Abstract
Background and objectives: Hyperglycemia and new-onset diabetes occurs frequently after kidney transplantation. The stress of surgery and exposure to immunosuppression medications have metabolic effects and can cause or worsen preexisting hyperglycemia. To our knowledge, hyperglycemia in the immediate posttransplantation period has not been studied.
Design, setting, participants, & measurements: We conducted a retrospective, observational study to characterize the prevalence and assess the pharmacologic management of hyperglycemia in kidney transplant recipients who underwent transplantation at our center between June 1999 and December 2006. Data were abstracted from electronic and pharmacy databases.
Results: The study cohort included 424 patients (mean age 51 yr; 58% men; 25% with pretransplantation diabetes). All patients with and 87% without pretransplantation diabetes had evidence of hyperglycemia (bedside glucose ≥200 mg/dl or physician-instituted insulin therapy), whereas the prevalence of hypoglycemia was low (4.5%). Hyperglycemia was sustained throughout hospitalization. All patients with and 66% without pretransplantation diabetes required insulin at hospital discharge. Patients with pretransplantation diabetes were treated primarily with short-acting insulin during the first 24 h after transplantation but were transitioned to long-acting insulin as the hospital stay progressed.
Conclusions: Investigators have historically attempted to identify hyperglycemia after hospital discharge. Our data indicate that a substantial number of patients without pretransplantation diabetes develop hyperglycemia and require insulin during the hospital phase of their care immediately after kidney transplantation. Prospective studies are needed to delineate factors that contribute to development of new-onset diabetes after transplantation among patients with transient hyperglycemia.
Hyperglycemia is a widely known complication after solid organ transplantation (1–6). Among kidney transplant recipients, it is of particular concern because approximately 23% have ESRD as a result of diabetes (7), and maintaining good glucose control after transplantation is necessary to prevent recurrent diabetic nephropathy (8); however, hyperglycemia may also occur de novo (i.e., among kidney transplant recipients without diabetes). Thus, in the inpatient setting after kidney transplantation, patients can be stratified into two hyperglycemic populations: (1) Patients with pretransplantation diabetes and (2) patients who do not have a history of diabetes and develop elevated glucose during hospitalization. The stress of surgery and exposure to immunosuppression medications have metabolic effects in both groups of patients that can cause or worsen hyperglycemia. Persistence of hyperglycemia beyond hospitalization in patients without preexisting diabetes has been termed new-onset diabetes after transplantation (NODAT). NODAT has been shown to have a negative impact on long-term graft and patient outcomes (9).
Recent studies have demonstrated an important relationship between inpatient hyperglycemia and adverse patient outcomes (e.g., higher mortality, greater costs) in several different clinical scenarios (10–12), and increased emphasis is now being placed on the need to manage inpatient hyperglycemia better (13). Previous studies of hyperglycemia among renal transplant patients typically focused on diabetic control after hospital discharge rather than during hospitalization; however, hyperglycemia is often evident in the immediate postoperative period, before the patient leaves the hospital, and may correlate with the future development of NODAT (14).
Kidney transplant recipients represent a segment of the inpatient population that may benefit from targeted interventions to improve inpatient hyperglycemia management. Early identification of hyperglycemia, ideally while patients are still hospitalized, and optimization of inpatient glycemic control may have clinically significant implications for long-term patient and graft survival. There are no published data on the prevalence of inpatient hyperglycemia or the management of hyperglycemia during the inpatient phase of care after transplantation. We therefore conducted a retrospective study to characterize better the prevalence of inpatient hyperglycemia and to assess the pharmacologic treatment of patients who undergo a first kidney transplant.
Materials and Methods
Case Selection
Our analysis included all adult patients who underwent a first kidney transplant between June 1999 and December 2006 at Mayo Clinic Hospital (Phoenix, AZ). After obtaining consent from the Mayo Clinic institutional review board, we identified the study cohort by conducting a systematic chart review. Available data included demographics (patient age, gender, and race/ethnicity), diagnosis of diabetes before transplantation, diagnosis of hepatitis C before transplantation, and the type of diabetes therapy before receipt of the transplanted organ. We further stratified patients with pretransplantation diabetes into two groups: (1) Patients who were treated with insulin before transplantation and (2) patients who were not treated with insulin before transplantation (i.e., patients who were taking oral agents or whose diabetes was managed by diet). We chose not to classify patients by diabetes type (type 1 or type 2) because the basis of classification made at initial diagnosis could not be verified. Pretransplantation diabetes status was documented as reported in the United Network for Organ Sharing form. Determination of diabetes therapy was made by reviewing the outpatient medication list.
