Abstract
Background: Because of the risk of performing renal biopsies in children with co-morbid conditions, we carried out this study to identify candidate protein biomarkers in the urine of HIV-infected children with renal disease.
Design, setting, participants & measurements: Urine samples from HIV-infected children with biopsy proven HIV-nephropathy (HIVAN; n = 4), HIV-associated Hemolytic Uremic Syndrome (HIV-HUS; n = 2), or no renal disease (n = 3) were analyzed by two-dimensional electrophoresis (2-DE) and proteomic methods. Positive findings were confirmed in HIV-infected children with (n = 20) and without (n = 10) proteinuria using commercially available assays.
Results: By 2-DE analysis, a single urine marker was not sufficient to distinguish children with HIVAN from the others. High urine levels of β2-microglobulin and retinol-binding protein (RBP) suggested the presence of tubular injury. In addition, we found elevated urine levels of iron and the iron-related proteins, transferrin, hemopexin, haptoglobin, lactoferrin, and neutrophil gelatinase-associated lipocalin (NGAL), in children with HIVAN and HIV-HUS. Furthermore, we detected a significant accumulation of iron in the urine and kidneys of HIV-transgenic (Tg) rats with renal disease.
Conclusion: These findings suggest that iron and iron-related proteins might be promising candidate urine biomarkers to identify HIV-infected children at risk of developing HIVAN and HIV-HUS. Moreover, based on the results of previous studies, we speculate that the release or accumulation of iron in the kidney of HIV-infected children may contribute to the rapid progression of their renal disease, and could become a new therapeutic target against HIVAN and HIV-HUS.
HIV-infected children can develop proteinuria secondary to several renal diseases, including immune complex glomerulopathies, thrombotic microangiopathies (TMA), and HIV-associated nephropathy (HIVAN) (1–5). In general, these renal diseases show a progressive clinical onset, and can have a significant clinical impact in the quality of life of HIV-infected children. Unfortunately, in most cases, the only way of making a definitive diagnosis of these renal diseases is to perform a renal biopsy (2,6–8). However, because of the risk of performing renal biopsies in HIV-infected children with a high viral load and other AIDS-related illness, it is important to identify reliable noninvasive biomarkers to aid in the early diagnosis, treatment, and follow up of these patients. In addition, since HIV-infected children do not typically have other co-morbidities or risk factors, such as atherosclerosis, hypertension, diabetes, or use of illegal drugs, they are an ideal group of patients for the identification of new renal biomarkers related to specific HIV-associated renal diseases.
Childhood HIVAN is typically seen in African American patients and is defined by the presence of heavy proteinuria, with mesangial hyperplasia or focal segmental glomerulosclerosis (FSGS) in association with microcystic tubular changes, leading to renal enlargement and rapid progression of the renal disease (1–5). HIV-infected children can also develop an atypical form of the Hemolytic Uremic Syndrome (HUS) characterized by a progressive clinical onset, lack of preceding diarrhea, preserved urine output with severe proteinuria, and rapid progression to end-stage renal disease or death due to infectious or bleeding complications (3,4,9). In addition, they can develop renal TMA lesions without the clinical manifestations of HUS, drug toxicity, and other glomerulopathies that cause proteinuria (3,10). The identification of new biomarkers that could facilitate the early diagnosis and distinction between HIVAN, HUS, and other renal diseases could have a significant impact in the clinical management of these patients. This knowledge will facilitate the identification of children at high risk of developing these diseases; the administration of disease-oriented fluid, hematologic, pharmacologic, and nutritional therapies; as well as the planning of early referrals to intensive care units with expertise in pediatric renal replacement therapies and plasmapheresis. Therefore, we carried out this study to identify new candidate protein urine biomarkers in HIV-infected children, and to determine whether several protein urine biomarkers used in HIV-negative adults to predict the diagnosis of other glomerular diseases were found in the urine of HIV-infected children (11).
Materials and Methods
Patients and Urine Collection
The study protocol, as well as all enrollment and consent documents, were approved by our Institutional Review Board in adherence to the declaration of Helsinki (CNMC-IRB # 1783). Urine samples were collected from control (n = 10) and HIV-infected children (n = 30) of approximately similar age and race during the period between January 1995 and December 2007. All HIV-infected patients acquired HIV-1 from their mothers through vertical transmission, with the exception of one child who was infected through a blood transfusion. Approximately 90% of the children were African Americans (27/30), whereas 6.7% and 3.3% of the remaining patients were White Hispanics (2/30) and Caucasians (1/30). The diagnosis of HIV-infection and AIDS was based on the criteria established by the Centers for Disease Control. All HIV-infected children were symptomatic at the moment the urine sample was collected, clinical categories B and C (approximately 35% and 65%, respectively), and received treatment with anti-retroviral therapies according to the criteria of the attending physicians. The patients ranged in age from 6 mo to 15 yr with an approximately equal ratio of males and females.
