Abstract
Background and objectives: To evaluate the inter- and intrareader reliability and the effect of gadolinium enhancement on kidney volume measurements obtained from pre- and postgadolinium T1 MR images in patients with autosomal dominant polycystic kidney disease (ADPKD).
Design, setting, participants, & measurements: Twenty subjects were randomly selected with approximately equal frequency from three kidney-size groups. Pre- and postgadolinium 3D T1 (pre-T1, post-T1) MR images were obtained. The stereology method was applied to segment and measure kidney volumes. The measurement process was repeated at two-wk intervals by two radiologists. Reliability was assessed with correlation coefficients. Intra- and inter-reader bias and measure differences were assessed with paired T-tests. The size effect on the pre- and post-T1 measurements was evaluated with one-way ANOVA.
Results: The intra- and inter-reader reliability was extremely high in all measurements. No systematic intrareader bias but a small inter-reader bias for the post-T1 measurements was observed. All kidney volumes measured on the pre- and post-T1 images were highly correlated with each other for both readers. The post-T1 volumes were significantly higher than pre-T1 volumes. While the post-pre volume differences were relatively constant across the three kidney-size groups, the post-pre percent volume differences were significantly smaller as the size of the kidney increased.
Conclusions: Kidney volume measurements can be made with minimum intra- and inter-reader variability on both pre- and post-T1 MR images. Kidney volumes measured on the pre-T1 were smaller than those on post-T1, and percent differences between pre-T1 and post-T1 kidney volumes decreased with increasing kidney size.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common single-gene renal disorder characterized by slow progressive growth of numerous cysts in the kidneys resulting in renal enlargement (1,2). Early in the course of ADPKD, noncystic renal parenchyma is mostly preserved and clearly discernible from the renal cysts. As the disease progresses, renal cysts grow in size and number and gradually occupy and enlarge the kidney. ADPKD ultimately results in renal failure in more than 50% of affected patients (3–6) and is the fourth leading cause of chronic kidney disease leading to end-stage renal disease in the United States (1).
The lack of a sensitive and reliable measure of disease progression early in ADPKD (while renal function is relatively preserved) has limited the ability to test the efficacy of potential therapeutic agents in ADPKD. Recent imaging studies (6–9), however, demonstrate that image-based methods can be used to reliably and accurately quantify kidney and liver cystic disease progression in ADPKD, and that increased kidney enlargement is associated with loss of kidney function.
Recently, nephrogenic systemic fibrosis, a condition marked by severe progressive skin thickening, joint contractures, and increased morbidity, was found associated with use of gadolinium contrast exposure in patients with reduced renal function and renal failure (10–12). These reports prompted clinicians and radiologists to re-assess the use of gadolinium contrast in clinical and research MR studies. Before the administration of gadolinium contrast for MR imaging, the clinical diagnostic benefits and risks of gadolinium contrast should be considered carefully for patients who are at high risk for nephrogenic systemic fibrosis. Given that some kidney volume measurements in ADPKD are obtained from MR images, it is possible that gadolinium contrast used during MR imaging contributes to kidney volume estimates from MR images. Thus, the purpose of this study was to assess and compare kidney volume measurements obtained from pre- and postgadolinium T1 MR images of patients with ADPKD, as well as to evaluate inter- and intrareader reliability of the measurements.
Materials and Methods
The study protocol for the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) has been previously described (6,8,9,13) and was approved by the institutional review board at each participating clinical center. Informed consent was obtained from all subjects who participated in the CRISP study.
Subjects
The CRISP study was implemented to acquire prospective, multi-year longitudinal measurements of renal and renal cyst volumes in a large cohort of ADPKD subjects with relatively intact renal function. The CRISP cohort consists of 241 ADPKD subjects between 15 and 46 yr old with relatively intact renal function, i.e., a 24 h creatinine clearance >70 ml/min/1.73 m2 or a Cockcroft-Gault estimate of creatinine clearance >70 ml/min, with a serum creatinine level of 1.6 mg/dl (141 μmol/L) or less in men and 1.4 mg/dl (124 μmol/L) or less in women. Detailed descriptions of the CRISP study protocol, the clinical characteristics of the cohort, and the baseline characteristics can be reviewed elsewhere (6,8,9,13).
