Abstract
A comprehensive genetic linkage map of the domestic cat X chromosome was generated with the goal of localizing the genomic position of the classic X-linked orange (O) locus. Microsatellite markers with an average spacing of 3 Mb were selected from sequence traces of the cat 1.9× whole genome sequence (WGS), including the pseudoautosomal region 1 (PAR1). Extreme variation in recombination rates (centimorgans per megabase) was observed along the X chromosome, ranging from a virtual absence of recombination events in a region estimated to be >30 Mb to recombination frequencies of 15.7 cM/Mb in a segment estimated to be <0.3 Mb. This detailed linkage map was applied to position the X-linked orange gene, placing this locus on the q arm of the X chromosome, as opposed to a previously reported location on the p arm. Fine mapping placed the locus between markers at positions 106 and 116.8 Mb in the current 1.9×-coverage sequence assembly of the cat genome. Haplotype analysis revealed potential recombination events that could reduce the size of the candidate region to 3.5 Mb and suggested multiple origins for the orange phenotype in the domestic cat. Furthermore, epistasis of orange over nonagouti was demonstrated at the genetic level.
THE domestic cat displays a broad diversity of phenotypic variation, including an array of coloration patterns resulting from interacting genotypes at multiple loci. Pigmentation genes have been identified on the basis of comparative genetic data supported by genetic linkage or association studies [agouti locus (melanism), ASIP (Eizirik et al. 2003); albino locus (siamese, burmese, and albino), TYR (O'Brien et al. 1986; Lyons et al. 2005; Schmidt-Küntzel et al. 2005; Imes et al. 2006); brown locus (chocolate and cinnamon), TYRP1 (Schmidt-Küntzel et al. 2005); dilute locus (dilute), MLPH (Ishida et al. 2006)]. Other genes involved in domestic cat pigmentation remain unknown, including the X-linked orange (O) locus (Searle 1968; Vella et al. 1999). This locus has attracted the attention of geneticists for over a century (e.g., Doncaster 1904; Wright 1918). Orange controls an unknown molecular mechanism that causes the suppression of black-brownish pigmentation (eumelanin) in favor of orange-yellowish coloration (pheomelanin) (Vella et al. 1999). The resulting orange phenotype is likely caused by the exclusive presence of pheomelanic pigments in the hair shaft (Figure 1A).
Figure 1.—
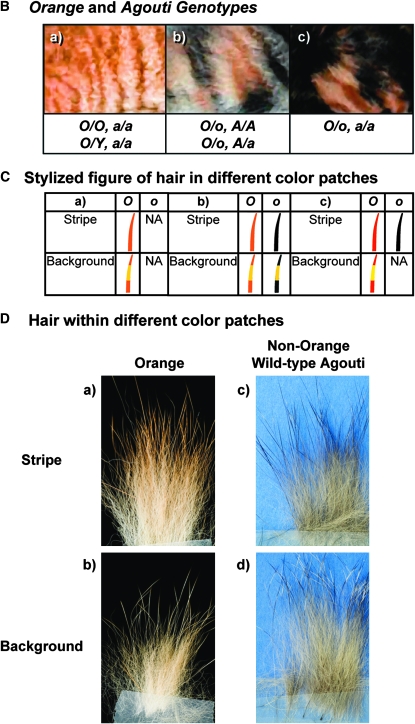
(A) Phenotypic variation at the orange locus. The top row depicts variation at the orange locus. Genotypes are presented for a female with representative coat color. From left to right: (a) nonorange, wild-type agouti; (b) nonorange, nonagouti; (c) orange heterozygote, nonagouti; (d) orange homozygote. No phenotype for the agouti locus is given for cat “d” as orange is epistatic over nonagouti. A female O/O, A/A or O/O, A/a would be indistinguishable from an O/O, a/a cat. The bottom row depicts the influence of the white spotting (S) locus on the size and appearance of color patches in female cats that are heterozygous at the O locus. From left to right: (e) heterozygote or homozygote for white spotting allele (S/s, S/S) and (f) wild type at the white spotting locus (s/s). O/o, orange alleles; A/a, agouti alleles; S/s, white spotting alleles. (B) Phenotypic epistasis of orange pattern over nonagouti. Orange and agouti genotypes are indicated under each picture representing phenotypic variation at the orange locus. (a) Skin patch from a domestic cat of orange coat color demonstrating pattern and for which the genotype at the agouti locus was determined to be nonagouti (O/O, a/a or O/Y, a/a). (b) Skin patch from a calico cat (O/o) demonstrating pattern in orange and nonorange fur parts on an agouti (A/A or A/a) background. It is seen that the pattern is continuous between the patches of different color. (C) Stylized representation of hairs within the different color patches. The types of hair responsible for a particular fur coloration are represented in a simplified way for each color patch. Of note, hair banding can show variation and some hairs may have multiple bands. Ca, Cb, and Cc correspond to the phenotypes represented in Ba, Bb, and Bc, respectively. O and o stand for orange and nonorange fur patches. (D) Tufts of hair from orange and nonorange individuals from patterned and background regions of the coat. (a) Orange patterned: hairs are largely uniformly pigmented a dark pheomelanin; note tips of some hairs exhibit narrow light pheomelanic bands. (b) Orange background: background hairs display dark pheomelanic base and lighter pheomelanic band. (c) Nonorange, patterned: hairs are largely uniformly pigmented with eumelanin; see occasional narrow agouti band at tips. (d) Nonorange, background: background hairs display eumelanic base, broad pheomelanic agouti band, eumelanic tips. Note that hairs can display multiple pheomelanic agouti bands.
Pheomelanic phenotypes have been reported in other species. In mice, cattle, horses, pigs, humans, dogs, bears, rabbits, and chickens, mutations in the Melanocortin 1 receptor (MC1R) have been reported as causative of red/yellow/white hair or plumage (Robbins et al. 1993; Klungland et al. 1995; Marklund et al. 1996; Kijas et al. 1998; Rees et al. 1999; Newton et al. 2000; Ritland et al. 2001; Kerje et al. 2003; Fontanesi et al. 2006). However, the autosomal location of MC1R, mapped to chromosome E2 in the domestic cat (Eizirik et al. 2003), eliminates this gene as causal for X-linked orange. An X-chromosomal region for the cat orange locus was previously proposed on the basis of exclusion mapping (Grahn et al. 2005). Only one other mammal, the Syrian hamster (Mesocricetus auratus), has been reported to have an X-linked pheomelanic phenotype (sex-linked yellow; Robinson 1966). Mapping of Sex-linked yellow in the hamster is presented in an accompanying article by Alizadeh et al. (2009, this issue).
Two striking phenotypic variants are seen in the tortoiseshell (mottled orange and nonorange) and the calico (mosaic pattern of large patches of orange, nonorange, and white) cats (Figure 1A). The coloration of the nonorange patches is influenced by the genetic background at other pigmentation loci, which segregate independently of the O locus (e.g., the brown locus in Lamoreux 1973). The tortoiseshell/calico phenotypes are a consequence of embryonic X inactivation whereby the alternative expression of orange vs. wild-type alleles in different skin patches creates a mosaic color patterning characteristic of female cats heterozygous at the O locus (Lyon 1999). Mosaic phenotypes were presented as a compelling argument for the establishment of the Lyon hypothesis for X inactivation (Lyon 1961). Due to the presence of both orange and nonorange coloration in heterozygous individuals, the orange allele (O) can be regarded as codominant with the wild-type allele. The mosaic phenotype is almost exclusive to females; rare occurrences in males have been explained by sex chromosome aneuploidy (XXY), chimerism, mosaicism, and somatic mutations (reviewed in Moran et al. 1984).
