Abstract
Loss of the zebrafish Nodal-related protein Squint causes a spectrum of phenotypes including cyclopia and midline bifurcations (MB). Here we examine MBs and their relation to cyclopia in maternal-zygotic squint (MZsqt) mutants. There is a concordance of MB with cyclopia in MZsqt embryos, and heat treatment or depletion of Hsp90a are “common” risk factors that increase the incidence of both phenotypes. Midline identity is specified on both sides of MBs, and deep-layer cells are initially lacking within bifurcations, whereas enveloping layer cells are intact. Bifurcations do not appear until the completion of gastrulation and are preceded by gaps in the expression of wnt5b, an essential regulator of dorsal convergence. The incidence of early MBs and wnt5b expression defects in heated MZsqt embryos is high, but there is also substantial recovery. Wnt5b depletion increases the incidence of MB, but not cyclopia, and as such Wnt5b is a “unique” risk factor for MB. Reciprocally, depletion of Wnt11 or Hsp90b increases cyclopia only. In summary, we find that MB arises after gastrulation in regions that fail to express wnt5b, and we show that two complex dysmorphologies – MB and cyclopia - can be promoted by either common or unique risk factors.
Keywords: Zebrafish, Cyclopia, Midline bifurcation, Squint, Nodal, Wnt5b, Wnt11, Hsp90
Introduction
In vertebrate development, the specification of mesoderm and endoderm depends largely on signaling by Nodal ligands of the TGFβ superfamily (Whitman, 2001). For instance, zebrafish carrying mutations in two Nodal-related ligands Squint/ndr2 (Sqt) and Cyclops/ndr1 (Cyc) fail to form any mesoderm or endoderm, except in the tail. Zebrafish carrying mutations in only one of these Nodal-related ligands have milder defects that can include cyclopia, an anomaly of the face and forebrain.
Holoprosencephaly (HPE), a human equivalent of cyclopia, affects approximately 1 in 15,000 human live births (Muenke and Beachy, 2000) and as many as 1 in 250 human embryos in utero (Matsunaga and Shiota, 1977). The most frequently disrupted pathway underlying human HPE is Shh signaling (Wallis and Muenke, 1999), whereas zebrafish cyclopia has been more commonly associated with Nodal pathway mutations (Blader and Strahle, 1998). However instances of Nodal pathway mutations being associated with human HPE (Roessler et al., 2008; Pei and Roessler, unpublished observations) and Shh pathway perturbations causing zebrafish cyclopia (Nasevicius and Ekker, 2000) have been documented. In zebrafish, mutations in certain genes acting in canonical and non-canonical WNT signaling, namely bozozok (boz), silberblick (sbl/wnt11), have also been found to cause cyclopia.
Loss of function mutations associated with human HPE are typically found in heterozygous states and display incomplete penetrance and variable expressivity, leading to the proposal that HPE is a genetically heterogeneous and complex disease whose etiology requires combinations of multiple genetic and environmental insults (Ming and Muenke, 2002). By contrast, a number of mutations causing zebrafish cyclopia are fully manifest (i.e., completely penetrant) when homozygous, such as cyc (Hatta et al., 1991). In contrast to the situation inferred from human patients, there are no known zebrafish cyclopia genes with heterozygous phenotypes. However some mutations, namely sqt (Aoki et al., 2002), schmalspur/foxh1 (Pogoda et al., 2000; Sirotkin et al., 2000) and boz (Solnica-Krezel et al., 1996), are variably penetrant in their homozygous states. We have previously shown that the incidence of cyclopia among sqt null embryos is modified by genetic and environmental factors (Pei et al., 2007).
In addition to cyclopia, three low-incident phenotypes have been reported in sqt mutants: ventralization of maternal-zygotic sqt mutants (Gore et al., 2005; Hagos et al., 2007), defects in anterior neural tube closure (Aquilina-Beck et al., 2007), and midline bifurcations (MB) (Pei et al., 2007). Here we further characterize the MB phenotype, focusing on its developmental onset and on the modifiers influencing its incidence. Unlike cyclopia and HPE, we do not recognize a homology between MB in zebrafish and any specific human developmental anomaly. However in general terms we note that aberrancies in dorsal midline closure underlie neural tube defects (NTDs; OMIM #182940), which comprise a significant fraction of human developmental disorders. Similar to HPE, NTDs are complex traits with multifactorial etiology.
We first observe bifurcations during early segmentation, at embryonic sites displaying an earlier reduction of wnt5b. Consistent with this, reduction of Wnt5b, which is required for normal convergence (Kilian et al., 2003), increases the incidence of MB in sqt mutants without concomitantly affecting cyclopia. By contrast, some previously characterized cyclopia risk factors, such as heat, Hsp90a reduction and loss of Sqt itself contribute to both phenotypes, while others, namely Hsp90b reduction and Wnt11 reduction affect only cyclopia (Heisenberg and Nusslein-Volhard, 1997; Pei et al., 2007).
Materials and Methods
Embryology and husbandry
The sqthi975 allele (Amsterdam et al., 2004) was introduced into our laboratory as three distinct clutches from related heterozygous parents in the Hopkins lab (MIT). The sqtcz35 allele used in this study was described previously (Feldman et al., 1998; Pei et al., 2007). Homozygous sqthi975/hi975 and sqtcz35/cz35 progeny were identified by genotyping as previously described and have been maintained thereafter for up to four generations by incrossing between these homozygous founders and/or their progeny. MZoep embryos were prepared as previously described from the oeptz57 mutant allele (Gritsman et al., 1999). All embryos were obtained by natural crosses and staged according to The Zebrafish Book (Westerfield, 1994). Unless otherwise indicated, MZsqt embryos used in this study had the sqthi975 allele. Whole-mount in situ hybridization was done as previously described (Thisse and Thisse, 1998). For histology analysis, wild type and MZsqthi975/hi975 embryos were heat treated at 34°C for 22h starting at the 64-cell stage, and then cultured at the standard temperature (28°C) for 48h. Three MZsqthi975/hi975 embryos with MB and two wild type embryos were used for paraffin embedding followed by transverse sectioning and hematoxylin and eosin (H&E) staining (American HistoLab, Gaithersburg, MD).
Microinjection
All microinjections were performed in one- to four-cell stage embryos. For the Hsp90 inhibitory drug experiment, wild type and MZsqthi975/hi975 embryos were injected with 0.7 nl of 27 mM radicicol in DMSO, 0.7 nl of 0.5 mM geldanamycin in DMSO, or with 0.7 nl DMSO alone. For the morpholino (MO) injections, wild type, MZsqthi975/hi975 and MZoep embryos were used. The morpholino sequences and injection doses were as follows: Hsp90a MO: 5′ CGCTGTATTTCCTCTTCTCCACCTG 3′; injection dose 3 ng. Hsp90b MO: 5′ TCAGGCATCTTGGTTAGTTTCGTTG 3′; injection dose 3 ng. Wnt5b MO: 5′ GTCCTTGGTTCATTCTCACATCCAT 3′ (Lele et al., 2001); injection dose 6 ng. Wnt11 MO: 5′ GTT CCT GTA TTC TGT CAT GTC GCTC 3′; injection dose 0.5 ng (Matsui et al., 2005). A standard morpholino was used as a general morpholino toxicity control (5′ CCTCTTACCTCAGTTACAATTTATA 3′) and was injected at equivalent doses.