Urine samples were tested for proteinuria and hematuria by Multistix 9 Reagent Strip (Bayer Corporation, Elkhart, IN). The urinary protein and creatinine levels were also measured by colorimetric assays from Wako Diagnostics (Richmond, VA) and R&D Systems (Minneapolis, MN), respectively. The results were reported as protein/creatinine ratios. Urine samples from HIV-infected children with orthostatic proteinuria, urinary tract infections, or acute systemic illness were excluded. All samples from patients with persistent proteinuria for more than 2 mo were included in the proteinuria group, stored at −70°C, and processed according to our institutional bioethical guidelines.
Two-Dimensional Gel Electrophoresis (2-DE) and Mass Spectrometry Analysis
Nine representative urine samples collected from HIV-infected children with biopsy proven HIV-HUS (n = 4), HIVAN (n = 2), or without renal disease (n = 3) were used to screen for relevant protein biomarkers by 2-DE. Samples were adjusted for protein/creatinine values and depleted of albumin using Proteome LabTM IgY spin column (Beckman Coulter, Inc., Fullerton, CA) (12). Albumin-depleted samples (200 μg total proteins) were desalted using p6 Bio-Spin columns (Bio-Rad, Richmond, CA), then dried by vacuum centrifugation and re-dissolved in rehydration buffer (12). First-dimension isoelectro-focusing (IEF) was performed on IPG strips (11 cm; pH 3–10) and the second dimension was performed on Criterion Tris-HCl (8%–16%) precast gels following the manufacturer settings (Bio-Rad). Protein spots visualized with Coomassie stains were scanned on a GS800 densitometer (Bio-Rad), imaged as a TIFF file for analysis, excised, and digested with trypsin as described before (12–13). Peptides were analyzed by matrix-assisted laser desorption time of flight mass spectrometry (MALDI-TOF) using an ABI 4700 TOF-TOF instrument (Applied Biosystems, Foster City, CA). The instrument was operated in reflectron-positive ion mode and calibrated using standard peptides mixture CalMix (Applied Biosystems). Proteins were identified using GPS explorer software (Applied Biosystems) by entering both the mass list of tryptic peptides and MS/MS data obtained for each protein spot.
Urinary Markers of Renal Dysfunction, Iron, and Iron-Related Proteins
Albumin was determined using a turbidimetric assay (Wako Diagnostics). β2-Microglobulin (R&D Systems) and RBP (ALPCO, Salem, NH) were measured by enzyme-linked immunoabsorbent assay (ELISA) as described by their manufacturers. Iron was determined by ferrozine staining as described previously (14). Haptoglobin, hemopexin, transferrin, lactoferrin, and neutrophil gelatinase-associated lipocalin (NGAL) were measured by ELISA (Assay Pro, St. Charles, MO; and Assay Designs, Inc., Ann Arbor, MI; respectively). The presence of iron in the kidney of HIV-1 infected children and HV-Tg rats was detected by Prussian blue staining using the Accustain iron stain kit (Sigma-Aldrich, Inc., St. Louis, MO). Whenever the urine values of control HIV-positive patients were borderline elevated, they were compared with the control urine values from non-HIV-infected children.
Urinary Iron Excretion in HIV-Tg Rats
To determine whether iron was released in the urine in correlation with the histologic progression of the renal disease, we used HIV-Tg rats carrying a defective HIV-1 proviral DNA, which develop similar renal histologic lesions to children with HIVAN (15). Urinary protein and iron levels/deposits were determined as described above. NGAL was measured by ELISA, adapting the protocol described by Kjeldsen et al. (16) using anti-rat lipocalin-2 antibodies and recombinant protein (R&D Systems).
Statistical Analysis
Comparisons between two groups were done with the t test. P values less than 0.05 were considered significant. When more than two means were compared, significance was determined by one-way ANOVA followed by multiple comparisons using the Student-Neuman-Keul's post hoc test. Data that were non-normal were either log-transformed before analysis or analyzed by the Kruskal-Wallis ANOVA, followed by Dunn's multiple comparisons post test. The Bartlett's test was used to test the homogeneity of variances, and the Mann-Whitney test was used whenever the variation between standard deviations from the different groups were significant (P < 0.05). All samples showing undetectable levels of the biomarker under investigation were assigned an arbitrary value corresponding to the lowest level of detection of the bioassay used.
