Abstract
Previous studies documenting an association between alcohol use and HIV medication nonadherence, have been unable to distinguish between-persons characteristics from within-person characteristics representing the temporally linked effects of alcohol. Hierarchical Linear Modeling (HLM) explored within- and between-person predictors of adherence during the past 14 days, as well as factors that moderate the event-level effects of alcohol consumption among 272 HIV-positive men and women with alcohol problems. On days in which participants drank, they had almost 9 times higher odds of medication nonadherence, with each drink increasing the odds by 20%. The cognitive and alcohol factors had significant between-person effects on adherence. Individuals with strong and rigid beliefs about the importance of strict medication adherence were significantly more affected by each dose of alcohol, while individuals with more alcohol use and problems were less affected by each drink. Regimen complexity increased the effects of having 1 or more drinks. These results highlight the importance of promoting medication adherence among alcohol-using adults, especially among patients with complex regimens or with high confidence and positive attitudes toward HIV medication.
Keywords: alcohol, HIV, adherence, self-efficacy, HLM
In a national probability survey of HIV-positive adults receiving medical care in the United States (Galvan et al., 2002), 53% of participants reported drinking in the past month. Although only 8% (or 15% of those reporting any drinking) were classified as heavy drinkers (defined as five or more drinks on 4 or more days during the previous month), this rate is approximately twice that estimated among the general population (Greenfield, Midanik, & Rogers, 2000). Rates of alcohol problems among people infected with HIV range from 8% to 41% (Cook et al., 2001; Lefevre et al., 1995; Tucker, Burnam, Sherbourne, Kung, & Gifford, 2003). One reason for the wide range is that each study uses different definitions of alcohol problems, uses different measurement tools, and categorizes levels of alcohol use uniquely. Nonetheless, alcohol misuse and use among HIV-positive persons is common, and alcohol consumption has been shown to decrease overall survival in this population (Braithwaite et al., 2007).
A major concern with alcohol use in HIV-infected people is the impact it has on HIV medication adherence. Adherence is the primary predictor of treatment success (Bangsberg 2006b). Poor adherence, once defined as less than 95% of doses taken, is now more specifically considered in relation to the class of medication in the regimen. Bangsberg (2006a) and his colleagues (Bangsberg et al., 2005) suggested that for single protease inhibitors, the window for viral suppression is 80–95% adherence, and for non-nucleoside reverse transcriptase inhibitors, the window is 54–100% adherence. The window has not yet been determined for other classes of HIV medication.
Alcohol use has been frequently linked with poor adherence (Chandler, Lau, & Moore, 2006; Parsons, Rosof, & Mustanski, 2007). In a study of 267 HIV-infected persons with a history of alcohol problems and taking antiretroviral medication, alcohol consumption was the most significant predictor of nonadherence (Samet, Horton, Meli, Freedberg, & Palepu, 2004). In another study, nearly half of problem drinkers reported taking medication off schedule, in comparison with 26% of those without problem drinking (Cook et al., 2001). In a study of 1,910 HIV-positive persons, those who drank at least moderately were significantly more likely to be nonadherent (Tucker et al., 2003). Fairly consistently, studies find that drinking is associated with nonadherence to HIV medications.
The true nature of the association between alcohol use and HIV medication adherence is yet to be understood. In most studies, the association between alcohol consumption and poor adherence may have been confounded by traits that may play a causal role in both frequency of alcohol consumption and poor adherence to medication, including personality factors (e.g., impulsivity and risk taking), psychiatric symptoms (e.g., anhedonia), cognitive or attitudinal factors (e.g., high value on health), or situational factors (e.g., chaotic and stressful environment). In our previous work—looking broadly at the role of social, cognitive, affective, and alcohol factors on adherence—it was the alcohol and cognitive factors that emerged as significant predictors of adherence (Parsons et al., 2007). Whether alcohol is actually driving nonadherence remains unclear.
To more definitively reveal the presence of a direct relationship between alcohol use and nonadherence, findings must establish whether or not these two behaviors occur simultaneously. As Braithwaite et al. (2005) have pointed out, “it is difficult to make inferences regarding the likely causality of this association because few studies controlled for these potential confounding variables and none performed detailed dose-response or temporal analyses.” Braithwaite et al. (2005) went on to do a temporal and dose response analysis for 2,702 HIV-positive and HIV-negative veterans and the impact of alcohol use on adherence to medication (both HIV and non-HIV medication). The unit of their analysis was “patient day” (level of drinking and missed medication on each day of the past 30 days assessed by using timeline followback), and analysis consisted of univariate logistic models that controlled for multiple observations by using cluster analysis. They found that alcohol consumption demonstrated a temporal and dose-response relationship to poor adherence. In other words, when the participants drank, they were more likely to miss medication, and when they drank more, they were even more likely to miss medication. They also found that HIV-positive people had poor adherence at lower levels of alcohol consumption than HIV-negative people, suggesting sensitivity to an alcohol-adherence relationship among this group.
