Abstract
Background and objectives: Kidney stones lead to chronic kidney disease (CKD) in people with rare hereditary disorders (e.g., primary hyperoxaluria, cystinuria), but it is unknown whether kidney stones are an important risk factor for CKD in the general population.
Design, setting, participants, & measurements: Among Olmsted County, MN, residents, all stone formers (n = 4774) whose condition was diagnosed in 1986 through 2003 were matched 1:3 to control subjects (n = 12,975). Cox proportional hazards models adjusted for age, gender, and comorbidities (hypertension, diabetes, obesity, dyslipidemia, gout, alcohol abuse, tobacco use, coronary artery disease, heart failure, cerebral infarct, and peripheral vascular disease) were used to assess the risk for incident CKD defined as a clinical diagnosis (diagnostic codes), ESRD or death with CKD, sustained (>90 d) elevated serum creatinine (>1.3 mg/dl in men, >1.1 mg/dl in women), or sustained estimated GFR <60 ml/min per 1.73 m2.
Results: During a mean of 8.6 yr of follow-up, stone formers were at increased risk for a clinical diagnosis of CKD, but an increased risk for ESRD or death with CKD was NS. Among patients with follow-up serum creatinine levels, stone formers were at increased risk for a sustained elevated serum creatinine and a sustained reduced GFR.
Conclusions: Kidney stones are a risk factor for CKD, and studies are warranted to assess screening and preventive measures for CKD in stone formers.
Kidney stones and chronic kidney disease (CKD) are common, affecting 5 and 13% of the adult population, respectively (1,2). CKD is a recognized complication of kidney stones as a result of rare hereditary disorders (e.g., primary hyperoxaluria, Dent disease, 2-8-hydroxyadenine crystalluria, cystinuria) (3–5), whereby nephrocalcinosis or renal crystal deposition can lead to progressive loss of GFR and ESRD at a young age. In addition, infection stones (struvite) can lead to an obstructive nephropathy with staghorn calculi and are the leading cause of ESRD attributed to nephrolithiasis (6). Although ESRD directly attributed to kidney stones is relatively modest, with an estimated prevalence of 3.2% among patients who start maintenance hemodialysis (6), kidney stones could still be a contributing factor in the development of CKD and its progression. A case-control study found that black patients who were on hemodialysis were three times more likely to have been stone formers than black individuals in the general population (7). In a cross-sectional study, renal function was reduced in stone clinic patients compared with normal individuals (8). In the general population, lower estimated GFR (eGFR) has been observed in overweight and obese stone formers (9), and a case-control study found an increased risk for CKD in stone formers without hypertension (10); however, population-based cohort studies assessing the risk for CKD with kidney stones are lacking. Taking advantage of the comprehensive medical record linkage system in Olmsted County, MN, the incidence of CKD in the general population was compared between a cohort of stone formers and a cohort of control subjects.
Materials and Methods
Study Population
Population-based research is feasible in Olmsted County because medical care is self-contained within the community. More than 95% of the population has at least one clinic visit with a health care provider in Olmsted County every 2 to 3 yr, allowing complete enumeration of the local population. Diagnostic codes (manually or automatically coded from the final diagnoses in clinical notes) dating back to 1935 are indexed and linked among virtually all Olmsted County providers through the Rochester Epidemiology Project (11). After institutional review board approval, all Olmsted County residents with their first diagnosis of a kidney stone from 1986 to 2003 were identified using International Classification of Diseases, Ninth Revision (ICD-9) codes 592, 594, and 274.11 and equivalent Hospital Adaptation of the International Classification of Diseases 8 (HICDA-8) codes. On manual review of 113 random charts with these codes, 70 had their first symptomatic kidney stone in Olmsted County with characteristic renal colic or with atypical pain and confirmation by imaging studies or hematuria, 25 had a kidney stone before living in Olmsted County, four had incidental (asymptomatic) kidney stones on imaging studies, five had bladder stones, and nine did not have verifiable stones. Stone formers were matched 1:3 to control subjects among all Olmsted County residents on index date (first stone episode for stone formers and nearest clinic visit for control subjects) ± 5 yr, duration of medical record before index date ± 5 yr, age ± 5 yr, and gender. Control subjects who had a subsequent kidney stone after their index date were censored at that point and subsequently included in the stone former cohort with their own matched control subjects.
Outcomes
CKD was identified using multiple approaches. A clinical diagnosis of CKD was defined by ICD-9 and equivalent HICDA-8 codes (see Appendix). Some specific codes (chronic renal failure, diabetic nephropathy, proteinuria, nephritis and nephropathy, and hypertensive renal disease) were analyzed individually. Deaths overall and with CKD listed as a cause were identified from Minnesota State death certificates. All maintenance dialysis (hemodialysis or peritoneal dialysis) and kidney transplants in Olmsted County were enumerated in clinical databases. A composite end point was defined by ESRD (dialysis or transplantation) or death with CKD (12).
