Abstract
Insulin-like growth factors (IGFs) control cell and organism growth through evolutionarily conserved signaling pathways. The mammalian acid-labile subunit (ALS) is a secreted protein that complexes with IGFs to modulate their activity. Recent work has shown that a Drosophila homolog of ALS, dALS, can also complex with and modulate the activity of a Drosophila IGF. Here we report the first mutations in the gene encoding dALS. Unexpectedly, we find that these mutations are allelic to a previously described mutation in convoluted (conv), a gene required for epithelial morphogenesis. In conv mutants, the tubes of the Drosophila tracheal system become abnormally elongated without altering tracheal cell number. conv null mutations cause larval lethality, but do not disrupt several processes required for tracheal tube size control, including septate junction formation, deposition of a lumenal/apical extracellular matrix, and lumenal secretion of Vermiform and Serpentine, two putative matrix-modifying proteins. Clearance of lumenal matrix and subcellular localization of clathrin also appear normal in conv mutants. However, we show that Conv/dALS is required for the dynamic organization of the transient lumenal matrix and normal structure of the cuticle that lines the tracheal lumen. These and other data suggest that the Conv/dALS-dependent tube size control mechanism is distinct from other known processes involved in tracheal tube size regulation. Moreover, we present evidence indicating that Conv/dALS has a novel, IGF-signaling independent function in tracheal morphogenesis.
INSULIN and insulin-like growth factors (IGFs) control energy homeostasis and growth through evolutionarily conserved signaling pathways (reviewed by Pavelic et al. 2007). Key regulators of these pathways are IGF binding proteins (IGFBPs) that modulate IGF activity, transport, and stability. The mammalian acid-labile subunit (ALS) forms ternary complexes with IGFs and IGFBP-3 or IGFBP-5 (reviewed by Boisclair et al. 2001). It has recently been shown that a Drosophila homolog of ALS, dALS, forms a ternary complex with Drosophila insulin-like peptide-2 (Dilp2) and the binding protein IMP-L2 (Arquier et al. 2008). Surprisingly, we find that the gene encoding dALS is allelic to convoluted (conv), a gene previously shown to be required for regulating the length of epithelial tubes in the Drosophila tracheal system (Beitel and Krasnow 2000).
The Drosophila tracheal system is a ramifying network of epithelial tubes that delivers oxygen directly to target tissues (reviewed by Kerman et al. 2006). The tracheal system is among the best characterized systems for investigating branching morphogenesis and for control of epithelial tube size, an essential feature of many vital organs such as lung and kidney. The dimensions of tracheal tubes are regulated by at least two distinct mechanisms, one of which involves a transient lumenal extracellular matrix (ECM) (Tonning et al. 2005) and the putative matrix-modifying proteins Vermiform (Verm) and Serpentine (Serp) (Luschnig et al. 2006; Wang et al. 2006). Importantly, lumenal secretion of Verm requires the septate junction (SJ), which restricts paracellular diffusion similar to the vertebrate tight junction, but is located in the basolateral membrane and contains polarity proteins that promote basal membrane identity (Wu and Beitel 2004; reviewed in Swanson and Beitel 2006). Mutations that disrupt the matrix or Verm secretion cause individual tracheal cells, and thus the overall tubes, to become too long (Araujo et al. 2005; Moussian et al. 2005, 2006; Luschnig et al. 2006; Tonning et al. 2006; Wang et al. 2006). It has not been established whether the ECM provides a signal to the epithelial cells that causes them to adjust their dimensions or whether the ECM serves as a mandrel that shapes the tubes by physical forces. However, in addition to the matrix-based size control mechanism, there is evidence that growth factor signaling constitutes a second mechanism that controls tracheal tube size because mutation of chico, which encodes the Drosophila homolog of the mammalian insulin receptor substrates 1–4, shortens tracheal tube length to match the reduced body size of the chico mutant larva (Beitel and Krasnow 2000).
Our analysis of the conv/dALS locus reveals that Conv activity defines a new step in the matrix-based size control process. Further, although Conv/dALS could potentially act through the insulin growth factor pathway to regulate tube size, our results suggest that the tracheal matrix organization function of Conv/dALS represents a distinct function from the IGF pathway function.
MATERIALS AND METHODS
Fly strains and genetics:
Fly strains were obtained from the Bloomington or Szeged stock centers except for convR278, convY58, and the ppl-Gal4 and cg-Gal4 fat body drivers. convR278 was created by EMS mutagenesis of al1 dpov1 b1 pr1 c1 px1 sp1 followed by backcrossing to remove the markers. The convR278 genomic sequence is CACCATCGATGTCTTGCACAATAACATCTCC (C → T change is underlined). convY58 was created by mobilization of transposable element insertion SelDSH1599. The primers 5′-GCCTAGTAGTCCGAATCCAGTA and 5′-TGGCCCATATAGACGAACTAG were used to identify convY58, which templates a 103-bp band, while the wild-type (WT) chromosome produces a 2.3-kb band. The sequence across the convY58 deletion endpoints is 5′-CCAGTAATATCGGTATGCCCAT. Fat body drivers ppl-Gal4 and cg-Gal4 were a gift from P. Léopold.
Molecular biology:
RNAi was performed as previously described (Kennerdell and Carthew 1998), using primers 5′-AAACGGGAAATTCTTTTTTCAG and 5′-AATTTTATATTAGCAATTGTAC for CG8561. A 6-kb fragment for CG8561 genomic rescue construct was amplified from pBACe3.6 (BACPAC resources), using primers 5′-CTGGATCCGAGCGAGATGTCACAACAGGGTTGTATTA and 5′-ATGCGGCCGCTGAATGCTGATTTATGCCATTCGCCAC, and cloned into pCasper5 (Le et al. 2007). The genomic rescue construct with the convR278 mutation was created by Quickchange (Stratagene, La Jolla, CA) of the WT construct and subcloning into pUASTattB (Bischof et al. 2007). The WT conv cDNA was amplified by RT–PCR from OregonR RNA, using primers 5′-GCGAACTTGGTTTTGGGCCATGGGATAA and 5′-TCTTGGTGCACAGTTTTCCCATGC, and cloned into pBluescriptC5 (Le et al. 2007) and then into pUASTattB. The 3.3-kb fragment was moved into the SacII site of the pAC5-YFP plasmid in frame with the C-terminal YFP tag and the tagged construct was cloned into the pUASTattB vector. Transgenic flies were made either using P transposase (Rubin and Spradling 1982; Spradling and Rubin 1982) or the φC31 integration systems (Bischof et al. 2007). The complete sequence of all constructs was determined using BigDye sequencing (Applied Biosystems, Foster City, CA). The accession number for conv cDNA is EU814872.