An interesting epistatic interaction involving X-linked orange is mediated by the autosomal white spotting (S) locus. Heterozygous orange females, without the mutant white spotting allele, will develop as tortoiseshell with tiny mottled spots (Figure 1A, f), while heterozygous orange females that carry a white spotting allele develop as calico cats with much larger patches (Figure 1A, e) (Searle 1968; Vella et al. 1999). The large calico patches have been inferred to result from a reduced number of melanoblasts in the skin, allowing for a spatial expansion of the clones leading to larger colored patches. The white areas in between are caused by an absence of mature melanocytes (Vella et al. 1999).
Another interesting epistatic situation involves the interaction of X-linked orange and the agouti locus. A deletion at the agouti locus changes nonorange agouti coats displaying tabby (which specifies striped, blotched, or spotted coats) or ticked to melanistic cats with a solid coat (Vella et al. 1999; Eizirik et al. 2003). In nonorange cats, a nonagouti (a/a) homozygote effectively masks the tabby pattern (Eizirik et al. 2003), but in orange (O/Y or O/O) cats the tabby pattern is easily observed as coat markings of dark and light orange regardless of the agouti status (Figure 2). In nonagouti (a/a) calico cats this leads to nonorange solid patches juxtaposed to orange patches displaying tabby patterning (Figure 1B, c). Epistasis of orange over nonagouti is investigated at a genotypic level in this study. Interestingly, cats of calico/tortoiseshell coat color carrying at least one agouti wild-type allele show continuity in the tabby pattern between orange and nonorange patches (Figure 1B, b) (Vella et al. 1999).
Figure 2.—
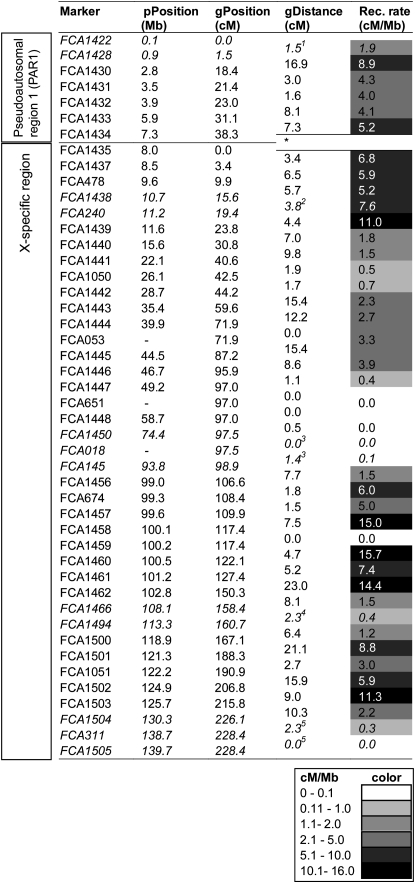
Domestic cat X chromosome linkage map. For each marker the physical location (pPosition) on the domestic cat X chromosome is indicated in megabases (column 2). Positions in the cat were determined using ABCC Get Trace Mapping Info, ABCC trace-centric tools (http://www.abcc.ncifcrf.gov/Genomes/Cat/index.php). The genetic location (gPosition) within the domestic cat linkage map is indicated in centimorgans (column 3). Pairwise genetic distance (gDistance in centimorgans) is represented in column 4. LOD scores are ≥3.0, unless otherwise noted [marker pairs that participate in a flip scored at LOD < 3.0 (odds of <1000:1) by CRI-MAP are represented in italics and the lower of the LOD scores estimated by CRI-MAP and Superlink are shown with superscript footnote numbers]. Recombination rates (Rec. rate) are indicated in centimorgans per megabase (column 5). The background color of the recombination rates is color coded to reflect the extreme variation in the values. *, linkage was determined between markers FCA1434 and FCA1435. However, the PAR1 and the X-specific region are kept separate since recombination mechanisms are different (PAR1 being able to recombine with the Y chromosome). 1, LOD = 1.58; 2, LOD = 0.17; 3, LOD = 1.21; 4, LOD = 1.19; 5, LOD = 1.56.
In addition to the O locus, the cat X chromosome contains at least two loci of biomedical interest: the locus for feline muscular dystrophy, encoding dystrophin, (Winand et al. 1994) and another locus implicated in skin dysplasia, believed to be X-linked in the domestic cat on the basis of breeding information (S. Pflueger, personal communication). The presence of additional feline hereditary X-linked diseases corresponding to human X-linked pathologies can be expected on the basis of the fact that X-linked traits are believed to be conserved in eutherian mammals (Ohno 1973). X-linked traits in the domestic cat are therefore also expected to be X-linked in humans, making the study of the domestic cat X chromosome of comparative genetic importance.
We report here the generation of a comprehensive genetic linkage map of microsatellites for the domestic cat X chromosome, which has been applied to the mapping of the O locus. Special attention has been given to include markers in the pseudoautosomal region (PAR), a segment that displays an autosomal pattern of inheritance undergoing recombination during male meiosis with the homologous region on the Y chromosome. The generation of a dense map (∼1 marker/3 Mb) provides an important addition toward the future mapping of X-linked traits in the domestic cat.
MATERIALS AND METHODS
Animals:
Linkage study:
The pedigree used for the linkage study is a multigeneration pedigree of 287 nonbreed cats generated by Nestlé Purina PetCare (Eizirik et al. 2003) of which 256 were genotyped. Orange coat color segregates in this pedigree, which has 109 informative meioses for this trait. Photographs of 55 individuals from this pedigree displaying orange coat color were used to evaluate presence/absence of pattern in orange coat/patches for the determination of epistasis of orange over nonagouti. An additional 88 informative meioses were obtained from a pedigree of 107 individuals. A portion of the 107-individual pedigree was generated at Michigan State University for the mapping of spinal muscular atrophy (Fyfe et al. 2006); a subset of the pedigree was generated at the National Institutes of Health (NIH) Animal Center for coat color segregation projects of the Laboratory of Genomic Diversity (LGD). Two individuals utilized in both subsets allow for merging into one pedigree for linkage analysis. DNA was extracted from blood samples with a QIAamp DNA Blood mini kit (QIAGEN, Valencia, CA) following the manufacturer's protocol.
Population study:
One hundred eleven unrelated male cats were sampled from a rural environment in Frederick, Maryland (53 orange, 13 wild type), and an urban setting in Porto Alegre, Rio Grande do Sul, southern Brazil (36 orange, 9 wild type). Male cats were chosen for the population study as they are hemizygous for the X chromosome and their haplotypes can be determined with certainty. Sampling of the U.S. cats was based on testicular tissue obtained as a by-product of routine neutering procedures, with permission of each pet owner. DNA was extracted from testis samples with a DNeasy blood and tissue kit (QIAGEN). Samples of the Brazilian cats were obtained from buccal swabs collected from individuals kept in private homes (with permission of owners) and veterinary clinics, as well as stray animals. Genomic DNA was extracted from freshly collected buccal swabs, using a simple salting-out method (Abrão et al. 2005).
