed multiple myeloma patients treated with high dose dexamethasone-containing regimes and the impact of novel agents. Haematologica 92: 546–549, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Chanan-Khan AA, Kaufman JL, Mehta J, Richardson PG, Miller KC, Lonial S, Munshi NC, Schlossman R, Tariman J, Singhal S: Activity and safety of bortezomib in multiple myeloma patients with advanced renal failure: A multicenter retrospective study. Blood 109: 2604–2606, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Leung N, Gertz MA, Zeldenrust SR, Rajkumar SV, Dispenzieri A, Fervenza FC, Kumar S, Lacy MQ, Lust JA, Greipp PR, Witzig TE, Hayman SR, Russell SJ, Kyle RA, Winters JL: Improvement of cast nephropathy with plasma exchange depends on the diagnosis and on reduction of serum free light chains. Kidney Int 73: 1282–1288, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Ritz E: Plasma exchange for acute renal failure of Myeloma—Logical, yet ineffective. J Am Soc Nephrol 17: 914–916, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Cserti C, Haspel R, Stowell C, Dzik W: Light-chain removal by plasmapheresis in myeloma-associated renal failure. Transfusion 47: 511–514, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Evans N, Hattersley J, Hutchison C, Mead GP, Bradwell AR, Chappel M. Modelling of haemodialysis in limiting serum free light chains in patients with renal failure, in Proceedings of the 6th IFAC Symposium on Modelling and Control in Biomedical Systems (D. Feng), Reims, September 20–22, 2006, Elsevier, Oxford, pp. 75–80
- 23.Vervoort G, Willems HL, Wetzels JF: Assessment of glomerular filtration rate in healthy subjects and normoalbuminuric diabetic patients: Validity of a new (MDRD) prediction equation. Nephrol Dial Transplant 17: 1909–1913, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Hutchison CA, Harding S, Mead G, Goehl H, Storr H, Bradwell AR, Cockwell P: Serum free light chain removal by high cut-off hemodialysis: Optimising removal and supportive care. Artif Organs 32: 910–917, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Bradwell AR, Carr-Smith HD, Mead GP, Tang LX, Showell PJ, Drayson MT, Drew R: Highly sensitive automated immunoassay for immunoglobulin FLCs in serum and urine. Clin Chem 47: 673–680, 2001 [PubMed] [Google Scholar]
- 26.Katzmann JA, Clark RJ, Abraham RS, Bryant S, Lymp JF, Bradwell AR, Kyle RA: Serum reference intervals and diagnostic ranges for free κ and free λ immunoglobulin light chains: Relative sensitivity for detection of monoclonal light chains. Clin Chem 48: 1437–1444, 2002 [PubMed] [Google Scholar]
- 27.Sanders PW, Booker BB: Pathobiology of cast nephropathy from human Bence Jones proteins. J Clin Invest 89: 630–639, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang PX, Sanders PW: Immunoglobulin light chains generate hydrogen peroxide. J Am Soc Nephrol 18(4): 1239–1245, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Sengul S, Zwizinski C, Simon EE, Kapasi A, Singhal PC, Batuman V: Endocytosis of light chains induces cytokines through activation of NF-κB in human proximal tubule cells. Kidney Int 62: 1977–1988, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Malani AK, Gupta V, Rangineni R: Bortezomib and dexamethasone in previously untreated multiple myeloma associated with renal failure and reversal of renal failure. Acta Haematologica 116: 255–258, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Nozza A, Siracusano L, Armando S: Bortezomib-dexamethasone combination in a patient with refractory multiple myeloma and impaired renal function. Clin Ther 28: 953–959, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Tanner GA, Evan AP: Glomerular and proximal tubular morphology after single nephron obstruction. Kidney Int 36: 1050–1060, 1989 [DOI] [PubMed] [Google Scholar]
- 33.Bachmann U, Schindler R, Storr M, et al.: Combination of bortezomib-based chemotherapy and extracorporeal free light chain removal for treating cast nephropathy in multiple myeloma. Nephrol Dial Transplant 1: 106–108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heyne N, Weisel KC, Hutchison C, Friedrich B, Goehl H, Kanz L, Risler T. Characterization of extracorporeal serum free light chain elimination kinetics via a high cut-off protein permeable membrane in light chain multiple myeloma. Nephrol Dial Transplant 22: vi123, 2007, [suppl 6] [Google Scholar]
- 35.Hutchison CA, Cook M, Heyne N, Weisel K, Billingham L, Bradwell A, Cockwell P: European Trial of Free Light Chain Removal by Extended Haemodialysis in Cast Nephropathy (EuLITE): A randomised control trial. Trials 9: 55, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.