MR Imaging Protocol
The MRI protocol for the CRISP study was standardized and implemented in 1.5 T MRI scanners. In each subject, a phased-array surface coil was positioned with its center over the inferior costal margin, estimated as the upper margin of the kidney. The field of view was maintained between 30 and 35 cm. The kidneys were imaged first posteroanterior in the coronal plane using the T2-weighted single-shot fast spin-echo (SSFSE/HASTE) sequence with fat saturation at 3 mm fixed slice thickness during breath-hold(s). For small kidneys or for a subject who can take a long breath-hold, one set of images obtained during a single breath-hold would be sufficient to cover the entire kidneys. For large kidneys, however, more than one set of images would be required, where neighboring image sets were overlapped by one slice. After the T2-weighted images were obtained, breath-hold coronal, three-dimensional spoiled gradient interpolated T1-weighted images without fat saturation at 3 mm fixed-slice thickness were obtained. Following this acquisition of pregadolinium T1 images, 0.1 mmol/kg gadolinium was injected at 1 ml/s. The same T1-weighted imaging sequence was repeated to acquire postgadolinium T1 images as prescribed in the CRISP study protocol (8).
Case Selection and Renal Volume Measurement
From the CRISP I database, we stratified participants into three groups based on their kidney size (combined right and left kidney volumes ≤750, 750 to 1500, >1500 ml). Using a random number generator, we randomly chose seven subjects from the small kidney-size group, six from the medium kidney-size group, and seven from the large kidney-size group for a total of 20 subjects. This kidney-size group stratification is the same scheme used in a recent CRISP study (6). Pre- and postgadolinium 3D gradient-echo T1 (pre- and postgadolinium T1) without fat saturation images of these 20 subjects were retrieved.
The volume of each kidney was measured from MR images using the stereology method (Figure 1). The stereology method has been widely used for image-based volumetric measurements of a variety of organs (7,14–21) because it provides a fast and reliable measurement of the area of an object. Its measurement is based on counting the number of intersections of a randomly oriented and positioned grid over the object. In our application, the area of the kidney in each image was calculated by converting the sum of the selected grid points overlaid the kidney to area pixel count (7,8). Total renal volume was calculated from the set of contiguous images by summing the products of the area measurements and the slice thickness.
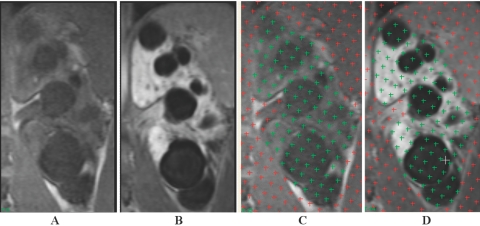
Figure 1.
Coronal T1-weighted magnetic resonance imaging (MRI) from a patient with ADPKD: (A and C) pregadolinium and (B and D) postgadolinium enhancement. Figure C corresponds to Figure A with superimposed stereology grids that were highlighted over the kidney regions by a reader, while Figure D corresponds to Figure B with superimposed stereology grids that were highlighted over the kidney regions by the same reader. With the administration of gadolinium, the kidney parenchyma in (B and D) enhanced brightly while the cysts remained unchanged. Note that the boundary of the kidney in the postgadolinium image (B) is better defined than that in the pregadolinium image (A), particularly near the spine and psoas muscle.
Two radiologists (CT, FZ), who had several years of experience each interpreting and analyzing MR images for kidney volume measurements, independently performed kidney volume measurements from the pre- and postgadolinium T1 images of the 20 cases (i.e., a total of 40 cases) twice using the stereology method. To reduce any potential order bias from the order of performing the measurements of the pre- and postgadolinium T1 images, the 40 cases were mixed and presented to the two readers for the measurement. More than a 2-wk interval was given between the initial and repeat measurements to reduce any memory effect.
Data and Statistical Analysis
The intrareader reliability in the right, left, and total (i.e., sum of right and left) kidney volume measurements was assessed using the intraclass correlation coefficient, while the inter-reader reliability was evaluated with the Pearson correlation coefficient. The first measurements of each reader were used for the inter-reader reliability computation. The differences between the repeated kidney volume measurements within and between the readers were calculated. The mean and SD of these differences were considered to represent the bias and variability, respectively, of the intra- and inter-reader kidney volume measurements. Patterns associated with the intra- and inter-reader bias for measuring the right, left, and total kidney volumes on the pre- and postgadolinium T1 images were tested for statistical significance with paired t test.