Marker development:
Testing of the previous candidate region:
A microsatellite (FCA1446; supplemental Table 1) was designed in the candidate region published by Grahn et al. (2005), to examine for linkage to orange in the Nestlé Purina pedigree.
Microsatellite linkage map:
Ten microsatellites described in previous publications were used in this study (Menotti-Raymond et al. 1999, 2003a,b). Forty-nine new microsatellite markers were genotyped for possible addition to the X chromosome linkage map (supplemental Table 1). Microsatellites were selected from the sequence traces of the cat 1.9× WGS for inclusion in the X chromosome map, on the basis of their conserved syntenic position on the dog X chromosome, following the method described by Ishida et al. (2006). Following the availability of a cat genome sequence assembly (Pontius et al. 2007), microsatellite markers were selected on the basis of their location on the cat X chromosome, using the algorithm ABCC Retrieve STRs (ABCC STR-centric tools, http://www.abcc.ncifcrf.gov/Genomes/Cat/index.php). Primers (supplemental Table 1) were designed with Primer 3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi; Rozen and Skaletsky 2000), including an M13 tail for fluorescent labeling of PCR products (Boutin-Ganache et al. 2001).
Fine-mapping of X-linked orange:
Thirty-six additional microsatellite markers were designed for the localization of the O locus according to the methods described above (see supplemental Table 2). In addition, a single-nucleotide polymorphism (SNP) was identified within the gene RAP2C, located in the candidate region, through exploratory sequencing. A primer pair was designed for amplification and genotyping of this SNP (supplemental Table 2).
The physical location on the domestic cat X assembly was determined for each marker. Markers identified from cat traces were localized on the cat X chromosome on the basis of the location attributed to the trace using the ABCC tool, “Get Trace Mapping Info” (ABCC trace-centric tools; http://www.abcc.ncifcrf.gov/Genomes/Cat/index.php) or the microsatellite flanking region using the ABCC tool “GMap a sequence to cat” (ABCC mapping and browsing tools; Wu and Watanabe 2005; http://www.abcc.ncifcrf.gov/Genomes/Cat/index.php). Markers from previous publications (obtained prior to the cat 1.9× WGS) were attributed to sequence traces with cross-species MegaBLAST (http://www.ncbi.nlm.nih.gov; Zhang et al. 2000), if possible. In the event that a marker could not be localized on the domestic cat assembly, its position in the cat was inferred using a comparative genomics approach. The locations of the microsatellite markers on the dog and human X chromosomes were obtained by applying a BLAT search (Kent 2002; UCSC genome browser, http://genome.ucsc.edu/) of the trace sequence containing the microsatellite or of the microsatellite flanking region. The location on the domestic cat radiation hybrid map was obtained from the literature for the previously published markers (Menotti-Raymond et al. 2003b).
Genotyping:
Microsatellite markers and SNP:
Primers of 95 microsatellite markers and one SNP were amplified in the Nestlé Purina PetCare pedigree (Eizirik et al. 2003) following touchdown PCR conditions as in Menotti-Raymond et al. (2005). Following initial mapping of orange, 6 microsatellite markers were amplified in a second pedigree of 107 individuals segregating for this trait in an attempt to reduce the candidate region. To establish haplotypes and investigate their association with orange, primers of 25 markers located in the region of zero recombination (between megabases 106 and 116.8 on the X chromosome physical map) were amplified in the 111 male cats of the population study. PCR products were analyzed as in Ishida et al. (2006). PCR product length was used as a surrogate for actual repeat number when determining allele identity. Inheritance was verified with Pedcheck (O'Connell and Weeks 1998) for genotypes of pedigree individuals.
ASIP locus:
Primers designed to genotype the 2-bp deletion in ASIP (agouti locus), causative of the melanistic phenotype in the domestic cat (Eizirik et al. 2003), were modified according to Boutin-Ganache et al. (2001) and amplified in 55 individuals of the Nestlé Purina pedigree. Individuals of orange and calico phenotype were selected on the basis of the availability of haplotype data and photographs providing unequivocal evaluation of pattern in the orange coat/patches. Tortoiseshell phenotypes were not evaluated due to the insufficient surface of undisturbed orange coat color. Genotyping at the agouti locus was determined on the basis of the size of the fluorescent amplicon as described by Eizirik et al. (2003).
Map construction:
Markers with an autosomal inheritance pattern, designed in the pseudoautosomal region 1 (PAR1), were analyzed independently from the markers with an X-specific mode of inheritance due to software limitations. Final linkage analysis was performed for 51 markers (7 markers inherited in an autosomal manner and 44 X-linked markers).
Two-point LOD scores between all marker pairs were computed with Superlink (Fishelson and Geiger 2002, 2004) for values of the recombination fraction (θ) between 0 and 0.4 in steps of 0.01. Two markers were considered linked if their peak LOD exceeded 3.0 (Ott 1991). Using this definition, single-linkage clustering determined that the PAR1 and X-specific markers each composed a single linkage group.
A preliminary order was suggested by treating the estimated Kosambi distance (Ott 1991) between markers as a distance, reducing the marker ordering problem to the traveling salesman problem (TSP) and using CONCORDE (Applegate et al. 2006) to solve TSP instances. The reduction to TSP is imperfect for linkage data of this type, so some rearrangement to get the optimal order was expected, especially in regions where markers are very close to one another in genetic distance. The order of linked markers was then iteratively tested and modified with the CRI-MAP version 2.4 (Lander and Green 1987) flips option until the order stabilized. During this process, five markers were dropped because they could not be ordered with any confidence or caused instability in the flips analysis. For flips that CRI-MAP scored at <1000:1 odds (LOD score <3.0), Superlink was used to examine support for alternative orders. For such low-scoring flips, we report the lower of the CRI-MAP and Superlink LOD scores.
Using CRI-MAP, the female θ was estimated between one pseudoautosomal marker (FCA1434) and one X-specific marker (FCA1435).
Linkage analysis for the mapping of the orange locus:
For mapping orange, LOD scores were computed with Superlink. Orange was coded as a fully penetrant X chromosome locus following the inheritance pattern described in the Introduction in which (unlike most traits) phenotype completely determines genotype at the locus. Linkage to orange was determined for the markers of the X-linkage map, followed by fine mapping with additional microsatellite markers designed for that purpose.