Immunohistochemistry and imaging:
Embryos were fixed and stained as previously described (Samakovlis et al. 1996). For clathrin stains, embryos were fixed in 4% paraformaldehyde and devitellinized in 80% ethanol to avoid the use of methanol. For assessment of lumenal clearance, WT and convR278 mutant embryos were collected in 1-hr lots and aged at 24°. Stage 16 embryos were predominantly found in the lot aged for 15 hr after collection, stage early 17 embryos were in the 16-hr lot, and stage mid-17 embryos were in the 17-hr lot. Primary antibodies used were anti-lumenal 2A12 1:5 (DSHB), anti-Coracle 1:500 (Fehon et al. 1994), anti-GFP 1:1000 (Abcam), rat anti-DE-cadherin DECAD2 1:20 (Oda et al. 1994), anti-Verm and Serp 1:300 (Luschnig et al. 2006), FITC-conjugated anti-Chitin binding probe 1:500 (New England Biolabs, Beverly, MA), and anti-clathrin 1:100 (Sigma, St. Louis). Secondary antibodies were used at 1:200 (Jackson Laboratories; Molecular Probes, Eugene, OR). To assess protein levels, heterozygous and homozygous embryos were imaged on the same slide in the same session. Electron micrograph (EM) images were obtained as in Wu et al. (2004). Paracellular barrier function was assessed using a 10-kDa dextran dye as described in Paul et al. (2003). None of nine convY58/Df(2R)7131 embryos showed dye leakage.
Sequence analysis and biochemistry:
Sequence alignment was done using ClustalW (Figure 2). Protein domains were predicted using SMART (Letunic et al. 2004). Western blotting used btl∷Gal4 UAS-Conv-YFP 0- to 20-hr embryos or Escherichia coli expressing GFP homogenized in lysis buffer (Genova and Fehon 2003) and probed with rabbit polyclonal anti-GFP (Abcam) at 1:10,000. WT is OregonR.
Figure 2.—
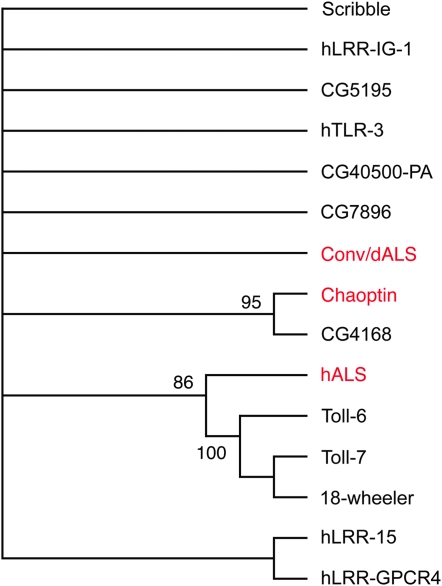
Convoluted is not closely related to other LRR proteins. A phylogenetic tree shows the relationship between Convoluted/dALS and other human and Drosophila LRR proteins as calculated using a “bootstrap” procedure that more robustly determines relationships of divergent proteins than do typical single-iteration approaches. When tested with the set of closest human (proteins starting with “h”) and Drosophila homologs of Convoluted identified by BLAST searches, Convoluted/dALS did not reproducibly show close relationships to any of the LRR proteins in 1000 samplings of the data set. Drosophila Scribbled was used as an outgroup for rooting the trees. Proteins were aligned using the ClustalW and bootstrap algorithms implemented by the MacVector 7 program (MacVector, Cary, NC).
RESULTS AND DISCUSSION
CG8561 encodes convoluted:
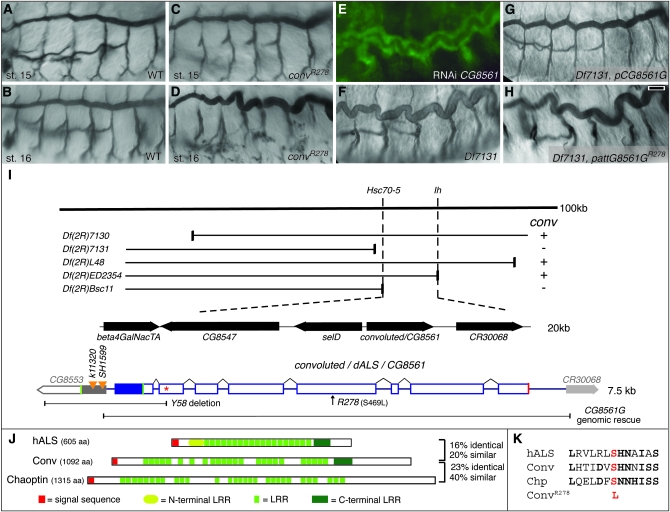
Although the original convK6507b allele was isolated from a screen of transposon-induced mutations, it is not associated with a transposable element (Beitel and Krasnow 2000). To obtain additional alleles, we performed a noncomplementation screen and isolated convR278, which fails to complement convK6507b and causes the same overly long tracheal phenotype as convK6507b (Figure 1D).