Heat treatment
Two methods were used for heat treatment. The first method was used for treating small number of embryos and reported previously (Pei et al., 2007). Briefly, 4 ml Wheaton glass vials were filled with 2 ml of egg water and up to 12 embryos. The vials were floated upright by tightly inserting their openings into Styrofoam racks and floated in a 34°C water bath. The second method was used for treating large number of embryos. Up to 50 embryos were transferred into 40 ml of 34°C pre-warmed egg water in 100 ml glass beakers. The beakers were then floated in a plastic rack in a 34°C water bath.
Cell labeling and time-lapse recording
Wild type and MZsqthi975/hi975 embryos were heat treated at 34°C starting at the 64-cell stage until the shield stage. The embryos were then dechorionated and stained with N-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)sphingosine (fluorescent bodipy ceramide) with all the buffers pre-warmed at 34°C. The stained embryos were embedded in 0.15% low-melting agarose in 0.3x Danieau’s buffer, and further cultured at 34°C in an air-chamber incubator for time-lapse recording and intermittently photographed at room temperature. For photographic monitoring by time lapse, embryos oriented with their dorsal views selected and intermittently imaged by fluorescent optical sections as well as by differential interference contrast, using a Zeiss LSM-510 Meta confocal microscope.
Canonical Wnt signaling activity assay
Wild type and MZsqthi975/hi975 embryos were used to measure canonical Wnt/β-catenin signaling activity using the TCF/LEF reporter assay. For the heat treatment experiment, six embryos per data point were injected with 23 pg of reporter plasmids (pLEF-luc and pRL-CMV at a ratio of 10:1 w/w), and cultured at standard (28°C) or heated (34°C) conditions starting at the 64-cell stage. For Wnt5b MO injection experiments, six embryos per data point of wild type and MZsqthi975/hi975 embryos were co-injected with 6 ng of Wnt5b MO and 23 pg of the reporter plasmids mentioned above. All injected embryos were lysed at the bud stage and assayed for luciferase activity with the Dual-luciferase reporter system (Promega).
Phenotype scoring and statistical analysis
Embryos were examined and scored on day 1–2 post fertilization for relevant phenotypes. Statistical analysis was performed by chi-square analysis (http://www.psych.ku.edu/preacher/chisq/chisq.htm) to calculate the p values between the numbers of embryos scored as wild type (WT) vs. MB under various conditions.
Results and Discussion
Midline bifurcation - a rare squint phenotype
We previously reported MB as a novel but infrequent phenotype seen in heat-treated maternal-zygotic sqt (MZsqt) embryos (Pei et al., 2007). MBs consist of a split in the dorsal midline on day two, typically within the trunk region, such that somites are present on both sides of the bifurcation. In this paper we examine the nature and origin of MBs, using fertile adult sqt null parents to breed clutches of homozygous null MZsqt embryos (Aoki et al., 2002; Pei et al., 2007).
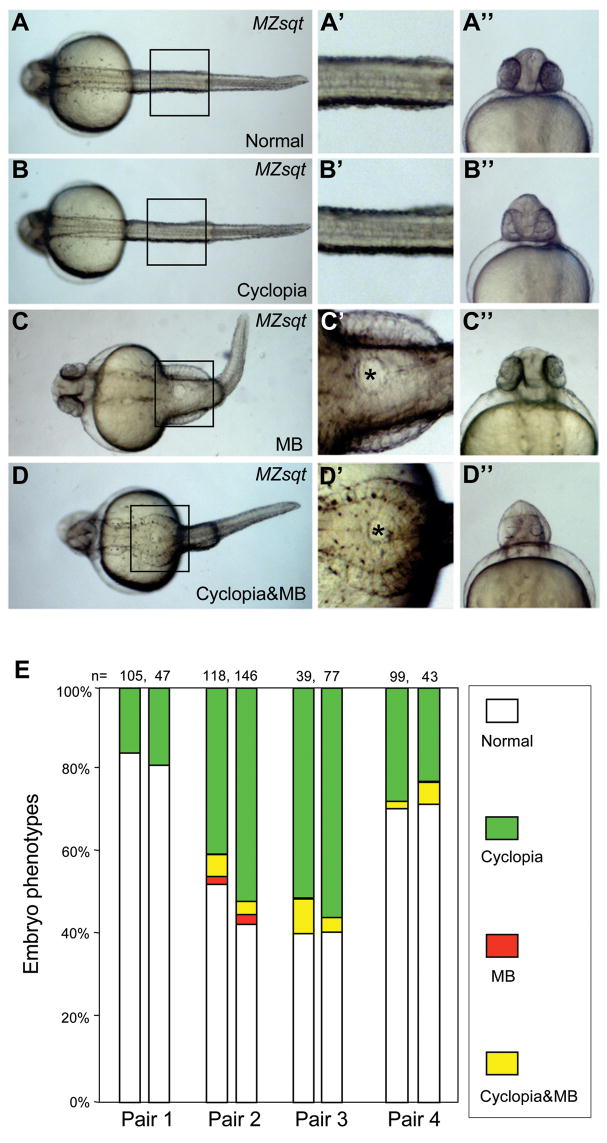
We first note that MB can also arise in MZsqt embryos at normal temperatures. We scored 3887 embryos from 42 pairs of MZsqt crosses. 4% of otherwise normal (Fig. 1C–C″) or cyclopean embryos (Fig. 1D–D″) displayed MB, while 8% displayed cyclopia (Fig. 1B–B″) alone and 88% appeared completely normal (Fig. 1A–A″). The bifurcations vary in size, with some spanning 1–2 somites along the anterior-posterior axis (e.g. Fig. 1D–D′), and others spanning more than a dozen somites (e.g., Fig. 1C–C′).
Fig. 1. Spontaneous midline bifurcations among MZsqt embryos.
(A–D) Various morphological phenotypes observed in MZsqt embryos. Four classes of embryos were observed in MZsqt embryos on 1 day post fertilization: normal-appearing embryos (A–A″), cyclopean embryos (B–B″), MB embryos (C–C″) and embryos with cyclopia and MB embryos (D–D″). The images in the central column (’) are magnifications of the boxes in the left column. The asterisks (*) label the bifurcated areas. The right hand column images (”) are frontal views of the same specimen. (E) Parental genetic background influences the incidence of MB manifestations. Repeat crosses were performed with four pairs of MZsqt parents. Embryo numbers are shown and the percentages of each embryonic phenotype indicated: normal appearing (white); cyclopia alone (green); MB alone (red) and MB + cyclopia (yellow). Pair 1, representative of the typical phenotypic spectrum, produced embryos with cyclopia but no MB. However, pairs 2 to 4 reproducibly yielded the MB phenotype.
Secondly, we note that the tendency for embryos to develop MB depends on their parents. Out of the 42 parental pairs studied, only 6 pairs produced embryos with MB. The frequency of MB and cyclopia were relatively constant on repeat crosses (Fig. 1E), as we have previously noted for cyclopia (Pei et al., 2007). This reproducibility of distinct transmission rates suggests a role for background genetic modifiers (or “risk factors”) of MB that vary between parental pairs.
A third finding is that MB tends to arise in the context of higher cyclopia rates. The average cyclopia rate among progeny of the 6 pairs that yielded embryos with MB was 32% (n=513), compared to 4.3% (n=3,374) among the 36 pairs that did not yield embryos with MB. At the level of individual embryos there is also a strong concordance of MB with cyclopia (Fig. 1E, yellow category). However a number of embryos also display MB and no cyclopia (Fig. 1C and E, red category). The overall low rate of MB in MZsqt embryos suggested to us that, like human HPE and cyclopia in sqt mutants, MBs are caused by synergies between multiple genetic and environmental risk factors.