Results
Proteomics
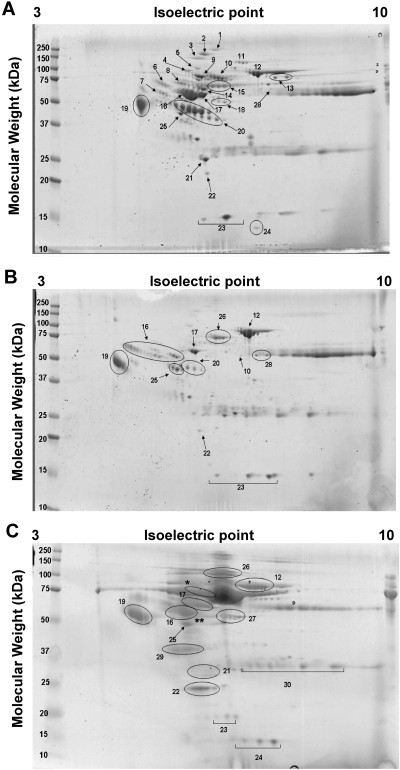
Our initial goal was to identify the presence of 11 urinary proteins previously used by Varghese et al. (11) to predict the diagnosis of several glomerular renal diseases in HIV-negative adult patients. By 2-DE, we found that all children with HIVAN and HIV-HUS showed high urinary levels of these 11 proteins, when compared with samples harvested from HIV-infected children without renal disease, and to the normal values reported in other studies for HIV-negative controls (17–24). These proteins are: orosomucoid, transferrin, α-1-microglobulin, zinc α-2-glycoprotein, α-1-antitrypsin, complement factor B, haptoglobin, transthyretin, RBP, albumin, and hemopexin (Figure 1 and Table 1). Hemopexin and haptoglobin were not detected in the urine of only one of the two children with biopsy proven HIV-HUS (Figure 1 and Table 1). The sequential changes seen in the proteomic maps from a child with biopsy proven HIVAN obtained at the time of diagnosis, and after one year of highly active antiretrovical therapy (HAART), are shown in Figure 1 and Table 1. We were unable to identify a single urine candidate biomarker to distinguish children with HIVAN or HIV-HUS, but found relevant changes in iron-related proteins that suggested a potential role of iron in the pathogenesis of these renal diseases, and therefore were explored in further detail.
Figure 1.
Representative 2-DE maps from urine samples harvested from children with HIVAN and HIV-HUS. Panel A shows the results from a urine sample collected from a child with biopsy proven HIVAN during the first visit to the hospital (SCr 2.7 mg/dl). Panel B shows the results from a urine sample collected from the same child 1 yr later, while receiving HAART (SCr 1.7 mg/dl). Panel C shows the results from a urine sample collected from a child with HIV-HUS. Albumin-depleted urinary samples were used for 2-DE analysis. Gels were stained with Bio-Safe Coomassie Blue and proteins were processed and analyzed as described in the Materials and Methods section. Numbers labeling spots in panels A–C correspond to protein identifications in Table 1.
Table 1.
Protein Identifications in Urine Collected from Child with HIVAN and HIV-HUS
| Spot | MW (kDa) | pI | Protein Identification | Protein Score
|
||
|---|---|---|---|---|---|---|
| HIVAN | HIV-HUS | |||||
| First visit | 1 yr later | |||||
| 1 | 143.7 | 6.3 | Complement factor H | 179 | – | – |
| 2 | 122.9 | 5.4 | Ceruloplasmin | 224 | – | – |
| 3 | 103.5 | 6.5 | Inter-α-trypsin inhibitor | 121 | – | – |
| 4 | 71.5 | 5.6 | Prothrombin | 262 | – | – |
| 5 | 71.0 | 5.6 | α-albumin | 122 | – | – |
| 6 | 47.8 | 5.3 | α-1-antichymotrypsin | 116 | – | – |
| 7 | 40.1 | 5.4 | Fetuin-A | 69 | – | – |
| 8 | 73.0 | 6.3 | Kininogen precursor | 91 | – | – |
| 9 | 54.8 | 5.6 | α-1-B glycoprotein | 169 | – | – |
| 10 | 52.4 | 6.6 | Hemopexin | 204 | 204 | * |
| 11 | 86.8 | 6.7 | Complement factor B | 84 | – | – |
| 12 | 79.3 | 6.8 | Transferrin | 368 | 348 | 114 |
| 13 | 188.6 | 6.0 | Complement C3 | 212 | – | – |
| 14 | 53.0 | 6.3 | Antithrombin-III | 145 | – | – |
| 15 | 38.5 | 6.1 | Ig α-1 chain C region | 156 | – | – |
| 16 | 46.8 | 5.4 | a-1-antitrypsin | 213 | 189 | 338 |
| 17 | 54.5 | 5.4 | Vitamin D-binding protein | 236 | 297 | 149 |
| 18 | 46.5 | 6.0 | Pigment epithelium-derived factor | 84 | – | – |
| 19 | 23.9 | 5.0 | Orosomucoid 2 | 227 | 227 | 227 |
| – | 23.7 | 4.9 | Orosomucoid 1 | 221 | 221 | 221 |
| 20 | 45.9 | 6.1 | Haptoglobin | 194 | 194 | ** |
| 21 | 30.8 | 5.6 | Apolipoprotein A-I | 193 | – | 200 |
| 22 | 23.4 | 5.8 | Plasma retinol-binding protein | 112 | 112 | 174 |
| 23 | 16.0 | 5.5 | Transthyretin | 160 | 160 | 160 |
| 24 | 13.8 | 6.1 | β-2-Microglobulin | 141 | – | 141 |
| 25 | 34.1 | 5.6 | Zn-a-2-glycoprotein | 41 | 201 | 124 |
| 26 | 71.3 | 5.9 | Albumin | – | 365 | 313 |
| 27 | 71.3 | 5.9 | Albumin | – | – | 261 |
| 28 | 36.6 | 8.5 | Ig γ-1 chain C region | 142 | 120 | – |
| 29 | 39.9 | 6.0 | α1-microglobulin | – | – | 95 |
| 30 | 11.8 | 5.6 | Ig-κ-chain | – | – | 62 |
MW, molecular weight; pI, isoelectric point.