Figure 1.—
Convoluted/dALS is an LRR protein required for tracheal tube morphogenesis. (A–D) The trachea in conv mutant embryos appear normal through stage 15 (C), but by stage 16 become overly long and “convoluted” (D). (E) RNAi knockdown of CG8561 causes the conv phenotype. (F–H) Embryos homozygous for Df(2R)7131 show the conv phenotype (F), which can be rescued by a genomic construct containing CG8561 (G) but not by the same construct bearing the convR278 point mutation (H). (I) Schematics of the CG8561 genomic region and transcript organization. Complementation tests between genetic deficiencies and convK6507b and convR278 located conv between Hsc70-5 and Ih. Sequence analysis and RNAi (E) identified CG8561 as conv. The mutations for convR278 and convY58 are indicated, as is the fragment used for the genomic rescue construct and the location of the stop codon that eliminates rescue ability of the construct (red asterisk). No base changes in the 6-kb rescue fragment were found in the convK6507b allele. Green boxes, start codons; red box, stop codon; open bars, open reading frame; solid bars, noncoding transcribed regions; orange triangles, P-element insertions in SelD. (J) The domain organization of Conv and its similarity to human ALS (hALS) and the Drosophila adhesion protein Chaoptin are shown. The percentage of similarity and identity is relative to Conv. (K) ClustalW alignments of Conv, hALS, and Chaoptin in the region of the convR278 mutation. Residues in common with Conv are in boldface type, and the convR278 mutation is in red. Trachea were visualized with btl∷Gal4 UAS-GFP in E and with mAb 2A12 in A–D and F–H. Bar in H (for A–H), 10 μm.
To clone conv, we used recombination mapping and deficiency complementation to define a region of ∼20 genes containing both convK6507b and convR278 (Figure 1I, supplemental Table S1). Sequencing and dsRNAi knockdown of all predicted genes in this interval revealed that CG8561 was the only gene that had a codon-altering base change in the convR278 strain and whose knockdown by dsRNAi injection caused a “long trachea” phenotype (Figure 1, E and K). To confirm that CG8561 encodes conv, we created transgenic flies bearing a 6-kb genomic fragment that entirely contained CG8561 but overlapped the RNAs of adjacent genes by <29 bp. This genomic fragment rescued the tracheal defects (24/24) and lethality caused by the convR278 and convk6507b mutations, as well as the tracheal defects caused by the small deficiency Df(2R)7131 that deletes CG8561 (Figure 1G and data not shown). Importantly, the rescue ability of this genomic fragment was eliminated by introduction of base changes that either correspond to the convR278 mutation or create a premature stop codon in the middle of the third exon (Figure 1H and data not shown). Together with the ability of a CG8561 cDNA to rescue tracheal defects (see below), these results demonstrate that CG8561 encodes conv.
Convoluted is a leucine-rich repeat protein with similarity to mammalian ALS and Drosophila adhesion and signaling proteins:
conv has very low embryonic expression levels, and no conv cDNAs were present in the publicly available cDNA libraries. We therefore used RT–PCR to assemble a complete cDNA and to confirm the predicted gene structure. The full-length cDNA is 3.3 kb long and has an open reading frame (ORF) of 1092 amino acids (aa) preceded by in-frame stop codons (Figure 1I and materials and methods). No evidence for alternative splicing was observed.
The Conv protein has a strongly predicted signal sequence at the N terminus and multiple leucine-rich repeats (LRR) that are commonly involved in protein–protein interactions. A BLASTP search revealed that the closest human homolog of Conv is ALS of the insulin growth factor binding complex (Boisclair et al. 2001). In strong support of Conv having functional as well as sequence similarity to human ALS, recent work by Arquier et al. (2008) has demonstrated that Conv/dALS can bind and antagonize Dilp2 and that altering Conv/dALS levels can affect metabolism. As we have previously shown that larval tracheal length is reduced in chico mutants, which have reduced insulin-like growth factor signaling (Beitel and Krasnow 2000), the increased length of trachea in conv mutants is consistent with conv functioning to regulate tracheal tube length through insulin-like peptide signaling. Furthermore, the R278 mutation is located in a region of significant similarity between ALS and Conv/dALS and could potentially disrupt Conv/dALS insulin binding functions (Figure 1K).
Alternatively, although ALS is the closest human homolog of Conv/dALS, there are substantial differences between the two proteins that have not previously been noted (Figure 1J). ALS is 605 aa long, has 19 tightly packed typical LRR domains, and has both N- and C-terminal class LRR domains. In contrast, Conv is 1092 aa long, has 23 somewhat dispersed LRR domains, and has a C- but not N-terminal class LRR domain. BLASTP searches of the Drosophila proteome using Conv/dALS reveal that Conv/dALS and human ALS (BLASTP score 181) have similarity that is less than or comparable to Conv/dALS and other Drosophila LRR proteins, including the cell adhesion protein Chaoptin (Reinke et al. 1988; Krantz and Zipursky 1990) (score 199), the Toll-7 and Toll-6 receptors (scores 181 and 179), and the 18-wheeler transmembrane protein (score 171) that controls salivary gland cell shape (Tauszig et al. 2000; Kolesnikov and Beckendorf 2007). Similarly, ClustalW alignment and Bootstrap tree analysis indicates that Conv/dALS is as related to Chaoptin and other LRR proteins as it is to ALS (Figure 2). Thus, while strong evidence supports Conv/dALS being the functional homolog of human ALS in IGF signaling, the divergence of human ALS and Conv/dALS is also consistent with Conv/dALS having an unanticipated IGF-independent function in tracheal morphogenesis.
convoluted is an essential gene:
Because Conv/dALS might be a multifunctional protein and it was unclear whether convK6507b and convR278 were null alleles, we created a conv null allele with which to definitively characterize Conv/dALS functions. Imprecise excision of the P-element SelDSH1599 created the small deficiency Df(2R)convY58 that deletes the entire intergenic region 5′ of conv as well as the first exon and a half of conv that include the transcriptional start site (Figure 1I, materials and methods). No conv transcript is detected by RT–PCR in convY58 homozygotes. Although convY58 also disrupts the adjacent gene SelD, SelD does not have a role in tracheal development because no tracheal defects are apparent in SelDK11320, SelDSH1599, or nine new excision alleles that disrupt SelD but complement convR278 (data not shown). More definitively, the 6-kb conv genomic fragment that does not include any SelD ORF completely rescues the tracheal defects of convY58 and Df(2R)7131 homozygous embryos (Figure 1, G and I). We therefore consider convY58 a molecular null allele of conv.