Synergy of squint and temperature in MB production
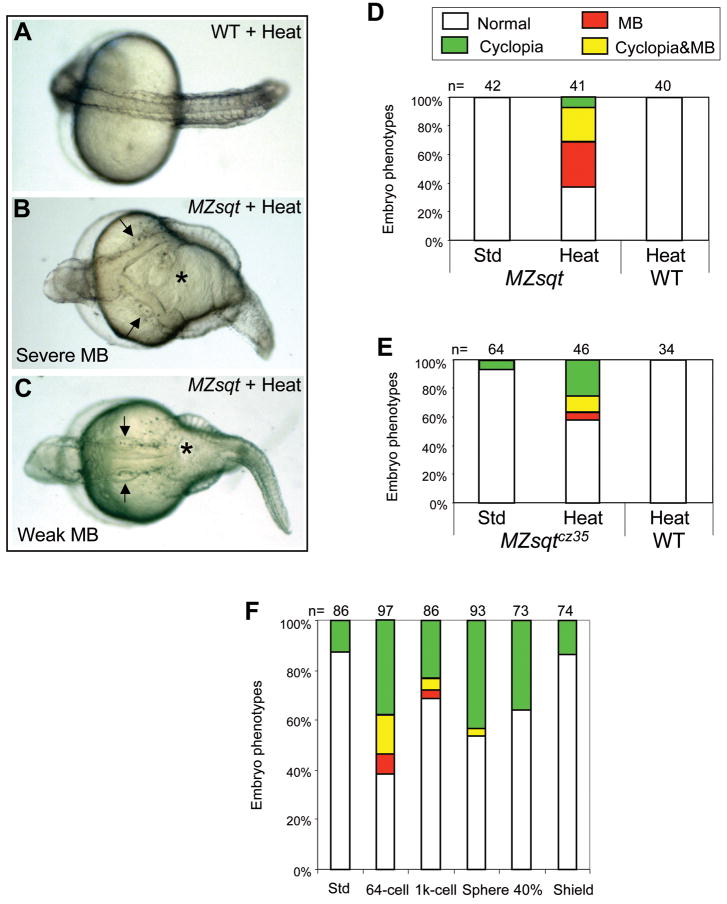
One such risk factor is heat, which modulates the incidence of cyclopia and can also induce MB in MZsqt embryos (Pei et al., 2007). To more precisely characterize how heat influences the incidence of MB, we performed a series of temperature shift experiments. We found that a short heat treatment - 1h at 34°C starting at the 256-cell stage, which increases cyclopia rates dramatically - did not increase MB incidence (data not shown). Heat treatment for 6h, beginning at the 64 cell stage, gave rise to a low incidence of MB in some clutches of embryos (data not shown), and an overnight (22h) heat treatment substantially increased the incidence and severity of MB (Fig. 2B–D). No MB was seen among identically-treated wild type (WT) embryos (Fig. 2A, D, E). Thus, both cyclopia and MB are synergistically increased by heat and loss of Sqt. As discussed later, we propose that the higher rate of MB in overnight heat-treated samples represents a reduction in the recovery rate, and that the induction of MB occurs prior to 5 hpf.
Fig. 2. Overnight heat treatment reliably induces midline bifurcations in MZsqt embryos.
(A–C) Embryonic phenotypes after overnight heat treatment. Overnight (22h) heat treatment caused no significant defect in WT embryos (A), but led to the MB phenotype in a fraction of MZsqt embryos from every cross tested. Severe MBs extended from the trunk to the hindbrain, as demarcated by the otic vesicles (arrows) (B). Weaker MBs were limited to the trunk (C). Arrows point to otic vesicles. (D–E) Overnight heat treatment increases MB incidence in MZsqt embryos. WT and MZsqthi975/hi975 (D) or MZsqtcz35/cz35 (E) embryos were cultured under standard conditions (28°C) or heated (34°C) overnight starting at the 64-cell stage, then scored for the MB phenotype on 1 day post fertilization. A significant difference was observed between control and heated MZsqthi975/hi975 embryos (p=3.00E-08), or control and heated MZsqtcz35/cz35 embryos (p=0.00126). (F) Early initiation of heat treatment is required to induce MB. MZsqt embryos pooled from several crosses were heated overnight, beginning at the indicated stages and scored for the MB phenotype on 1 day post-fertilization. Numbers of embryos scored are shown. Percentages for each phenotype are indicated: normal appearing (white); cyclopia alone (green); MB alone (red) and MB + cyclopia (yellow).
The synergy of overnight heat exposure and loss of Sqt is quite robust; we observed embryos with MB in every 64-cell through overnight heated clutch from sqt−/− parents we scored, including parents carrying the sqtcz35 allele (Fig. 2E). This association of MB with either the sqthi975 or sqtcz35 allele, which has formally been proven to be null (Bennett et al., 2007), adds weight to our argument, published elsewhere (Pei et al., 2007), that sqthi975 is also null.
We also could produce MB in heat-treated progeny of heterozygous sqtcz35/+ mothers crossed with homozygous sqthi975/hi975 fathers from entirely different genetic backgrounds (data not shown). This indicates that MB is not dependent upon the loss of maternal Squint or on background-associated homozygous recessive mutations. Overnight heat treatment did not induce MB in embryos depleted for the Nodal-related ligand Cyc, either genetically (cycm294/m294 homozygotes (Schier et al., 1996)) or using a morpholino (Karlen and Rebagliati, 2001) (data not shown), suggesting that MB reflects a particular requirement for Sqt activity.
An explanation for why Sqt but not Cyc protects against MB could be that Nodal activity is required prior to the mid-blastula stages, when Squint is the only Nodal expressed (Rebagliati et al., 1998). Consistent with this idea, we were only able to produce MB via overnight treatment of MZsqt embryos when heat was initiated prior to 5 hpf (40% epiboly) (Fig. 2F). To explain these various findings, we propose that early heat treatment initiates events leading to MB by disrupting processes or factors that are normally protected by Sqt, and that later-stage heat prevents recovery from this initial insult.