The differences between the pre- and postgadolinium T1 kidney volume measurements were also compared by calculating the difference (post − pre) divided by the pre-gadolinium kidney volume measurements for each of the two readers, i.e. “post-pre percent volume difference.” To investigate the effect of the kidney size on the pre- and postgadolinium T1 measurement differences, the post-pre volume differences and percent differences were compared among the three kidney-size groups (total kidney volumes ≤750, 750 to 1500, >1500 ml) with one-way ANOVA. The statistical analysis was performed with SPSS Statistical Software (SAS Institute, Inc., Cary, NC). Alpha was set at 0.05.
Results
Intra- and Inter-reader Reliability, Bias, and Variability
The intrareader reliability for both readers in the pre- and postgadolinium T1 measurements was extremely high: the correlation coefficients were all 0.99 (for reader 1 and reader 2; for the right, left, and total kidney volumes). Likewise, the inter-reader reliability for both pre- and postgadolinium T1 measurements was extremely high: the correlation coefficients were all 0.99 (for the right, left, and total kidney volumes). The intra- and inter-reader bias and variability in the right, left, and total kidney volume measurements are summarized in Table 1. T-test analysis of these measurements indicated no systemic intrareader bias but a small inter-reader bias for the postgadolinium T1 measurements (P < 0.01). Overall, substantial variability in the differences across all of the measurements implies lack of systematic differences within and between the readers. A similar trend was also observed across the three kidney-size groups, suggesting that the intra- and inter-reader bias was not affected by the kidney size.
Table 1.
Intra- and inter-reader bias and variability in kidney volume measurements
| Intra-reader Differences in Kidney Volume Measurements (mL)
|
Inter-reader Differences in Kidney Volume Measurements (mL)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pregadolinium
|
Postgadolinium
|
Pregadolinium
|
Postgadolinium
|
|||||||||
| Right | Left | Total | Right | Left | Total | Right | Left | Total | Right | Left | Total | |
| Mean (bias) | 10.88 | 3.74 | 14.62 | −4.15 | −5.34 | −9.49 | 6.64 | 2.91 | 9.56 | 14.55 | 15.80 | 30.36 |
| SD (variability) | 14.1 | 16.5 | 22.7 | 10.5 | 13.8 | 22.1 | 16.0 | 21.7 | 30.0 | 18.0 | 20.0 | 34.4 |
| P value | 0.003 | 0.323 | 0.010 | 0.093 | 0.101 | 0.070 | 0.078 | 0.554 | 0.171 | 0.002 | 0.002 | 0.001 |
| Mean % Change (bias) | 1.71 | 0.52 | 1.06 | −0.78 | −0.68 | −0.69 | 1.24 | 0.85 | 1.04 | 2.99 | 2.85 | 2.89 |
| SD % Change (variability) | 2.3 | 1.8 | 1.5 | 1.6 | 1.8 | 1.46 | 3.3 | 2.8 | 2.5 | 3.1 | 2.7 | 2.4 |
| P value | 0.003 | 0.206 | 0.004 | 0.043 | 0.115 | 0.047 | 0.106 | 0.188 | 0.083 | 0.000 | 0.000 | 0.000 |
Comparison of the Pre- and Postgadolinium T1 Measurements
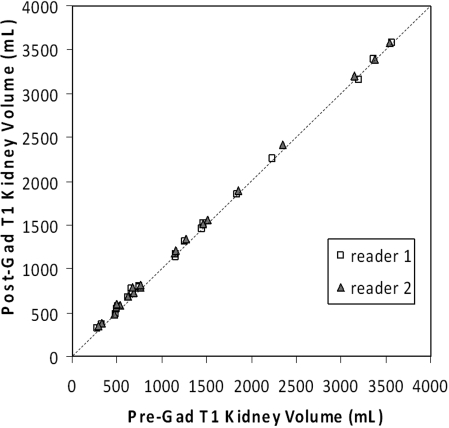
All three (right, left, total) kidney volumes measured on the pre- and postgadolinium T1 images were highly correlated with each other for both readers (Table 2). The Pearson correlation coefficients ranged 0.96 to 1.00. In particular, the correlation coefficients between the pre- and postgadolinium total kidney volume measurements were 1.00 for both readers (Figure 2).
Table 2.