The current study used hierarchical linear modeling (HLM) to investigate temporal and dose response relationships between alcohol use and medication adherence in an HIV- positive sample experiencing alcohol problems. The hierarchical models fit to these data took into account the “nesting” of multiple reports from the same individual; failure to take this dependency into account would have violated the assumption of independent observations. Our models simultaneously tested the temporal, within-person association between alcohol use and medication adherence; the between-persons main effects of individual-level cognitive, alcohol, and regimen factors; and for the first time, the moderating effects of individual-level factors on the relationship between alcohol consumption and medication adherence. As in Braithwaite et al. (2005), we explore the effects of alcohol both as a dichotomous variable (i.e., at least one drink) and as a continuous variable (i.e., number of standard drinks). Our study strengthens the literature both for the use of HLM and because the sample is one of the largest studies exploring the effect of alcohol on adherence with an exclusive sample of problem alcohol users. The analyses build on our previous work using regression analyses and structural equation modeling to evaluate the factors that predict adherence by using event-level analyses to test for a temporal association between the consumption of alcohol and medication adherence on the same day. We similarly build on our previous work, which identified latent variables that underlie multiple components of alcohol use and problems and cognitive factors related to medication adherence and which found no association between affective (e.g., depression and anxiety) and social factors (e.g., social support) and adherence (Parsons et al., 2007). We utilize factor scores in our analyses to better represent these broad constructs and also to reduce the effects of measurement error (Bollen, 1989).
Method
Participants
Participants were 272 HIV-positive men and women living in the greater New York City metropolitan area who were currently taking antiretroviral medication, reported problem-level drinking, and agreed to be part of a randomized clinical trial comparing motivational interviewing (MI) and cognitive behavioral therapy (CBT) to education for increasing medication adherence and reducing alcohol use. Data were collected between 2002 and 2005. Two recruitment methods were used: (a) interested patients contacted us in response to flyers placed in clinic waiting rooms and were then screened by telephone (n = 179, 65.8%) and (b) interested patients completed an on-site screener during HIV-related community events (n = 93, 34.2%). Inclusion criteria were: age greater than 18 years, a score of 8 or above on the Alcohol Use Disorder Identification Test (AUDIT), and currently on a highly active anti-retroviral therapy (HAART) regimen. A score of 8 on the AUDIT suggests problem-level drinking (Maisto, Carey, Carey, Gordon, & Gleason, 2000). People for whom their drug-related problems were more severe than were their alcohol-related problems and those with active psychosis were excluded.
A total number of 1,285 individuals phoned the project line for screening. Of these, 898 were excluded because they failed to meet eligibility criteria at the time of phone screening or upon secondary screening during the initial visit. The most common reasons for ineligibility were greater problems associated with other drug use in comparison with alcohol (n = 564), a score of less than 8 on the AUDIT (n = 308), and no alcohol use in the past 30 days (n = 61). A total of 105 failed to show for their first appointment, resulting in 282 eligible participants. However, 10 of these had incomplete baseline data, so the final sample for analysis was 272. The research was reviewed and approved by the Institutional Review Board of the investigators.
Procedure
All participants underwent a baseline interview intended to examine sociodemographic and biopsychosocial variables such as mental health, adherence-related social support and social norms, decision-making processes regarding adherence and alcohol use, regimen characteristics, motivation to change current behavior, and viral load and CD4 counts. The findings reported in this article represent data from the baseline interviews. The majority of the assessment was completed on an audio computer-assisted self-interview (ACASI) in which the participant responded to automated questions on a computer screen that they could either read or listen to with headphones. ACASI has been found to be an effective interview method for people of diverse educational backgrounds, and because they have audio assistance it eliminates the effects that reading ability has on internal validity (Gribble, Miller, Rogers, & Turner, 1999; Turner et al., 1998). Viral load and CD4 counts were obtained through an on-site blood draw by a certified phlebotomist. The interview generally lasted about 3 hr, and participants were paid $30.
Level 2 (Between-Subjects) Measures
Demographics
Participants were asked a series of demographic questions including age, gender, ethnicity, relationship status, sexual identity, and employment status.
