INTRODUCTION
Odontogenic myxoma is a slow-growing, painless, and site-aggressive tumor.1 Large-sized lesions may cause tooth dislodgement and cortical bone expansion.2,3 Since pain and hypoesthesia are not common, the lesion may reach a considerable size before the patient perceives its existence and seeks treatment.4
This lesion is not encapsulated, thus promoting significant infiltration into the adjacent medullar bone. In addition, this condition carries a high recurrence rate. The treatment of choice for this condition is surgical excision by either enucleation, curettage, or block resection.5 The average recurrence rate is 25%, especially when more conservative treatments are used.1,6
Cryotherapy has been used in the maxillofacial region for the removal of neoplasias or abnormal cell elements with no need for extensive segmental resection.7–9 Liquid nitrogen is the most efficient type of freezing spray available for bone cryosurgery.10 The most commonly observed complications associated with cryotherapy are pathologic fractures7,10,11 and bone sequestra.7,10
This paper reports the case of a recurrent mandibular myxoma diagnosed 30 years after the initial lesion treatment, and discusses the possibility of conservative management of extensive odontogenic lesions with high recurrence rates.
CASE REPORT
A 47-year-old Caucasian female patient was referred to the Oral and Maxillofacial Trauma and Surgery Service (Brazil) in January, 1995. The patient complained of a symptomatic volume increase in the left mandibular body that had existed for one month. The patient reported that she underwent a surgical intervention for the removal of a tumor in the same area 30 years before. The histopathological diagnosis of the biopsy performed in 1965 was “edematous fibroma.”
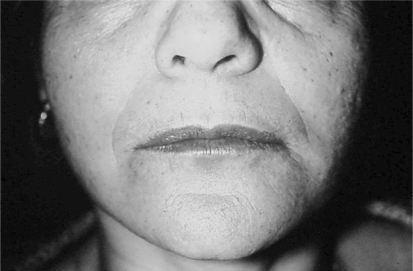
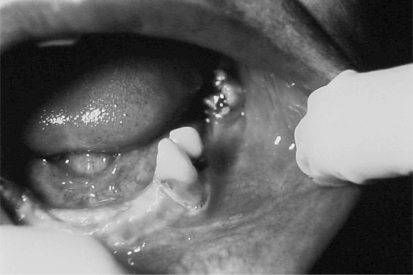
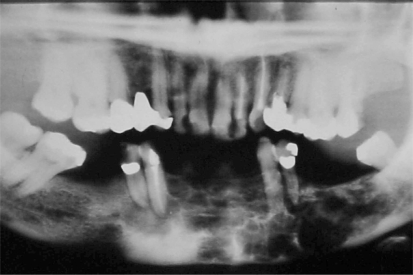
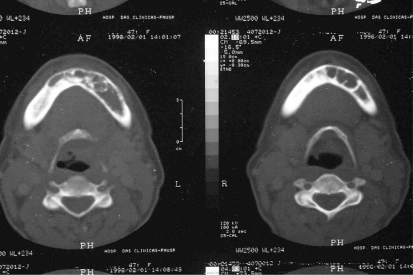
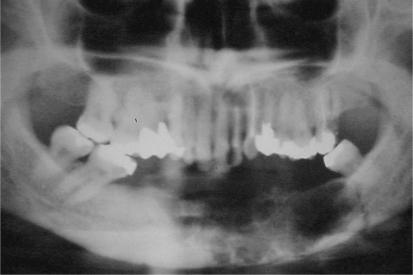
On extraoral clinical examination, a slight crowning was observed in the left mandibular body region (Figure 1) and the patient described local pain. An intraoral clinical examination showed light swelling that was firm on palpation. There was an overlying intact mucosa and a discrete loss of definition of the left inferior vestibular fornix. There were no tooth displacements or rotations and no related sensory disturbances (Figure 2). The panoramic radiograph was not pathognomonic, and revealed an extensive radiolucent, multilocular area with imprecise borders that extended from the left posterior mandibular body to the anterior contralateral mandibular body, and exhibited a “soap bubble” appearance (Figure 3). Computed axial tomography imaging showed an area of infiltration in the medullar bone with a discrete expansion of the external mandibular cortical layer and a thin trabecula along the entire lesion area. No cortical bone or tooth root reabsorbtion were seen. Thus, the lesion did not penetrate the periosteum, and was not contiguous with the alveolar mucosa (Figure 4).