Postoperative Management after Kidney Transplantation
After a brief admission (typically hours), patients undergo renal transplantation. After transplantation, patients are started on intravenous fluid with normal saline (substituting 1 ml/1 ml urine output for the first 24 h; after that it is reduced to 0.5 ml/1 ml urine output) and subsequently discontinued once the patient is able to tolerate adequate oral intake. They are started on a clear liquid diet on postoperative day 1 and are advanced as tolerated to an appropriate diet (usually an 1800-calorie American Diabetes Association [ADA] diet for patients with a history of diabetes or a regular diet for those without pretransplantation diabetes). Standard immunosuppression for the study cohort included possible induction therapy with either rabbit antithymocyte Ig or basiliximab. All patients receive a 5-d tapering course of glucocorticoids (methylprednisolone intravenous 500 mg on day 1, 250 mg on day 2, and 125 mg on day 3, followed by oral prednisone 60 mg on day 4 and 30 mg on day 5), after which a majority of patients receive an early steroid-withdrawal immunosuppression regimen using mycophenolate mofetil and tacrolimus as maintenance immunosuppressants. The few patients who receive ongoing steroid therapy receive the same initial 5-d tapering course in an identical manner to the course given to those who are on early steroid withdrawal, and they are subsequently maintained on low dosages of glucocorticoids. Tacrolimus is initiated when there is a >30% drop in serum creatinine. The day of tacrolimus initiation is variable for each patient; however, all patients, including patients with delayed graft function, are started on tacrolimus before discharge.
The nursing staff collects bedside blood glucose values every 6 to 8 h to obtain preprandial values. Our common protocol for hyperglycemia is a sliding scale of subcutaneous short-acting insulin that starts at 2 U and increases by 2 U for every 50-mg/dl increase in blood sugar >200 mg/dl. Long-acting insulin is started at the discretion of the physician.
Assessment of Inpatient Hyperglycemia and Glycemic Control
To capture episodes of hyperglycemia, we computed a composite average bedside glucose measurements obtained during the first 24 h after admission (F24BedGlucavg) during which time the kidney transplant was performed, the last 24 h before discharge (L24BedGlucavg), and for the middle (median) day of the hospital stay (Mid24BedGlucavg). The first 24-h period was calculated forward from the admission time. We similarly averaged bedside glucose measurements for all three time periods (first, middle, and last days of hospital stay) to calculate a composite average (BedGlucavg). We calculated the overall frequency of hyperglycemia over the first, middle, and last days of hospitalization.
Threshold for hyperglycemia was defined as bedside blood glucose measurement ≥200 mg/dl; however, because many patients received one or more doses of insulin after a first episode of blood sugar ≥200 mg/dl, an isolated frequency of blood sugar ≥200 mg/dl does not capture the true prevalence of hyperglycemia had insulin not been administered. Thus, to capture the true prevalence of hyperglycemia, we further defined hyperglycemia as a bedside glucose ≥200 mg/dl on at least one bedside glucose measurement or as a physician order for insulin administration as therapy for expected hyperglycemia. The renal transplant database was linked by patient identifiers to our electronic laboratory database to retrieve information on bedside glucose values by methods described previously (15,16). In addition, we calculated the overall prevalence of hypoglycemia (blood glucose <70 mg/dl) during the hospital stay.
To assess diabetic control, we linked case identifiers to our inpatient pharmacy database to determine types of insulin pharmacotherapy actually administered to the patient for treatment of hyperglycemia. We further examined the insulin treatment strategies by classifying the type of regimen as “long-acting” (when only long-acting insulin was administered) or as “short-acting” (when only short-acting insulin was administered) and as long-acting and short-acting when both kinds of insulin were administered.
Statistical Analysis
We first analyzed data among patients without pretransplantation diabetes to determine the prevalence of hyperglycemia; F24BedGlucavg, Mid24BedGlucavg, L24BedGlucavg, and BedGlucavg values; and insulin use. These analyses were repeated for the subset of patients who had pretransplantation diabetes, comparing the subset of patients who were treated with insulin before transplantation with those who were not treated with insulin before transplantation.