Inhibition of Hsp90 activity promotes midline bifurcations in MZsqt embryos
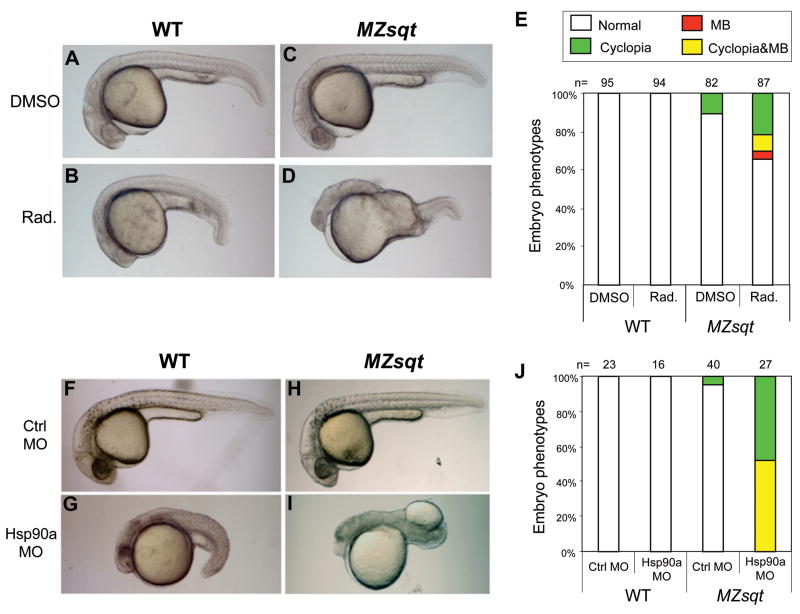
Hsp90 has been found to modify phenotypic expression in the sense that its removal can uncover phenotypes caused by mutations that would otherwise be silent. This has been observed in various model organism contexts (Queitsch et al., 2002; Richter and Buchner, 2001; Rutherford and Lindquist, 1998; Sangster et al., 2004) including our own observed increase of cyclopia in MZsqt embryos subjected to Hsp90 disruption (Pei et al., 2007). Given that Sqt disruption and heat are shared risk factors for MB and cyclopia, we also examined the effect of Hsp90 disruption on MB. There are two Hsp90 paralogs in zebrafish: Hsp90a and Hsp90b. The incidence of MB in MZsqt embryos was indeed increased when both Hsp90s were targeted with the drug radicicol (Fig. 3A–E) or by injection of an antisense morpholino oligonucleotide targeting Hsp90a (Fig. 3F–J). This increase in MB was seen above the background of standard loss of Hsp90 phenotypes seen both in WT and MZsqt embryos, namely: shortened axes, small heads and dark regions in the heads of Hsp90a morphants, which presumably demarcate regions of cell death. Thus, Hsp90a is a modifier of both MB and cyclopia incidence in MZsqt embryos. We do note, however, that we were unable to increase MB rates in MZsqt embryos with an antisense morpholino oligonucleotide against Hsp90b or the Hsp90-targeting drug geldanamycin (data not shown) although these treatments increased cyclopia (Pei et al., 2007). The ability of Hsp90a MOs but not Hsp90b MOs to induce MB could relate to the fact that hsp90a mRNA, but not hsp90b mRNA, is enriched in the paraxial mesoderm, which flanks the sites of MB (Du et al., 2008; Etard et al., 2007). Regarding the differences between radicicol and geldanamycin, we do not have a precise explanation, but note that radicicol has been reported to out-perform geldanamycin in other contexts, for instance in its unique ability to buffer oxidative stress in human retinal pigment epithelial cells (Ryhanen et al., 2008; Sano, 2001).
Fig. 3. Inhibition of Hsp90 function increases the incidence of midline bifurcations.
(A–D) Phenotypes of embryos injected with the Hsp90-inhibitory chemical radicicol. WT and MZsqt embryos were injected with 0.7 nl of either 27 mM radicicol in DMSO or DMSO only, and scored for the MB phenotype on 1 day post fertilization. Injection of DMSO caused no significant defects in both WT (A) and MZsqt embryos (C). Injection of radicicol in WT embryos caused slightly dark, small heads and short tails (B). Injection of radicicol in MZsqt embryos, caused these same defects, but also induced cyclopia (image not shown) and MB (D). (E) Incidence of cyclopia and MB among WT and MZsqt embryos injected with radicicol. Numbers of embryos scored are shown and correspond to a highly significant difference (p=0.000484) in the MB rates of DMSO and radicicol treated MZsqt embryo groups. (F–I) Phenotypes of the embryos injected with an Hsp90a MO. WT and MZsqt embryos were injected with 3 ng of either control MO or an Hsp90a-targeting MO, and scored for the MB phenotype on 1 day post fertilization. The control MO caused no obvious defects in WT (F) or MZsqt embryos (H). Injection of Hsp90a MO into WT embryos generated severe defects, including dark heads and very short tails, but no MB phenotype was seen (G). Injection of the Hsp90a MO into MZsqt embryos, caused these same defects, but also induced cyclopia (image not shown) and MB (I). (J) Incidence of cyclopia and MB among WT and MZsqt embryos injected with the Hsp90a MO. Numbers of embryos scored are shown and correspond to a highly significant difference (p=0.000001) in the MB rates of control and HSP90a MO-injected MZsqt embryos. Percentages for each phenotype are indicated: normal appearing (white); cyclopia alone (green); MB alone (red) and MB + cyclopia (yellow).
Tissue on both sides of midline bifurcations acquires midline identity
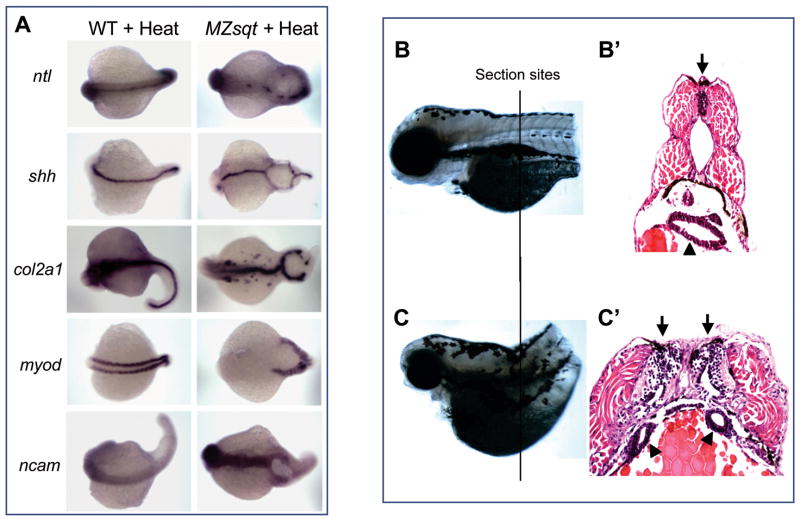
We performed in situ marker analysis to determine which tissue markers are expressed in and around MBs. For these studies, we heat treated MZsqt progeny to obtain a sufficient number of specimens. Two questions this study addressed are whether complete midline specification occurs in the context of MB and, if so, whether such specification is limited to one side of the bifurcation? We found that tissue on both sides of bifurcations express all dorsal midline markers we tested (Fig. 4A). We detected shh (Strahle et al., 1996) and col2α1(Yan et al., 1995), indicating that floor plate identities are intact. We also detected patchy expression of ntl (Schulte-Merker et al., 1994) on both sides of bifurcations, indicating that notochord identity arises, but not consistently. Somites on both sides of bifurcations express the early muscle marker myod as normal (Weinberg et al., 1996), and ncam (Mizuno et al., 2001) expression on both sides of bifurcations confirms that neural specification is intact (Fig. 4A).
Fig. 4. Tissue on both sides of midline bifurcations acquires midline identity.
(A) Whole-mount in situ analysis of gene expression. WT and MZsqt embryos were heat treated overnight (22h) starting at the 64-cell stage and fixed on 1 day post fertilization for whole-mount in situ analysis. All embryos are oriented with a dorsal view, anterior on the left. (B–C) Histological sections of WT and MB embryos. WT and MZsqt embryos were heat treated for 22h starting at the 64-cell stage and then reared for 2 more days, fixed with 4% paraformaldehyde, paraffin embedded, sectioned (5 um/section) and stained with hematoxylin and eosin. The approximate section sites for the WT embryo (B) and the MZsqt embryo (C) are indicated. Apostrophes (’) indicate the sections from the same specimen. Arrows indicate neural tubes. Arrowheads indicate guts.
To better visualize MB architecture, we prepared transverse sections, revealing the clear presence of duplicated guts and neural tubes on both sides of the bifurcation (Fig. 4C′). Thus, tissue on each side of MB displays a full spectrum of midline fates. What of the tissue within the bifurcations? If MB were akin to spina bifida (OMIM:#182940), as previously reported in sqt mutants (Aquilina-Beck et al., 2007) as well as another zebrafish model (Sumanas et al., 2005), one would expect the tissue within the lesion to be neural, yet neither neural markers (ncam, col2a1 or shh) nor other markers (myod and ntl) were detected.
