Abstract
OBJECTIVES
We developed a prosthesis for open pleurostomy cases where pulmonary decortication is not indicated, or where post-pneumonectomy space infection occurs. The open pleural window procedure not only creates a large hole in the chest wall that is shocking to patients, also results in a permanent deformation of the thorax. prosthesis for open pleurostomy is a self-retained silicone tube that requires the removal of 3 cm of one rib for insertion, and acts as a mature conventional open pleural window. Herein, we report our 13–year experience with this device in the management of different kinds of pleural empyema.
METHODS
Forty-four consecutive patients with chronic empyema were treated. The etiology of empyema was diverse: pneumonia, 20; lung resections, 12 (pneumonectomies, 7; lobectomies, 4; non-anatomical, 1); mixed-tuberculous, 6; and mixed-malignant pleural effusion, 6. After debridment of both pleural surfaces, the prosthesis for open pleurostomy was inserted and attached to a small recipient plastic bag.
RESULTS
Infection control was achieved in 20/20 (100%) of the parapneumonic empyemas, in 3/4 (75%) of post-lobectomies, in 6/7 (85%) of post-pneumectomies, in 6/6 (100%) of mixed-tuberculous cases, and in 4/6 (83%) of mixed-malignant cases. Lung re-expansion was also successful in 93%, 75%, 33%, and 40% of the groups, respectively.
CONCLUSIONS
Prosthesis for open pleurostomy insertion is a minimally invasive procedure that can be as effective as conventional open pleural window for management of chronic empyemas. Thus, we propose that the use of prosthesis for open pleurostomy should replace the conventional method.
Keywords: Open Pleural Window, Pleurostomy, Empyema, Pleural Effusion, Pulmonary Decortication
INTRODUCTION
Chronic empyema, regardless of its cause, usually poses a difficult challenge to the thoracic surgeon.1 In practice, it most commonly originates from pleuro-pulmonary infection, lung resections associated with bronchopleural fistulas, and complicated esophageal surgery.2,3 When there is a healthy lung that can be re-expanded and the patient can tolerate a major operation, pulmonary decortication is the treatment of choice, with or without the use of VATS.4–8 However, there are many patients for whom this operation cannot be indicated, and the most commonly adopted option for these patients is the creation of an open pleural window (OPW),9 an old procedure also known as pleurostoma or thoracostoma. Because most surgeons resect 6 cm to 20 cm of two to four ribs,9–12,14,16,17,19 the final result is a large defect in the patient’s chest wall, which gives an unpleasant appearance. In 1997, we reported a new technique for the management of chronic empyema13 that seemed to be a good alternative to the usual OPW. In brief, we created a small pleural window whose dimensions are comparable with those of a mature conventional thoracostomy, and whose diameter is maintained thereafter by the insertion of a self-restrained tube into the drainage tract. We thus named this device “prosthesis for open pleurostomy” (POP). In the present report, we summarize our 13-year experience in our institution with the use of this device for management of chronic empyemas of varied etiology. We also present a new version of the original model. This is not an experimental study as the technique has been approved since 199713 by the ethical committee, and all patients signed an informed consent to participate. The conventional indication of OPW was given to all patients, but we decided to change the treatment to POP management.
METHODS
The tube originally described in our first article was remodeled to make it lighter and to provide a more delicate appearance (Figure 1). In brief, it is a corrugated 10 cm long silicone tube (ID, 2.0 cm; OD, 2.5 cm) with three wings at its base. The wings should stay close to the parietal pleura, thus avoiding spontaneous extrusion of the tube. Externally, the tube has a movable ring, which is fixed to the corrugations closest to the chest wall, thus preventing it from falling into the pleural cavity. Those corrugations are fashioned in such a way that the ring is allowed to advance smoothly, but is not able to retract (“fish skin”).
Figure 1.