Embryonic morphogenesis appears normal in both convR278 and convY58, with the notable exception of tracheal tube size-control defects (see below). Conv/dALS function is largely or entirely dispensable for neural and muscle function as larvae homozygous for convR278 or convY58 hatch and begin crawling. However, neither these homozygous larvae nor larvae trans-heterozygous for convR278/convY58 survive past the second larval stage, which is presumably due to the 100% penetrant failure of conv mutant trachea to fill with air and thereby provide oxygen to target tissues (Figure 3, B and D). Thus conv/dALS is an essential gene under normal conditions, which is in contrast to the viable null phenotype of Imp-L2, the insulin-binding protein required for Conv/dALS to bind Dilp2-containing complexes, and in contrast to the viable null phenotype of chico, the fly homolog of human insulin receptor substrates 1–4 (Bohni et al. 1999; Honegger et al. 2008).
Figure 3.—
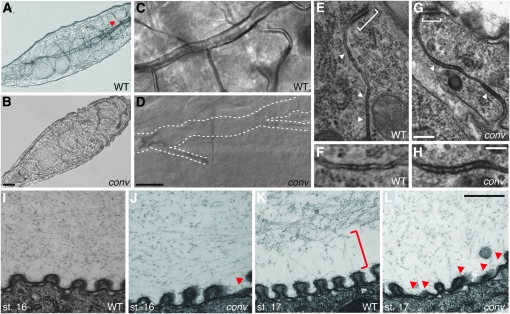
Convoluted is required for extracellular matrix organization and for gas filling, but not for septate junction ultrastructure. (A–D) Both WT (A and C) and conv (B and D) larvae hatch and move, but most regions of the trachea in conv mutants are not filled with air and are poorly visualized (trachea in A indicated with red arrowhead and in D with dotted lines). (E–H) Adherens junctions (brackets) and septate junctions (arrowheads in E and G; higher magnification in F and H) appear normal in EM images of stage 17 embryos. (I–L) EM images show that at stage 16 the lumenal matrix is more dense in WT (I) than in conv embryos (J) and that some taenidial ridges (red arrowheads) are abnormal in conv mutants. By stage 17, conv mutants lack the normal gap (red bracket in K) between the matrix and the lumen wall and the taenidial defects are more pronounced. Genotypes: conv is convR278/convY58 in B and D and convR278/Df(2R)7131 in G, H, J, and L. Bars: B (for A and B), 25 μm; D (for C and D), 10 μm; G (for E and G), 200 nm; H (for F and H), 100 nm; and L (for I–L), 500 nm.
Convoluted is required for tracheal tube-size control, but not for SJ assembly, chitin matrix deposition, or chitin deacetylase secretion:
The trachea of embryos homozygous or trans-heterozyogous for conv mutations becomes abnormally long during stage 16 (Figure 1D). The onset and severity of the phenotypes caused by convR278, convY58, Df(2R)7131, or convR278 in trans to convY58 or Df(2R)7131 are indistinguishable, suggesting that convR278 is null for the tracheal functions of conv.
To understand the role of Conv/dALS in tracheal tube-size control, we asked if conv mutations affect known mechanisms of tube-size control (reviewed in Swanson and Beitel 2006). Localization of apical polarity and SJ markers appears normal in conv null mutants, as is the ultrastructure of intercellular septa that form paracellular diffusion barriers (Figures 3, G and H, and 4E and data not shown). Consistent with this, conv mutants have no paracellular barrier defects as evidenced by wild-type-like impermeability of trachea and salivary glands in convY58/Df(2R)7131 embryos to a 10-kDa dextran dye (data not shown). Chitin biosynthesis is not affected, as chitin accumulates normally in the tracheal lumen (Figure 4F), as do the putative chitin deacetylases Verm and Serp (Figure 4, G and H). Together, these results indicate that Conv/dALS is not required for the known tracheal tube-size control mechanisms that operate before stage 16.
Figure 4.—
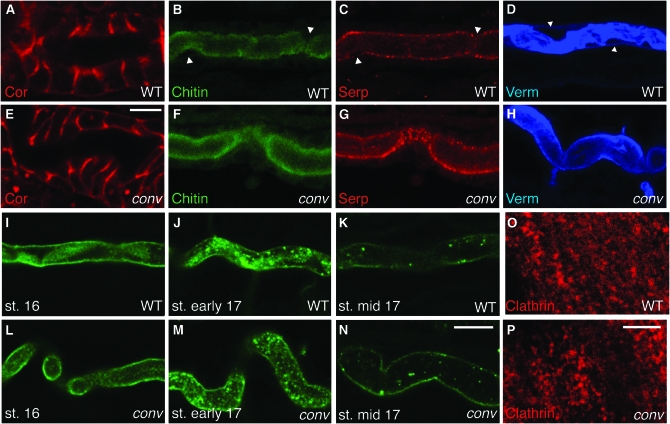
Convoluted is not required for junction formation or secretion. (A and B) Localization of the canonical SJ marker Coracle is normal in conv mutants. (B–H) Lumenal matrix components chitin, Serp, and Verm are present at similar levels in stage 17 WT (B–D) and conv (F–H) embryos, but the gaps that normally appear between the lumenal walls and the matrix (white arrowheads) are missing in conv mutants. (I–N) Clearance of the lumenal antigen 2A12 proceeds equivalently in WT (I–K) and conv (L–N) embryos. (O and P) Clathrin shows diffuse cytoplasmic staining in the epidermis of both WT (O) and conv (P) embryos. Clathrin staining in the trachea was also cytoplasmic in both WT and conv embryos (data not shown). Genotypes: conv is convR278/convY58 in E–H and is convR278/convR278 in I–P. WT, OregonR; st., stage (see materials and methods). Bars: E (for A and E), 5 μm; N (for B–N), 10 μm; and P (for O and P), 5 μm.