Continuous variables were compared using t test and ANOVA, and non-normally distributed variables were compared using the Wilcoxon rank sum test. The χ2 test was used to compare proportions. Logistic regression models were used to determine risk conferred by traditional risk factors (age, gender, race, hepatitis C, pretransplantation body mass index [BMI]) on presence of hyperglycemia on the day of hospital discharge. In addition, incidence of postsurgical wound complications (including wound dehiscence, wound infection, and wound hematoma) and other infections within 1 mo after transplantation was determined in the two groups. All statistical analyses were conducted using Stata 8.0 statistical software (Stata Corp., College Station, TX).
Results
General Patient Characteristics
Our analytic cohort consisted of 424 adult patients who received a first solitary kidney transplant between June 1999 and December 2006. A total of 105 (25%) patients had a pretransplantation diagnosis of diabetes, 75 of whom were treated with insulin before transplantation. The mean age of patients at transplantation was 51 ± 9 yr; 58% were men. The mean length of hospital stay was 4.6 ± 2.2 d. Patients with pretransplantation diabetes were older than those without pretransplantation diabetes, and a greater proportion of patients with pretransplantation diabetes were of Native American or Hispanic ethnicity (Table 1). White patients with pretransplantation diabetes were more likely to be treated with insulin compared with nonwhite patients (Table 1).
Table 1.
Descriptive analyses of kidney transplant cohort by presence or absence of pretransplantation diabetesa
| Variable | Diabetes Present before Transplantation (n = 105)
|
No Diabetes before Transplantation (n = 319) | |
|---|---|---|---|
| Treated with Insulin before Transplantation (n = 75) | Treated with Diet or Oral Agents before Transplantation (n = 30) | ||
| Age (yr; mean [SD])b | 57 (9) | 60 (9) | 49 (14) |
| Male gender (%) | 64.0 | 63.0 | 56.0 |
| Race (%)c | |||
| black | 2.7 | 10.0 | 8.0 |
| white | 66.2 | 36.7 | 69.0 |
| Native American | 10.8 | 23.3 | 5.0 |
| Hispanic | 20.3 | 23.3 | 14.0 |
| BMI (kg/m2; mean [SD]) | 29.7 (5.7) | 27.9 (5.2) | 26.8 (5.6) |
| Donor type, deceased (%)b | 47.0 | 57.0 | 30.0 |
n = 424. BMI, body mass index.
P < 0.05 between patients with and without diabetes.
P < 0.05 between patients with diabetes treated with insulin and patients with diabetes treated with diet or oral agents before transplantation.
Prevalence of Inpatient Hyperglycemia
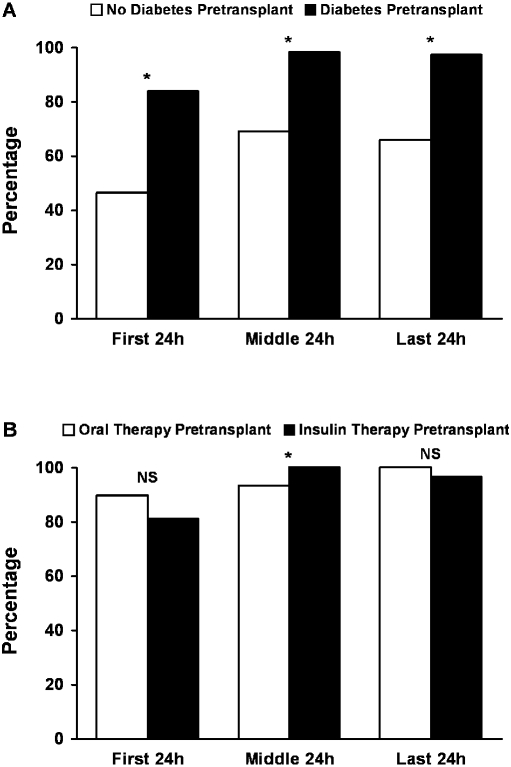
Figure 1 shows the distribution of hyperglycemia, defined as blood sugar ≥200 mg/dl or a physician order for administration of insulin during the first, middle, or last 24 h of the hospital stay. Overall, 87% of patients without diabetes and 100% with pretransplantation diabetes had blood sugar ≥200 mg/dl or received a dose of insulin during their entire hospitalization. Factors including age at transplantation, gender, BMI, ethnicity, and presence of hepatitis C were not significantly associated with the presence of hyperglycemia before discharge (during the last 24 h of hospital stay) among the cohort who did not have diabetes before transplantation. Univariate and multivariate logistic regression models demonstrated no significant risk for hyperglycemia conferred by the various traditional risk factors for diabetes, including age, gender, race, positive hepatitis C status, and pretransplantation BMI in the two groups.