Haplotype determination for the orange locus candidate region:
Haplotypes were directly obtained from the genotyping data for the 111 male individuals collected for the population study. For the individuals from the Nestlé Purina PetCare pedigree, 22 haplotypes were inferred by ferret (G. Nelson, unpublished results), an in-house implementation of the expectation-maximization (EM) algorithm (Excoffier and Slatkin 1995), that was modified for this project to allow input of monosome (male X) data to improve the accuracy of the inference of the female haplotypes. Because ferret considers all possible haplotypes consistent with the genotypes, and was limited to considering ∼300 million distinct haplotypes in an inference, the analysis was first performed for three overlapping shorter sequences (loci FCA1466–FCA1474, FCA1474–FCA1489, and FCA1482–FCA1498); these haplotypes were then assembled into larger segments for all 33 loci by an additional haplotype inference. Ferret tests haplotype inferences by bootstrap resampling of the genotype data; inferences for the three segments as well as the ultimate assembly of these segments had >99% bootstrap replicability. Inheritance for the haplotype segments and final haplotypes was verified with Pedcheck (O'Connell and Weeks 1998).
RESULTS AND DISCUSSION
Two approaches are classically used for mapping of genetic loci of interest, a candidate gene approach and genome scans. The sole candidate gene for orange, MC1R was mapped in the cat in a previous study to an autosome, chromosome E2 (Eizirik et al. 2003). Additionally, a candidate region on the X chromosome was proposed for the O locus in the vicinity of megabase 46.7 (Grahn et al. 2005) on the basis of exclusion analysis utilizing the available X chromosome genetic linkage maps (Menotti-Raymond et al. 1999, 2003a) containing two linkage groups spanning an estimated 25 cM, along with three unmapped loci. A microsatellite (FCA1446, see supplemental Table 1), was designed in the proposed candidate region. A demonstrated lack of linkage between FCA1446 and orange (LOD = −55, θ = 0.01), at a LOD score below the frequently used exclusion criterion of −2 (Ott 1991), led to our development of a comprehensive linkage map of the X chromosome for the mapping of orange.
Linkage map:
To generate a comprehensive map of the cat X chromosome, microsatellites were selected as described in Marker development (materials and methods). A total of 59 microsatellite markers (supplemental Table 1) were amplified in a multigeneration domestic cat pedigree. Forty-six of the loci (FCA1435–FCA1503; supplemental Table 1) demonstrated an X-linked pattern of inheritance.
Thirteen microsatellite markers (FCA1422–FCA1434; supplemental Table 1), selected on the basis of the homology of their flanking segments to regions located near the telomeric region of the p arm of the dog X chromosome, demonstrated an autosomal inheritance pattern. These markers were determined to be located in PAR1 of the X chromosome on the basis of criteria recommended for the identification of pseudoautosomal genes (Burgoyne 1982): (1) failure to map to an autosome [marker FCA1433 demonstrated lack of linkage with ∼500 autosomal markers in a third-generation linkage map of the cat (Menotti-Raymond et al. 2008)], (2) linkage to an X-specific marker [marker FCA1434 linked to the X-specific marker FCA1435 with a female recombination fraction estimated at 0.02 (LOD > 85)], and (3) physical mapping to the X chromosome [12 of the 13 markers are placed on the cat X chromosome in the current assembly (Garfield browser 12.2; Pontius and O'Brien 2007; http://lgd.abcc.ncifcrf.gov/cgi-bin/gbrowse/cat)]. Indirect physical mapping was obtained by selecting 2 markers, FCA1432 and FCA1434, within introns of NLGN4X and TBL1X, respectively, that were previously mapped to the pseudoautosomal region of the domestic cat radiation hybrid (RH) map (Murphy et al. 2007). Additionally, sequence traces containing marker FCA1426 (data not shown) align to homologous regions on the human X and Y chromosomes.
The final genetic recombination map (Figure 2) comprises 46 microsatellite markers (7 pseudoautosomal and 39 X-specific markers) spanning the estimated 140 Mb of the domestic cat X chromosome (Garfield browser 12.2; Pontius and O'Brien 2007). Eight microsatellites located in high marker density regions were excluded from the map, and 5 others were dropped during the analysis (see materials and methods). Genetic distances between adjacent loci range from 0 to 23.0 cM (Figure 2), and the total genetic length of the domestic cat X chromosome was estimated to be 268 cM (38 cM for PAR1, 2 cM between FCA1434 and FCA1435, and 228 cM for the X-specific region). Evidence for complete coverage of the X chromosome includes demonstration of linkage between a pseudoautosomal (FCA1434) and an X-specific marker (FCA1435). The most distal cat marker is located at megabase 139.7, <0.3 Mb from the end of the current cat X sequence assembly. The total genetic length of the cat X chromosome is larger than that reported in other mammals [34 cM in the silver fox (covering two-thirds of the X chromosome; Kukekova et al. 2007), 65 cM in the horse (Swinburne et al. 2006), 71 cM in the mouse (Shifman et al. 2006), 78 cM in the rat (Bihoreau et al. 2001), 121 cM in the sheep (Maddox et al. 2001), 147 cM in the cow (Ihara et al. 2004), and 195 cM in humans (Matise et al. 2007)]. Additionally, some of the older maps may underestimate the size if they do not cover the entirety of the X chromosome. Similarly, the recombination length of the domestic cat autosomal map is longer than that observed in most reported mammalian maps (Menotti-Raymond et al. 2008).
The order of microsatellite markers on the X chromosome inferred by genetic linkage data is consistent with their physical coordinates in the domestic cat genome assembly (Figure 2). Only one difference was detected between the genetic linkage and radiation hybrid maps: marker FCA53, which follows an X-linked inheritance pattern, was mapped to chromosome B1 in the first genetic linkage map of the cat (Menotti-Raymond et al. 1999). This first genetic linkage map was generated in a hybrid pedigree between the domestic cat the Asian leopard cat (Prionailurus bengalensis) (Menotti-Raymond et al. 1999). The high incidence of “null” alleles in hybrid individuals for this locus was misinterpreted as an autosomal inheritance pattern. FCA53 was mapped on cat B2 in the cat RH map (Menotti-Raymond et al. 2003b); however, its location has presently been determined to be on the X chromosome (W. J. Murphy, personal communication). Of note, FCA053 could not be placed on the cat, dog, or human assemblies. For other cat X markers the colinearity between the domestic cat, dog, and human X chromosome coordinates is shown in supplemental Figure 1 and confirms the conserved synteny of the mammalian X chromosome between dog, human, and cat, as previously reported by Murphy et al. (1999).
Extreme variation of recombination rates was apparent across the X chromosome, ranging from 0 cM/Mb, extending over 34 Mb, to 15.7 cM/Mb localized within 300 kb (supplemental Figure 2). Several phenomena have been suggested to be causative of variation in recombination frequencies; among these are differences in GC content, gene density, and location on the chromosome (telomeric vs. centromeric; Nachman 2002). No evidence for a correlation between GC content and recombination frequencies was found utilizing the domestic cat genome browser (Garfield browser 12.2; Pontius and O'Brien 2007). The centromere, a region that is expected to have suppressed recombination (Nachman 2002), is located approximately at position 53 Mb of the domestic cat X chromosome (Garfield browser 12.2; Pontius and O'Brien 2007) and is indeed situated within the region of zero recombination. Interestingly, the telomeric portion of the q arm also exhibits low recombination rates (0 and 0.3 cM/Mb) in the terminal 10 Mb of the domestic cat X chromosome. This is an unusual observation, as telomeric regions are classically reported as chromosomal areas undergoing frequent recombination (Nachman 2002). Suppression of recombination has also been attributed to chromosomal rearrangements such as inversions, in which recombination occurring in the inverted region in a heterozygous individual leads to nonviable recombinants (Griffiths et al. 1996). However, a large inversion is unlikely to account for these observations, because there is no ambiguity in marker order associated either with the linkage analysis or from previous cytogenetic in situ hybridization studies (T. Raudsepp, personal communication).