Pearson correlation coefficients between various paired kidney volume measurements by two readers (reader 1/reader 2)
| Volumes | Pregadolinium | Pregadolinium | Postgadolinium | Postgadolinium | Postgadolinium |
|---|---|---|---|---|---|
| Right Kidney | Total Kidney | Left Kidney | Right Kidney | Total Kidney | |
| Pregadolinium Left Kidney | 0.964/0.961 | 0.991/0.990 | 0.999/0.999 | 0.962/0.961 | 0.991/0.991 |
| Pregadolinium Right Kidney | – | 0.991/0.990 | 0.961/0.956 | 1.00/1.00 | 0.990/0.989 |
| Pregadolinium Total Kidney | – | – | 0.989/0.988 | 0.990/0.990 | 1.00/1.00 |
| Postgadolinium Left Kidney | – | – | – | 0.960/0.957 | 0.990/0.989 |
| Postgadolinium Right Kidney | – | – | – | – | 0.990/0.989 |
Figure 2.
Plot of the pre- versus postgadolinium T1 kidney volume measurements by two readers along the line of identity. The data points represent the total kidney volumes. The correlation coefficients were 1.00 for both readers (Table 2).
The kidney volumes measured on the postgadolinium T1 images were significantly greater than those on the pregadolinium T1 images for the right, left, and total kidney measurements for both readers (P < 0.05) (Table 3). For example, the mean and SD of the post-pre differences in the total kidney volume were 28.5 ± 31.0 ml for reader 1 and 49.3 ± 26.3 ml for reader 2. The mean and SD of the post-pre percent differences in the total kidney volume were 4.6 ± 5.2% for reader 1 and 6.6 ± 6.2% for reader 2. One reader's magnitude of the post-pre differences and percentage differences of the kidney volumes was slightly greater than the other reader (P < 0.05).
Table 3.
(Post-pre) kidney volume differences and percent differences in the right, left and total kidney measurements made by two readers. The postgadolinium volumes were significantly higher than the pregadolinium volumes
| Kidney | Reader | Volume difference (post − pre) (mL)
|
Volume %difference [(post − pre)/pre]x100
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | P value | P value (reader effect) | Mean | SD | P value | P value (reader effect) | ||
| Right | R1 | 16.49 | 14.84 | 0.001 | 0.006 | 4.98 | 6.26 | 0.002 | 0.010 |
| R2 | 24.41 | 14.69 | 0.000 | – | 6.87 | 6.51 | 0.001 | – | |
| Left | R1 | 11.99 | 22.59 | 0.028 | 0.029 | 4.34 | 5.51 | 0.002 | 0.020 |
| R2 | 24.87 | 21.11 | 0.000 | – | 6.56 | 7.11 | 0.001 | – | |
| Total | R1 | 28.48 | 31.00 | 0.001 | 0.003 | 4.57 | 5.24 | 0.001 | 0.005 |
| R2 | 49.28 | 26.27 | 0.000 | – | 6.59 | 6.25 | 0.000 | – | |
The post-pre differences and percentage differences of the kidney volumes were computed and compared in three kidney-size groups (Table 4). The within reader post-pre volume differences were not statistically different among the three groups. For example, the mean and SD of the post-pre volume differences for the total kidney volume in (small, medium, large) kidney groups were (36.7 ± 22.1, 38.7 ± 41.5, 11.5 ± 24.5 ml; P = 0.20) for reader 1 and (50.4 ± 28.3, 57.9 ± 34.1, 40.8 ± 15.9 ml; P = 0.52) for reader 2.
Table 4.