Figure 1.
(A and B) Inpatient hyperglycemia prevalence after kidney transplantation in patients with and without pretransplantation diabetes (A; *P < 0.001) and in the subset of patients with known diabetes according to outpatient pretransplantation insulin use (B; *P < 0.05). Hyperglycemia was defined as blood sugar >200 mg/dl or physician-ordered administration of insulin therapy.
Bedside Blood Glucose Measurements during Hospitalization
Patients with pretransplantation diabetes had a significantly higher frequency of bedside blood glucose values ≥200 mg/dl during their entire hospitalization (median 9; confidence interval [CI] 8 to 11) compared with patients who did not have pretransplantation diabetes (median 1; 95% CI 1 to 1; P < 0.001). Bedside blood glucose measurements were significantly higher at every time point during hospitalization among patients with pretransplantation diabetes compared with those without pretransplantation diabetes (Table 2). In addition, the BedGlucavg level was significantly higher among those who were treated with insulin before transplantation versus those who were on oral or diet therapy before transplantation (Table 2). Overall, among all subcohorts, bedside blood glucose worsened in the middle 24 h of the hospital stay.
Table 2.
Average blood sugar at various time points after kidney transplantationa
| Bedside Glucose (mg/dl; mean [SD])b | Diabetes Present before Transplantation (n = 105)
|
No Diabetes before Transplantation (n = 319) | |
|---|---|---|---|
| Treated with Insulin before Transplantation (n = 75) | Treated With Diet or Oral Agents before Transplantation (n = 30) | ||
| F24BedGlucavg | 176 (56) | 168 (50) | 143 (32) |
| Mid24BedGlucavg | 224 (57) | 208 (51) | 150 (29) |
| L24BedGlucavg | 202 (52) | 185 (54) | 142 (32) |
| BedGlucavg | 203 (37) | 184 (30) | 145 (22) |
n = 424. BedGlucavg, bedside glucose composite average; F24BedGlucavg, composite average bedside glucose measurements obtained during the first 24 h after admission; L24BedGlucavg, composite average bedside glucose measurements obtained during the last 24 h before discharge; Mid24BedGlucavg, bedside glucose composite average (BedGlucavg) during the middle (median) of the hospitalization.
P < 0.05 between patients with and without diabetes. P < 0.05 between patients with diabetes treated with insulin and patients with diabetes treated with diet or oral agents before transplantation.
Prevalence of Inpatient Hypoglycemia
Overall, only 4.5% patients had at least one documented episode of bedside glucose <70 mg/dl during their hospital stay. On further analysis, 3.7% had one episode of hypoglycemia and close to 1% had two or more episodes of hypoglycemia during the hospital stay.
Patterns of Blood Glucose Monitoring and Insulin Therapy
Among patients with pretransplantation diabetes versus those without pretransplantation diabetes, the mean number of bedside glucose measurements was 24 ± 16 versus 16 ± 18 (P = 0.08) during hospitalization. Among patients with diabetes treated with insulin therapy versus those not treated with insulin therapy before transplantation, the mean number of bedside glucose measurements was 26 ± 17 versus 20 ± 8 (P < 0.001) during hospitalization.
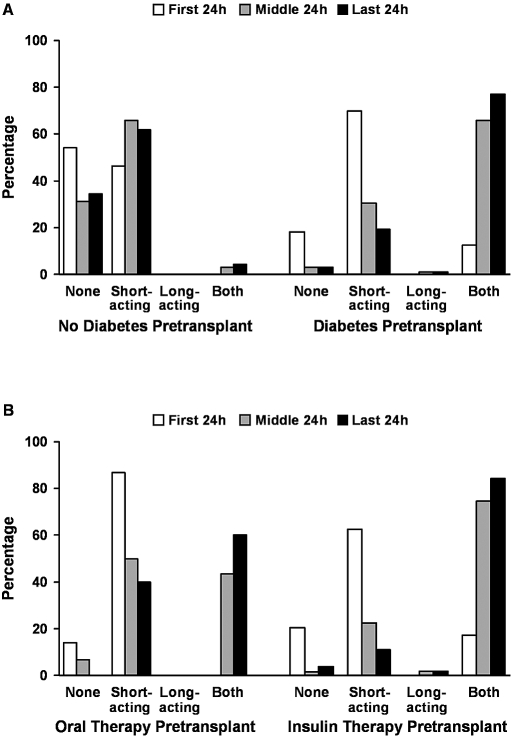
Insulin regimens differed among patients with pretransplantation diabetes compared with those without a history of diabetes (Figure 2). Patients without pretransplantation diabetes were administered predominantly short-acting insulin throughout their hospital stay (Figure 2A), with only a small group receiving any form of long-acting insulin. Patients with pretransplantation diabetes experienced a shift from a predominant use of short-acting insulin during the first 24 h of the hospital stay to a regimen consisting of a combination of long-acting and short-acting therapy as their hospitalization progressed (Figure 2B).