Midline bifurcations arise after the completion of gastrulation
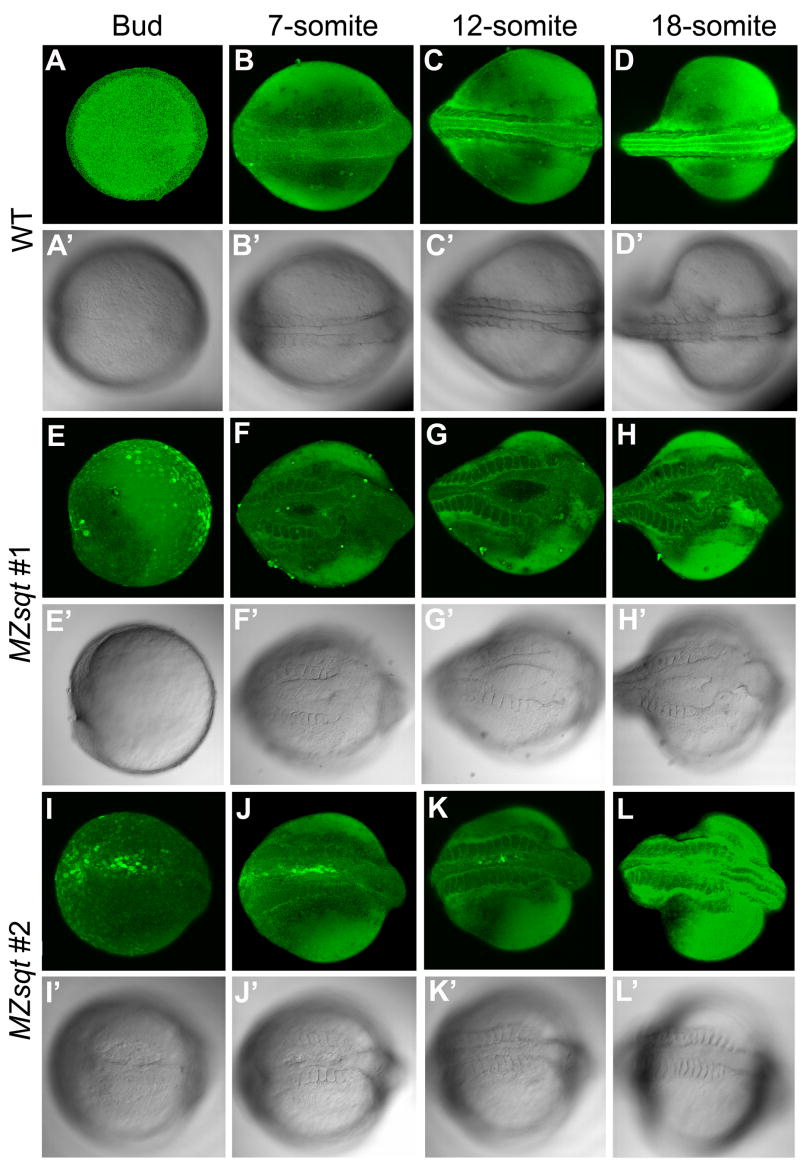
To determine when and where MBs first arise, we imaged fifteen heat-treated MZsqt embryos vitally stained with fluorescent bodipy ceramide at multiple stages of development, and eleven were observed to form MB. Each of these embryos completed gastrulation normally and MB was not detectable until the bud stage (7/11 embryos - Fig. 5I–I′) or later at the 7-somite stage (4/11 embryos Fig. 5E–H). Careful examination of the region inside MBs reveals cell-cell boundaries typical of large enveloping-layer (EVL) cells with no labeling of smaller deep layer (DEL) cells (Fig. 5F–H). In other instances, EVL-sized cells within MBs were cytoplasmically-labeled, but labeling of DEL-sized cells was similarly absent (Fig. 5I). In further support of the EVL being intact in MBs, expression of the EVL marker Type I cytokeratin was unperturbed in all bud-stage heat-treated MZsqt embryos we examined (data not shown). We conclude that newly-formed MBs are characterized by a local deficiency of DEL cells with the EVL layer intact.
Fig. 5. Time-lapse imaging of midline bifurcation formation and recovery.
Embryos vitally-stained with fluorescent bodipy ceramide were mounted in soft agarose and incubated at 34°C except for ~ 15 minute interruptions at room temperature (~22°C) for microphotography, performed every ~2 hours. (A–D) Midline formation in heated WT embryos. Heated WT embryos developed well-defined dorsal midlines during the recording. (E–L) Midline formation in two heated MZsqt embryos. (E–H) MZsqt #1 embryos formed a large bifurcation, first visible at the 7-somite stage. (I–L) MZsqt #2 embryos displayed a small bifurcation, first visible at the bud stage (I), which recovered by the 12-somite stage (K). Because embryos were incubated and photographed at temperatures (34°C and 22°C) causing distinct developmental rates, the indicated stages are based on morphology, rather than time. Apostrophes (’) indicate bright field images of the same specimen. Dorsal views are given throughout, with the anterior to the right.
Live imaging also revealed a high incidence of recovery, whereby MBs gradually close and heal, a process that we observed in 8 of the 11 embryos that displayed MB (Fig. 5J–L). This healing would explain why DEL cells are seen throughout the sectioned MB in Fig. 4C. The high incidence (11 of 15) of MB formation and recovery (8 of 11) also supports our earlier hypothesis that effective recovery decreases final MB rates of embryos subjected only to early heat.
In summary, our in vivo experiments show that MBs first arise during early segmentation, that EVL cells remain intact above bifurcations and that there is a high incidence of recovery. Because we did not capture the moment of MB formation, we could not distinguish whether MBs arise via a local separation of DEL cells or via a failure to deliver sufficient numbers of DEL cells to the embryo’s dorsal side.
Dorsal gaps in wnt5b and ntl expression precede MB formation
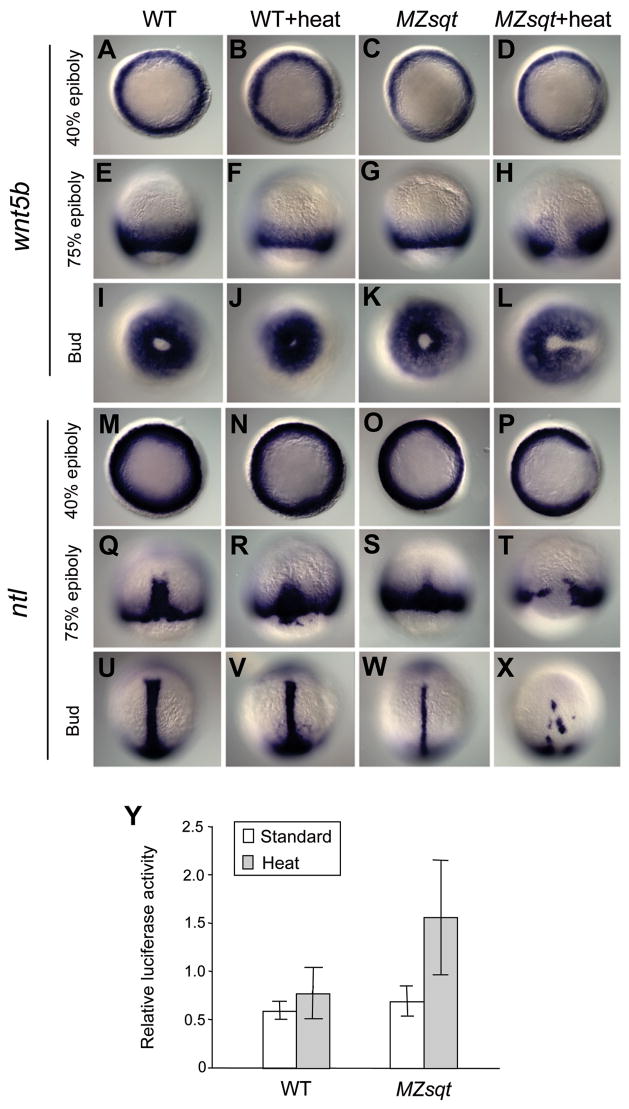
MB has broad similarities to human dysmorphological syndromes such as spina bifida, that are considered to be closure defects. To explore the possibility of an underlying convergence defect, we examined expression of wnt5b/pipetail, a non-canonical Wnt signaling ligand that is essential for convergence movements (Kilian et al., 2003; Marlow et al., 2004; Matsui et al., 2005). Of particular interest, wnt5b/pipetail mutants sometimes display a “split somite” defect that strongly resembles MB (Westfall et al., 2003a; Westfall et al., 2003b). We found that wnt5b expression was specifically reduced in dorsal domains of heat-treated MZsqt gastrulas, and we propose that these dorsal domains are the sites of future MBs (Fig. 6A–L). We observed no heat- or Sqt-dependent changes in the expression of another Wnt family member, wnt11 (data not shown), which is expressed in the dorsal anterior of gastrula-stage embryos (Heisenberg et al., 2000).