Cognitive factor
Multiple scales measuring attitudes toward HIV medication adherence were used to measure the cognitive factor. The Adherence Attitudes Scale is an 18-item scale developed for a previous study on medication adherence (Halkitis, Kutnick, & Slater, 2005). It is intended to measure risk perception regarding vulnerability to treatment failure and other perceived negative health outcomes resulting from nonadherence. In our sample, the scale demonstrated good reliability (α = .95). The Decisional Balance Scale was constructed to measure attitudes toward adherence through a 22-item measure that includes perceived pros and cons of adhering. This measure is based on the Decisional Balance Inventory (Velicer, DiClemente, Prochaska, & Brandenburg, 1985) and adapted for pros and cons of taking HIV medication. The measure demonstrated good reliability (α = .89) in our sample. The Confidence for Adherence Scale consists of 11 items that were specifically developed for HIV medication adherence self-efficacy through pilot work with HIV-positive adults (Parsons, Rosof, Punzalan, & DiMaria, 2005). The measure asks participants to rate on a 5-point scale how confident they are that they could take their HIV medications on time under several circumstances (e.g., on vacation and out at night). The scale demonstrated good internal consistency in this sample (α = .91). Maximum likelihood (ML) factor analysis found the existence of one underlying factor that explained 40.22% of the total variance in these scales and provided a good fit to the data (Parsons et al., 2007). A score created using the regression method functioned as our measure of the Cognitive factor in all analyses.
Alcohol factor
Two different scales measuring alcohol-related problems and one measure of alcohol consumption were used to constitute the alcohol factor. First, during the screening interview we used the AUDIT, a 10-item survey, which measures alcohol consumption, dependence symptoms, and personal and social harm related to drinking over the past 30 days. The AUDIT has demonstrated good content, criterion, and construct validity and reliability (Bohn, Babor, & Kranzler, 1995). Second, during the ACASI, we assessed the negative consequences of alcohol use for the past 90 days on specific domains of the participants' lives using the Drinker Inventory of Negative Consequences (DrinC). The DrinC was used in Project MATCH and demonstrates good psychometric properties (Miller & Tonigan, 1995). Third, total current alcohol consumption was assessed using a timeline follow-back (TLFB) interview (Sobell & Sobell, 1992), during which participants reflected back on the past 30 days to report the number of standard drinks consumed each day. Research staff assisted with the TLFB interview to mark memorable events on the calendar as anchor points and then assisted the participant to recall day by day the number of standard drinks consumed. ML factor analysis found one factor that explained 38.92% of the total variance in each of these alcohol scales (see Parsons et al., 2007). A factor score created using the regression method was used as our measure of the Alcohol factor in all analyses.
HIV medication side effects
Two variables were used. The first was created by summing the total number of side effects attributed to HIV medications out of 13 possible effects, and the second examined the level of distress reported by the participant regarding these side effects on a scale from 0 (not at all) to 3 (extremely). These items we combined into a single variable by computing individual z scores and then summing them together.
HIV medication regimen complexity
This variable was created by summing the total number of dosing times and the total number of HAART pills taken per day.
Level 1 (Within-Subject) Measures
Alcohol use
Data on the number of standard drinks consumed each day for the past 14 days were obtained from the TLFB. The TLFB has demonstrated good test–retest reliability, convergent validity, and agreement with collateral reports and urine assays for alcohol and drug abuse (Demarce, Burden, Lash, Stephens, & Brambow, 2007; Fals-Stewart, O'Farrell, Freitas, McFarlin, & Rutigliano, 2000; Rice, 2007). TLFB reports of drinking behaviors are comparable to those obtained via daily diaries, palm computers, and interactive voice-response systems (Carney, Tennen, Affleck, DelBoca, & Kranzler, 1998; Toll, Cooney, McKee, & O'Malley, 2006). Furthermore, previous studies using a multilevel analytic approach have utilized the TLFB to examine temporal relationships between alcohol use and victimization (Parks & Fals-Stewart, 2004), alcohol use and sexual behavior (Irwin, Morgenstern, Parsons, Wainberg, & Labouvie, 2006; Weinhardt, Carey, Carey, Maisto, & Gordon, 2001), and alcohol use and HIV medication adherence (Braithwaite et al., 2005).