Hemopexin and
haptoglobin were not detected in this 2-DE gel; detection was made by ELISA.
Urinary Iron Levels in HIV-Infected Children with Renal Disease
Under normal circumstances, little or no iron is detectable in the urine of humans (25). However, as shown in Figure 2A, all urine samples harvested from HIV-infected children showed higher iron levels when compared with HIV-negative children without renal disease. HIV-infected children with proteinuria showed the most significant increase in iron excretion, when compared with all other groups (Figure 2A). In the kidney, iron accumulation was found in children with HIV-HUS (Figure 3, E and F). Among 25 HIV-infected children studied for the presence of systemic iron overload, only one child with HIV-HUS, two children with HIVAN, and one child with proteinuria showed evidence of iron overload, based on the presence of a decreased total iron binding capacity (TIBC) in association with a high transferrin saturation, and high iron or ferritin serum levels (data not shown).
Figure 2.
Mean ± SEM values for total iron (A), haptoglobin (B), hemopexin (C), transferrin (D), lactoferrin (E), and NGAL (F) in urine samples harvested from HIV-negative children without proteinuria (Control); HIV-infected children without proteinuria (HIV+-C); HIV-infected with trace proteinuria (HIV+-T); and HIV-infected with >1 + proteinuria (HIV+-P) (n = 10, 10, 11, and 9 in each group, respectively). All measurements were done as described in the Materials and Methods section. Urine values were expressed as a urinary creatinine (UCR) ratio. P values less than 0.05 were considered significant. (+, * and # indicate a P < 0.05; and ++, ** and ## indicate a P < 0.001 when compared with the HIV -C, HIV +-C, and HIV+-T groups, respectively).
Figure 3.
Prussian blue staining demonstrating the accumulation of iron in representative renal sections harvested from HIV-Tg rats without renal disease (A) and with renal disease (B–C). Panel D shows a control staining in a representative renal section from an HIV-Tg rat with renal disease. Panels E and F show the Prussian blue staining in representative renal sections harvested from an HIV-infected child without renal disease (E) and a child with HIV-HUS (F). Magnification, ×200.
Urinary Iron-related Proteins in HIV-Infected Children with Renal Disease
The 2-DE results showing elevated levels of the iron-related proteins haptoglobin, hemopexin, and transferrin (Figure 1 and Table 1) were confirmed by ELISA. We found high urinary levels of all these proteins in children with biopsy proven HIVAN and HIV-HUS, relative to the samples from HIV-infected children without renal disease and the normal range control values. Moreover, the urinary levels of haptoglobin, hemopexin, and transferrin were significantly increased in the urine of HIV-infected children with proteinuria, when compared with HIV-infected patients with trace or no proteinuria (Figure 2, B–D). Subsequently, we performed more experiments to determine whether two additional iron-related proteins, lactoferrin and NGAL, an established urine biomarker in children with acute renal failure (26–27), were also increased in the urine of HIV-infected children with proteinuria, when compared with all other groups (Figure 2, E and F) (P < 0.05).
Glomerular and Tubular Injury in HIV Renal Disease
By 2-DE, we found elevated levels of albumin, β2-microglobulin, and RBP in the urine of children with biopsy proven HIVAN and HIV-HUS. Proteinuria detected by dipstick was confirmed by a colorimetric assay (Figure 4A). Microalbuminuria, a marker of glomerular injury and/or endothelial dysfunction (28), was detected in HIV-infected children with trace and >1+ proteinuria by dipstick (Figure 3B). In a similar manner, two low-molecular-weight proteins that are considered markers of renal proximal tubular injury (29), β2-microglobulin and RBP, were elevated in the urine of HIV-infected children with proteinuria (Figure 4, C–D), confirmed by Elisa. RBP showed a significant increase in HIV-infected children with trace proteinuria (Figure 4, C–D), but future longitudinal studies are needed to define whether it could be a more sensitive biomarker than β2-microglobulin. Overall, these findings support the notion that proximal tubular injury is an early event in the pathogenesis of childhood HIV-related renal diseases.
Figure 4.