Fig. 6. Wnt5b and no tail expression prior to formation of midline bifurcations.
(A–X) wnt5b (A–L) and ntl (M–X) expression during gastrulation of standard and heat-treated WT and MZsqt embryos. Embryos were cultured at 28°C or heated (34°C) starting at the 64-cell stage and then fixed at different stages for whole-mount in situ analysis. No difference in wnt5b expression was seen between control and heated WT embryos at all tested stages. However, heated MZsqt embryos showed reduced wnt5b expression in the dorsal region at all stages tested. Similarly, no differences in ntl expression was seen between control and heated WT embryos at all stages. However, reduced dorsal expression of ntl is obvious in control MZsqt embryos. Heated MZsqt embryos display further reduced dorsal expression of ntl at the 40% epiboly stage (P) and the 70% epiboly stage (T), and bifurcated ntl expression is often seen at the bud stage (X). Embryo orientations: 40% epiboly, animal pole view, dorsal on the right; 75% epiboly, dorsal view, animal pole on the top; bud stage, vegetal pole view, dorsal on the right for wnt5b embryos and dorsal view, animal pole on the top for ntl embryos. (Y) Up-regulated Wnt/β-catenin signaling in heated MZsqt embryos. WT and MZsqt embryos injected with 23 pg of reporter plasmids were reared at 28°C or heated (34°C) starting at the 64-cell stage and lysed at the bud stage for the TCF/LEF reporter assay. Average values and standard deviations from triplicate experiments are shown.
wnt5b/pipetail has been shown to genetically interact with the T-box gene no tail (ntl) (Marlow et al., 2004) and early dorsal gaps in no tail expression have previously been reported in sqt;cyc double mutants (Feldman et al., 1998). Accordingly, we also examined ntl expression in heat-treated MZsqt embryos and found that it was reduced on the dorsal side of all MZsqt embryos, and typically absent on the dorsal side heat-treated MZsqt embryos (Fig. 6M–X). This raises the possibility that MB is induced by the combinatorial loss of both Ntl and Wnt5b. wnt5b and ntl gaps were observed in the vast majority of heat-treated MZsqt embryos, whereas only about three quarters of the embryos we live imaged developed MB, with most recovering, and a much smaller fraction of the heat-treated MZsqt embryos we documented at later stages displayed MB. This indicates (1) that a fraction of embryos with gaps in wnt5b or ntl expression never form bifurcations, and (2) that recovery is quite frequent, as also observed in our live imaging (Fig. 5).
To obtain a measure of how the local reduction of wnt5b transcripts might affect Wnt signaling at the global level, we employed a functional assay. Non-canonical Wnt signaling by factors such as Wnt5b is thought to negatively regulate canonical Wnt/β-catenin signaling, seen for instance by increased β-catenin expression and increased expression of boz in wnt5b/ppt mutants (Slusarski et al., 1997; Westfall et al., 2003a; Westfall et al., 2003b). We measured the Wnt/β-catenin signaling activity of heat-treated MZsqt embryos and controls, using a TCF/LEF luciferase reporter system, and detected a substantial increase of Wnt/β-catenin activity specifically in heated MZsqt embryos (Fig. 6Y). This increase is consistent with the hypothesis that reductions in wnt5b expression cause a global reduction in non-canonical Wnt signaling in heated MZsqt embryos.
Decreased Wnt5b signaling increases midline bifurcation incidence
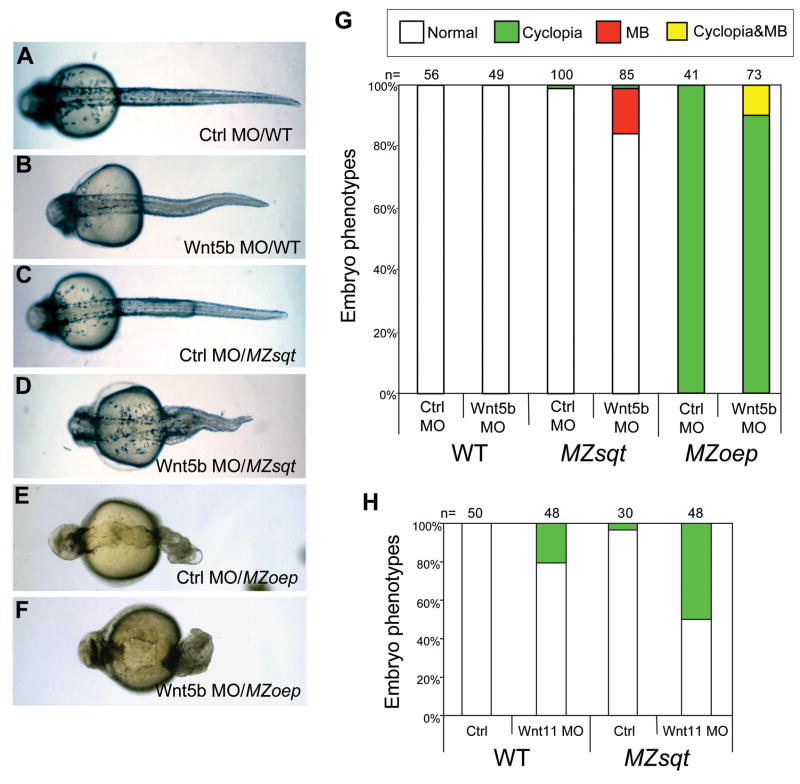
To more directly assess whether loss of Wnt5b synergizes with loss of Sqt, we injected MZsqt embryos with an antisense morpholino oligonucleotide targeting Wnt5b translation (Lele et al., 2001). Injection of the Wnt5b MO into WT embryos caused shortened axes and curled tails (Fig. 7B). Injection of the Wnt5b MO into MZsqt embryos caused the same phenotypes, but also induced MB (Fig. 7D, G). Interestingly, and distinct from heat and Hsp90 perturbations, these Wnt5b alterations caused no change in the rate of cyclopia (Fig. 7G). As a point of comparison, we also injected WT and MZsqt embryos with a Wnt11 MO. Wnt11 MO injection caused cyclopia in WT embryos, consistent with the reported phenotype of wnt11/silberblick mutants (Heisenberg and Nusslein-Volhard, 1997), and increased the incidence of cyclopia in MZsqt embryos (Fig. 7H), consistent with the previously reported synergy of sqt and wnt11/silberblick (Heisenberg and Nusslein-Volhard, 1997). However no MB was seen in either WT or MZsqt embryos injected with the Wnt11 MO at this dose (0.5 ng/embryo; Fig. 7H) nor at a higher dose (1.5 ng) where >95% cyclopia is seen in MZsqt embryos (n=36), suggesting loss of Wnt11 exclusively modifies cyclopia.