Photograph of the device
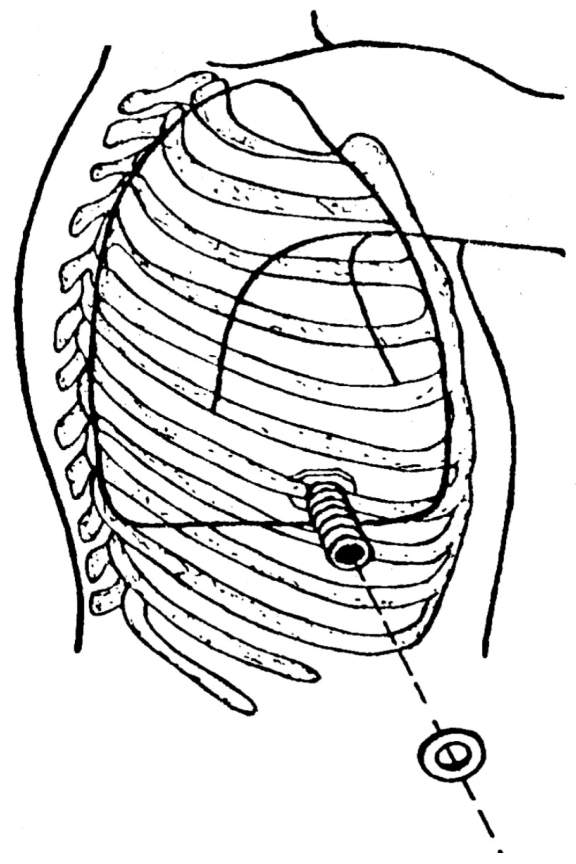
More often than not, POP insertion is done under general endotracheal anesthesia, although some very ill or debilitated patients have been operated on under local anesthetic infiltration. A skin incision of about 4 to 5 cm is made, tissues are dissected, and the prosthesis is inserted into the pleural space after a 3 cm segment of the rib closest to the most dependent portion of the empyematic cavity is resected (Figure 2). The appropriate site for insertion can be determined by chest X-rays and CT scans, finger palpation through a pre-existing chest tube tract, or by brief thoracoscopic visualization either using the video thoracoscope or a Carlens’ mediastinoscope. Sometimes, it is necessary to provide partial debridement of the pleural content and pleural surfaces, which can also be easily accomplished by thoracoscopy.
Figure 2.
Schematic representation of POP and its fixation after a 3 cm segment of a rib had been removed
In all patients, there was an underlying parenchymal process limiting compliance, the complete debridement of the visceral and parietal pleura was impossible, and chronic drainage was necessary. After the POP was in place and secured by the external ring, its excess was trimmed away leaving only 2 cm of the tube out of the incision. Usually, one or two stitches were used to close the skin on each side of the tube. No bandages were necessary, and a small recipient plastic bag was adapted around the external end of the tube. After discharge, patients were advised to change the bag daily and to wash the pleural cavity with saline if there was copious purulent secretion but no bronchopleural fistula. After that, a small dressing was applied, and the wound was left to close much like wounds of ordinary chest tubes.
From September 1995 through December 2006, 44 patients with chronic empyema who were not suitable for pulmonary decortication were treated by this method and followed until July 2007. Two additional patients have been treated to date, but the follow-up was too short, thus they were not included in this paper. There were 34 males and 10 females (78% and 22%) whose age ranged from 16 to 79 years (mean, 45.9 ± 8.3 yrs). The etiology of empyemas was: parapneumonic in 20 (45%), post-lung resection in 12 (27%), mixed-tuberculous in 6 (14%), and mixed-malignant in 6 (14%).
RESULTS
Given the heterogeneity of our patients and the etiology of their empyemas, we report the results for each group separately. No surgical complications were specifically related to this method.