Appendix.
| Clinical Diagnoses | ICD-9 Codes |
|---|---|
| Clinical CKD | |
| diabetes with renal manifestations | 250.4 |
| gouty nephropathy | 274.10, 274.19 |
| hypertensive renal disease | 403 |
| hypertensive heart and renal disease | 404 |
| Goodpasture syndrome | 446.21 |
| renal vein thrombosis | 453.3 |
| hepatorenal syndrome | 572.4 |
| nephrotic syndrome | 581 |
| chronic glomerulonephritis | 582 |
| nephritis and nephropathy | 583 |
| chronic renal failure | 585 |
| unspecified renal failure | 586 |
| renal sclerosis | 587 |
| disorder of kidney and ureter | 593.89 |
| unspecified disorder of kidney and ureter | 593.9 |
| cystic kidney disease | 753.1 |
| renal agenesis | 753.0 |
| congenital kidney anomalies | 753.3 |
| proteinuria | 791.0 |
| Obesity | 278.0, 278.1, 278.8 |
| Dyslipidemia | 272.0, 272.1, 272.2, 272.3, 272.4 |
| Alcohol dependence | 291, 303, 305, V79.1 |
| Tobacco use | 305.1, 989.84, V15.82 |
| Diabetes | 250, 357.2, 362.01, 362.02, 366.41, 648.8, 790.2 |
| Hypertension | 401 |
| Coagulation defects | 286 |
| Gout | 274.0, 274.81, 274.82, 274.89, 274.9 |
| Coronary artery disease | 410, 411, 412, 413 |
| Heart failure | 402.91, 428.0, 428.1, 428.9 |
| Cerebral infarct | 430, 431, 432.9, 433, 434, 435.0, 435.1, 435.8, 435.9, 436, 437.0, 437.1, 437.5, 437.9 |
| Peripheral vascular disease | 440, 441, 442, 443, 444, 557.0, 557.1, 557.9, 593.81 |
Independently, CKD was assessed via serum creatinine (SCr) levels available from the Mayo Clinic electronic medical records from 1983 to 2006. SCr levels were standardized by exchanging 146 frozen serum samples collected from 1999 through 2000 with the Cleveland Clinic (standardized SCr = −0.265 + 1.092 * Mayo Clinic SCr) (13). CKD was classified by an elevated SCr level (>97.5th percentile in regional kidney donors: >1.3 mg/dl in men and >1.1 mg/dl in women) (13,14), which identifies individuals with a GFR less than expected with normal aging (15,16). CKD was also classified by an eGFR <60 ml/min per 1.73 m2 using the SCr-based Modification of Diet in Renal Disease equation (17). To distinguish CKD from acute renal failure, analyses required the first SCr level >90 d after the initial SCr elevation (or eGFR <60 ml/min per 1.73 m2) and all intervening SCr levels to meet the threshold requirement.
Comorbidities
The initial date of clinical diagnoses that have been associated with kidney stones or CKD (hypertension, diabetes, obesity, dyslipidemia, gout, alcohol dependence, tobacco use, coronary artery disease, heart failure, cerebral infarct, and peripheral vascular disease) were identified from ICD-9 codes (see Appendix) and equivalent HICDA-8 codes.
Statistical Analyses
Patients were excluded from analyses for incident CKD when they had clinical CKD or ESRD before the index date or within 90 d after the index date or when they had an elevated mean SCr during the 3 yr to 1 mo before the index date. In addition, patients with no follow-up clinic visits >90 d after the index date were excluded. Patients without incident CKD were censored as of their last clinic visit or death or on December 31, 2006, whichever came first. Incident SCr elevations were based on follow-up SCr tests with dates >90 d after the index date. Additional analyses assessed the incidence of eGFR <60 and <30 ml/min per 1.73 m2 during follow-up, and these analyses excluded patients with prevalent eGFR <60 ml/min per 1.73 m2 instead of prevalent elevated SCr at baseline. To assess for detection bias (18) between stone formers and control subjects, we compared the proportion with at least one follow-up SCr test. To address potential detection bias, we performed a subset analysis by limiting the sample to patients with at least one follow-up SCr test and with censoring by the last SCr test date.