Fig. 7. Wnt5b uniquely modifies midline bifurcation incidence; Wnt11 uniquely modifies cyclopia incidence.
(A–F) Phenotypes of Wnt5b MO-injected embryos. Injection of a control MO did not cause obvious defects in either WT embryos (A), MZsqt embryos (C) or MZoep embryos (E). Injection of 6 ng of a Wnt5b MO caused shortened tails in WT (B) and MZsqt embryos (D); however, it also caused MB in MZsqt embryos (D) and MZoep embryos (F). (G) Quantification of increased MB in Wnt5b MO-injected MZsqt and MZoep embryos. Significant differences were observed in the MB rates of control MO versus Wnt5b MO-injected MZsqt embryos (p=0.00005) and control MO versus Wnt5b MO-injected MZoep embryos (p=0.04068), but no significant differences were seen in the cyclopia rates of control MO versus Wnt5b MO-injected MZsqt embryos (p=0.90922), and all MZoep embryos had cyclopia. (H) Quantification of increased cyclopia in Wnt11 MO-injected MZsqt and WT embryos. Significant differences were observed in the cyclopia rate of control MO versus Wnt11 MO-injected MZsqt (p=0.0002) and control MO versus Wnt11 MO-injected WT (p=0.00066) embryos, but MB was not seen in any Wnt11 MO injected embryos. Numbers of embryos scored are shown and embryo percentages are indicated as follows: normal appearing (white); cyclopia alone (green); MB alone (red) and MB + cyclopia (yellow).
Based on our data, we conclude that insults in two major embryonic pathways, Nodal signaling (Sqt) and non-canonical Wnt (Wnt5b), synergize to produce a specific phenotype (MB). This led us to wonder whether MB is simply the product of two gastrulation movement deficiencies: convergence, which requires non-canonical Wnt signaling, and internalization, which requires Nodal signaling. If this were the case, depletion of Wnt5b in embryos completely deficient for internalization should lead to increased incidence of MB. To address this question, we knocked Wnt5b down in MZoep embryos, which do not undergo internalization because they suffer a complete loss of Nodal signaling (Gritsman et al., 1999). We observed MB in Wnt5b-depleted MZoep embryos at rates comparable to Wnt5b-depleted MZsqt embryos (Fig. 7F and G), indicating that MB is not the result of a simple compounding of defective gastrulation movements. Since the principle difference between MZsqt and MZoep embryos is the additional absence of Cyc signaling, these results also lend support to our argument that MB is specifically induced by loss of Sqt, with its early signaling, and not by loss of Cyc. Because MZoep embryos do not form endoderm or non-tail mesoderm (Gritsman et al., 1999), the development of MB in Wnt5b-depleted MZoep embryos also reveals that MB is independent of local mesendoderm differentiation, such as trunk somite formation (Fig. 7F).
In summary, we have provided evidence that under enhanced conditions (heated MZsqt embryos), MB occurs frequently, is preceded by a regional decrease in wnt5b expression, and frequently recovers. We have demonstrated that MZsqt mutant embryos are an excellent system for elucidating biological principles underlying complex disease. We have used this system to discern shared and unique classes of genetic and environmental risk factors underlying two complex phenotypes. Of particular interest is the specific intersection of insults in the Nodal and non-canonical Wnt pathways in the production of MB.
Acknowledgments
We thank Bo Xiong and Anming Meng for the LEF-luc and control DNA constructs; Jamie Brown and Sung-Kook Hong for technical advice; Erich Roessler for critical reading of the manuscript and inspiring discussions; Maximilian Muenke for permission to cite unpublished data. This work was supported by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amsterdam A, Nissen RM, Sun Z, Swindell EC, Farrington S, Hopkins N. Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci U S A. 2004;101:12792–7. doi: 10.1073/pnas.0403929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki TO, Mathieu J, Saint-Etienne L, Rebagliati MR, Peyrieras N, Rosa FM. Regulation of nodal signalling and mesendoderm formation by TARAM-A, a TGFbeta-related type I receptor. Dev Biol. 2002;241:273–88. doi: 10.1006/dbio.2001.0510. [DOI] [PubMed] [Google Scholar]
- Aquilina-Beck A, Ilagan K, Liu Q, Liang JO. Nodal signaling is required for closure of the anterior neural tube in zebrafish. BMC Dev Biol. 2007;7:126. doi: 10.1186/1471-213X-7-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JT, Stickney HL, Choi WY, Ciruna B, Talbot WS, Schier AF. Maternal nodal and zebrafish embryogenesis. Nature. 2007;450:E1–2. doi: 10.1038/nature06314. discussion E2-4. [DOI] [PubMed] [Google Scholar]
- Blader P, Strahle U. Casting an eye over cyclopia. Nature. 1998;395:112–3. doi: 10.1038/25836. [DOI] [PubMed] [Google Scholar]
- Du SJ, Li H, Bian Y, Zhong Y. Heat-shock protein 90alpha1 is required for organized myofibril assembly in skeletal muscles of zebrafish embryos. Proc Natl Acad Sci U S A. 2008;105:554–9. doi: 10.1073/pnas.0707330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etard C, Behra M, Fischer N, Hutcheson D, Geisler R, Strahle U. The UCS factor Steif/Unc-45b interacts with the heat shock protein Hsp90a during myofibrillogenesis. Dev Biol. 2007;308:133–43. doi: 10.1016/j.ydbio.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, Sirotkin HI, Schier AF, Talbot WS. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature. 1998;395:181–5. doi: 10.1038/26013. [DOI] [PubMed] [Google Scholar]
- Gore AV, Maegawa S, Cheong A, Gilligan PC, Weinberg ES, Sampath K. The zebrafish dorsal axis is apparent at the four-cell stage. Nature. 2005;438:1030–5. doi: 10.1038/nature04184. [DOI] [PubMed] [Google Scholar]
- Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot WS, Schier AF. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell. 1999;97:121–32. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- Hagos EG, Fan X, Dougan ST. The role of maternal Activin-like signals in zebrafish embryos. Dev Biol. 2007;309:245–58. doi: 10.1016/j.ydbio.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Hatta K, Kimmel CB, Ho RK, Walker C. The cyclops mutation blocks specification of the floor plate of the zebrafish central nervous system. Nature. 1991;350:339–41. doi: 10.1038/350339a0. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Nusslein-Volhard C. The function of silberblick in the positioning of the eye anlage in the zebrafish embryo. Dev Biol. 1997;184:85–94. doi: 10.1006/dbio.1997.8511. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Karlen S, Rebagliati M. A morpholino phenocopy of the cyclops mutation. Genesis. 2001;30:126–8. doi: 10.1002/gene.1046. [DOI] [PubMed] [Google Scholar]
- Kilian B, Mansukoski H, Barbosa FC, Ulrich F, Tada M, Heisenberg CP. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Dev. 2003;120:467–76. doi: 10.1016/s0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Lele Z, Bakkers J, Hammerschmidt M. Morpholino phenocopies of the swirl, snailhouse, somitabun, minifin, silberblick, and pipetail mutations. Genesis. 2001;30:190–4. doi: 10.1002/gene.1063. [DOI] [PubMed] [Google Scholar]