Figure 2.
(A and B) Pattern of inpatient insulin administration after kidney transplantation in patients with and without pretransplantation diabetes (A) and in the subset of patients with known diabetes according to outpatient pretransplantation insulin use (B).
By the last 24 h of the hospital stay, 66% of patients who had no diagnosis of pretransplantation diabetes and 100% of patients with a history of pretransplantation diabetes received insulin. Furthermore, among patients with pretransplantation diabetes, 28% who were not receiving insulin before transplantation were on this therapy by hospital discharge. All pretransplantation insulin users continued on insulin at hospital discharge.
Postsurgical Wound Complications and Infections
The incidence of postsurgical wound complications (including wound dehiscence, wound infection, and wound hematoma) within 1 mo after transplantation was 22 versus 14% (P = 0.05) among patients who did require insulin versus those who did not require insulin on the last day of hospital discharge. In addition, no statistical difference was observed in the rates of other infections within 1 mo after transplantation in the two groups.
Discussion
NODAT has received considerable attention in the medical literature (1–7,17,18); however, in each publication, hyperglycemia was defined during the outpatient phase of care, yet the inpatient setting is an optimal place to diagnose and treat hyperglycemia and to begin or reinforce efforts to educate the patient in the self-management skills that will be used in the outpatient arena (19). Using the kidney transplant population as our model, we conducted a retrospective analysis to define better the prevalence and to start to address the pharmacologic management strategies for optimum glucose control.
Our analyses indicate that the prevalence of inpatient hyperglycemia was high not only in patients with pretransplantation diabetes but also among patients without pretransplantation diabetes mellitus. We also observed that the middle 24-h period of the hospital stay was the point at which we observed the highest level of glucose values; this may be explained by the peak effect of steroids, because all transplant patients received high dosages of steroids for the first 5 d after transplantation.
We also elucidated patterns of insulin use in our hospitalized kidney transplant population. In general, a basal-bolus regimen (a long-acting insulin at bedtime and a short-acting insulin with meals or for correction) is the favored and most effective management approach to inpatient hyperglycemia (10,13,20). We observed that there was predominant use of short-acting insulin to manage hyperglycemia in patients without pretransplantation diabetes, perhaps reflecting beliefs on the part of their physicians that hyperglycemia would be short-lived. Because we do not know what proportion of patients ultimately resolves their hyperglycemia, all of these patients require education in self-monitoring of blood glucose and insulin administration before hospital discharge, a potentially increased burden on limited hospital staff that provide such services.
The significance of management of inpatient glucose in transplant patients can be viewed from two perspectives, short-term and long-term. In the short term, control of inpatient hyperglycemia may prove to be important for immediate surgical outcome. It is now recognized that inpatient hyperglycemia is associated with a number of adverse outcomes in several different clinical scenarios, including higher rates of surgical wound infections (10,13,21). Several national and regional quality improvement initiatives are under way to increase awareness and to develop strategies to improve care of hospitalized patients with diabetes or hyperglycemia (10,13,19,21–26). The transplant population represents a subset of patients who should be included in these efforts. In the long term, inpatient hyperglycemia may affect both graft and patient outcomes. Little is known about the outcomes of transplant patients who experience inpatient hyperglycemia (e.g., how many require long-term insulin therapy, the relationship between hyperglycemia and graft and patient survival). Future studies are required to determine the influence of inpatient hyperglycemia during the immediate postoperative period on the development of NODAT and its impact on long-term outcomes, including graft survival, infections, and cardiovascular disease. Identifying patients early, while still in the hospital, allows construction of a patient cohort that can be tracked prospectively.