Parapneumonic Empyemas
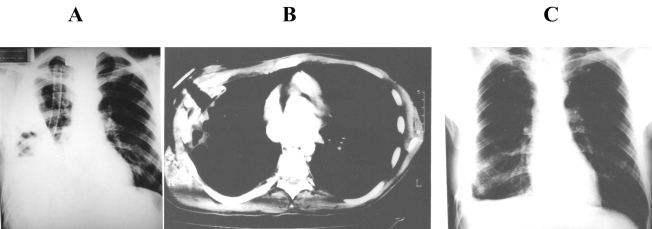
THE complete control of pleural infection was achieved in all 20 patients of this group after a mean period of 206 days of drainage with POP (range, 80 to 408 days). In 14 of the 15 survivors (93%), there was also complete lung re-expansion. A typical example of such cases is presented in Figure 3.
Figure 3.
A: Massive parapneumonic empyema in the right hemithorax. Although adequately drained, the lung did not re-expand and there was some remaining air-fluid. B: CT-scan four months after POP insertion. The lung has been completely re-expanded. POP was removed at this occasion. C: Chest X-Ray one month after POP removal
In one patient, a 63-year old man, the tube remained in place after 350 days due to incomplete lung re-expansion. Five patients died due to their primary diseases (systemic lupus, 1; carcinomatosis, 1; lung abscess, 1; chronic obstructive lung disease, 1; massive pneumonia, 1), but without pleural sepsis, after a mean drainage period of 91 days (range, 63 to 147 days).
Post-Lung Resection Empyemas
Of these 12 cases, four occurred after a lobectomy (bronchiectasis, 1; lung cancer, 1; aspergiloma, 1; metastatic thymoma, 1), one after a non-anatomical resection of a congenital lung cyst, and seven after a pneumonectomy: six on the right (lung cancer, 2; bronchiectasis, 3; pleural mesothelioma, 1), and one on the left (tuberculosis). None of these 12 patients developed signs of bronchopleural fistula; however, after a non-anatomical resection, three of the four post-lobectomy empyema patients as well as the one with empyema achieved both complete lung re-expansion and resolution of the empyema after a mean drainage period of 197 days (range: 58 to 570 days). One patient with pleural empyema after a lobectomy for aspergiloma had to undergo complete pneumonectomy associated with muscle flap transposition in order to achieve definitive control of the infection. POP was utilized as a bridge between this major operation and an additional one in five of the seven post-pneumonectomy empyema patients. Those patients were later submitted either to thoracomyoplasty (2 cases) or thoraco-omentoplasty (3 cases) when their empyematic cavity was considered sufficiently clean. In those cases, open drainage with POP was maintained for a mean period of 581 days (range: 253 to 1095 days), although infection control was achieved sooner. Delays in surgery occurred due to individual particularities. In the meantime, patients stayed at home and were able to improve their nutritional and psychological status before undergoing the next major operation. One of the seven pneumonectomy patients (with bronchiectasis) achieved complete control of the pleural infection, but died of pulmonary embolism 46 days after POP insertion. The patient with pleural mesothelioma died 67 days after drainage with POP due to cancer dissemination.
Mixed-Tuberculous Empyemas
These six patients had tuberculous effusion complicated by secondary infection. In all six, pleural sepsis was controlled after open pleural drainage with POP was instituted. In two, complete lung re-expansion occurred after a drainage period of 180 and 571 days, respectively. Two other patients with complete empyema control are still under drainage (for 60 and 756 days, respectively) due poor lung expansion arising from extensive disease and scarring. Another patient of this subset underwent a successful procedure similar to Clagett’s. Thus, his pleurostoma was surgically closed after the remaining clean cavity was filled with saline and neomycin 126 days after drainage with POP. The last patient in this group died of progressive tuberculosis and respiratory insufficiency 160 days after being drained with POP in place.