Comparison among three kidney-size groups of the (post-pre) kidney volume differences and percent differences in the right, left, and total kidney measurements made by two readers. The percent difference was larger when the total kidney volume was smaller
| Kidney | Reader | Small Kidney Group Mean (SD) | Medium Kidney Group Mean (SD) | Large Kidney Group Mean (SD) | P value | Group Pairwise Relationship | |
|---|---|---|---|---|---|---|---|
| Volume difference (post − pre) (mL) | Right | R1 | 20.36 (11.48) | 21.09 (25.58) | 21.03 (17.63) | 0.054 | – |
| R2 | 26.84 (18.54) | 28.06 (21.00) | 20.18 (25.67) | 0.781 | – | ||
| Left | R1 | 16.35 (15.69) | 17.62 (17.63) | −4.17 (21.68) | 0.975 | – | |
| R2 | 23.55 (12.54) | 29.85 (17.15) | 20.60 (15.27) | 0.542 | – | ||
| Total | R1 | 36.70 (22.07) | 38.70 (41.51) | 11.51 (24.52) | 0.204 | – | |
| R2 | 50.39 (28.29) | 57.91 (34.14) | 40.78 (15.92) | 0.523 | – | ||
| Volume %difference [(post − pre) pre]x100 | Right | R1 | 8.26 (4.76) | 4.75 (6.09) | 0.06 (1.72) | 0.011 | Small > Large |
| R2 | 11.61 (8.80) | 5.94 (5.35) | 2.37 (2.18) | 0.030 | Small > Large | ||
| Left | R1 | 8.13 (8.00) | 5.93 (5.35) | 1.36 (1.15) | 0.122 | – | |
| R2 | 11.20 (6.75) | 7.71 (6.50) | 1.83 (1.17) | 0.015 | Small > Large | ||
| Total | R1 | 8.01 (4.90) | 5.07 (5.96) | 2.04 (2.18) | 0.021 | Small > Large | |
| R2 | 11.16 (6.79) | 6.72 (5.66) | 1.92 (0.98) | 0.012 | Small > Large |
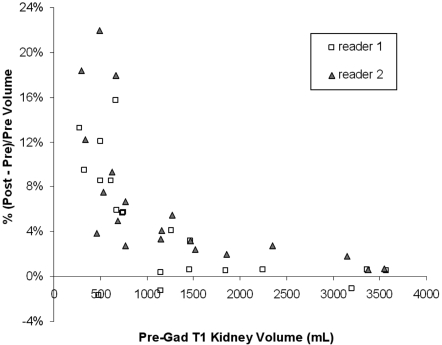
While the post-pre volume differences were relatively constant across the three kidney-size groups, the percent volume differences (i.e., the post-pre volume difference normalized by the pregadolinium kidney size) was significantly different among the three groups. The mean and SD of the post-pre percent volume differences for the total kidney volume in (small, medium, large) kidney groups were (8.1 ± 4.9, 5.1 ± 6.0, 2.0 ± 2.2%; P = 0.02) for reader 1 and (11.2 ± 6.8, 6.7 ± 5.7, 1.9 ± 1.0%; P = 0.01) for reader 2. This indicates that the post-pre percent volume differences were smaller as the size of the kidney increased (i.e., a larger kidney size resulting in a greater normalization factor) (Figure 3).
Figure 3.
Plot of the percent volume difference [i.e., (post − pre)/pre x 100] against the pregadolinium T1 kidney volume. The data points represent the total kidney volumes. The postgadolinium T1 kidney volume measurements tended to be larger than the pregadolinium measurements, and the percent volume differences tended to be smaller with increases in the kidney volume.
Discussion
It is well established that increased kidney size in ADPKD is associated with the severity of renal functional impairment (4,22) and with clinical complications including hypertension and gross hematuria (23). Recent imaging studies of CRISP and other populations demonstrated that renal volume and the rate of renal volume growth could be reliably measured and used to monitor the disease progression in the early stages of ADPKD when GFR is preserved (6,8,24).
Different imaging modalities such as ultrasound (25–28), CT (29,30), and MRI have been used to quantify the size of the kidney in ADPKD. MRI has increasingly been used because it provides high-resolution 3D images with excellent tissue contrast without exposure to ionizing radiation or iodinated contrast medium. It has been widely used for morphologic and functional analyses of various organs, notably the brain, heart, kidney, and liver. Some of the limitations of MR imaging include relatively long image- acquisition times and variability in the quality of images that can be produced from different MR scanners. Recent multi-center clinical trials that used imaging studies to monitor the disease progression demonstrated that MR imaging was well suited to assess renal structures and changes in kidney volume and cysts volume (6–8).
Gadolinium contrast is commonly used in clinical renal MR imaging studies. It helps detect renal masses and differentiate renal cysts from the background renal parenchyma. Gadolinium-enhanced MR angiography provides a superb visualization of renal vessels and is used to evaluate renal vascular abnormality such as renal artery stenosis. Gadolinium-enhanced T1 MR imaging provides excellent delineation of the renal structures and boundary that expedites the segmentation of the kidney from the surrounding anatomical structure and the measurement of the renal and renal cyst volume (7,8).
Until recently it was believed that gadolinium contrast media were safe for both the kidneys and all other organs within the dose range up to 0.3 mmol/kg body weight and that gadolinium-based contrast media could be used in place of iodinated agents for radiologic examinations in patients with significant renal impairment (31). This practice, however, has been changed. Nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy, which was described in 1997, was linked to exposure to gadolinium contrast media in 2006 (12). Because NSF is associated with patients with renal impairment (10,11,32), measurement of serum creatinine/eGFR before the administration of gadolinium is routinely performed in clinical MR imaging. In patients with impaired renal function, the clinical diagnostic benefits and risks of gadolinium enhancement should be considered carefully. Specifically, for the purpose of the quantification of the kidney size in patients with ADPKD, who may be subjected to renal functional impairment, it would be desirable to measure the kidney volume on MR imaging without gadolinium.