- Marlow F, Gonzalez EM, Yin C, Rojo C, Solnica-Krezel L. No tail cooperates with non-canonical Wnt signaling to regulate posterior body morphogenesis in zebrafish. Development. 2004;131:203–16. doi: 10.1242/dev.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Raya A, Kawakami Y, Callol-Massot C, Capdevila J, Rodriguez-Esteban C, Izpisua Belmonte JC. Noncanonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development. Genes Dev. 2005;19:164–75. doi: 10.1101/gad.1253605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga E, Shiota K. Holoprosencephaly in human embryos: epidemiologic studies of 150 cases. Teratology. 1977;16:261–72. doi: 10.1002/tera.1420160304. [DOI] [PubMed] [Google Scholar]
- Ming JE, Muenke M. Multiple hits during early embryonic development: digenic diseases and holoprosencephaly. Am J Hum Genet. 2002;71:1017–32. doi: 10.1086/344412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Kawasaki M, Nakahira M, Kagamiyama H, Kikuchi Y, Okamoto H, Mori K, Yoshihara Y. Molecular diversity in zebrafish NCAM family: three members with different VASE usage and distinct localization. Mol Cell Neurosci. 2001;18:119–30. doi: 10.1006/mcne.2001.1007. [DOI] [PubMed] [Google Scholar]
- Muenke M, Beachy PA. Genetics of ventral forebrain development and holoprosencephaly. Curr Opin Genet Dev. 2000;10:262–9. doi: 10.1016/s0959-437x(00)00084-8. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–20. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Pei W, Williams PH, Clark MD, Stemple DL, Feldman B. Environmental and genetic modifiers of squint penetrance during zebrafish embryogenesis. Dev Biol. 2007;308:368–78. doi: 10.1016/j.ydbio.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogoda HM, Solnica-Krezel L, Driever W, Meyer D. The zebrafish forkhead transcription factor FoxH1/Fast1 is a modulator of nodal signaling required for organizer formation. Curr Biol. 2000;10:1041–9. doi: 10.1016/s0960-9822(00)00669-2. [DOI] [PubMed] [Google Scholar]
- Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417:618–24. doi: 10.1038/nature749. [DOI] [PubMed] [Google Scholar]
- Rebagliati MR, Toyama R, Fricke C, Haffter P, Dawid IB. Zebrafish nodal-related genes are implicated in axial patterning and establishing left-right asymmetry. Dev Biol. 1998;199:261–72. doi: 10.1006/dbio.1998.8935. [DOI] [PubMed] [Google Scholar]
- Richter K, Buchner J. Hsp90: chaperoning signal transduction. J Cell Physiol. 2001;188:281–90. doi: 10.1002/jcp.1131. [DOI] [PubMed] [Google Scholar]
- Roessler E, Ouspenskaia MV, Karkera JD, Velez JI, Kantipong A, Lacbawan F, Bowers P, Belmont JW, Towbin JA, Goldmuntz E, Feldman B, Muenke M. Reduced NODAL signaling strength via mutation of several pathway members including FOXH1 is linked to human heart defects and holoprosencephaly. Am J Hum Genet. 2008;83:18–29. doi: 10.1016/j.ajhg.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–42. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- Ryhanen T, Mannermaa E, Oksala N, Viiri J, Paimela T, Salminen A, Atalay M, Kaarniranta K. Radicicol but not geldanamycin evokes oxidative stress response and efflux protein inhibition in ARPE-19 human retinal pigment epithelial cells. Eur J Pharmacol. 2008;584:229–36. doi: 10.1016/j.ejphar.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Sangster T, Spence M, Sinclair P, Payne R, Smith C. Unexpected observation of ion suppression in a liquid chromatography/atmospheric pressure chemical ionization mass spectrometric bioanalytical method. Rapid Commun Mass Spectrom. 2004;18:1361–4. doi: 10.1002/rcm.1477. [DOI] [PubMed] [Google Scholar]
- Sano M. Radicicol and geldanamycin prevent neurotoxic effects of anti-cancer drugs on cultured embryonic sensory neurons. Neuropharmacology. 2001;40:947–53. doi: 10.1016/s0028-3908(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Schier AF, Neuhauss SC, Harvey M, Malicki J, Solnica-Krezel L, Stainier DY, Zwartkruis F, Abdelilah S, Stemple DL, Rangini Z, Yang H, Driever W. Mutations affecting the development of the embryonic zebrafish brain. Development. 1996;123:165–78. doi: 10.1242/dev.123.1.165. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Hammerschmidt M, Beuchle D, Cho KW, De Robertis EM, Nusslein-Volhard C. Expression of zebrafish goosecoid and no tail gene products in wild-type and mutant no tail embryos. Development. 1994;120:843–52. doi: 10.1242/dev.120.4.843. [DOI] [PubMed] [Google Scholar]
- Sirotkin HI, Gates MA, Kelly PD, Schier AF, Talbot WS. Fast1 is required for the development of dorsal axial structures in zebrafish. Curr Biol. 2000;10:1051–4. doi: 10.1016/s0960-9822(00)00679-5. [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Yang-Snyder J, Busa WB, Moon RT. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev Biol. 1997;182:114–20. doi: 10.1006/dbio.1996.8463. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L, Stemple DL, Mountcastle-Shah E, Rangini Z, Neuhauss SC, Malicki J, Schier AF, Stainier DY, Zwartkruis F, Abdelilah S, Driever W. Mutations affecting cell fates and cellular rearrangements during gastrulation in zebrafish. Development. 1996;123:67–80. doi: 10.1242/dev.123.1.67. [DOI] [PubMed] [Google Scholar]
- Strahle U, Blader P, Ingham PW. Expression of axial and sonic hedgehog in wildtype and midline defective zebrafish embryos. Int J Dev Biol. 1996;40:929–40. [PubMed] [Google Scholar]
- Sumanas S, Zhang B, Dai R, Lin S. 15-zinc finger protein Bloody Fingers is required for zebrafish morphogenetic movements during neurulation. Dev Biol. 2005;283:85–96. doi: 10.1016/j.ydbio.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B. High resolution Whole-Mount in situ Hybridization. The Zebrafish Science Monitor. 1998;5:8–9. [Google Scholar]
- Wallis DE, Muenke M. Molecular mechanisms of holoprosencephaly. Mol Genet Metab. 1999;68:126–38. doi: 10.1006/mgme.1999.2895. [DOI] [PubMed] [Google Scholar]
- Weinberg ES, Allende ML, Kelly CS, Abdelhamid A, Murakami T, Andermann P, Doerre OG, Grunwald DJ, Riggleman B. Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development. 1996;122:271–80. doi: 10.1242/dev.122.1.271. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: a guide for the laboratory use of zebrafish (Danio rerio) 1994 [Google Scholar]
- Westfall TA, Brimeyer R, Twedt J, Gladon J, Olberding A, Furutani-Seiki M, Slusarski DC. Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/beta-catenin activity. J Cell Biol. 2003a;162:889–98. doi: 10.1083/jcb.200303107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall TA, Hjertos B, Slusarski DC. Requirement for intracellular calcium modulation in zebrafish dorsal-ventral patterning. Dev Biol. 2003b;259:380–91. doi: 10.1016/s0012-1606(03)00209-4. [DOI] [PubMed] [Google Scholar]
- Whitman M. Nodal signaling in early vertebrate embryos: themes and variations. Dev Cell. 2001;1:605–17. doi: 10.1016/s1534-5807(01)00076-4. [DOI] [PubMed] [Google Scholar]
- Yan YL, Hatta K, Riggleman B, Postlethwait JH. Expression of a type II collagen gene in the zebrafish embryonic axis. Dev Dyn. 1995;203:363–76. doi: 10.1002/aja.1002030308. [DOI] [PubMed] [Google Scholar]