Mixed-Malignant Empyemas
This group included six patients with infected malignant pleural effusions secondary to cancer of varied origin: breast, 4; esophagus, 1; stomach, 1. After being drained with POP, complete infection control was achieved in four patients. In two, there was also complete pulmonary re-expansion, and POP was removed after 38 days and 67 days, respectively. Two other patients underwent a successful procedure similar to Clagett’s as mentioned above, after 148 days and 258 days of drainage. One esophagectomy patient died due to systemic complications 11 days after POP insertion. In this case, neither lung re-expansion nor empyema cure could be evaluated. The last patient of this group died due to gastric cancer dissemination 158 days after drainage. At that time, POP was still in place, but pulmonary re-expansion was still incomplete.
COMMENTS
Although there are some variations in skin incisions used by different surgeons,9–11,14,16,17,19 the open pleural window is always recommended to be made wide for three main reasons: first, to enable debridement and promote ample drainage of the pleural cavity; second, to allow frequent irrigation and packing of the purulent surface; and third, because a small pleurostoma tends to close with time due to scarring and chest wall shrinkage. Eloesser (1935),14 one of the first proponents of the OPW, resected 6 cm of three ribs; Clagett and Geraci,15 who revived this operation in the early 1960s, also resected 6 cm of two or three ribs. From the more recent literature, Postmus et al.16 resected 8 cm of two to three ribs; Galvin et al.,17 Hurvitz and Tucker18 and Jacques and Deslauriers9 resected 10 cm of two ribs; and Weissberger19 resected 15 to 20 cm of three or four ribs.
Although effective for the treatment of chronic empyemas, the resulting major resection of the chest wall induced by open pleural windows should, in our opinion, be avoided, especially considering the current popularity of minimally invasive operations.5,7,8,9,21 The gross defect generated by the conventional OPW gives such a marred appearance to patients who more often than not become quite disappointed postoperatively. Most nurses and interns of our hospital also consider this to be an outdated and difficult operation.
Thus, the senior author (LTBF) of the present study visualized the benefit of POP and reasoned that if a pleurostoma is larger than necessary and shrinks with time, it would be best to fabricate a dedicated tube that would maintain the necessary orifice for long term drainage (a mature pleurostoma) from the beginning. Complete pleural debridement can be easily accomplished by thoracoscopy at the time of POP insertion and repeated afterwards as many times as needed.5,7,8 POP also allows intermittent or continuous irrigation of the pleural cavity when indicated. Additionally, large OPWs have also been associated with other major complications such as herniation of abdominal content.20
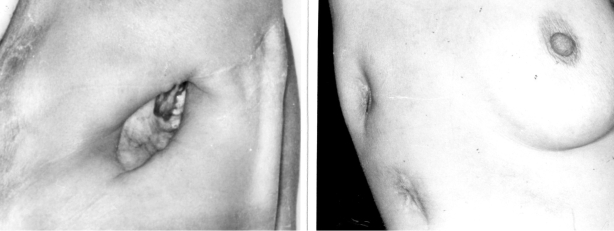
In the present study, treatment of chronic empyemas with POP provided the same results we used to observe when performing the large OPW, both in terms of infection control and lung re-expansion. The use of this new device, however, offers additional advantages over the conventional operation: 1) insertion takes only about 20 minutes, compared with at least 60 minutes usually necessary to create a large pleurostoma; 2) it can be done under local anesthesia; 3) it does not require multiple postoperative dressings or the close attention of nurses. The main advantage for the patient, however, is that the use of POP precludes major chest wall demolition, thus avoiding gross aesthetic compromise. (Figure 4).
Figure 4.
On the left, a conventional open pleural window scar in a male patient. On the right, the upper scar is after POP removal, and below is a scar after removal of a regular 36FR chest tube
When the patient has a re-expandable lung, it will gradually replenish the whole pleural cavity in about 2 to 6 months. In this case, the tube is removed during the patient’s next visit to the clinic, after applying a careful rotation and a gentle pull on the tube in the outpatient clinic.
Another indication for POP was found for critically ill or debilitated patients who were in need of an OPW, but who could not tolerate the extent of the conventional procedure, especially the general anesthesia. POP can be performed with sedation and local anesthesia.