The results of our study demonstrated that renal volume measurements on the T1 MR images with and without gadolinium were extremely highly correlated and closely matched, indicating that the use of gadolinium is not required for the quantification of size of the kidney. Compared with gadolinium-enhanced T1 images, however, T1 images without gadolinium are of lower signal and lower intrinsic tissue-contrast (Figure 1). Determining the kidney border in large kidneys without gadolinium is more difficult than that with gadolinium. Subsequently, the measurement of kidney volumes without gadolinium would require more careful comparison of T1 and T2 images and will take more time to analyze. Our preliminary study (data not presented) indicates that the measurement of kidney volumes without gadolinium would take about 25% more time than that with gadolinium. Alternatively, one may consider using T2 MR imaging for the quantification of the kidney volume, because high kidney tissue-contrast and hyperintense renal cysts in T2 images would help delineate the kidney boundaries against the background tissues. Compared with T1 imaging, however, T2 imaging requires longer scanning time and is subjected to increased variations in image quality. Thus, T2 imaging of the entire kidney usually requires multiple breath-hold scanning and is more prone to misregistration, motion artifacts, and heterogeneous tissue signal intensities, which may result in a greater imprecision in the kidney volume measurement.
We observed that the kidney volumes measurements were highly repeatable within and between the readers and could be made in an unbiased fashion with minimum variability on both T1 images with and without gadolinium enhancement. This implies that either unenhanced or gadolinium-enhanced T1 images could be used to measure the changes in kidney size in patients with ADPKD. We also found that reader 2 had larger differences between pre- and postgadolinium measurements than reader 1. This is important as we explore ways to trend the kidney-size changes over time when use of gadolinium changes during the study.
Our study demonstrated that the kidney volumes measured on the pregadolinium T1 were significantly smaller than those on the postgadolinium T1. Although the exact cause of this trend is uncertain, we postulate that the administration of gadolinium resulted in “blooming” (i.e., amplified signal intensity of the renal parenchyma due to gadolinium-induced T1-shortening) of the boundaries of the kidneys. With 3-mm slice thickness in the T1 images, each kidney boundary voxel represents a 3-mm averaged boundary tissue between the kidney and background perinephric fat. The background perinephric fat is usually brighter than the pregadolinium kidney parenchyma but darker than the postgadolinium kidney parenchyma. It is conceivable that some averaged boundary voxels, which would be deemed as the background tissue on the pregadolinium T1 images, may be considered as the kidney parenchyma on the postgadolinium T1 images because of their relative brightness. As a result, the postgadolinium kidney volume measurements include more boundary voxels and appear larger than the pregadolinium measurements. A similar blooming effect with the use of gadolinium was observed in MR angiography in that gadolinium enhancement increased the apparent diameter of the renal artery with a higher signal-to-noise ratio, compared with unenhanced MR imaging (33). The kidney volume was likely measured higher with gadolinium than without gadolinium because of this apparent blooming.
The kidney volumes measured with gadolinium were larger than without gadolinium by a mean of 28.5 ml for reader 1 and 49.3 ml in our study cohort. This appears smaller than the increase in the kidney volume on average over the course of a year (i.e., a mean of 204 ml over a 3-yr period in the CRISP study) (6). Nevertheless, this “systemic” difference in the kidney volume measurements between T1 with and without gadolinium should be considered when kidney volume is used to quantify the progression of disease in ADPKD. An adjustment factor between the unenhanced and gadolinium-enhanced kidney volumes may be obtained using various approaches. One potential approach is building separate regression models for the three different kidney-size groupings.
Our study result also demonstrated that the percent differences between the kidney volume measured on unenhanced and enhanced T1 images tended to be smaller with increase in kidney size. This trend was expected because, while the post-pre volume differences were relatively constant across the three kidney-size groups, the relative contribution of the increased kidney volume due to the enhanced boundary voxels in a larger kidney was less than that in a smaller kidney when the percent difference (i.e., normalized by the kidney size) was calculated.