Because of these exclusions, matching was not retained in analyses. Logistic regression was used to calculate the odds ratios (OR) for prevalent CKD in stone formers compared with control subjects. Survival free of subsequent CKD was estimated using the Kaplan-Meier method. The association of kidney stones with subsequent CKD events was assessed using Cox proportional hazards models with adjustments for age, gender, and comorbidities. Comorbidities were considered as fixed covariates (present before the index date). Results were similar with adjustment for comorbidities occurring after the index date (considered as time-dependent covariates; data not shown). Models also evaluated CKD events with death as a competing risk. Hazard ratios (HR; i.e., CKD event rate in stone formers relative to control subjects) and 95% confidence intervals (CI) were reported.
Results
Baseline Characteristics
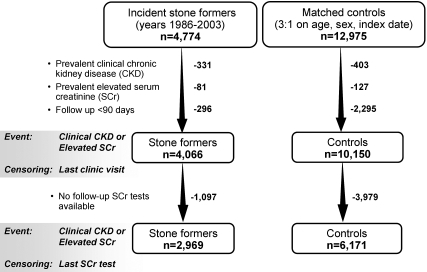
Figure 1 diagrams the sampling framework. A total 4774 stone formers and 12,975 control subjects were identified from the Olmsted County general population between 1986 and 2003. After exclusion of patients with prevalent clinical CKD or prevalent elevated SCr and those who lacked clinic visits 90 d after the index date, 4066 stone formers and 10,150 control subjects were followed for incident CKD. Only 2.1% of these patients were nonwhite, consistent with the racial distribution of the community (96% white in 1990). As a consequence of the matching, stone formers and control subjects were similar with respect to age (mean 44 yr), gender (59% male), and length of medical record documentation before the index date (mean 18 yr). The mean ± SD (range) follow-up to last clinic visit or death was 8.6 ± 5.4 yr (0.25 to 20.93 yr) in stone formers and 8.7 ± 5.3 yr (0.25 to 20.96 yr) in control subjects. As shown in Table 1, stone formers were more likely than control subjects to have a baseline diagnosis of hypertension, diabetes, obesity, dyslipidemia, gout, or coronary artery disease and were less likely to have a diagnosis of alcohol dependence. Stone formers were also more likely to have at least one SCr test >90 d after the index date (73 versus 61%; P < 0.0001). A total of 2969 stone formers and 6171 control subjects in the subset analysis were censored by last SCr test (Figure 1).
Figure 1.
Sampling framework among Olmsted County, MN, residents. The sample size in stone former and control cohorts varied depending on whether follow-up and censoring were by clinic visits in Olmsted County or by serum creatinine (SCr) tests at the Mayo Clinic.
Table 1.
Baseline comorbidities in Olmsted County, MN, stone former and control cohorts
| Comorbidities at Baseline | Stone Formers (n = 4066; n [%]) | Control Subjects (n = 10,150; n [%]) | P |
|---|---|---|---|
| Hypertension | 787 (19.4) | 1703 (16.8) | 0.0003 |
| Diabetes | 391 (9.6) | 754 (7.4) | <0.0001 |
| Obesity | 978 (24.1) | 2097 (20.7) | <0.0001 |
| Dyslipidemia | 890 (21.9) | 1931 (19.0) | 0.0001 |
| Gout | 130 (3.2) | 230 (2.3) | 0.0015 |
| Alcohol dependence | 233 (5.7) | 796 (7.8) | <0.0001 |
| Tobacco use | 622 (15.3) | 1634 (16.1) | 0.2400 |
| Coronary artery disease | 299 (7.4) | 649 (6.4) | 0.0400 |
| Cerebral infarct | 121 (3.0) | 268 (2.6) | 0.2700 |
| Heart failure | 89 (2.2) | 180 (1.8) | 0.1000 |
| Peripheral vascular disease | 180 (4.4) | 417 (4.1) | 0.3900 |
Prevalence of CKD
Clinical diagnosis of CKD was more prevalent in stone formers than in control subjects (6.9 versus 3.1%; OR 2.32; 95% CI 2.00 to 2.70). Prevalent clinical CKD varied with respect to the index date: >5 yr before index date, OR 1.30 (95% CI 0.99 to 1.72); 1 to 5 yr before index date, OR 1.36 (95% CI 1.02 to 1.80); 0 to 1 yr before the index date, OR 2.73 (95% CI 1.81 to 4.12); and 0 to 90 d after the index date, OR 6.99 (95% CI 5.11 to 9.58). At least one SCr level was available in 2123 stone formers and 4340 control subjects between 3 yr and 1 mo before the index date (45 versus 33%; P < 0.0001). Stone formers were also more likely than control subjects to have prevalent CKD assessed by elevated SCr (3.5 versus 1.9%; OR 1.81; 95% CI 1.49 to 2.22) or reduced eGFR (4.6 versus 3.0%; OR 1.55; 95% CI 1.31 to 1.84). The increased prevalence of elevated SCr among stone formers did not change substantively after adjustment for all comorbidities (OR 1.77; 95% CI 1.44 to 2.18).