Mean ± SEM values for total urinary protein (A), albumin (B), β2-microglobulin (C), and RBP in urine samples harvested from HIV-negative children without proteinuria (Control); HIV-infected children without proteinuria (HIV+-C); HIV-infected with trace proteinuria (HIV+-T); and HIV-infected with >1 + proteinuria (HIV+-P) (n = 10, 10, 11, and 9 in each group, respectively). All measurements were done as described in the methods section. Urine values were expressed as a urinary creatinine (UCR) ratio. P values less than 0.05 were considered significant. (+, * and # indicate a P < 0.05; and ++, ** and ## indicate a P < 0.001 when compared with the HIV−-C, HIV+-C, and HIV+-T groups, respectively).
Iron and NGAL Urine Levels in HIV-1 Transgenic Rats with Renal Disease
To determine whether iron was released in the urine in correlation with the histologic progression of the renal disease, we used HIV-Tg rats that develop renal disease similar to the one seen in children, including HIVAN and renal TMA lesions (15). We found increased urinary levels of iron (8.7-fold, P = 0.0032) and NGAL (3.8-fold, P = 0.0491) in HIV-Tg rats with proteinuria, both during the early and late stages of their renal disease, when compared with the corresponding HIV-Tg rat littermates without renal disease (Figure 5, A–C) or wild type rats (data not shown). In addition, HIV-Tg rats showing high levels of iron and NGAL in the urine progressively developed renal iron deposits (Figure 3, A–D), mimicking the accumulation of iron seen in children with HIV-HUS (Figure 3F).
Figure 5.

Mean ± SEM values for total urinary protein (A), total iron (B), and NGAL (C) secretion in HIV-Tg rats without proteinuria (HIV-Tg-C) and with >1 + proteinuria (HIV-Tg-P) (n = 15 and 13, respectively). All measurements were done as described in the Materials and Methods section. Urine values were expressed as a urinary creatinine (UCR) ratio. P values less than 0.05 were considered significant.
Progressive Changes in Selected Urine Candidate Biomarkers in a Child with HIVAN
Finally, we followed the changes in total protein, iron, β2-microglobulin, transferrin, and NGAL in a child with biopsy proven HIVAN who responded successfully to HAART therapy. As shown in Figure 6, the urinary levels of all selected biomarkers decreased in correlation with the recovery of renal function and response to HAART, but remained elevated above the normal range throughout the follow-up period. This child showed a low serum iron and low TIBC, but the transferrin saturation and ferritin serum levels were above the normal range (data not shown). Nevertheless, no iron deposits were found in the renal biopsy (data not shown).
Figure 6.
Progressive changes in selected candidate biomarkers in urine samples collected through a period of 79 d from a child with biopsy proven HIVAN. A decrease in viral load (A), total protein (B), total iron (C), NGAL (D), β2-microglobulin (E), and transferrin (F) levels is observed in correlation with an improvement in renal function (A: serum creatinine) in response to HAART. Dotted lines indicate normal control levels.
Discussion
To the best of our knowledge, this study reports for the first time the presence of high levels of iron in the urine of HIV-infected children and HIV-Tg rats with renal disease. These findings, when taken in the context of the results of previous studies (30–32), suggest that iron and iron-related proteins should be considered as potential candidate urine biomarkers to identify HIV-infected children at risk of developing renal disease.
Initially, we used a proteomic approach to screen for the presence of candidate urine biomarkers in a selected group of HIV-infected children with biopsy proven HIVAN and HIV-HUS. We were unable to identify a single candidate protein that could distinguish between these diseases. However, we found the 11 candidate protein biomarkers that have been used by Varghese et al. to predict the diagnosis of FSGS, lupus nephritis, membranous nephropathy, and diabetic nephropathy in adult HIV-negative patients, using an artificial neural network analysis (11). Thus, it remains to be determined whether a similar analysis could be used to identify HIV-infected children that might have HIVAN.
Considering that many of the proteins identified in the proteomic approach are associated with the transport or metabolism of iron, we subsequently focused our study on defining whether iron alone, or in combination with other iron-related proteins, could become candidate urine biomarkers in HIV-infected children. We found high levels of iron, haptoglobin, hemopexin, transferrin, lactoferrin, and NGAL in HIV-infected children with proteinuria, and in children with biopsy proven HIVAN and HIV-HUS. Haptoglobin, is a plasma protein with hemoglobin-binding capacity that forms a stable complex aiding in the recycling of heme iron (33). Hemopexin, a heme-binding plasma glycoprotein, forms the second line of defense against hemoglobin-mediated oxidative damage during intravascular hemolysis (21). Transferrin is the most important plasma protein involved in the transport of iron between sites of absorption and storage (22). Lactoferrin is an iron-binding glycoprotein that can function as an immune modulator, antimicrobial, or antioxidant (34). NGAL is involved in iron trafficking (16) and, as shown by Prasad and colleagues, is an excellent urine biomarker to predict the outcome of acute renal failure in children after cardiovascular bypass surgery (26–27).