Figure 1 -.
Slight left parasymphisis and mandibular body enlargement
Figure 2 -.
Discrete loss of definition on the left inferior vestibular fornix. The oral expansion of the mandibular bone was due to slowly increase in the size of the central lesion
Figure 3 -.
Image of a extensive multilocular radiolucency from the lower left molar region to the right premolar region. The radiographic appearance resemble soap bubble patterns
Figure 4 -.
Computed axial tomography scan showing the lesion with thin residual bony trabeculae or septa dividing the lesion in various compartments and slight expansion of mandibular external cortical
An incisional biopsy was made and a histopathological examination of the tissue sample exhibited rounded, spindled, and stellate cells arranged in a loose, myxoid stroma with few collagen fibrils. These results confirmed the clinical hypothesis of odontogenic myxoma (Figure 5). Lesion excision was performed under general anesthesia, followed by vigorous curettage of the bone lodge and three 1 min liquid nitrogen sprayings with a defrosting interval between applications (Figure 6).
Figure 5 -.
Photomicrograph showing rounded, stellate and spindle-shaped mesenchymal cells arranged in abundant loose myxoid stroma with few collagen fibrils (hematoxylin-eosin, original magnification X100)
Figure 6 -.
Frozen bone after liquid nitrogen application
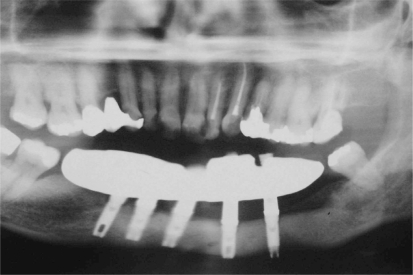
Postoperative complications included hypoesthesia in the mental region, suture dehiscence, and bone sequestra. A pathologic fracture occurred in this area 2 ½ months postoperatively (Figure 7). The sequestrated bone was removed 6 months after surgery, and the mandibular fracture was treated with local care (irrigation) and a diet consisting of soft foods. Five years after the surgical procedure, there were no radiographic or clinical signs of recurrence, and the patient’s ultimate rehabilitation was completed by the insertion of osteointegrated implants. Five titanium implants with bicortical anchors were placed in the mandible. After a four-month osteointegration period, an implant-supported denture was installed.
Figure 7 -.
Panoramic radiograph 10 weeks after the surgery. Image suggesting pathological fractures in the left molar region. Teeth within the lesional area were extracted
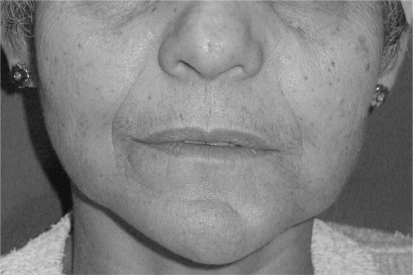
After 10 years of postoperative follow up, the patient is rehabilitated with no clinical or radiographic signs of lesion recurrence (Figures 8–10).
Figure 8 -.
Ten-year postoperative aspect showing preserved mandible contour and clear chin form without any enlargement. There were no sensitive or motor nerve impairment
Figure 10 -.
Panoramic radiograph taken after 10 years of follow-up showing absence of lesion recurrence
DISCUSSION
There are no clinical or radiological signs that would allow a physician to distinguish myxoma from odontogenic or non-odontogenic lesions; however, histological analysis shows several lesions that could be misinterpreted as myxoma. Differential diagnosis, like ameloblastoma, ameloblastic fibroma, or odontogenic fibroma, could be listed as initial diagnostic hypotheses based on the clinical and imaginological findings. Nevertheless, histologically, such lesions present a marked epithelial presence and a scarcely gelatinous texture.