We should note that each of the 241 patients in the CRISP study had up to four separate measurements of kidney volume with gadolinium enhancement over an interval of three yrs, yet no signs of nephrogenic systemic fibrosis were observed. All of these subjects had GFR values that fell within normal limits at baseline, and this may account for the failure to experience this complication with repeated exposure to gadolinium.
In conclusion, our study demonstrated that kidney volume measurements can be made in an unbiased fashion with minimum intra- and intervariability on both pre- and postgadolinium T1 MR images. Kidney volumes measured with the pregadolinium images were smaller than those with the postgadolinium images, and the percent differences were smaller with increasing kidney size. When serial kidney volume measurements are used to quantify the progression of disease in PKD, a consistent use of the same type of MR images (with or without gadolinium) is desirable to reduce measurement variations. Given a concern for NSF in patients with renal impairment, serial MR images without gadolinium would be preferred in measuring volume progression over time in PKD.
Disclosures
None.
Acknowledgments
This study was supported by the CRISP Study U01 cooperative agreements (DK56956, DK56943, DK56957, and DK56961) of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, and the institutional GCRCs. We are indebted to the study coordinators who worked with professional grace and high competence to bring this project to conclusion: Beth Stafford, Kristin Cornwell, Diane Watkins, Sharon Langley, and Teresa Chacana. We thank Paul Commean, Stan Philips, Steve Moore, Paul Koppel, and Fred Prior for image data transfer, and Johana Schafer for managerial assistance. ANALYZE software was provided by Dr. Richard Robb of the Mayo Biomedical Imaging Resource in Rochester, MN.
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Gabow PA: Autosomal dominant polycystic kidney disease. N Engl J Med 329: 332–342, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Wilson PD: Polycystic kidney disease. N Engl J Med 350: 151–164, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Choukroun G, Itakura Y, Man NK, Christophe JL, Albouze G, Jungers P, Grünfeld JP: The rate of progression of renal failure in ADPKD. Contrib Nephrol 115: 28–32, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Gabow PA, Johnson AM, Kaehny WD, Kimberling WJ, Lezotte DC, Duley IT, Jones RH: Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int 41: 1311–1319, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Grantham JJ: Mechanisms of progression in autosomal dominant polycystic kidney disease. Kidney Int Suppl 63: S93–97, 1997 [PubMed] [Google Scholar]
- 6.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF Jr, Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP; CRISP Investigators: Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bae KT, Commean PK, Lee J: Volumetric measurement of renal cysts and parenchyma using MRI: Phantoms and patients with polycystic kidney disease. J Comput Assisted Tomogr 24: 614–619, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Chapman AB, Guay-Woodford LM, Grantham JJ, Torres VE, Bae KT, Baumgarten DA, Kenney PJ, King BF Jr, Glockner JF, Wetzel LH, Brummer ME, O’Neill WC, Robbin ML, Bennett WM, Klahr S, Hirschman GH, Kimmel PL, Thompson PA, Miller JP; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort.: Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int 64: 1035–1045, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Bae KT, Zhu F, Chapman AB, Torres VE, Grantham JJ, Guay-Woodford LM, Baumgarten DA, King BF Jr, Wetzel LH, Kenney PJ, Brummer ME, Bennett WM, Klahr S, Meyers CM, Zhang X, Thompson PA, Miller JP; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP): Magn Reson Imaging Evaluation of Hepatic Cysts in Early Autosomal-Dominant Polycystic Kidney Disease: The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease Cohort. Clin J Am Soc Nephrol 1: 64–69, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Thomsen HS, Marckmann P: Extracellular Gd-CA: differences in prevalence of NSF. European journal of radiology 66: 180–183, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Bellin MF, Van Der Molen AJ: Extracellular gadolinium-based contrast media: An overview. European journal of radiology 66: 160–167, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Thomsen HS: ESUR guideline: Gadolinium-based contrast media and nephrogenic systemic fibrosis. European radiology 17: 2692–2696, 2007 [DOI] [PubMed] [Google Scholar]