Convoluted is required for tracheal lumenal and apical extracellular matrix organization:
The defect common to most currently identified mutations affecting tracheal tube-size control is that they disrupt organization of the tracheal apical and/or lumenal extracellular matrix (reviewed in Swanson and Beitel 2006). In conv mutants, EMs show that at stage 16, the lumenal matrix appears to be less dense and somewhat grainier than in WT (Figure 3, I and J). By stage 17 the lumenal matrix in conv mutants appears even more sparse (Figure 3, K and L), and while WT embryos create a gap between the lumenal matrix and the tracheal tube surface (Figure 3K, bracket), in conv mutants the matrix still extends to the tracheal surface (Figure 3L). The failure of conv mutants to create a gap is also detectable by immunohistochemical staining of Verm. In stage 17 WT embryos, gaps are visible between the apical surface and the Verm-stained lumenal matrix (Figure 4D, spaces adjacent to arrowheads that mark the apical surface of the tracheal cell), while in conv mutants Verm continues to occupy the entire lumenal space and is not organized into fibrils (Figure 4H).
In addition to lumenal matrix defects, conv mutants have grossly abnormal morphogenesis of the apical matrix (cuticle) that lines the tracheal surface. In conv mutants, the taenidia, which normally are stereotyped periodic ridges in the highly organized apical matrix, are frequently misshapen and flattened (Figure 3, I–L). Thus, Conv/dALS is required for normal organization and modulation of apical and lumenal matrices that control tracheal tube size. This result is unexpected because neither human ALS nor components of the Drosophila IGF pathway have been observed to be required for apical extracellular matrix organization (Bohni et al. 1999; Honegger et al. 2008) and we have not observed aberrant embryonic tracheal morphologies in either chico or Imp-L2 mutants (Beitel and Krasnow 2000 and data not shown).
Convoluted defines a new step in the matrix-based tracheal tube-size control process:
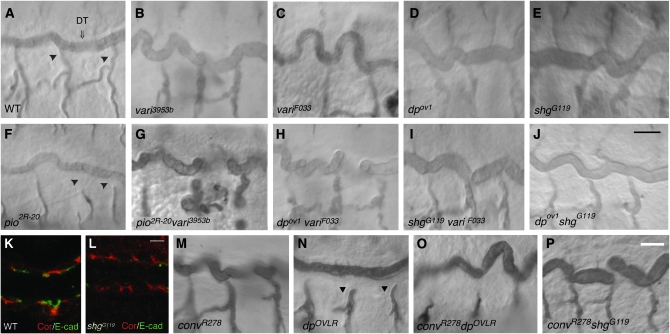
As mutations in genes encoding SJ proteins and conv both cause lumenal matrix defects, we performed additional genetic tests to determine whether there were differences between the effects of conv and SJ mutations on the extracellular matrix. We examined the genetic interactions of varicose (vari), which encodes an adaptor protein critical for SJ formation (Wu et al. 2007), and of conv with piopio (pio) and dumpy (dp). Pio and Dp are extracellular matrix proteins deposited in the tracheal lumen that contain ZP domains (Wilkin et al. 2000; Jazwinska et al. 2003; Bokel et al. 2005). ZP domains are named after proteins that form the zona pelucida, a gel-like substance surrounding mammalian oocytes (Jovine et al. 2005). Intriguingly, ZP domains can polymerize to form strands (Jovine et al. 2002), which in the trachea could potentially play a role parallel to that of the chitin-based fibrillar matrix. Alternatively, Pio and Dp are transmembrane proteins and thus could act as mediators of chitin-fibril-based signaling or scaffolding. Pio and Dp are required for the cell intercalation that produces unicellular tracheal branches (Jazwinska et al. 2003; Ribeiro et al. 2004). In strong pio and dp mutants intercalation fails to stop and unicellular tracheal branches become disconnected (Figure 5, F and N). As the multicellular tracheal tubes in pio and dp mutants have normal length and diameter, these ZP proteins were not thought to have a role in tracheal tube-size control. However, we find that both a strong mutation in pio, pio2R-20, and a viable mutation in dp that does not cause branch breaks, dpov1 (Figure 5D), significantly enhanced the tracheal tube elongation defects of both weak and strong mutations in vari (Figure 5, G and H). This enhancement was specific because a lethal mutation affecting DE-cadherin, shgG119, that reduces DE-cadherin levels by >50% (Figure 5L) and causes sporadic tracheal branch breaks, did not enhance the vari, dp, or pio mutant phenotypes (Figure 5, I and J, and data not shown).
Figure 5.—
Mutations in the ZP proteins Pio and Dp enhance Vari but not Conv/dALS mutations. (A–H) Whereas in vari (B and C) and conv (M) mutant embryos the tracheal dorsal trunk (DT) is long and tortuous compared to wild type (WT) (A), pio (F) and dp mutants have straight trachea (C and N). Strong pio and dp mutants have disconnected tracheal branches (arrowheads in F and N). The tracheal length defects caused by a strong vari mutation (C) are enhanced (I) by a weak mutation in dp that does not by itself cause length increases or branch breaks (D). The trachea length defects caused by weak mutation in vari (B) are also enhanced (G) by a pio mutation (F). (I–L) The specificity of the interactions of vari with dp and pio is demonstrated by the failure of the viable shgG119 allele (E) to enhance the tracheal length defects of a null vari allele (I) despite the fact that shgG119 homozygotes have significantly reduced tracheal E-cadherin staining (K and L) and have sporadic tracheal branch breaks (not shown). (M–P) Neither a strong mutation in dp (N) nor the weak DE-cadherin mutation shgG119 (E) enhance the tracheal length defects caused by a strong conv mutant (O and P). Bars: J (for A–J), 10 μm; L (for K and L), 2.5 μm; and P (for M–P), 10 μm.
In contrast to the enhancement of the vari length defects by dp and pio, double-mutant combinations of a strong conv allele and dp or pio did not have increased tracheal length (Figure 5, O and P), even when the dp conv double mutant was constructed with a stronger allele of dp, dpOVLR, that causes a fully penetrant branch-break phenotype (Figure 5, N and P). Thus, although the exact role of pio and dp in tracheal length control remains to be determined, these results provide genetic evidence that conv has distinct effects from a SJ mutant on lumen matrix and size control. This possibility is further supported by double-mutant combinations of conv and vari and of conv and coracle showing enhanced tracheal length defects (data not shown). Thus, the interactions of conv mutations with mutations in ZP and SJ genes indicate that Conv has a distinct role from SJ proteins controlling tracheal tube size.
