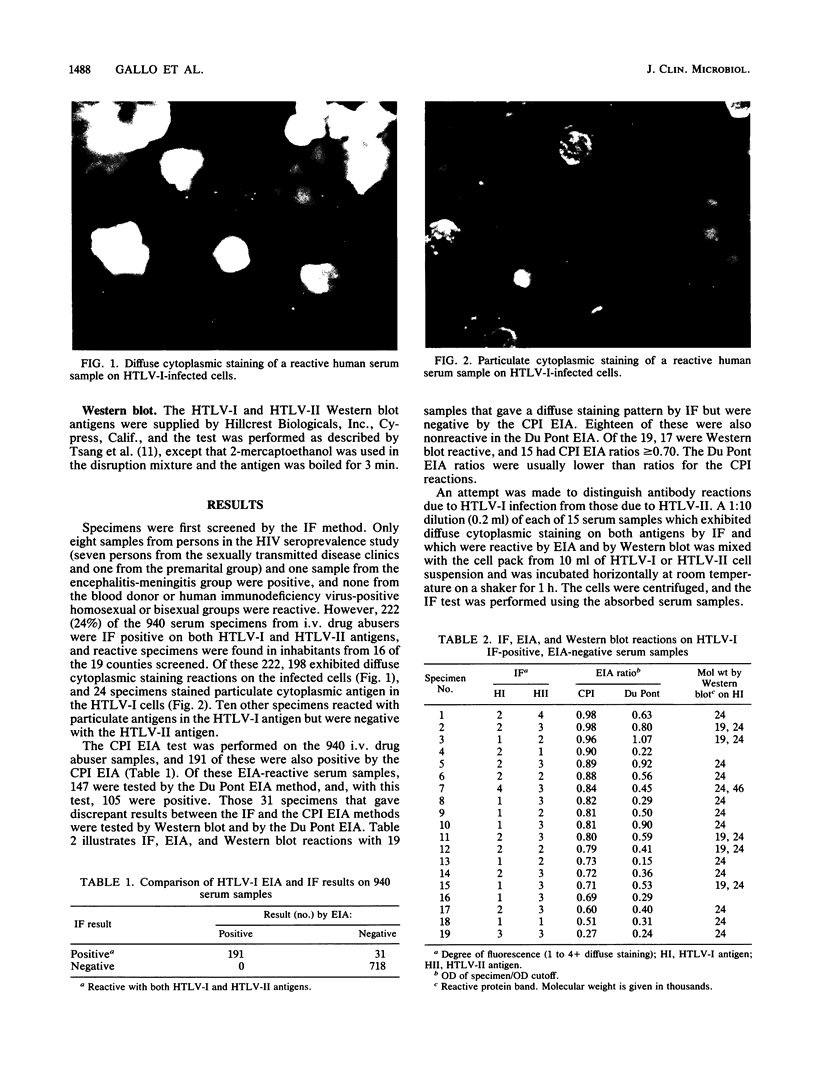
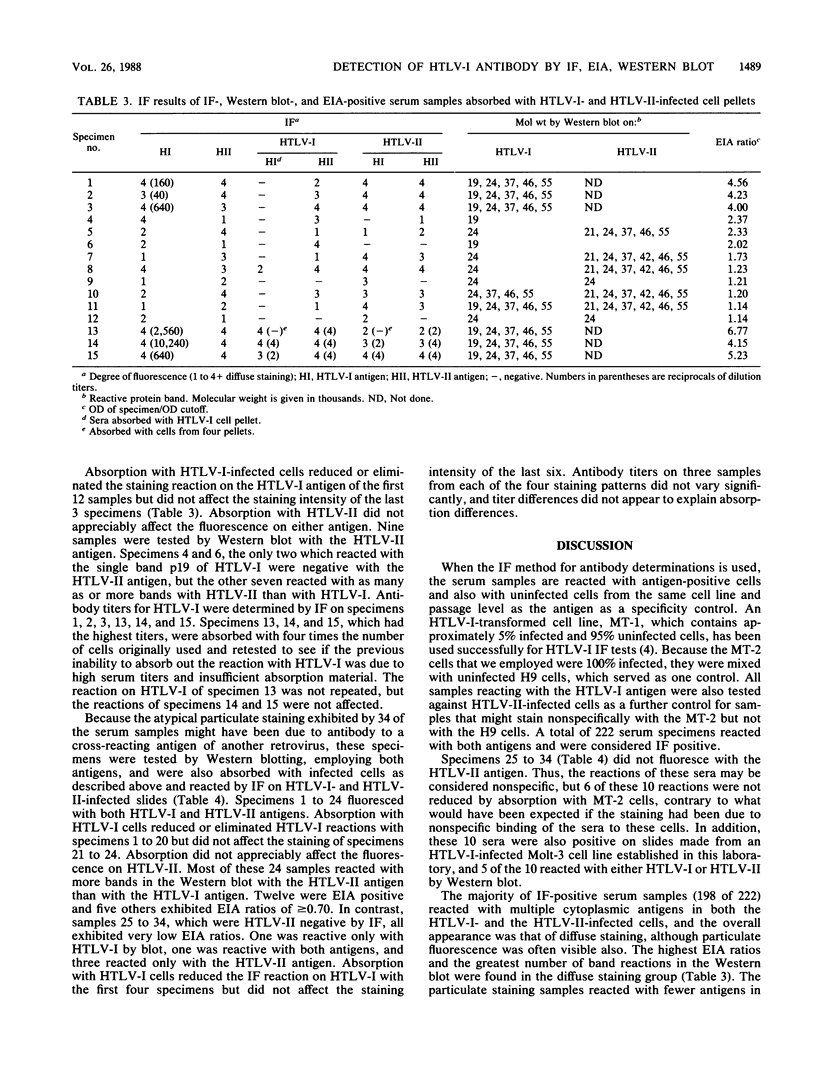
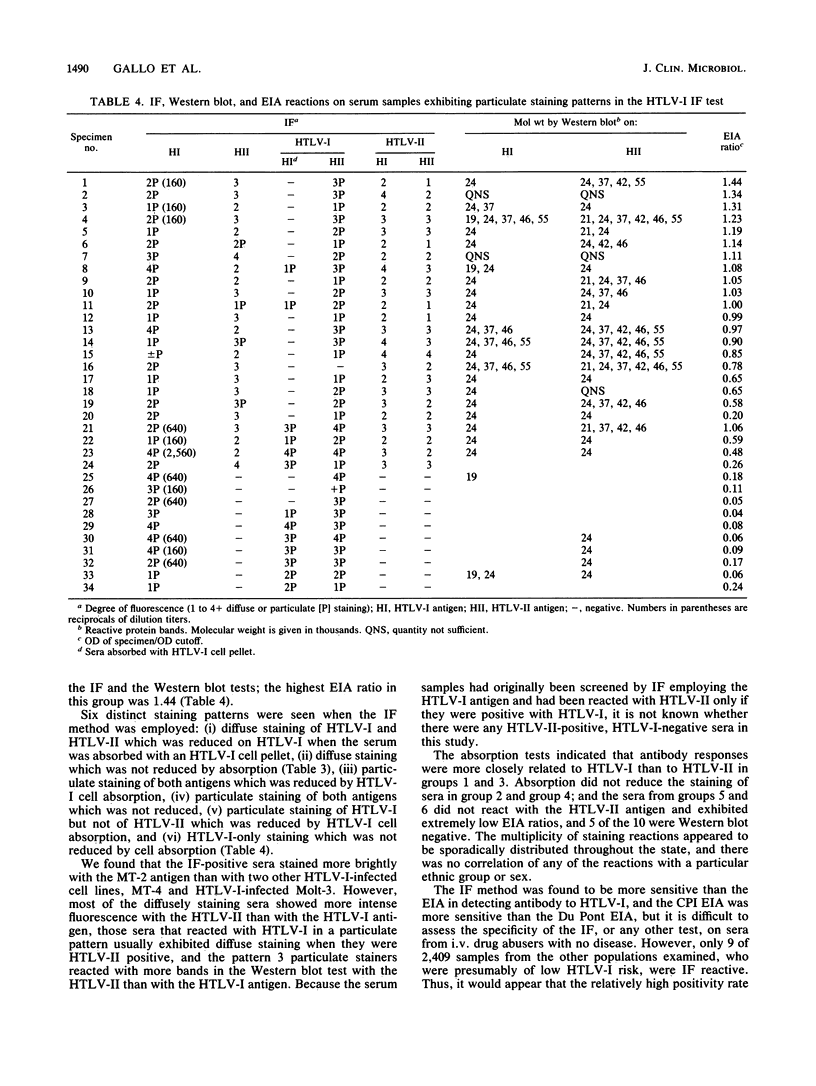
Abstract
A total of 3,349 serum samples were screened by the immunofluorescence (IF) method for antibody to human T-cell leukemia virus type I (HTLV-I). Only 9 of 2,409 specimens from selected individuals, blood bank donors, patients with encephalitis-meningitis, and human immunodeficiency virus antibody-positive homosexual or bisexual men were reactive by IF. In addition, 940 serum samples from intravenous drug abusers were tested by IF and also by an HTLV-I enzyme immunoassay (EIA) method. Of these, 222 (24%) were positive for both HTLV-I and HTLV-II antigens by IF, and 191 of these 222 were also reactive in the HTLV-I EIA. Of the 31 IF-positive, EIA-negative serum samples, 20 exhibited optical density readings greater than or equal to 70% of the positive cutoff in the EIA, and 29 samples reacted with 1 or more bands in the Western blot (immunoblot) test. An additional 10 specimens that were EIA negative reacted only with HTLV-I by IF. Differences in staining morphology and in reactions on HTLV-I and HTLV-II antigens before and after absorption of the serum specimens with HTLV-I and HTLV-II-infected cell pellets revealed six distinct serological patterns by IF. These results indicate that infections by HTLV-I or by another