Although odontogenic myxomas are always surgically managed, questions have been raised in regard to the type of treatment modality that should be applied to each case. It is important to emphasize that, as long as the patient is properly followed-up, an early-diagnosed recurrent lesion does not imply treatment failure; the lesion may be removed using a relatively simple procedure, without resorting to extensive mutilating surgical interventions that pose difficulties to the patient’s rehabilitation. A minimum of five years of surveillance is required to confirm that the lesion has healed, and periodical clinical and radiographic follow-up should be maintained indefinitely.12
The conservative treatment established for the case of recurrent mandibular myxoma reported in this paper was lesion excision with curettage and cryotherapy, which contrasts with the radical treatment of block resection that is advised by most authors.1,2,4,13–15 This is due to the invasive nature, large size, and recurrence history of this lesion. The conservative approach has several advantages over a more invasive treatment, which would consist of segmental mandibulectomy and mandibular reconstruction with a fibular microsurgical flap. The treatment performed represented a less morbid intervention, the possibility of intraoral access, the absence of a donor area, a shorter hospitalization time, and a lower procedural cost. In pediatric patients, this treatment modality has the additional advantage of not interfering with facial growth,16 while the functional and aesthetic results are less incapacitating.
The criterion used to make the decision between a radical treatment (block resection) and a conservative treatment (excision, curettage, and cryotherapy) involved the presence of the remaining basal mandibular bone, which provided a framework for mandibular re-structuring. Several authors have indicated block resection as the treatment of choice for extensive lesions17,18 or for those that have failed to heal after conservative management.18 Other authors advise resection with a safety margin for any case due to the high recurrence rate of these lesions.19
Bone freezing was used to eliminate any remaining neoplastic cells, without the need for extensive segmental resection.7–9 The reported complications in this case did not interfere with treatment success. Liquid nitrogen seems to provide local bone devitalization without affecting the inorganic structure, thereby yielding new bone formation.8 Therefore, before liquid nitrogen application, the tumoral mass should be excised and the surgical site should be carefully curetted.
The conservative management of myxomas by lesion excision and curettage with liquid nitrogen cryotherapy is an alternative to radical block resection. Although complications due to bone freezing may occur, they do not compromise the success of the treatment. The proposed therapy enhances patient rehabilitation by maintaining a satisfactory facial contour, thereby significantly minimizing the psychosocial impact to the patient. In addition, this treatment represents a less expensive intervention compared to more radical procedures.
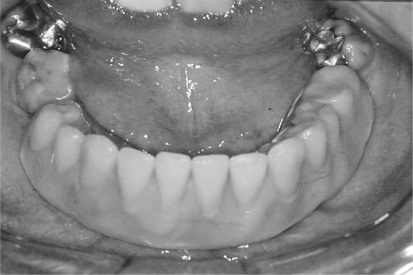
Figure 9 -.