Mapping of the orange locus:
Linkage analysis:
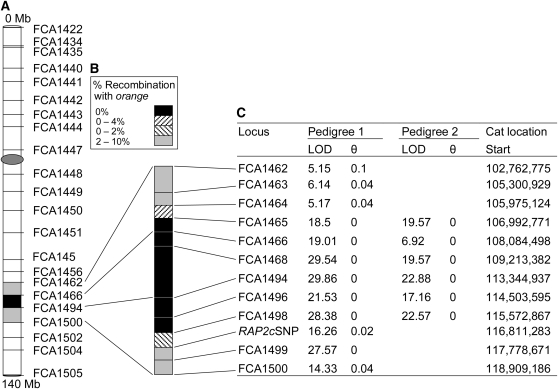
The markers placed on the domestic cat X chromosome linkage map were utilized to identify linkage to the O locus in a multigeneration nonbreed domestic cat pedigree (Eizirik et al. 2003), segregating for orange. Significant linkage to the O locus was detected for markers FCA1466 and FCA1494, (θ = 0, LOD = 19.0 and 29.9, respectively) (Figure 3).
Figure 3.—
Genetic linkage mapping of the orange locus on the cat X chromosome. (A) The majority of the markers of the linkage map are represented on the domestic cat X chromosome. (B) Markers used for fine mapping (FCA1462–FCA1500) are represented on the magnified chromosome section. Solid areas and hatched areas represent the candidate region (0% recombination and transition between 0% and 2 or 4%, respectively). (C) Specific recombination values are represented. Optimal recombination fractions (θ) between the markers and the O locus are shown in columns 3 and 5. LOD scores at the optimal θ (LOD) are represented in columns 2 and 4. Values are given for pedigree 1 (columns 2 and 3; Nestlé Purina PetCare) and for pedigree 2 (columns 4 and 5; spinal muscular atrophy and LGD combined) when available. The position of each locus is represented in column 6 (determined using ABCC Get Trace Mapping Info, ABCC trace-centric tools; http://www.abcc.ncifcrf.gov/Genomes/Cat/index.php).
Fine-scale mapping utilizing six additional microsatellite markers (Figure 3, supplemental Table 2) and one SNP reduced the candidate region to 10.8 Mb, between markers FCA1464 (106.0 Mb) and RAP2cSNP (116.8 Mb). The extent of the zero-recombination region could not be reduced further despite the addition of 88 informative meioses from an additional pedigree segregating for orange (Figure 3).
The candidate region for X-linked orange in the domestic cat corresponds to an interval between 120 and 131 Mb in the human X chromosome. Interestingly the candidate region for Sex-linked yellow in the Syrian hamster was mapped in a nonhomologous region, situated between 46 and 54 Mb on the human X chromosome (Alizadeh et al. 2009). Additional in situ hybridization data provided by Alizadeh et al., utilizing mouse BAC probes from the conserved syntenic position in the cat orange region, provide further support that the orange loci in the Syrian hamster and the domestic cat are located in nonhomologous regions. However, we cannot rule out the possibility that microrearrangements have occurred in either the hamster or the cat genome that have not been detected by our methodologies and that the two orange loci could be orthologs. Alternatively, the two genes could be independent members of the same pathway or encode gene products with similar function.
Haplotype analysis:
An attempt was made to reduce the size of the orange candidate region through identification of ancestral recombination events within the orange interval delimited by the linkage analysis, using a haplotype-based approach. The logic followed here is that recombination between an orange and a nonorange haplotype could “erode” the length of the orange haplotype, further refining the size of the orange candidate region.
Haplotype analysis was performed in a collection of 367 cats, including the Nestlé Purina pedigree and a population sample set of 111 male individuals for which haplotypes could be unambi guously determined. Individuals in the population survey were sampled from two distant locales, one in southern Brazil and the other in the eastern United States. The broad geographic scope of the survey, including populations that have not been in direct genetic contact for many generations, should increase the probability of sampling distantly related alleles, implying an enhanced likelihood of spanning ancestral recombination events within a narrow chromosomal interval. Additional markers (n = 22; supplemental Table 2) were designed to increase the marker density in the candidate region.
The haplotypes generated here included 25 microsatellite markers (FCA1466–FCA1498) located across the region of zero recombination (supplemental Figure 3). One hundred unique haplotypes were characterized, including 36 haplotypes observed in association with the wild-type or nonorange phenotype (subsequently referred to as nonorange haplotypes) and 64 haplotypes associated with the orange phenotype (referred to subsequently as orange haplotypes; supplemental Figure 3).
To identify alleles that are exclusive or prevalent in orange haplotypes relative to nonorange haplotypes (which would thus be useful for detecting ancestral recombination events), the alleles were color coded with respect to their relative frequency of occurrence in the two alternative color phenotypes (supplemental Figure 3). Three major groups were identified in both the orange (groups I–III) and nonorange (groups 1–3) haplotypes on the basis of their allele composition. Orange haplotype groups I and II are more similar to the nonorange groups 1 and 2, respectively, than to any other haplotype group (see supplemental Figure 3). Haplotype group III appears distinct, being represented in only one nonorange haplotype (hap17; supplemental Figure 3).
Group III (hap43–100; supplemental Figure 3) comprises 91% of the orange haplotypes and is characterized by five “signature” alleles (at loci FCA1470, FCA1472, FCA1482, FCA1486, and FCA1494; A–E in supplemental Figure 3), which are found almost exclusively in orange haplotypes. We identified 6 haplotypes (hap43–48) that, at locus A, exhibited a change from the 194-bp signature allele observed in the remaining group III haplotypes. The additional introduction of the adjacent 243-bp allele at the locus FCA1466 in hap44 and -48 is suggestive of a recombinatorial origin of the flanking marker alleles 243 and 168 (see hap19 and -21). At the telomeric boundary of the candidate region, 14 haplotypes (hap47–60) exhibited a change at locus E from the 268-bp signature allele observed in all other group III haplotypes. Both signature loci, A and E, show little allelic variation relative to the major nonorange and orange haplotype groups, suggesting a recombination-based vs. mutational origin of the nonsignature alleles. Signature loci C (FCA1482) (hap59) and D (FCA1486) (hap55–60) display a high level of allele complexity; nonsignature alleles may therefore represent mutational or gene conversion origins. Ancestral recombination events with nonorange haplotypes affecting signature loci A and E would decrease the orange candidate region to 3.5 Mb, extending from marker FCA1470 at 109.8 Mb to marker FCA1494 at 113.3 Mb. Nonetheless, the possibility of mutational events at loci A and E cannot be excluded. The identified region contains no obvious candidate genes and the annotation of this genomic segment is still unclear. Within the 3.5 Mb, 4 putative genes were predicted by the cat genome browser (Garfield browser 12.2; Pontius and O'Brien 2007) and >20 RefSeq genes align to the dog genome (UCSC genome browser; www.genome.ucsc.edu).