Incidence of CKD by Clinical Diagnosis
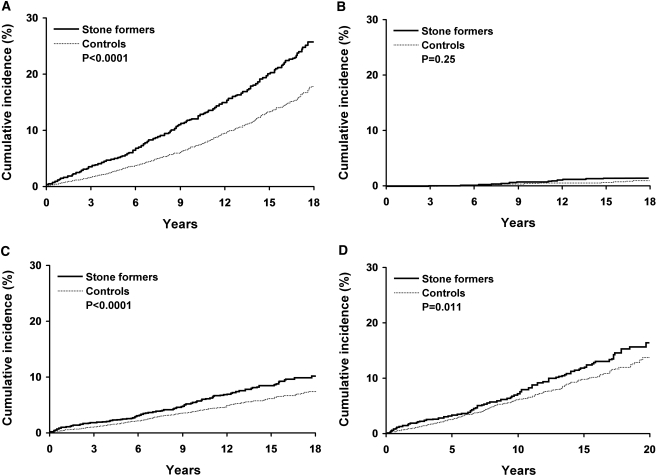
After exclusion of patients with prevalent CKD, stone formers were more likely to receive a subsequent diagnosis of clinical CKD compared with control subjects (HR 1.67; 95% CI 1.48 to 1.88; Figure 2A). Accounting for the competing risk for death only lowered clinical CKD event rates slightly (2 percentage points in both stone formers and control subjects at 18 yr). Some specific diagnostic code categories (chronic renal failure, diabetic nephropathy, and proteinuria) were increased among the stone formers (Table 2). Stone formers trended toward a higher risk for ESRD or death with CKD (HR 1.34; 95% CI 0.81 to 2.23), but this was not statistically significant (P = 0.25; Figure 2B). In the subset with follow-up SCr levels, the risk for clinical CKD remained increased in stone formers (HR 1.50; 95% CI 1.32 to 1.70). The increased risk for clinical CKD among the stone formers remained statistically significant and did not change substantively after adjustment for age, gender, and comorbidities (Table 3). Unlike previous reports (9,10), the risk for clinical CKD did not differ with stone formers who were obese compared with stone formers who were not obese (HR 1.49 versus 1.74; P = 0.22 for interaction) or with stone formers who had hypertension compared with stone formers who did not have hypertension (HR 1.70 versus 1.62; P = 0.62 for interaction).
Figure 2.
Risk for chronic kidney disease (CKD) among Olmsted County, MN, stone formers and control subjects. (A through D) Cumulative incidence for any CKD diagnostic code (518 stone formers and 189 control subjects at risk at 18 yr, A); ESRD by dialysis or kidney transplant or death with CKD (577 stone formers and 229 control subjects at risk at 18 yr, B); and a sustained elevated SCr level with censoring by last clinic visit in Olmsted County (610 stone formers and 236 control subjects at risk at 18 yr, C) or with censoring by last SCr level at the Mayo Clinic (226 stone formers and 102 control subjects at risk at 18 yr, D).
Table 2.
Risk for CKD by diagnostic codes and ESRD among Olmsted County, MN, stone formers and control subjectsa
| Outcome | Stone Formers (n = 4066)
|
Control Subjects (n = 10,150)
|
HR (95% CI) | ||
|---|---|---|---|---|---|
| Events | 15 yr (%)b | Events | 15 yr (%)b | ||
| Any CKD diagnostic code | 440 | 20.0 | 684 | 13.0 | 1.67 (1.48 to 1.88) |
| Individual CKD diagnoses (ICD-9 code) | |||||
| chronic renal failure (585) | 163 | 7.8 | 283 | 5.9 | 1.46 (1.20 to 1.77) |
| diabetic nephropathy (250.4) | 93 | 4.2 | 130 | 2.6 | 1.81 (1.38 to 2.36) |
| proteinuria (791.0) | 61 | 3.0 | 93 | 1.7 | 1.66 (1.20 to 2.29) |
| nephritis and nephropathy (583) | 37 | 2.4 | 66 | 1.5 | 1.41 (0.94 to 2.10) |
| hypertensive renal disease (403) | 12 | 0.5 | 38 | 0.8 | 0.78 (0.41 to 1.50) |
| other (mostly nonspecific renal failure and azotemia) | 145 | 7.3 | 201 | 4.0 | 1.83 (1.48 to 2.27) |
| ESRD or death with CKD | 23 | 1.4 | 43 | 0.7 | 1.34 (0.81 to 2.23) |
| maintenance dialysis | 12 | 0.8 | 26 | 0.4 | 1.15 (0.58 to 2.29) |
| kidney transplant | 14 | 0.8 | 21 | 0.5 | 1.67 (0.85 to 3.29) |
| death with CKD | 5 | 0.2 | 5 | 0.1 | 2.53 (0.73 to 8.74) |
| Mortality | 355 | 14.0 | 944 | 16.0 | 0.95 (0.84 to 1.07) |
CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio; ICD-9, International Classification of Diseases, Ninth Revision.