An intraoral ten-years postoperative view showing an implant-supported denture over a health attached alveolar mucosa
REFERENCES
- 1.Dezotti MS, Azevedo LR, Fontao FN, Capelozza AL, Sant’ana E. Odontogenic myxoma–a case report and clinico-radiographic study of seven tumors. J Contemp Dent Pract. 2006:117–24. [PubMed] [Google Scholar]
- 2.Allphin AL, Manigilia AJ, Gregor RT, Sawyer R. Myxomas of the mandible and maxilla. Ear Nose Throat J. 1993;72:280–4. [PubMed] [Google Scholar]
- 3.Rocha AC, Cavalcante M, Araújo VC. Mixoma Odontogênico: Relato de caso com considerações clínicas, radiográficas e histopatológicas. Revista de Pós-Graduação da Faculdade de Odontologia da Universidade de São Paulo; São Paulo. 1996. pp. 246–9. [Google Scholar]
- 4.Spencer KR, Smith A. Odontogenic myxoma: case report with reconstructive considerations. Aust Dent J. 1998;43:209–12. doi: 10.1111/j.1834-7819.1998.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 5.Ogütcen-Toller M, Sener I, Kasap V, Cakir-Ozkan N. Maxillary myxoma: surgical treatment and reconstruction with buccal fat pad flap: a case report. J Contemp Dent Pract. 2006:107–16. [PubMed] [Google Scholar]
- 6.Chinellato LEM, Damante JH, Alvares LC. Mixoma odontogênico (fibromixoma) Rev Paul Odontol. 1987:2–8. [Google Scholar]
- 7.Bradley PF. Modern trends in cryosurgery of bone in the maxillo-facial region. Int J Oral Surg. 1978:405–15. doi: 10.1016/s0300-9785(78)80116-1. [DOI] [PubMed] [Google Scholar]
- 8.Pogrel MA. The use of liquid nitrogen cryotherapy in the management of locally aggressive bone lesions. J Oral Maxillofac Surg. 1993:269–73. doi: 10.1016/s0278-2391(10)80172-7. [DOI] [PubMed] [Google Scholar]
- 9.Curi MM, Dib LL, Pinto DS. Management of solid ameloblastoma of the jaws with liquid nitrogen spray cryosurgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997:339–44. doi: 10.1016/s1079-2104(97)90028-7. [DOI] [PubMed] [Google Scholar]
- 10.Bradley PF, Fisher AD. The cryosurgery of bone. An experimental and clinical assessment. Br J Oral Surg. 1975:111–27. doi: 10.1016/0007-117x(75)90001-3. [DOI] [PubMed] [Google Scholar]
- 11.Fisher AD, Williams DF, Bradley PF. The effect of cryosurgery on the strength of bone. Br J Oral Surg. 1978:215–22. doi: 10.1016/0007-117x(78)90003-3. [DOI] [PubMed] [Google Scholar]
- 12.Colombo CS, Boivin Y. Myxoma of the jaws. Oral Surg Oral Med Oral Pathol. 1966:431–6. doi: 10.1016/0030-4220(66)90399-9. [DOI] [PubMed] [Google Scholar]
- 13.Arienza B, Arienza F, Garzón JC, Hass E, Arana G. Mixoma de maxilar superior; caso clínico. Rev Assoc Odontol Argent. 1986:46–50. [PubMed] [Google Scholar]
- 14.Gonçales ES, Lorandi CS, Yurguel LS, Cardoso CFR. Mixoma odontogênico mandibular: relato de caso. Rev Inst Cienc Saude. 1999:139–43. [Google Scholar]
- 15.Andrews T, Kountakis SE, Maillard AA. Myxomas of the head and neck. Am J Otolaryngol. 2000:184–9. doi: 10.1016/s0196-0709(00)85022-x. [DOI] [PubMed] [Google Scholar]
- 16.Wachter BG, Steinberg MJ, Darrow DH, McGinn JD, Park AH. Odontogenic myxoma of the maxilla: a report of two pediatric cases. Int J Pediatr Otorhinolaryngol. 2003:389–93. doi: 10.1016/s0165-5876(02)00349-x. [DOI] [PubMed] [Google Scholar]
- 17.Fenton S, Slootweg PJ, Dunnebier EA, Mourits MP. Odontogenic myxoma in a 17- month-old child: a case report. J Oral Maxillofac Surg. 2003:734–6. doi: 10.1053/joms.2003.50121. [DOI] [PubMed] [Google Scholar]
- 18.Harder F. Myxomas of the jaws. Int J Oral Surg. 1978:148–55. doi: 10.1016/s0300-9785(78)80017-9. [DOI] [PubMed] [Google Scholar]
- 19.Simon EN, Merkx MA, Vuhahula E, Ngassapa D, Stoelinga PJ. Odontogenic myxoma: a clinicopathological study of 33 cases. Int J Oral Maxillofac Surg. 2004:333–7. doi: 10.1016/j.ijom.2003.12.004. [DOI] [PubMed] [Google Scholar]