closely related retrovirus(es) occur in California. Further studies utilizing statistically valid sampling methods are needed to estimate true prevalence rates among various groups. IF and Western blot tests should supplement the EIA method to maximize sensitivity and specificity of test procedures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blattner W. A., Kalyanaraman V. S., Robert-Guroff M., Lister T. A., Galton D. A., Sarin P. S., Crawford M. H., Catovsky D., Greaves M., Gallo R. C. The human type-C retrovirus, HTLV, in Blacks from the Caribbean region, and relationship to adult T-cell leukemia/lymphoma. Int J Cancer. 1982 Sep 15;30(3):257–264. doi: 10.1002/ijc.2910300302. [DOI] [PubMed] [Google Scholar]
- Gallo D., Diggs J. L., Shell G. R., Dailey P. J., Hoffman M. N., Riggs J. L. Comparison of detection of antibody to the acquired immune deficiency syndrome virus by enzyme immunoassay, immunofluorescence, and Western blot methods. J Clin Microbiol. 1986 Jun;23(6):1049–1051. doi: 10.1128/jcm.23.6.1049-1051.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Robert-Guroff M., Miyoshi I., Golde D., Gallo R. C. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science. 1982 Nov 5;218(4572):571–573. doi: 10.1126/science.6981847. [DOI] [PubMed] [Google Scholar]