Cumulative incidence over 15 yr.
Table 3.
Risk for CKD among Olmsted County, MN, stone formers compared with control subjects with adjustment for baseline comorbiditiesa
| Adjusting Factors | Event Type (HR [95% CI])
|
||
|---|---|---|---|
| Clinical CKD by Any Diagnostic Code | ESRD or Death with CKD | Sustained Elevated SCr | |
| All patients with censoring by last clinic visit in Olmsted County (4066 stone formers and 10,150 control subjects) | |||
| age, gender | 1.68 (1.49 to 1.89) | 1.31 (0.79 to 2.18) | 1.44 (1.21 to 1.72) |
| age, gender, hypertension | 1.66 (1.47 to 1.87) | 1.29 (0.78 to 2.15) | 1.41 (1.18 to 1.69) |
| age, gender, diabetes | 1.59 (1.41 to 1.80) | 1.26 (0.76 to 2.10) | 1.41 (1.18 to 1.69) |
| age, gender, obesity | 1.63 (1.45 to 1.84) | 1.29 (0.78 to 2.14) | 1.40 (1.17 to 1.68) |
| age, gender, all comorbidities (Table 1) | 1.56 (1.39 to 1.77) | NAb | 1.36 (1.13 to 1.62) |
| Patients with follow-up SCr levels at the Mayo Clinic with censoring by last SCr test (2969 stone formers and 6171 control subjects) | |||
| age, gender | 1.59 (1.40 to 1.80) | 1.30 (0.78 to 2.17) | 1.31 (1.10 to 1.57) |
| age, gender, hypertension | 1.58 (1.39 to 1.79) | 1.29 (0.77 to 2.16) | 1.29 (1.08 to 1.55) |
| age, gender, diabetes | 1.52 (1.34 to 1.73) | 1.27 (0.76 to 2.12) | 1.29 (1.07 to 1.54) |
| age, gender, obesity | 1.57 (1.38 to 1.78) | 1.30 (0.78 to 2.17) | 1.29 (1.07 to 1.54) |
| age, gender, all comorbidities (Table 1) | 1.51 (1.33 to 1.71) | NAb | 1.25 (1.04 to 1.49) |
Results similar with adjustment for comorbidities (i.e., risk factors) as time-dependent covariates. NA, not applicable; SCr, serum creatinine.
Too few events (66 or 63) in a model with 13 covariates.
Incidence of CKD by SCr
There was a higher incidence of sustained elevated SCr (Figure 2, C and D) and sustained reduced eGFR among stone formers compared with control subjects. Table 4 compares the risk for SCr events with CKD defined by elevated SCr versus reduced eGFR, with event censoring by last clinic visit versus last SCr test, and with CKD defined with any duration versus a sustained duration. Regardless of approach, stone formers had an increased risk for CKD (P ≤ 0.01 for all). The increased risk for developing an elevated SCr among stone formers remained statistically significant and did not change substantively with adjustment for age, gender, and any comorbidity (Table 3). Compared with the risk for a sustained eGFR <60 ml/min per 1.73 m2 in stone formers, the magnitude of risk was similar for a sustained eGFR <30 ml/min per 1.73 m2, although not statistically significant (HR 1.42 [95% CI 1.24 to 1.63] versus 1.53 [95% CI 0.91 to 2.56], respectively).
Table 4.
Risk for CKD by SCr-based events among Olmsted County, MN, stone formers and control subjects
| Event Typea | Event Duration | Stone Formers
|
Control Subjects
|
HR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Events | At Risk | 15 yr (%)b | Events | At Risk | 15 yr (%)b | |||
| All patientsc | ||||||||
| elevated SCr | 4066 | 10,150 | ||||||
| Any | 655 | 24.0 | 1073 | 18.0 | 1.63 (1.48 to 1.80) | |||
| Sustained | 189 | 8.4 | 331 | 6.0 | 1.46 (1.22 to 1.74) | |||
| reduced eGFR | 4017 | 10,039 | ||||||
| Any | 862 | 33.0 | 1504 | 25.0 | 1.55 (1.42 to 1.68) | |||
| Sustained | 310 | 13.0 | 562 | 10.0 | 1.42 (1.24 to 1.63) | |||
| Patients with SCr testsd | ||||||||
| elevated SCr | 2969 | 6171 | ||||||
| Any | 655 | 36.0 | 1073 | 31.0 | 1.41 (1.28 to 1.56) | |||
| Sustained | 189 | 13.0 | 331 | 11.0 | 1.26 (1.05 to 1.51) | |||
| reduced eGFR | 2924 | 6070 | ||||||
| Any | 862 | 49.0 | 1504 | 42.0 | 1.34 (1.23 to 1.45) | |||
| Sustained | 310 | 21.0 | 562 | 18.0 | 1.22 (1.06 to 1.40) | |||
Elevated SCr >1.3 mg/dl in men and >1.1 mg/dl in women; reduced estimated GFR (eGFR) <60 ml/min per 1.73 m2.