Previous studies, done in humans and experimental animal models, support the notion that the accumulation of iron can accelerate the progression of renal disease (30–32). Iron accumulation per se, has been considered an independent predictor of both functional and structural renal damage (30). Proximal tubular iron accumulation correlated independently with protein excretion and impairment of GFR (30–32). Therefore, we hypothesize that the induction of glomerular damage by HIV-1 facilitates the filtration of plasma proteins and the accumulation of iron-containing proteins in the renal tubules. In addition, HIV-1 can induce direct tubular damage, and these changes may affect the tubular reabsorption and/or catabolism of filtered iron containing proteins, leading to the release and accumulation of soluble iron (Fe2+) in renal tubules. Soluble iron is able to catalyze the formation of radical oxygen species (ROS) (35–39) and can also enhance the replication of HIV-1 (40) in macrophages that are recruited to these renal injury sites, causing further renal tubular injury.
At the present time, we do not know the exact mechanisms responsible for the renal accumulation of iron in HIV-infected children or HIV-Tg rats with renal disease. The pathogenesis of iron accumulation in HIV-infection is probably multifactorial and not well understood (41–44). Only four children with renal disease in our study showed serologic evidence of iron overload. Children with proteinuria without hematuria also exhibited high levels of iron and iron-related proteins. These findings suggest that several mechanisms acting together might contribute to this process. The renal iron deposits in the kidney of HIV-infected children and HIV-Tg rats with renal disease could not be explained entirely on the basis of the development of proteinuria, since not all children/rats with massive proteinuria develop renal iron deposits. Nevertheless, HIV-Tg rats show predominately tubular iron deposits, a finding consistent with the proposed mechanism of iron accumulation resulting from increased filtration of iron. Alternatively, the presence of TMA lesions could induce intravascular hemolysis, and we have found TMA lesions in children with HIV-HUS and in HIV-Tg rats with renal disease (4,15). Intravascular hemolysis leads to the release of free plasma hemoglobin in excess of haptoglobin, with the subsequent filtration and absorption of hemoglobin by renal glomerular and tubular cells. Heme is a breakdown by heme oxygenase, and iron is consequently stored in these cells in the form of ferritin and hemosiderin. In agreement with this notion, children with HIV-HUS show glomerular iron deposits in association with the TMA lesions. In addition, the chronic renal inflammatory changes induced by HIV-1 can also facilitate the renal recruitment of phagocytic cells and the sequestration of iron by these cells (44). In support of this notion, an increased deposition of iron-ferritin and/or hemosiderin was found in the bone marrow, brain, skeletal muscle, and liver of patients with AIDS (41–43). Finally, too much iron supplementation could be an additional factor contributing to the renal accumulation of iron in children with HIV-associated renal diseases (45–47). Iron deficiency anemia is a common finding in HIV-infected children and is associated with increased progression to AIDS (48). HIV-infected children frequently develop renal disease during the late stages of HIV-infection, when the use of the most common laboratory indicators of the iron status is somewhat limited, because these markers are involved in the acute phase response to HIV-1 infection (49–50). Thus, it is difficult to determine how much iron supplementation these children need, and some patients may receive too much iron. Overall, based on these studies, we speculate that the accumulation of iron may be an additional factor contributing to the rapid progression of the renal disease in HIV-infected children.
Conclusion
In summary, we conclude that iron, in combination with haptoglobin, hemopexin, transferrin, lactoferrin, or NGAL, could be considered as potential candidate urine biomarkers to monitor the outcome and progression of the renal injury in HIV-infected children. Many questions related to the mechanisms responsible for the accumulation of iron in the kidney of HIV-infected children, as well as the potential role that iron may play in the pathogenesis of childhood HIVAN, remain unanswered at this point. Thus, future studies to determine how HIV-1 facilitates the renal accumulation of iron, as well as the role of iron-related proteins in the pathogenesis of childhood HIV-associated renal diseases are warranted. Finally, our findings suggest that the prevention of renal iron accumulation and/or toxicity should be considered as an additional goal in the treatment of HIV-infected children with proteinuria.
Disclosures
None.
Acknowledgments
This study was supported in part by the National Institutes of Health grants: R01-HL55605-08S1, R01-DK049419-10S2, R21 NICAN-AT002278, and 5R24 HD050846 (Integrated Molecular Core for Rehabilitation Medicine). Part of these data were presented in the 2008 Pediatric Academies Society Meeting (May 3 through 6, 2008, Honolulu, HI).