Mutations in conv cause tracheal tube-length and matrix defects similar to those caused by mutations in the wurst locus, which encodes a J-domain transmembrane protein that is required for clathrin-mediated endocytosis of lumenal material (Behr et al. 2007). However, in contrast to wurst mutants, lumenal clearance of the 2A12 marker (Figure 4, I–N) and Verm (data not shown) in the conv mutant was the same as in wild type. Similarly, in conv mutants, clathrin had a dispersed cytoplasmic localization in epidermal and tracheal cells that was indistinguishable from that of wild-type embryos (Figure 4, O and P, and data not shown) and markedly different from the striking membrane localization observed in wurst mutant epidermis (Behr et al. 2007). Therefore, Conv/dALS is not required for lumenal clearance and does not appear to regulate endocytosis. Taken together, our data suggest that in the temporal sequence of events, Conv/dALS acts after SJ proteins but before Wurst in controlling tracheal tube size and defines a new step in this process.
Expression of Convoluted in the trachea but not the fat body rescues epithelial morphogenesis defects:
If Conv/dALS functions as a matrix-organizing or cell-adhesion protein, one would expect it to be localized to the tracheal apical cell surface or lumen. Unfortunately, our attempts to raise antibodies to Conv/dALS protein have been unsuccessful. We therefore attempted to determine the subcellular localization of Conv/dALS by expressing a YFP-tagged protein in the tracheal system using the ubiquitous da-Gal4 that efficiently expresses and rescues many tracheal genes (Wodarz et al. 1995; Wu et al. 2004, 2007; Paul et al. 2007). When expressed with da-Gal4, Conv∷YFP showed little accumulation in the tracheal system and its subcellular localization could not be reliably determined in tracheal cells by YFP fluorescence or by anti-YFP immunofluorescent staining (data not shown). Interestingly, although little Conv∷YFP was evident in the trachea, da-Gal4 driving Conv∷YFP almost completely rescued embryonic tracheal morphological defects (90%, n = 20; no rescue of adult viability, n > 100), suggesting either that very little Conv/dALS is required in the embryonic tracheal system or that Conv/dALS does not act in the embryonic tracheal system.
To more directly address whether Conv/dALS acts in the tracheal system, we expressed Conv/dALS and Conv∷YFP using the btl-Gal4 driver that expresses only in the tracheal system and in some glia in the central nervous system (Shiga et al. 1996). With this driver, Conv∷YFP localized to the tracheal cytoplasm, suggesting that it was inefficiently trafficked to the cell surface (Figure 6B). Similar results were obtained by Arquier et al. (2008) with a myc∷dALS construct expressed in the fat body and in cultured S2 cells. For the Conv∷YFP fusion, the cytoplasmic localization was not an artifact of cleavage of the C-terminal YFP tag as Western blots showed that almost all detectable GFP immunoreactivity was in a high molecular weight band that corresponds to the correct size of the Conv∷YFP fusion protein (Figure 6C). There was no obvious accumulation of tagged protein in the tracheal lumen, apical surfaces, or basal surfaces.
Figure 6.—
Expression of Convoluted/dALS in the trachea rescues tracheal defects. (A and B) Specific expression of YFP-tagged Conv in the trachea can rescue conv mutants (note straight trachea in B), although anti-GFP staining shows a predominantly cytoplasmic localization (B). (C) Western blotting using anti-GFP shows that the immunoreactivity is Conv-YFP fusion protein and not just cleaved tag. Full-length Conv-YFP and YFP are predicted to be 149 and 29 kDa, respectively. Molecular weight markers are indicated. (D and E) Expression of untagged Conv/dALS in the tracheal system also rescues the tracheal defects of convY58 homozygotes (E). (F–H) Expression of untagged (F and H) or tagged (G) Conv in the fat body using the ppl-Gal4 (F and G) or cg-Gal4 drivers does not rescue the tracheal defects of the convY58 homozygotes. The genotype in B is btl∷Gal4, convR278/UAS-Conv-YFP, convY58; that in E is btl∷Gal4, convR278/UAS-Conv, convY58; and that in G is ppl∷Gal4, convR278/UAS-Conv-YFP, convR278. Bars: B (for A and B), 5 μm; and H (for D–H), 10 μm.
Despite poor trafficking of Conv∷YFP in the tracheal system, both tagged- and untagged-expression constructs fairly efficiently rescued embryonic tracheal defects in conv mutants (66%, n = 18, and 80%, n = 25, respectively; Figure 6, B and E), but did not rescue the viability defects. As this rescue was achieved using the btl-Gal4 driver, this result is consistent with Conv/dALS acting as a cell-adhesion or matrix-organizing factor in the tracheal lumen. However, this result is also consistent with local rescue of IGF pathway function since Honegger et al. (2008) have shown that ectopic expression of Imp-L2 in imaginal eye cell clones can locally reduce ommatidia size. We therefore expressed Conv/dALS in the fat body using the ppl-Gal4 driver (Colombani et al. 2003). Although this driver was successfully used in combination with Conv/dALS constructs by Arquier et al. (2008) to alter IGF signaling pathway functions, no rescue of the tracheal defects was observed using either the untagged or the tagged constructs (Figure 6, F and G). A similar lack of rescue was observed using the cg-Gal4 driver (Hennig et al. 2006), which expresses in the fat body as well as in hemocytes (Figure 6H). These results suggest that Conv/dALS acts autonomously in the tracheal system.