The six remaining orange haplotypes (groups I and II) contain one (hap37) or none (hap38–42) of the five signature alleles mentioned above (supplemental Figure 3). The high degree of similarity observed between the orange haplotypes of groups I and II and the nonorange haplotypes of groups 1 and 2, respectively, suggests the occurrence of additional independent mutational events causative of orange, leading to the orange haplotypes of groups I and II. To identify the number of times orange arose in this data set, investigation of variation at the nucleotide level is required once the gene responsible for the orange phenotype is identified.
A noteworthy observation is that each of the orange haplotype groups is represented in both the American and the Brazilian data sets. This could be explained by recent gene flow between the populations due to ease of modern travel or by an old origin of the haplotype groups, preceding the colonization of domestic cats into Brazil and the United States, in both cases originating from European gene pools.
Epistasis of orange over nonagouti:
We have previously shown that the nonagouti mutation in domestic cats is caused by a 2-bp deletion in exon 2 of the Agouti Signaling Protein (ASIP) gene, leading to an early frameshift and premature truncation (Eizirik et al. 2003). Nonorange cats, homozygous for nonagouti (a/a), are completely black, similar to laboratory mice that carry null alleles of agouti. In most mammals, the phenotypic difference between agouti and nonagouti individuals is the presence of pheomelanic-banding in all hairs in the former but not the latter. However, the situation in domestic cats is more complicated due to the presence of tabby patterns. In nonorange cats, banding of hairs is suppressed in the striped or spotted regions of tabby pattern; these hairs are more uniformly pigmented, and hence “stand out” against the lighter-colored background of banded (agouti) hairs (see hairs in Figure 1D, c and d). The overall effect, generated by regions of darker largely unbanded hairs juxtaposed with regions of lighter-banded hairs, creates pattern in the coat. In orange cats, banding of hairs is also suppressed in striped and spotted regions; however, the hair in these regions is a dark orange (as opposed to black in wild-type cats), and the light-colored background hairs are a dark orange with light-colored orange banding (Figure 1C, b and c, and 1D, a and b).
Another particularity of orange cats is that visibility of tabby pattern does not seem to be affected by the genotype of the agouti locus. While nonagouti, nonorange cats exhibit no pattern (other than occasional faint “ghost patterns”), nonagouti, orange cats do exhibit pattern (Figure 1B). Thus, to investigate this epistatic relationship between orange and nonagouti, we determined agouti genotypes for orange or calico individuals (n = 55) in our pedigree and confirmed orange status at a genotypic level using haplotype data.
In calico cats, tabby pattern was exhibited in orange fur independent of the presence or absence of pattern in nonorange patches (Table 1; Figure 1B). In nonorange fur patches, tabby pattern was fully hidden in presence of the nonagouti (a/a) genotype (Figure 1B) All orange cats exhibited pattern. On the basis of a molecular genetic test for the agouti locus (Eizirik et al. 2003), 45 of the 55 individuals with orange coat demonstrated a wild-type agouti genotype (A/A or A/a), and 10 demonstrated a nonagouti genotype (a/a) (Table 1). Thus, genotype status at the agouti locus exhibited no influence on the presence or absence of tabby pattern in orange-colored fur, but genotype at ASIP did correlate with the presence of tabby pattern in nonorange fur as assessed in individuals of calico phenotype (n = 19; Table 1). Independence of tabby pattern from the agouti genotype in areas of orange coat color, and therefore epistasis of orange over nonagouti, is clearly demonstrated by these results. This observation illustrates the complexity of the molecular mechanisms underlying domestic cat (and mammalian) coat color, with multiple processes interacting to generate different combinations of phenotypes.
TABLE 1.
Interaction of orange with tabby pattern and nonagouti
| Tabby pattern in orange
|
||||
|---|---|---|---|---|
| Yes
|
No
|
|||
|
Agouti genotype
| ||||
| Phenotype | a/a | A/A or A/a | a/a | A/A or o/a |
| Orange (O/O or O/Y) | 4 | 32 | 0 | 0 |
| Calico (O/o) | 6a | 13b | 0 | 0 |
| Tortoiseshell (O/o) | NA | NA | NA | NA |
| Nonorange (o/o or o/Y) | NA | NA | NA | NA |
| Total | 10 | 45 | 0 | 0 |
Genotypes at the agouti locus (a/a, A/a, or A/A) are represented in columns 2–5, based on the phenotype in the orange fur parts of domestic cats. No cats of orange coat color lacked tabby pattern (columns 4 and 5) and cats with tabby pattern in orange were of both agouti and nonagouti genotype.
All 6 individuals homozygous for the agouti deletion (a/a) displayed an absence of pattern in the nonorange fur parts.
All 13 individuals carrying at least one wild-type allele (A/A or A/a) displayed tabby pattern in the nonorange fur parts.
Summary:
We developed a comprehensive genetic linkage map of the domestic cat X chromosome, including its pseudoautosomal region 1 (46 markers spanning 38 cM in the PAR1 region and 228 cM in the X-linked region). The map was successfully used to define a candidate interval of 10.8 Mb (delineated by markers FCA1464 and RAP2cSNP) for the X-linked orange locus. Additional haplotype analysis suggested multiple origins for orange in the domestic cat and the potential of historic recombination events at the telomeric and centromeric boundary of our orange candidate region, which would reduce the orange interval to 3.5 Mb.
The linkage map will provide a valuable resource for future localization of other X-linked traits. We recommend including the markers of the pseudoautosomal region in whole genome scans seeking loci controlling traits that have an autosomal inheritance pattern as the inheritance patterns of pseudoautosomal and autosomal loci are indistinguishable. The O locus and its influence on the poorly understood pheomelanic pathway are currently unknown. To better understand the process, as well as the genetic basis for the epistasis of orange over nonagouti, the causative gene for this trait needs to be identified. A solid genomic basis for this in-depth search has been laid out by this study, which will hopefully foster additional investigation on this trait, its functional implications, and its relevance for mammalian pigmentation genetics in general.
Acknowledgments
We thank Gregory Barsh for discussion and sharing of unpublished results, Ana Carolina Garcia Escobar for the collection and extraction of the DNAs obtained from Brazil, the Laboratory of Genomic Diversity core lab for the extraction of the local samples, Joan Pontius and the Advanced Biomedical Computing Center for their advice regarding the whole genome sequencing of the cat, Carlos Driscoll for his suggestion of using microsatellites for haplotype analysis, Solveig Pflueger for her advice as a cat breeding specialist, Richa Agarwala and James Tomlin for assistance with map computation, and John Fyfe (Michigan State University) for making available to this study samples from a feline spinal muscular atrophy pedigree that also segregated for orange. We also acknowledge the photographers who generated the images used in Figure 1A: Susan Feingold (Fulton County Animal Services), Joanna Harkin (Alliance for Stray Animals and People), Bill Hopkins (pet owner), Andrea Thompson (pet owner), Anthony Griffith (author of Introduction to Genetic Analysis), and Martin Feather and Cristy Bird (feral tortoiseshell cat living on the grounds of a wat in Bangkok, Thailand). We also thank Marti Welch (Scientific Publication, Graphics and Media; Advanced Technology Program, Science Applications International Corporation, Frederick, MD) for excellent assistance in generating figures and photographs. This publication has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health (NIH), under contract no. NO1-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research is supported in part by the Intramural Research Program of the NIH, National Library of Medicine.