In patients with post-pneumonectomy empyema and in those for whom the lung cannot re-expand completely, even after many months of open drainage, POP has been used as a temporary measure. In cases where a thoracoplasty or a thoracomyoplasty could be anticipated,22–24 POP acted as a bridge between the acute state and the definitive operation. Moreover, prolonged drainage with POP usually favors a significant reduction of the empyematic cavity, thus allowing performance of a less extensive operation. Nonetheless, these are still major procedures that may represent a threat to senior or debilitated patients. Therefore, in cases where clinical conditions or patient’s refusal precludes such operations, it is our impression that a permanent pleurostomy, as provided by POP, could be a safer option. We did not use specific quality of life questionnaires to evaluate the results of this operation, but rather compared it with the classical treatment (OPW), the appearance was better and the patients and family expressed satisfaction with the “POP” prosthesis and because it was easier to carry out normal activities.
Finally, we would like to state that our only intention in presenting this report was to demonstrate that chronic empyemas can now be treated with a much less invasive and non-deforming operation, with the same result as with the conventional technique.
ACKNOWLEDGEMENT
We acknowledge the cooperation and help of Prof. Dr. Fábio Biscegli Jatene, in our institution, without whose support this study could not have been completed.
REFERENCES
- 1.Cham CH, Haq SM, Rahamin J. Empyema thoracis: a problem with late referral? Thorax. 1993;48:925–7. doi: 10.1136/thx.48.9.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Light RW, Girard WM, Jenkinson SG, George RB. Parapneumonic effusions. Am J Med. 1980;69:507–12. doi: 10.1016/0002-9343(80)90460-x. [DOI] [PubMed] [Google Scholar]
- 3.Lemmer JH, Botham MJ, Orringer MB. Modern management of adult thoracic empyema. J Thorac Cardiovasc Surg. 1985;90:849–55. [PubMed] [Google Scholar]
- 4.LeMense GP, Strange C, Sahn SA. Empyema thoracis: therapeutic management and outcome. Chest. 1995;107:1532–7. doi: 10.1378/chest.107.6.1532. [DOI] [PubMed] [Google Scholar]
- 5.Cassina PC, Hauser M, Hillejan L, Greschuchna D, Stamatis G. Video-assisted thoracoscopy in the treatment of pleural empyema: stage-based management and outcome. J Thorac Cardiovasc Surg. 1999;117:234–8. doi: 10.1016/S0022-5223(99)70417-4. [DOI] [PubMed] [Google Scholar]
- 6.Kim BY, Oh BS, Jang W, Min YI, Park YK, Park JC. Video-assisted thoracoscopic decortication for management of postpneumonic pleural empyema. Am J Surg. 2004;188:321–4. doi: 10.1016/j.amjsurg.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Petrakis IE, Kogerakis NE, Drositis IE, Lasithiotakis KG, Bouros D, Chalkiadakis GE. Video-assisted thoracoscopic surgery for thoracic empyema: primarily, or after fibrinolytic therapy failure. Am J Surg. 2004;187:471–4. doi: 10.1016/j.amjsurg.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 8.Chan DTL, Sihoe ADL, Chan S, Tsang DSF, Fang B, Lee TW, et al. Surgical treatment for empyema thoracis: is video-assisted thoracic surgery better than thoracotomy? Ann Thorac Surg. 2007;84:225–31. doi: 10.1016/j.athoracsur.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Jacques LF, Deslauriers J. Open drainage. In: Pearson FG, Deslauriers J, Ginsberg R, Hiebert CA, McKneally MF, Urshel HC Jr, editors. Thoracic Surgery. New York: Churchill Livingstone; 1995. pp. 1136–40. [Google Scholar]