Concluding remarks:
Drosophila Conv/dALS has been characterized as an important player in the IGF signaling pathway where it forms a ternary complex containing Imp-L2 (Arquier et al. 2008). Here we report the first characterization of mutations in the gene encoding Drosophila ALS and find that in contrast to Imp-L2, conv/dALS is an essential gene. Surprisingly, Conv/dALS has an important role in tracheal epithelial morphogenesis, where it limits tube elongation. Although these results raise the fascinating possibility that Conv/dALS could act by dampening IGF signaling to prevent abnormal tracheal growth, the observations that Imp-L2 is not required for tracheal morphogenesis and that Conv/dALS appears to act autonomously in the tracheal system suggest that Conv/dALS has a tracheal-matrix organizing function that is distinct from its IGF-binding function. The exact role of Conv/dALS in matrix organization is unclear, but the apparently low level of embryonic Conv expression suggests that Conv/dALS acts as an important regulator rather than a structural component of the lumenal extracellular matrix.
Acknowledgments
We thank S. Paul for performing paracellular diffusion assays; V. Wu for DE-cadherin confocal microscopy; R. Carroll and H. Hong for technical assistance; K. Basler, R. Fehon, E. Hafen, S. Luschnig, M. Krasnow and P. Léopold for reagents; and I. T. Helenius and T. Krupinski for comments on the manuscript. This work was supported by a postdoctoral fellowship from the Canadian Institutes of Health Research (CIHR) (to P.L.) and a predoctoral fellowship from the National Institutes of Health (NIH) Cell and Molecular Basis of Disease training grant (T32 GM008061 to K.S.N.). Operating support was provided by the CIHR (to U.T.) and the NIH (R01 GM069540 to G.J.B.).
References
- Araujo, S. J., H. Aslam, G. Tear and J. Casanova, 2005. mummy/cystic encodes an enzyme required for chitin and glycan synthesis, involved in trachea, embryonic cuticle and CNS development—analysis of its role in Drosophila tracheal morphogenesis. Dev. Biol. 288 179–193. [DOI] [PubMed] [Google Scholar]
- Arquier, N., C. Geminard, M. Bourouis, G. Jarretou, B. Honegger et al., 2008. Drosophila ALS regulates growth and metabolism through functional interaction with insulin-like peptides. Cell Metab. 7 333–338. [DOI] [PubMed] [Google Scholar]
- Behr, M., C. Wingen, C. Wolf, R. Schuh and M. Hoch, 2007. Wurst is essential for airway clearance and respiratory-tube size control. Nat. Cell Biol. 9 847–853. [DOI] [PubMed] [Google Scholar]
- Beitel, G. J., and M. A. Krasnow, 2000. Genetic control of epithelial tube size in the Drosophila tracheal system. Development 127 3271–3282. [DOI] [PubMed] [Google Scholar]
- Bischof, J., R. K. Maeda, M. Hediger, F. Karch and K. Basler, 2007. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 104 3312–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohni, R., J. Riesgo-Escovar, S. Oldham, W. Brogiolo, H. Stocker et al., 1999. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1–4. Cell 97 865–875. [DOI] [PubMed] [Google Scholar]
- Boisclair, Y. R., R. P. Rhoads, I. Ueki, J. Wang and G. T. Ooi, 2001. The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: an important but forgotten component of the circulating IGF system. J. Endocrinol. 170 63–70. [DOI] [PubMed] [Google Scholar]
- Bokel, C., A. Prokop and N. H. Brown, 2005. Papillote and Piopio: Drosophila ZP-domain proteins required for cell adhesion to the apical extracellular matrix and microtubule organization. J. Cell Sci. 118 633–642. [DOI] [PubMed] [Google Scholar]
- Colombani, J., S. Raisin, S. Pantalacci, T. Radimerski, J. Montagne et al., 2003. A nutrient sensor mechanism controls Drosophila growth. Cell 114 739–749. [DOI] [PubMed] [Google Scholar]
- Fehon, R. G., I. A. Dawson and S. Artavanis-Tsakonas, 1994. A Drosophila homologue of membrane-skeleton protein 4.1 is associated with septate junctions and is encoded by the coracle gene. Development 120 545–557. [DOI] [PubMed] [Google Scholar]
- Genova, J. L., and R. G. Fehon, 2003. Neuroglian, Gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila. J. Cell Biol. 161 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig, K. M., J. Colombani and T. P. Neufeld, 2006. TOR coordinates bulk and targeted endocytosis in the Drosophila melanogaster fat body to regulate cell growth. J. Cell Biol. 173 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger, B., M. Galic, K. Kohler, F. Wittwer, W. Brogiolo et al., 2008. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J. Biol. 7 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinska, A., C. Ribeiro and M. Affolter, 2003. Epithelial tube morphogenesis during Drosophila tracheal development requires Piopio, a luminal ZP protein. Nat. Cell Biol. 5 895–901. [DOI] [PubMed] [Google Scholar]
- Jovine, L., H. Qi, Z. Williams, E. Litscher and P. M. Wassarman, 2002. The ZP domain is a conserved module for polymerization of extracellular proteins. Nat. Cell Biol. 4 457–461. [DOI] [PubMed] [Google Scholar]
- Jovine, L., C. C. Darie, E. S. Litscher and P. M. Wassarman, 2005. Zona pellucida domain proteins. Annu. Rev. Biochem. 74 83–114. [DOI] [PubMed] [Google Scholar]
- Kennerdell, J. R., and R. W. Carthew, 1998. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell 95 1017–1026. [DOI] [PubMed] [Google Scholar]