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Chaparro AI, Mitchell CD, Abitbol CL, Wilkinson JD, Baldarragi G, Lopez E, Zilleruelo G: Proteinuria in children infected with the human immunodeficiency virus. J Pediatr 152: 844–849, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Ray PE, Lian X, Rakusan T, Liu XH: A 20-year history of childhood HIV-associated nephropathy. Pediatr Nephrol 19: 1075–1092, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Ray PE, Rakusan T, Loechelt BJ, Selby DM, Liu XH, Chandra RS: Human immunodeficiency virus (HIV)-associated nephropathy in children from Washington, DC area: 12 years’ experience. Semin Nephrol 18: 396–405, 1998 [PubMed] [Google Scholar]
- 4.Turner ME, Kher K, Rakusan T, D'Angelo L, Kapur S, Selby D, Ray PE: Atypical hemolytic uremic syndrome in human immunodeficiency virus-1-infected children. Pediatr Nephrol 11: 161–163, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Strauss J, Abitol C, Zilleruelo G, Scott G, Paredes A, Malaga S, Montane B, Mitchell C, Parks W, Pardo V: Renal disease in children with acquired immunodeficiency syndrome. N Engl J Med 321: 625–630, 1989 [DOI] [PubMed] [Google Scholar]
- 6.Kopp JB, Winkler C: HIV-associated nephropathy in African Americans. Kidney Int 83: S43–S49, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Winston J, Klotman PE: HIV-associated nephropathy. Mt Sinai J Med 65: 27–32, 1998 [PubMed] [Google Scholar]
- 8.Kimmel PL, Phillips TM, Ferreira-Centeno A, Farkas-Szallasi T, Abraham AA, Garrett CT: Brief report: Idiotypic IgA nephropathy in patients with HIV infection. N Engl J Med 327: 702–704, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Ray PE, Liu XH: Pathogenesis of Shiga toxin-induced hemolytic uremic Syndrome. Pediatr Nephrol 16: 823–839, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Alpers CE: Light at the end of the TUNEL: HIV-associated thrombotic microangiopathy. Kidney Int 63: 385–396, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Varghese SA, Powell TB, Budisavljevic MN, Oates JC, Raymond JR, Almeida JS, Arthus JM: Urine biomarkers predict the cause of glomerular disease. J Am Soc Nephrol 18: 913–922, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanderver A, Schiffmann E, Timmons M, Kellersberger KA, Fabris D, Hoffman EP, Maletkovic J, Hathout Y: Decreased asialotransferrin in cerebrospinal fluid of patients with childhood-onset ataxia and central nervous system hypomyelination/vanishing white matter disease. Clin Chem 51: 2031–2042, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Jensen ON, Wilm M, Shevchenko A, Mann M: Sample preparation methods for mass spectrometry peptide mapping directly from 2-DE gels. Methods Mol Biol 112: 513–530, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Fish WW: Rapid colorimetric micromethod for the quantitation of complexed iron in biological samples. Methods Enzymol 54: 357–364, 1988 [DOI] [PubMed] [Google Scholar]
- 15.Ray PE, Liu X-H, Robinson LR, Reid W, Xu J, Owens JW, Jones OD, Denaro F, Davis HG, Bryant JL: A novel HIV-1 transgenic rat model of childhood HIV-1 associated nephropathy. Kidney Int 63: 2242–2253, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Kjeldsen L, Koch C, Arnljots K, Borregaard N: Characterization of two ELISAs for NGAL, a newly described lipocalin in human neutrophils. J Immunol Methods 198: 155–164, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Tobal D, Olascoaga A, Moreira G, Kurdián M, Sánchez F, Roselló M, Alallón W, González Martínez, Noboa O: Rust urine after intense hand drumming is caused by extracorpuscular hemolysis. Clin J Am Soc Nephrol 3: 1022–1027, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao PV, Lu X, Standley M, Pattee P, Neelima G, Girisesh G, Dakshinamurthy KV, Roberts Jr. CT, Nagalla SR: Proteomic identification of urinary biomarkers of diabetic nephropathy. Diabetes Care 30: 629–637, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Narita T, Sasaki H, Hosoba M, Miura T, Yashioka N, Morii T, Shimotomai T, Koshimura J, Fujita H, Kakei M, Ito S: Parallel increase in urinary excretion rates of immunoglobulin G, ceruloplasmin, transferrin, and orosomucoid in normoalbuminuric type 2 diabetic patients. Diabetes Care 27: 1176–1181, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Tamano M, Fuke Y, Endo M, Ohsawa I, Fujita T, Ohi H: Urinary complement factor H in renal disease. Nephron 92: 705–707, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Delanghe JR, Langlois MR: Hemopexin: A review of biological aspects and the role in laboratory medicine. Clin Chim Acta 312: 13–23, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Prinsen BHCMT, De Sain-Vander Velden MGM, Kaysen GA, Straver HWHC, Van Rijn HJM, Stellaard F, Berger Ruud, Rabelink TJ: Transferrin synthesis is increased in nephritic patients insufficiently to replace urinary losses. J Am Soc Nephrol 12: 1017–1025, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Norden AGW, Scheiman SJ, Deschodt-Lanckman MM, Laosley M, Nortier JL, Thakker RV, Unwin RJ, Wrong O: Tubular proteinuria defined by a study of Dent's (CLCN5 mutation) and other tubular diseases. Kidney Int 57: 240–249, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Dengler R, Plewan A, Münstermann U, Busch R, Eger G, Emmerich B: Urinary excretion of protelyzed α1-antitrypsin: specificity, quantification, and relation to therapy response in patients with acute myeloid leukemia. Clin Cancer Res 1: 199–205, 1995 [PubMed] [Google Scholar]