- 10.Molnar TF. Current surgical treatment of thoracic empyema in adults. Eur J Cardiovasc Surg. 2007;32:422–30. doi: 10.1016/j.ejcts.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Smole-Juttner F, Beuster W, Pinter H, Pongratz M, Friehs G. Open-Window thoracostomy in pleural empyema. Eur J Cardiothorac Surg. 1992:635–8. doi: 10.1016/1010-7940(92)90186-2. [DOI] [PubMed] [Google Scholar]
- 12.Katsuragi N, Nakajima Y, Shiraishi Y, Hashizume M, Takahashi N. [Chronic tuberculous methicillin-resistant Staphylococcus aureus (MRSA) empyema with bronchopleural fistulae treated by open window thoracostomy followed by thoracoplasty and latissimus dorsi muscle transposition] Kyobu Geka. 2005;58:1121–4. [PubMed] [Google Scholar]
- 13.Filomeno LTB, Campos JRM, Almeida AW, Werebe EC, Jatene FB, Leirner AA. A dedicated prosthesis for open thoracostomy. Ann Thorac Surg. 1997;63:1494–6. doi: 10.1016/s0003-4975(97)00316-0. [DOI] [PubMed] [Google Scholar]
- 14.Eloesser L. An operation for tuberculous empyema. Surg Gynecol Obstet. 1935;60:1096–7. [Google Scholar]
- 15.Clagett OT, Geraci JE. A procedure for the management of postpneumonectomy empyema. J Thorac Cardiovasc Surg. 1963;45:141–5. [PubMed] [Google Scholar]
- 16.Postmus PE, Kerstjens JM, de Boer WJ, Homan van der Heide JN, Koeter GH. Treatment of post-pneumectomy pleural empyema by open window thoracostomy. Eur Resp J. 1989;2:853–5. [PubMed] [Google Scholar]
- 17.Galvin IF, Gibbons JRP, Maghout MH. Bronchopleural fistula: a novel type of window thoracostomy. J Thorac Cardiovasc Surg. 1988;96:433–5. [PubMed] [Google Scholar]
- 18.Hurvitz FJ, Tucker BL. The Eloesser flap: past and present. J Thorac Cardiovasc Surg. 1986;92:958–61. [PubMed] [Google Scholar]
- 19.Weissberger D. Empyema and bronchopleural fistula: experience with open window thoracostomy. Chest. 1982;82:447–50. doi: 10.1378/chest.82.4.447. [DOI] [PubMed] [Google Scholar]
- 20.Ampollini L, Bobbio A, Cattelani L, Carbognani P. Abdominal visceral hernia through a chest wall defect secondary to open-window thoracostomy. Eur J Cardiothorac Surg. 2006;29:601. doi: 10.1016/j.ejcts.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 21.Cheng YJ, Wu HH, Chou SH, Kao EL. Video-assisted thoracoscopic surgery in the treatment of chronic empyema thoracis. Surg Today. 2002;32:19–25. doi: 10.1007/s595-002-8107-2. [DOI] [PubMed] [Google Scholar]
- 22.Okumura Y, Takeda S, Asada H, Inoue M, Sawataba N, Shiono H, et al. Surgical results for chronic empyema using omental pedicled flap: long-term follow-up study. Ann Thorac Surg. 2005;79:1857–61. doi: 10.1016/j.athoracsur.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Yuste M, Ramos G, Duque JL, Heras F, Castanedo M, Cerezal LJ, et al. Open-window thoracostomy and thoracomyoplasty to manage chronic pleural empyema. Ann Thorac Surg. 1998;65:818–22. doi: 10.1016/s0003-4975(97)01386-6. [DOI] [PubMed] [Google Scholar]
- 24.Seify H, Mansour K, Miller J, Douglas T, Burke R, Losken A, et al. Single-stage muscle flap reconstruction of the postpneumonectomy empyema space: the Emory experience. Past Reconstr Surg. 2007;120:1886–91. doi: 10.1097/01.prs.0000256051.99115.fb. [DOI] [PubMed] [Google Scholar]