- Kerman, B. E., A. M. Cheshire and D. J. Andrew, 2006. From fate to function: the Drosophila trachea and salivary gland as models for tubulogenesis. Differentiation 74 326–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov, T., and S. K. Beckendorf, 2007. 18 wheeler regulates apical constriction of salivary gland cells via the Rho-GTPase-signaling pathway. Dev. Biol. 307 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz, D. E., and S. L. Zipursky, 1990. Drosophila chaoptin, a member of the leucine-rich repeat family, is a photoreceptor cell-specific adhesion molecule. EMBO J. 9 1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, T., M. Yu, B. Williams, S. Goel, S. M. Paul et al., 2007. CaSpeR5, a family of Drosophila transgenesis and shuttle vectors with improved multiple cloning sites. Biotechniques 42(164): 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic, I., R. R. Copley, S. Schmidt, F. D. Ciccarelli, T. Doerks et al., 2004. SMART 4.0: towards genomic data integration. Nucleic Acids Res. 32 D142–D144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig, S., T. Batz, K. Armbruster and M. A. Krasnow, 2006. Serpentine and vermiform encode matrix proteins with chitin binding and deacetylation domains that limit tracheal tube length in Drosophila. Curr. Biol. 16 186–194. [DOI] [PubMed] [Google Scholar]
- Moussian, B., H. Schwarz, S. Bartoszewski and C. Nusslein-Volhard, 2005. Involvement of chitin in exoskeleton morphogenesis in Drosophila melanogaster. J. Morphol. 264 117–130. [DOI] [PubMed] [Google Scholar]
- Moussian, B., E. Tang, A. Tonning, S. Helms, H. Schwarz et al., 2006. Drosophila Knickkopf and Retroactive are needed for epithelial tube growth and cuticle differentiation through their specific requirement for chitin filament organization. Development 133 163–171. [DOI] [PubMed] [Google Scholar]
- Oda, H., T. Uemura, Y. Harada, Y. Iwai and M. Takeichi, 1994. A Drosophila homolog of cadherin associated with armadillo and essential for embryonic cell-cell adhesion. Dev. Biol. 165 716–726. [DOI] [PubMed] [Google Scholar]
- Paul, S. M., M. Ternet, P. M. Salvaterra and G. J. Beitel, 2003. The Na+/K+ ATPase is required for septate junction function and epithelial tube-size control in the Drosophila tracheal system. Development 130 4963–4974. [DOI] [PubMed] [Google Scholar]
- Paul, S. M., M. J. Palladino and G. J. Beitel, 2007. A pump-independent function of the Na,K-ATPase is required for epithelial junction function and tracheal tube-size control. Development 134 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavelic, J., T. Matijevic and J. Knezevic, 2007. Biological & physiological aspects of action of insulin-like growth factor peptide family. Indian J. Med. Res. 125 511–522. [PubMed] [Google Scholar]
- Reinke, R., D. E. Krantz, D. Yen and S. L. Zipursky, 1988. Chaoptin, a cell surface glycoprotein required for Drosophila photoreceptor cell morphogenesis, contains a repeat motif found in yeast and human. Cell 52 291–301. [DOI] [PubMed] [Google Scholar]
- Ribeiro, C., M. Neumann and M. Affolter, 2004. Genetic control of cell intercalation during tracheal morphogenesis in Drosophila. Curr. Biol. 14 2197–2207. [DOI] [PubMed] [Google Scholar]
- Rubin, G. M., and A. C. Spradling, 1982. Genetic transformation of Drosophila with transposable element vectors. Science 218 348–353. [DOI] [PubMed] [Google Scholar]
- Samakovlis, C., N. Hacohen, G. Manning, D. C. Sutherland, K. Guillemin et al., 1996. Development of the Drosophila tracheal system occurs by a series of morphologically distinct but genetically coupled branching events. Development 122 1395–1407. [DOI] [PubMed] [Google Scholar]
- Shiga, Y., M. Tanaka-Matakatsu and S. Hayashi, 1996. A nuclear GFP/beta-galactosidase fusion protein as a marker for morphogenesis in living Drosophila. Dev. Growth Differ. 38 99–106. [Google Scholar]
- Spradling, A. C., and G. M. Rubin, 1982. Transposition of cloned P elements into Drosophila germ line chromosomes. Science 218 341–347. [DOI] [PubMed] [Google Scholar]
- Swanson, L. E., and G. J. Beitel, 2006. Tubulogenesis: an inside job. New work shows that a dynamic and highly patterned apical extracellular matrix regulates epithelial cell shape and tube size from within the lumen of the Drosophila tracheal system. Curr. Biol. 16 R51–R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauszig, S., E. Jouanguy, J. A. Hoffmann and J. L. Imler, 2000. Toll-related receptors and the control of antimicrobial peptide expression in Drosophila. Proc. Natl. Acad. Sci. USA 97 10520–10525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonning, A., J. Hemphala, E. Tang, U. Nannmark, C. Samakovlis et al., 2005. A transient luminal chitinous matrix is required to model epithelial tube diameter in the Drosophila trachea. Dev. Cell 9 423–430. [DOI] [PubMed] [Google Scholar]
- Tonning, A., S. Helms, H. Schwarz, A. E. Uv and B. Moussian, 2006. Hormonal regulation of mummy is needed for apical extracellular matrix formation and epithelial morphogenesis in Drosophila. Development 133 331–341. [DOI] [PubMed] [Google Scholar]
- Wang, S., S. A. Jayaram, J. Hemphala, K. A. Senti, V. Tsarouhas et al., 2006. Septate-junction-dependent luminal deposition of chitin deacetylases restricts tube elongation in the Drosophila trachea. Curr. Biol. 16 180–185. [DOI] [PubMed] [Google Scholar]
- Wilkin, M. B., M. N. Becker, D. Mulvey, I. Phan, A. Chao et al., 2000. Drosophila dumpy is a gigantic extracellular protein required to maintain tension at epidermal-cuticle attachment sites. Curr. Biol. 10 559–567. [DOI] [PubMed] [Google Scholar]
- Wodarz, A., U. Hinz, M. Engelbert and E. Knust, 1995. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell 82 67–76. [DOI] [PubMed] [Google Scholar]
- Wu, V. M., and G. J. Beitel, 2004. A junctional problem of apical proportions: epithelial tube-size control by septate junctions in the Drosophila tracheal system. Curr. Opin. Cell Biol. 16 493–499. [DOI] [PubMed] [Google Scholar]
- Wu, V. M., J. Schulte, A. Hirschi, U. Tepass and G. J. Beitel, 2004. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J. Cell Biol. 164 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, V. M., M. H. Yu, R. Paik, S. Banerjee, Z. Liang et al., 2007. Drosophila Varicose, a member of a new subgroup of basolateral MAGUKs, is required for septate junctions and tracheal morphogenesis. Development 134 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]