Cumulative incidence over 15 yr.
Censoring by last clinic visit in Olmsted County.
Censoring by last SCr test at the Mayo Clinic.
Discussion
In this population-based cohort study, the risk for developing CKD among stone formers was increased compared with control subjects. Risk for a clinical diagnosis of CKD was 50 to 67% higher, risk for a sustained elevated SCr was 26 to 46% higher, and risk for a sustained reduced eGFR was 22 to 42% higher. These increased risks were independent of comorbidities associated with CKD. During an average of 8.6 yr of follow-up, there was no statistically significant increased risk for ESRD or death with CKD.
It is interesting that there was an increased risk for CKD before the first diagnosis of kidney stones. The manual chart review showed that approximately 22% of the stone formers had a kidney stone before their residence in Olmsted County. Furthermore, the charted diagnosis of kidney stones sometimes occurred months or even years after symptoms. Thus, undocumented kidney stones may explain this increased risk for CKD before the first documented stone. The higher risk for clinical CKD in stone formers in the year before their initial kidney stone diagnosis (OR 2.73) compared with >5 yr before (OR 1.30) is consistent with this hypothesis. Another complicating factor is that CKD is usually asymptomatic and may remain undiagnosed for years until a clinician has an indication (e.g., kidney stone) to obtain a SCr or a urinalysis. The observed increased risk for clinical CKD in the first 90 d after the stone episode (OR 6.99) may represent this detection bias or possibly acute renal failure misclassified as CKD. For addressing these potential biases, clinical CKD was conservatively analyzed as preexisting (prevalent) when diagnosed within the first 90 d after the index date (results were similar using 2 yr instead of 90 d).
Identifying CKD can be elusive because the disorder is usually asymptomatic, and diagnosis relies on biomarkers that do not cleanly demarcate between normal and disease, namely SCr (or eGFR) and urinary protein. Thus, multiple approaches were used to classify CKD in this study. Diagnostic codes have the advantage of contributing clinical context and judgment in the interpretation of laboratory and radiologic studies that are used to diagnose CKD, but chronicity may be unclear and knowledge of previous kidney stones may influence the diagnosis. Individual codes can also help characterization of the disease, and, in this study, stone formers seemed to be at increased risk for a renal disease with proteinuria. Individual codes should also be interpreted with caution. Any patient who has diabetes and develops CKD will likely receive a “diabetic nephropathy” diagnosis. “Hypertensive renal disease” may be preferentially diagnosed in patients who have hypertension and develop CKD with no other risk factors. ESRD is the “hardest” end point for CKD, but this event only occurred in 66 patients, and longer follow-up may be needed to detect an association.
Several factors need consideration with a SCr-based classification of CKD (Table 4). It is debatable whether an eGFR <60 ml/min per 1.73 m2 in older individuals with normal SCr levels is an adequate criterion for a disease (15,16,19–25). This study found that stone formers were at increased risk for CKD defined by either an elevated SCr or a reduced eGFR; however, stone formers may be more likely to have SCr tests obtained during follow-up and, thus, be more likely to have their CKD detected. There was indeed evidence of a detection bias, with 73% of stone formers having at least one follow-up SCr test compared with 61% of the control subjects; however, when limiting the sample to only patients with follow-up SCr tests at the Mayo Clinic (censoring by last SCr test), stone formers were still at an increased risk for CKD. This alternative approach addressed the detection bias but likely led to a selection bias, particularly in control subjects. Patients with follow-up SCr levels at the Mayo Clinic (the only provider of urology and nephrology subspecialty care in the county) were likely at higher risk for CKD than patients who did not have follow-up SCr levels or had follow-up SCr levels only at other providers.