There are some limitations to our study. First, our electronic database does not necessarily distinguish between a preprandial and a postprandial bedside glucose; however, ADA guidelines recommend no excursions of glucose ≥200 mg/dl, even after meals, so our definition of hyperglycemia is consistent with that of the ADA (27). In addition, current suggestions do not really seek to discriminate between pre- and postprandial values. Some type of mean value is currently favored (28). Second, we chose to divide patients with pretransplantation diabetes by their form of therapy (oral agents or insulin), a distinction that does not capture the underlying cause of diabetes. Third, blood glucose values were not captured as a continuum; instead, we had three periods during which the blood glucose and insulin usage were captured (first 24 h, middle 24 h, and the last 24 h). It should be noted that because the median length of hospital stay was 4 d, we anticipate that the three periods used to capture the data sufficiently capture the information about hyperglycemia. Fourth, a retrospective review of electronic medical records does not allow assessment of reasons underlying decision-making behavior of clinicians (e.g., why they did or did not change therapy). Finally, our study lacks data on pretransplantation glycemic control as determined, for example, by glycosylated hemoglobin (HbA1c) values. There are few data on whether prehospital glucose control is associated with inpatient hyperglycemia, although recent data indicate that preoperative HbA1c levels are correlated with outcome in coronary artery bypass patients (29). Until recently, there has not been an emphasis on obtaining HbA1c values at admission; however, recent Joint Commission guidelines on inpatient diabetes management require an HbA1c value if one has not been done within 60 d of before admission (24). Consequently, our hospital is in the process of implementing these guidelines, and future analysis should help answer the question about whether the severity of pretransplantation hyperglycemia is correlated with hospital inpatient hyperglycemia in these patients.
Conclusions
Investigators have typically attempted to delineate hyperglycemia after hospital discharge. Our data indicate that a substantial number of patients without pretransplantation diabetes develop hyperglycemia and require insulin during the inpatient phase of their care. Long-term follow-up will be required to determine which of these patients have hyperglycemia that will actually evolve into diabetes and which ones will recover from their hyperglycemic episode. Moreover, the long-term impact of inpatient hyperglycemia, including its effect on cardiovascular, allograft, and overall patient outcomes, needs to be studied. Overall quality improvement efforts are needed to better manage inpatient hyperglycemia after kidney transplantation.
Disclosures
None.
Acknowledgments
This publication was made possible by grant 1 KL2 RR024151 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. This publication was also supported in part by the intramural research program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases. Information on the NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov.
Published online ahead of print. Publication date available at www.cjasn.org.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of National Center for Research Resources or the National Institutes of Health.
References
- 1.Baid S, Cosimi AB, Farrell ML, Schoenfeld DA, Feng S, Chung RT, Tolkoff-Rubin N, Pascual M: Posttransplant diabetes mellitus in liver transplant recipients: Risk factors, temporal relationship with hepatitis C virus allograft hepatitis, and impact on mortality. Transplantation 72: 1066–1072, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Davidson J, Wilkinson A, Dantal J, Dotta F, Haller H, Hernandez D, Kasiske BL, Kiberd B, Krentz A, Legendre C, Marchetti P, Markell M, van der Woude FJ, Wheeler DC: New-onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation 75: SS3–SS24, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Hur KY, Kim MS, Kim YS, Kang ES, Nam JH, Kim SH, Nam CM, Ahn CW, Cha BS, Kim SI, Lee HC: Risk factors associated with the onset and progression of posttransplantation diabetes in renal allograft recipients. Diabetes Care 30: 609–615, 2007 [DOI] [PubMed] [Google Scholar]
- 4.John PR, Thuluvath PJ: Outcome of patients with new-onset diabetes mellitus after liver transplantation compared with those without diabetes mellitus. Liver Transpl 8: 708–713, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ: Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 3: 178–185, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Reisaeter AV, Hartmann A: Risk factors and incidence of posttransplant diabetes mellitus. Transplant Proc 33: 8S–18S, 2001 [DOI] [PubMed] [Google Scholar]