- 13.King BF, Torres VE, Brummer ME, Chapman AB, Bae KT, Glockner JF, Arya K, Felmlee JP, Grantham JJ, Guay-Woodford LM, Bennett WM, Klahr S, Hirschman GH, Kimmel PL, Thompson PA, Miller JP; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP): Magnetic resonance measurements of renal blood flow as a marker of disease severity in autosomal-dominant polycystic kidney disease. Kidney Int 64: 2214–2221, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Bartsch G: Stereology, a new quantitative morphological approach to study prostatic function and disease. Eur Urol 3: 85–95, 1977 [DOI] [PubMed] [Google Scholar]
- 15.Brugghe J, Baak JP, Meijer GA, van Diest PJ, Brinkhuis M: Rapid and reliable assessment of volume percentage of epithelium in borderline and invasive ovarian tumors. Anal Quant Cytol Histol 20: 14–20, 1998 [PubMed] [Google Scholar]
- 16.Doherty CP, Fitzsimons M, Holohan T, Mohamed HB, Farrell M, Meredith GE, Staunton H: Accuracy and validity of stereology as a quantitative method for assessment of human temporal lobe volumes acquired by magnetic resonance imaging. Magn Reson Imaging 18: 1017–1025, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Garden AS, Roberts N: Fetal and fetal organ volume estimations with magnetic resonance imaging. Am J Obstet Gynecol 175: 442–448, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Graves MJ, Dommett DM: Comparison of cardiac stroke volume measurement determined using stereological analysis of breath-hold cine MRI and phase contrast velocity mapping. Br J Radiol 73: 825–832, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Mazonakis M, Damilakis J, Maris T, Prassopoulos P, Gourtsoyiannis N: Comparison of two volumetric techniques for estimating liver volume using magnetic resonance imaging. J Magn Reson Imaging 15: 557–563, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Ronan L, Doherty CP, Delanty N, Thornton J, Fitzsimons M: Quantitative MRI: A reliable protocol for measurement of cerebral gyrification using stereology. Magn Reson Imaging 24: 265–272, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Walton JM, Roberts N, Whitehouse GH: Measurement of the quadriceps femoris muscle using magnetic resonance and ultrasound imaging. Br J Sports Med 31: 59–64, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalgaard OZ: Bilateral polycystic disease of the kidneys: A follow-up of two hundred eighty-four patients and their families. Acta Med Scand 328(suppl): 1–233, 1957 [PubMed] [Google Scholar]
- 23.Gabow PA, Chapman AB, Johnson AM, Tangel DJ, Duley IT, Kaehny WD, Manco-Johnson M, Schrier RW: Renal structure and hypertension in autosomal dominant polycystic kidney disease. Kidney Int 38: 1177–1180, 1990 [DOI] [PubMed] [Google Scholar]
- 24.Fick-Brosnahan GM, Belz MM, McFann KK, Johnson AM, Schrier RW: Relationship between renal volume growth and renal function in autosomal dominant polycystic kidney disease: A longitudinal study. Am J Kidney Dis 39: 1127–1134, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Parfrey PS, Bear JC, Morgan J, Cramer BC, McManamon PJ, Gault MH, Churchill DN, Singh M, Hewitt R, Somlo S, et al.: The diagnosis and prognosis of autosomal dominant polycystic kidney disease. N Engl J Med 323: 1085–1090, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM: Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343: 824–827, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Gabow PA, Ikle DW, Holmes JH: Polycystic kidney disease: Prospective analysis of nonazotemic patients and family members. Ann Intern Med 101: 238–247, 1984 [DOI] [PubMed] [Google Scholar]
- 28.Gabow PA, Kaehny WD, Johnson AM, Duley IT, Manco-Johnson M, Lezotte DC, Schrier RW: The clinical utility of renal concentrating capacity in polycystic kidney disease. Kidney Int 35: 675–680, 1989 [DOI] [PubMed] [Google Scholar]
- 29.Thomsen HS, Madsen JK, Thaysen JH, Damgaard-Petersen K: Volume of polycystic kidneys during reduction of renal function. Urol Radiol 3: 85–89, 1981 [DOI] [PubMed] [Google Scholar]
- 30.King BF, Reed JE, Bergstralh EJ, Sheedy PF 2nd, Torres VE: Quantification and longitudinal trends of kidney, renal cyst, and renal parenchyma volumes in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 11: 1505–1511, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Thomsen HS: How to avoid CIN: Guidelines from the European Society of Urogenital Radiology. Nephrol Dial Transplant 20 Suppl 1: i18–22, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Thomsen HS, Marckmann P: MRI contrast media are used to improve visualization of abnormal structures or lesions in various parts of the body. Introduction. Eur J Radiol 66: 153–159, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Bass JC, Prince MR, Londy FJ, Chenevert TL: Effect of gadolinium on phase-contrast MR angiography of the renal arteries. AJR 168: 261–266, 1997 [DOI] [PubMed] [Google Scholar]