The mechanism by which kidney stones could increase the risk for CKD is not fully clear. Characteristics of stone formers that have been associated in other studies with risk for ESRD include hereditary stone disease, struvite stones, urinary tract infections, frequent stone episodes, obstructive uropathy, and urinary tract anomalies (6,26). Before ESRD, different factors may mediate an increased risk for CKD. Hypertension, diabetes, obesity, dyslipidemia, and gout all were associated with kidney stones, as others have reported (27–30). The HR remained relatively constant adjusting for these factors, and there was little evidence that these factors explained the increased risk for CKD. Further studies are needed to determine whether the risk for CKD in community stone formers is related to medications (e.g., thiazide diuretics [31,32], citrate [33], analgesic nephropathy), stone type (8), urinary tract infections (34), shock wave lithotripsy (35,36), recurrent obstruction with high stone burden (37), or damage from chronic crystalluria (particularly calcium oxalate) (26).
There were several potential limitations to this study. Laboratory measures of urinary protein and SCr levels from Olmsted County providers besides the Mayo Clinic were not available. Kidney stone events that occurred outside Olmsted County were not detected. The sample was predominantly white, and the risk for CKD with kidney stones may differ in other race groups (7,38). Information on stone type, stone burden, medications (e.g., thiazide diuretics), lithotripsy, and other surgeries were not available, and the risk for CKD may vary depending on these factors. Alternative methods to classify comorbidities besides diagnostic codes could lead to different results. Finally, stone formers were more likely to have >90 d of follow-up than control subject (93 versus 82%). Including patients with <90 d of follow-up would have increased the risk estimates for CKD in stone formers.
Conclusions
Kidney stones were predictive of developing CKD whether judged by a clinical diagnosis, elevated SCr, or reduced eGFR. There was not a statistically significant increased risk for ESRD. Few stone formers are medically evaluated and treated to prevent recurrent stones. CKD is an important independent predictor of cardiovascular disease and mortality before ESRD (19,39). Patients with kidney stones may warrant more aggressive screening for subclinical CKD, and, if identified, measures to ameliorate progression may be important (e.g., angiotensin blockade) (40). It is also possible that aggressive medical treatment to prevent recurrent kidney stones may decrease the risk for CKD.
Disclosures
None.
Acknowledgments
This project was supported by research grants (Mayo Clinic O'Brien Urology Center P50 DK77321, DK78229, and AR30582) from the National Institutes of Health, US Public Health Service.
Part of this work was presented at the annual meeting of the American Society of Nephrology; November 4 through 9, 2008; Philadelphia, PA.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC: Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int 63: 1817–1823, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Frymoyer PA, Scheinman SJ, Dunham PB, Jones DB, Hueber P, Schroeder ET: X-linked recessive nephrolithiasis with renal failure. N Engl J Med 325: 681–686, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Leumann EP: Primary hyperoxaluria: An important cause of renal failure in infancy. Int J Pediatr Nephrol 6: 13–16, 1985 [PubMed] [Google Scholar]
- 5.Worcester EM, Coe FL, Evan AP, Parks JH: Reduced renal function and benefits of treatment in cystinuria vs other forms of nephrolithiasis. BJU Int 97: 1285–1290, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Jungers P, Joly D, Barbey F, Choukroun G, Daudon M: ESRD caused by nephrolithiasis: Prevalence, mechanisms, and prevention. Am J Kidney Dis 44: 799–805, 2004 [PubMed] [Google Scholar]
- 7.Stankus N, Hammes M, Gillen D, Worcester E: African American ESRD patients have a high pre-dialysis prevalence of kidney stones compared to NHANES III. Urol Res 35: 83–87, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Worcester EM, Parks JH, Evan AP, Coe FL: Renal function in patients with nephrolithiasis. J Urol 176: 600–603, discussion 603, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Gillen DL, Worcester EM, Coe FL: Decreased renal function among adults with a history of nephrolithiasis: A study of NHANES III. Kidney Int 67: 685–690, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Vupputuri S, Soucie JM, McClellan W, Sandler DP: History of kidney stones as a possible risk factor for chronic kidney disease. Ann Epidemiol 14: 222–228, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Melton LJ 3rd: History of the Rochester Epidemiology Project. Mayo Clin Proc 71: 266–274, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J: Risk factors for chronic kidney disease: A prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol 14: 2934–2941, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Rule AD, Larson TS: Do we need another equation to estimate GFR from serum creatinine in renal allograft recipients? Nephrol Dial Transplant 23: 2427–2428, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rule AD, Rodeheffer RJ, Larson TS, Burnett JC Jr, Cosio FG, Turner ST, Jacobsen SJ: Limitations of estimating glomerular filtration rate from serum creatinine in the general population. Mayo Clin Proc 81: 1427–1434, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Poggio ED, Rule AD: Can we do better than a single estimated GFR threshold when screening for chronic kidney disease? Kidney Int 72: 534–536, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Wetzels JF, Kiemeney LA, Swinkels DW, Willems HL, den Heijer M: Age- and gender-specific reference values of estimated GFR in Caucasians: The Nijmegen Biomedical Study. Kidney Int 72: 632–637, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Last JM: A Dictionary of Epidemiology, Oxford, Oxford University Press, 2001