- 7.US Renal Data System: USRDS 2007 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2007
- 8.Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M: Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 339: 69–75, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Cosio FG, Kudva Y, van der Velde M, Larson TS, Textor SC, Griffin MD, Stegall MD: New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int 67: 2415–2421, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, Hirsch IB: Management of diabetes and hyperglycemia in hospitals. Diabetes Care 27: 553–591, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Laird AM, Miller PR, Kilgo PD, Meredith JW, Chang MC: Relationship of early hyperglycemia to mortality in trauma patients. J Trauma 56: 1058–1062, 2004 [DOI] [PubMed] [Google Scholar]
- 12.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R: Intensive insulin therapy in the critically ill patients. N Engl J Med 345: 1359–1367, 2001 [DOI] [PubMed] [Google Scholar]
- 13.ACE/ADA Task Force on Inpatient Diabetes: American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control. Endocr Pract 12: 458–468, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Chakkera H, Weil J, Khan A, Cook C, Mazur M, Mulligan D, Reddy S, Moss A, McKeel K, Heilman R: Early post-transplant hyperglycemia is predictive of the development of new onset diabetes mellitus in kidney transplant recipients [Abstract]. Am J Transplant 7[Suppl 2]: 462, 2007 [Google Scholar]
- 15.Cook CB, Castro JC, Schmidt RE, Gauthier SM, Whitaker MD, Roust LR, Argueta R, Hull BP, Zimmerman RS: Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum. J Hosp Med 2: 203–211, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Knecht LA, Gauthier SM, Castro JC, Schmidt RE, Whitaker MD, Zimmerman RS, Mishark KJ, Cook CB: Diabetes care in the hospital: Is there clinical inertia? J Hosp Med 1: 151–160, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Cosio FG, Pesavento TE, Osei K, Henry ML, Ferguson RM: Post-transplant diabetes mellitus: Increasing incidence in renal allograft recipients transplanted in recent years. Kidney Int 59: 732–737, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Montori VM, Basu A, Erwin PJ, Velosa JA, Gabriel SE, Kudva YC: Posttransplantation diabetes: A systematic review of the literature. Diabetes Care 25: 583–592, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Roman SH, Chassin MR: Windows of opportunity to improve diabetes care when patients with diabetes are hospitalized for other conditions. Diabetes Care 24: 1371–1376, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Umpierrez GE, Smiley D, Zisman A, Prieto LM, Palacio A, Ceron M, Puig A, Mejia R: Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 30: 2181–2186, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Garber AJ, Moghissi ES, Bransome ED Jr, Clark NG, Clement S, Cobin RH, Furnary AP, Hirsch IB, Levy P, Roberts R, Van den Berghe G, Zamudio V: American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract 10: 77–82, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Institute for Healthcare Improvement: Getting Started Kit: Prevent Surgical Site Infections. Available at: http://www.ihi.org/NR/rdonlyres/00EBAF1F-A29F-4822-ABCE-829573255AB8/0/SSIHowtoGuideFINAL.pdf. Accessed February 7, 2007
- 23.Institute for Healthcare Improvement: Implement Effective Glucose Control. Available at: http://www.ihi.org/IHI/Topics/CriticalCare/IntensiveCare/Changes/ImplementEffectiveGlucoseControl.htm. Accessed August 29, 2007
- 24.Joint Commission on Accreditation of Healthcare Organizations: Inpatient Diabetes Certification. Available at: http://www.jointcommission.org/CertificationPrograms/Inpatient+Diabetes/. Accessed February 7, 2007 [PubMed]
- 25.Pham PT, Pham PC, Lipshutz GS, Wilkinson AH: New onset diabetes mellitus after solid organ transplantation. Endocrinol Metab Clin North Am 36: 873–890, vii, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Cook CB, Stockton L, Baird M, Osburne RC, Davidson PC, Steed RD, Bode BW, Reid J, McGowan KA: Working to improve care of hospital hyperglycemia through statewide collaboration: The Georgia Hospital Association Diabetes Special Interest Group. Endocr Pract 13: 45–50, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Postprandial blood glucose. American Diabetes Association. Diabetes Care 24: 775–778, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Schnipper JL, Magee M, Larsen K, Inzucchi SE, Maynard G: Society of Hospital Medicine Glycemic Control Task Force summary: Practical recommendations for assessing the impact of glycemic control efforts. J Hosp Med 3: 66–75, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Halkos ME, Puskas JD, Lattouf OM, Kilgo P, Kerendi F, Song HK, Guyton RA, Thourani VH: Elevated preoperative hemoglobin A1c level is predictive of adverse events after coronary artery bypass surgery. J Thorac Cardiovasc Surg 136: 631–640, 2008 [DOI] [PubMed] [Google Scholar]