- 25.Yang J, Mori K, Li JY, Barasch J: Iron, lipocalin, and kidney epithelia. Am J Physiol Renal Physiol 285: F9–F18, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, Parikh CR, Goldsten SL: Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: A prospective cohort study. Critical Care 11: R84–R94, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P: Identification of neutrophil-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543. 2003 [DOI] [PubMed] [Google Scholar]
- 28.Szczech LA, Grunfeld C, Scherzer R, Canchola JA, van der Horst C, Sidney S, Wohl D, Shlipak MG: Microalbuminuria in HIV infection. AIDS 11: 1003–1009, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guder WG, Hofman W: Markers for the diagnosis and monitoring of renal tubular lesions. Clin Nephrol 38: [Suppl 1]: S3–S7. 1992 [PubMed] [Google Scholar]
- 30.Shah SV: Role of iron in progressive renal disease. Am J Kidney Dis 37: S30–S33, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Alfrey AC: Toxicity of tubule fluid iron in the nephritic syndrome. Am J Physiol 263: F637–F641, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Sponsel HT, Alfrey AC, Hammond WS, Durr JA, Ray C, Anderson RJ: Effect of iron on renal tubular epithelial cells. Kidney Int 50: 436–444, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Fagoonee S, Gburek J, Hirsch E, Marro S, Moestrup SK, Lauberg JM, Chritensen EI, Silengo L, Altruda F, Tolosano E: Plasma protein haptoglobin modulates renal iron loading. Am J Path 166: 973–983, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamauchi K, Tomita M, Giehl TJ, Ellison RT III: Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect Immun 61: 719–728, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Machlin LJ, Bendich A: Free radical tissue damage: Protective role of antioxidants. FASEB 1: 441–445, 1987 [PubMed] [Google Scholar]
- 36.Lim CS, Vaziri ND: The effects of iron dextran on the oxidative stress in cardiovascular tissues of rats with chronic renal failure. Kidney Int 65: 1802–1809, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Zhou XJ, Laszik Z, Wang QX, Silva FG, Vaziri NS: Association of renal injury with increased oxygen free radical activity and altered nitiric oxide metabolism in chronic experimental hemosiderosis. Lab Invest 12: 1905–1914, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV: In vitro and in vivo evidence suggesting a role for iron in ciplastin-induced nephrotocity. Kidney Int 53: 394–401, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Baliga R, Ueda N, Shah SV: Increase in bleomycin-detectable iron in ischemia/reperfusion injury to rat kidneys. Biochem J 191: 901–905, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Georgiou NA, van der Bruggen T, Oudshoorn M, Nottet HSLM, Marx JJ, van Asbeck BS: Inhibition of human immunodeficiency virus type 1 replication in human mononuclear blood cells by the iron chelators deferoxime, deferiprone and bleomycin. J Infect Dis 181: 484–490, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Gordeuk VR, Delanghe JR, Langlois MR, Boelaert JR: Iron status and the outcome of HIV-infection: An overview. J Clin Virol 20: 111–115, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Al-Khafaji B, Kralovic S, Smith RD: Increased hepatic iron in the acquired immunodeficiency syndrome: An autopsy study. Mol Pathol 10: 474–480, 1997 [PubMed] [Google Scholar]
- 43.De Monye C, Karcher DS, Boelaer JR, Gordeuk VR: Bone marrow macrophage iron grade and survival of HIV-seropositive patients. AIDS 13: 375–380, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Savarino A, Pescarmona GP, Boelaert JR: Iron metabolism and HIV infection: Reciprocal interactions with potentially harmful consequences? Cell Biochem Funct 17: 279–287, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Jacobus DP: Randomization to iron supplementation of patients with advanced human immunodeficiency virus disease: An inadvertent but controlled study with results important for patient care. J Infect Dis 173: 1044–1045, 1996 [DOI] [PubMed] [Google Scholar]
- 46.McDermid JM, Jaye A, Schim vand der Loeff MF, Todd J, Bates C, Austin S, Jeffries D, Awasana AA, Whittlex AA, Prentice A: Elevated iron status strongly predicts mortality in West African adults with HIV infection. J Acquir Immune Defic Syndr 46: 498–507, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Ianotti LL, Tielsch HM, Black MM, Black RE: Iron supplementation in early childhood: Health benefits and risks. Am J Clin Nutr 84: 1261–1276, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sembra RD, Gray GE: Pathogenesis of anemia during human immunodeficiency virus infection. J Invest Med 49: 225–239, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Gupta S, Imam A, Licorish K: Serum ferritin in acquired immune deficiency syndrome. J Clin Lab Immunol 20: 11–13, 1986 [PubMed] [Google Scholar]
- 50.Lewis DK, Witty CJ, Epino H, Letsky EA, Mukibi JM, van den Broek NR: Interpreting tests for iron deficiency among adults in a high HIV prevalence African setting: Routine tests may lead to misdiagnosis. Trans R Soc Trop Med Hyg 101: 613–617, 2007 [DOI] [PubMed] [Google Scholar]