References
- Abrão, M. G., A. E. C. Billerbeck, M. Y. Nishi, S. Marui and B. B. Mendonça, 2005. Padronização da técnica de extraçao de DNA de células de mucosa oral com NaCl: aplicação no estudo do gene PROP1. Arq. Bras. Endocrinol. Metabol. 49 978–982. [DOI] [PubMed] [Google Scholar]
- Alizadeh, A., L. Z. Hong, C. B. Kaelin, H. Manuel and G. S. Barsh, 2009. Genetics of Sex-linked yellow in the Syrian hamster. Genetics 181 1427–1436. [DOI] [PMC free article] [PubMed]
- Applegate, D., R. Bixby, V. Chvátal and W. Cook, 2006. The Traveling Salesman Problem: A Computational Study. Princeton University Press, Princeton, NJ.
- Bihoreau, M.-T., L. Sebag-Montefiore, R. F. Godfrey, R. H. Wallis, J. H. Brown et al., 2001. A high-resolution consensus linkage map of the rat, integrating radiation hybrid and genetic maps. Genomics 75 57–69. [DOI] [PubMed] [Google Scholar]
- Boutin-Ganache, I., M. Raposo, M. Raymond and C. F. Deschepper, 2001. M13-tailed primers improve the readability and usability of microsatellite analyses performed with two different allele-sizing methods. Biotechniques 31 24–26, 28. [PubMed] [Google Scholar]
- Burgoyne, P. S., 1982. Genetic homology and crossing over in the X and Y chromosomes of mammals. Hum. Genet. 61 85–90. [DOI] [PubMed] [Google Scholar]
- Doncaster, L., 1904. On the inheritance of tortoiseshell and related colors in cats. Proc. Camb. Philos. Soc. 13 35–38. [Google Scholar]
- Eizirik, E., N. Yuhki, W. E. Johnson, M. Menotti-Raymond, S. S. Hannah et al., 2003. Molecular genetics and evolution of melanism in the cat family. Curr. Biol. 13 1–20. [DOI] [PubMed] [Google Scholar]
- Excoffier, L., and M. Slatkin, 1995. Maximum-likelihood estimation of molecular haplotype frequencies in a diploid population. Mol. Biol. Evol. 12 921–927. [DOI] [PubMed] [Google Scholar]
- Fishelson, M., and D. Geiger, 2002. Exact genetic linkage computations for general pedigrees. Bioinformatics 18(Suppl. 1): S189–S198. [DOI] [PubMed] [Google Scholar]
- Fishelson, M., and D. Geiger, 2004. Optimizing exact genetic linkage computations. J. Comput. Biol. 11 263–275. [DOI] [PubMed] [Google Scholar]
- Fontanesi, L., M. Tazzoli, F. Beretti and V. Russo, 2006. Mutations in the melanocortin 1 receptor (MC1R) gene are associated with coat colours in the domestic rabbit (Oryctolagus cuniculus). Anim. Genet. 37 489–493. [DOI] [PubMed] [Google Scholar]
- Fyfe, J. C., M. Menotti-Raymond, V. A. David, L. Brichta, A. A. Schäffer et al., 2006. An ∼140-kb deletion associated with feline spinal muscular atrophy implies an essential LIX1 function for motor neuron survival. Genome Res. 16 1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn, R. A., B. M. Lemesch, L. V. Millon, T. Matise, Q. R. Rogers et al., 2005. Localizing the X-linked orange colour phenotype using feline resource families. Anim. Genet. 36 67–70. [DOI] [PubMed] [Google Scholar]
- Griffiths, A. J. F., J. H. Miller, D. T. Suzuki, R. C. Lewontin and W. M. Gelbart, 1996. An Introduction to Genetic Analysis. W. H. Freeman, New York.
- Ihara, N., A. Takasuga, K. Mizoshita, H. Takeda, M. Sugimoto et al., 2004. A comprehensive genetic map of the cattle genome based on 3802 microsatellites. Genome Res. 14 1987–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imes, D. L., L. A. Geary, R. A. Grahn and L. A. Lyons, 2006. Albinism in the domestic cat (Felis catus) is associated with a tyrosinase (TYR) mutation. Anim. Genet. 37 175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida, Y., V. A. David, E. Eizirik, A. A. Schäffer, B. A. Neelam et al., 2006. A homozygous single-base deletion in MLPH causes the dilute coat color phenotype in the domestic cat. Genomics 88 698–705. [DOI] [PubMed] [Google Scholar]
- Kent, W. J., 2002. BLAT—the BLAST-like alignment tool. Genome Res. 12 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerje, S., J. Lind, K. Schütz, P. Jensen and L. Andersson, 2003. Melanocortin 1-receptor (MC1R) mutations are associated with plumage colour in chicken. Anim. Genet. 34 241–248. [DOI] [PubMed] [Google Scholar]
- Kijas, J. M. H., R. Wales, A. Törnsten, P. Chardon, M. Moller et al., 1998. Melanocortin receptor 1 (MC1R) mutations and coat color in pigs. Genetics 150 1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klungland, H., D. I. Våge, L. Gomez-Raya, S. Adalsteinsson and S. Lien, 1995. The role of melanocyte-stimulating hormone (MSH) receptor in bovine coat color determination. Mamm. Genome 6 636–639. [DOI] [PubMed] [Google Scholar]
- Kukekova, A. V., L. N. Trut, I. N. Oskina, J. L. Johnson, S. V. Temnykh et al., 2007. A meiotic linkage map of the silver fox, aligned and compared to the canine genome. Genome Res. 17 387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoreux, M. L., 1973. Brown-orange tortoiseshell cat. J. Hered. 64 240. [DOI] [PubMed] [Google Scholar]
- Lander, E. S., and P. Green, 1987. Construction of multilocus genetic linkage maps in humans. Proc. Natl. Acad. Sci. USA 84 2363–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon, M. F., 1961. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature 190 372–373. [DOI] [PubMed] [Google Scholar]
- Lyon, M. F., 1999. X-chromosome inactivation. Curr. Biol. 9 R235–R237. [DOI] [PubMed] [Google Scholar]
- Lyons, L. A., D. L. Imes, H. C. Rah and R. A. Grahn, 2005. Tyrosinase mutations associated with Siamese and Burmese patterns in the domestic cat (Felis catus). Anim. Genet. 36 119–126. [DOI] [PubMed] [Google Scholar]
- Maddox, J. F., K. P. Davies, A. M. Crawford, D. J. Hulme, D. Vaiman et al., 2001. An enhanced linkage map of the sheep genome comprising more than 1000 loci. Genome Res. 11 1275–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund, L., M. Johansson Moller, K. Sandberg and L. Andersson, 1996. A missense mutation in the gene for melanocyte-stimulating hormone receptor (MC1R) is associated with the chestnut coat color in horses. Mamm. Genome 7 895–899. [DOI] [PubMed] [Google Scholar]