- Karpas A., Malik K., Lida J. Studies of human retroviruses in relation to adult T-cell leukaemia, acquired immune deficiency syndrome, and multiple sclerosis. Arch Virol. 1987;95(3-4):237–249. doi: 10.1007/BF01310783. [DOI] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Guroff M., Weiss S. H., Giron J. A., Jennings A. M., Ginzburg H. M., Margolis I. B., Blattner W. A., Gallo R. C. Prevalence of antibodies to HTLV-I, -II, and -III in intravenous drug abusers from an AIDS endemic region. JAMA. 1986 Jun 13;255(22):3133–3137. [PubMed] [Google Scholar]
- Rodgers-Johnson P., Gajdusek D. C., Morgan O. S., Zaninovic V., Sarin P. S., Graham D. S. HTLV-I and HTLV-III antibodies and tropical spastic paraparesis. Lancet. 1985 Nov 30;2(8466):1247–1248. doi: 10.1016/s0140-6736(85)90778-0. [DOI] [PubMed] [Google Scholar]
- Saxinger W., Blattner W. A., Levine P. H., Clark J., Biggar R., Hoh M., Moghissi J., Jacobs P., Wilson L., Jacobson R. Human T-cell leukemia virus (HTLV-I) antibodies in Africa. Science. 1984 Sep 28;225(4669):1473–1476. doi: 10.1126/science.6089348. [DOI] [PubMed] [Google Scholar]
- Schüpbach J., Kalyanaraman V. S., Sarngadharan M. G., Blattner W. A., Gallo R. C. Antibodies against three purified proteins of the human type C retrovirus, human T-cell leukemia-lymphoma virus, in adult T-cell leukemia-lymphoma patients and healthy Blacks from the Caribbean. Cancer Res. 1983 Feb;43(2):886–891. [PubMed] [Google Scholar]