- 19.O'Hare AM, Bertenthal D, Covinsky KE, Landefeld CS, Sen S, Mehta K, Steinman MA, Borzecki A, Walter LC: Mortality risk stratification in chronic kidney disease: One size for all ages? J Am Soc Nephrol 17: 846–853, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Poggio ED, Rule AD: A critical evaluation of chronic kidney disease: Should isolated reduced estimated glomerular filtration rate be considered a ‘disease’? Nephrol Dial Transplant December 22, 2008. [epub ahead of print] [DOI] [PMC free article] [PubMed]
- 21.Glassock RJ, Winearls C: An epidemic of chronic kidney disease: Fact or fiction? Nephrol Dial Transplant 23: 1117–1121, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Bauer C, Melamed ML, Hostetter TH: Staging of chronic kidney disease: Time for a course correction. J Am Soc Nephrol 19: 844–846, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Couser WG: Chronic kidney disease the promise and the perils. J Am Soc Nephrol 18: 2803–2805, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Coresh J, Stevens LA, Levey AS: Chronic kidney disease is common: What do we do next? Nephrol Dial Transplant 23: 1122–1125, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Stevens LA, Coresh J, Levey AS: CKD in the elderly: Old questions and new challenges—World Kidney Day 2008. Am J Kidney Dis 51: 353–357, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Gambaro G, Favaro S, D'Angelo A: Risk for renal failure in nephrolithiasis. Am J Kidney Dis 37: 233–243, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Taylor EN, Stampfer MJ, Curhan GC: Diabetes mellitus and the risk of nephrolithiasis. Kidney Int 68: 1230–1235, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Taylor EN, Stampfer MJ, Curhan GC: Obesity, weight gain, and the risk of kidney stones. JAMA 293: 455–462, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Cappuccio FP, Strazzullo P, Mancini M: Kidney stones and hypertension: Population based study of an independent clinical association. BMJ 300: 1234–1236, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soucie JM, Coates RJ, McClellan W, Austin H, Thun M: Relation between geographic variability in kidney stones prevalence and risk factors for stones. Am J Epidemiol 143: 487–495, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Hawkins RG, Houston MC: Is population-wide diuretic use directly associated with the incidence of end-stage renal disease in the United States? A hypothesis. Am J Hypertens 18: 744–749, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Reungjui S, Hu H, Mu W, Roncal CA, Croker BP, Patel JM, Nakagawa T, Srinivas T, Byer K, Simoni J, Wesson D, Sitprija V, Johnson RJ: Thiazide-induced subtle renal injury not observed in states of equivalent hypokalemia. Kidney Int 72: 1483–1492, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Evan AP, Lingeman JE, Coe FL, Shao Y, Parks JH, Bledsoe SB, Phillips CL, Bonsib S, Worcester EM, Sommer AJ, Kim SC, Tinmouth WW, Grynpas M: Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int 67: 576–591, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Kristensen C, Parks JH, Lindheimer M, Coe FL: Reduced glomerular filtration rate and hypercalciuria in primary struvite nephrolithiasis. Kidney Int 32: 749–753, 1987 [DOI] [PubMed] [Google Scholar]
- 35.Williams CM, Thomas WC Jr: Permanently decreased renal blood flow and hypertension after lithotripsy. N Engl J Med 321: 1269–1270, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Liou LS, Streem SB: Long-term renal functional effects of shock wave lithotripsy, percutaneous nephrolithotomy and combination therapy: A comparative study of patients with solitary kidney. J Urol 166: 36, discussion 36–37, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Marangella M, Bruno M, Cosseddu D, Manganaro M, Tricerri A, Vitale C, Linari F: Prevalence of chronic renal insufficiency in the course of idiopathic recurrent calcium stone disease: Risk factors and patterns of progression. Nephron 54: 302–306, 1990 [DOI] [PubMed] [Google Scholar]
- 38.Sarmina I, Spirnak JP, Resnick MI: Urinary lithiasis in the black population: An epidemiological study and review of the literature. J Urol 138: 14–17, 1987 [DOI] [PubMed] [Google Scholar]
- 39.Foster MC, Hwang SJ, Larson MG, Parikh NI, Meigs JB, Vasan RS, Wang TJ, Levy D, Fox CS: Cross-classification of microalbuminuria and reduced glomerular filtration rate: Associations between cardiovascular disease risk factors and clinical outcomes. Arch Intern Med 167: 1386–1392, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS, Group AS: Progression of chronic kidney disease: The role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition—A patient-level meta-analysis. Ann Intern Med 139: 244–252, 2003 [DOI] [PubMed] [Google Scholar]