- Matise, T. C., F. Chen, W. Chen, F. M. De La Vega, M. Hansen et al., 2007. A second-generation combined linkage physical map of the human genome. Genome Res. 17 1783–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menotti-Raymond, M., V. A. David, L. A. Lyons, A. A. Schäffer, J. F. Tomlin et al., 1999. A genetic linkage map of microsatellites in the domestic cat (Felis catus). Genomics 57 9–23. [DOI] [PubMed] [Google Scholar]
- Menotti-Raymond, M., V. A. David, M. E. Roelke, Z. Q. Chen, K. A. Menotti et al., 2003. a Second-generation integrated genetic linkage/radiation hybrid maps of the domestic cat (Felis catus). J. Hered. 94 95–106. [DOI] [PubMed] [Google Scholar]
- Menotti-Raymond, M., V. A. David, R. Agarwala, A. A. Schäffer, R. Stephens et al., 2003. b Radiation hybrid mapping of 304 novel microsatellites in the domestic cat genome. Cytogenet. Genome Res. 102 272–276. [DOI] [PubMed] [Google Scholar]
- Menotti-Raymond, M., V. A. David, L. L. Wachter, J. M. Butler and S. J. O'Brien, 2005. An STR forensic typing system for genetic individualization of domestic cat (Felis catus) samples. J. Foren. Sci. 50 1061–1070. [PubMed] [Google Scholar]
- Menotti-Raymond, M., V. A. David, A. A. Schäffer, J. F. Tomlin, E. Eizirik et al., 2008. An autosomal genetic linkage map of the domestic cat, Felis silvestris catus. Genomics (in press). [DOI] [PMC free article] [PubMed]
- Moran, C., C. B. Gillies and F. W. Nicholas, 1984. Fertile male tortoiseshell cats. Mosaicism due to gene instabilitiy? J. Hered. 75 397–402. [DOI] [PubMed] [Google Scholar]
- Murphy, W. J., S. Sun, Z.-Q. Chen, J. Pecon-Slattery and S. J. O'Brien, 1999. Extensive conservation of sex chromosome organization between cat and human revealed by parallel radiation hybrid mapping. Genome Res. 9 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, W. J., B. Davis, V. A. David, R. Agarwala, A. A. Schäffer et al., 2007. A 1.5-Mb-resolution radiation hybrid map of the cat genome and comparative analysis with the canine and human genomes. Genomics 89 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman, M. W., 2002. Variation in recombination rate across the genome: evidence and implications. Curr. Opin. Genet. Dev. 12 657–663. [DOI] [PubMed] [Google Scholar]
- Newton, J. M., A. L. Wilkie, L. He, S. A. Jordan, D. L. Metallinos et al., 2000. Melanocortin-1 receptor variation in the domestic dog. Mamm. Genome 11 24–30. [DOI] [PubMed] [Google Scholar]
- O'Brien, S. J., M. E. Haskins, C. A. Winkler, W. G. Nash and D. F. Patterson, 1986. Chromosomal mapping of beta-globin and albino loci in the domestic cat: a conserved mammalian chromosome group. J. Hered. 77 374–378. [DOI] [PubMed] [Google Scholar]
- O'Connell, J. R., and D. E. Weeks, 1998. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am. J. Hum. Genet. 63 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno, S., 1973. Ancient linkage groups and frozen accidents. Nature 244 259–262. [DOI] [PubMed] [Google Scholar]
- Ott, J., 1991. Analysis of Human Genetic Linkage, Ed. 2. Johns Hopkins University Press, Baltimore.
- Pontius, J. U., and S. J. O'Brien, 2007. Genome annotation resource fields—GARFIELD: a genome browser for Felis catus. J. Hered. 98 386–389. [DOI] [PubMed] [Google Scholar]
- Pontius, J. U., J. C. Mullikin, D. R. Smith, Agencourt Sequencing Team, K. Lindblad-Toh et al., 2007. Initial sequence and comparative analysis of the cat genome. Genome Res. 17 1675–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees, J. L., M. Birch-Machin, N. Flanagan, E. Healy, S. Phillips et al., 1999. Genetic studies of the human melanocortin-1 receptor. Ann. NY Acad. Sci. 885 134–142. [DOI] [PubMed] [Google Scholar]
- Ritland, K., C. Newton and H. D. Marshall, 2001. Inheritance and population structure of the white-phased “Kermode” black bear. Curr. Biol. 11 1468–1472. [DOI] [PubMed] [Google Scholar]
- Robbins, L. S., J. H. Nadeau, K. R. Johnson, M. A. Kelly, L. Roselli-Rehfuss et al., 1993. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell 72 827–834. [DOI] [PubMed] [Google Scholar]
- Robinson, R., 1966. Sex-linked yellow in the Syrian hamster. Nature 212 824–825. [DOI] [PubMed] [Google Scholar]
- Rozen, S., and H. Skaletsky, 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132 365–386. [DOI] [PubMed] [Google Scholar]
- Schmidt-Küntzel, A., E. Eizirik, S. J. O'Brien and M. Menotti-Raymond, 2005. Tyrosinase and Tyrosinase Related Protein 1 alleles specify domestic cat coat color phenotypes of the albino and brown loci. J. Hered. 96 289–301. [DOI] [PubMed] [Google Scholar]
- Searle, A. G., 1968. Comparative Genetics of Coat Color in Mammals. Logos Press, London.
- Shifman, S., J. T. Bell, R. R. Copley, M. S. Taylor, R. W. Williams et al., 2006. A high-resolution single nucleotide polymorphism genetic map of the mouse genome. PLoS Biol. 4 2227–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinburne, J. E., M. Boursnell, G. Hill, L. Pettitt, T. Allen et al., 2006. Single linkage group per chromosome genetic linkage map for the horse, based on two three-generation, full-sibling, crossbred horse reference families. Genomics 87 1–29. [DOI] [PubMed] [Google Scholar]
- Vella, C. M., L. M. Shelton, J. J. McGonagle and T. W. Stanglein, 1999. Robinson's Genetics for Cat Breeders and Veterinarians. Butterworth-Heinemann, Oxford/Boston.
- Winand, N. J., M. Edwards, D. Pradhan, C. A. Berian and B. J. Cooper, 1994. Deletion of the dystrophin muscle promoter in feline muscular dystrophy. Neuromuscul. Disord. 4 433–445. [DOI] [PubMed] [Google Scholar]
- Wright, S., 1918. Color inheritance in mammals: X. The cat–curious association of deafness with blue-eyed white color and or femaleness with tortoise-shelled color, long known-variations of tiger pattern present interesting features. J. Hered. 9 139–144. [Google Scholar]
- Wu, T. D., and C. K. Watanabe, 2005. GMAP: a genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics 21 1859–1875. [DOI] [PubMed] [Google Scholar]
- Zhang, Z., S. Schwartz, L. Wagner and W. Miller, 2000. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 7 203–214. [DOI] [PubMed] [Google Scholar]