In order to investigate the complexities of the putative relationship between alcohol and medication adherence, alcohol use was defined in two ways. First, in order to measure whether consumption of any amount of alcohol was related to medication nonadherence, a dichotomous consumption variable was created with 0 representing no alcohol consumption and 1 representing consumption of one or more alcoholic drinks. Second, in order to investigate a dose-response relationship between alcohol consumption and medication adherence, a continuous- consumption variable was created, which represented the total number of standard drinks consumed on each day.
Adherence
Adherence to HIV medication was also assessed using the TLFB to help participants recall day by day all medication doses taken and missed during the past 2 weeks. The TLFB has been used in previous studies to assess HIV medication adherence (Braithwaite et al., 2005), and such self-reports have been significantly correlated with viral load and CD4 counts (Parsons, Rosof, & Mustanski, 2005). Self-report measures of adherence are robust and compare well to electronic measures (Pearson, Simoni, Hoff, Kurth, & Martin, 2007; Simoni et al., 2006). Research staff assisted with the TLFB interview to mark memorable events on the calendar as anchor points and then assisted the participant to recall day by day the number of doses missed. A period of 14 days was used to have the opportunity to capture 2 weeks of both weekday and weekend activity. Adherence was defined as at least 95% of all doses taken on a single day. This variable was scored so that 0 = adherent and 1 = nonadherent. It is important to note that during the time participants were enrolled in the study (2002–2005), HIV treatment providers and researchers were working under the premise that 95% adherence was necessary to successfully treat HIV infection and achieve maximal viral suppression, and participants were being instructed to maintain that level of adherence.
Analytic Strategy
Multilevel modeling using HLM v. 6.0 (Raudenbush & Bryk, 2002) was used to analyze the daily diary data. Multilevel modeling is an analytic procedure developed to account for the dependency in observations when data have a nested, multilevel structure, such as days (Level 1) nested within person (Level 2). In this case, the Level 1 relationship between alcohol consumption and medication adherence is modeled individually for each participant, and the average relationship, across participants, is reported. It is also possible to include Level 2 variables in the model to account for differences between participants in the average level of medication adherence or as moderators of the relationship between alcohol consumption and adherence. For example, we include the alcohol factor both as a predictor of average level of medication adherence and as a moderator of the relationship between daily alcohol consumption and medication adherence. Because the outcomes are binary, the Bernoulli outcome with LaPlace estimation was used as recommended by Raudenbush and Bryk (2002), which produces estimates of the odds ratio of nonadherence occurring conditionally on alcohol consumption. None of the Level 1 variables were centered, because they were either dichotomous or had a meaningful value for zero (e.g., number of drinks). Similarly, the Level 2 variables did not need to be centered within the HLM program, because they either had a meaningful value at zero (e.g., regimen complexity) or they were standardized and already had a mean of approximately zero.
Although the TLFB approach to assessing substance use has been found to have high retest reliability, convergent validity, and agreement with collateral reports and biological data (e.g., Fals-Stewart et al., 2000), we tested for one type of systematic reporting bias that could lead us to fail to identify a true association (i.e., Type II error) or wrongly identify a false associations (i.e., Type I error). If participants systematically tended to underreport one behavior (e.g., alcohol use) and overreport the other behavior (e.g., medication adherence) as they went further back through the timeline, it would result in an underestimate of the true relationship between alcohol consumption and medication adherence. On the other hand, if participants tended to increasingly overreport or underreport both behaviors as they went back through their timeline, it would result in an overestimate of the true relationship between alcohol consumption and medication adherence that was actually attributable to day in the timeline. To test for this possibility, we used a multilevel model with day in timeline (1 to 14) as an independent Level 1 predictor and alcohol consumption and medication adherence as dependent variables. If a significant linear effect of day exists, it suggests that systematic error in reporting has occurred and that day in timeline should be regressed out of reports of alcohol consumption and medication adherence before the relationship between these two variables is computed. Of course this analysis only tests for an important type of systematic self-report bias that could result in some kinds of false conclusions, but does not test for unsystematic inaccuracies in the TLFB data that would require another kind of design.
Results
The sample was predominantly men (78.3%, n = 213) and was ethnically diverse with 57.7% (n = 157) of the sample identifying as African American and 24.7% (n = 67) as Hispanic (see Table 1). Over half the sample (59.9%, n = 163) identified as gay or bisexual. Mean age was 43.7 (SD = 7.23) and ranged from 26 to 66 years. Gender, age, ethnicity, education, and sexual orientation were not significantly predictive of medication adherence (Parsons et al., 2007) and were not included in analyses. Furthermore, for within-person analyses, individuals are treated as their own controls, eliminating confounding effects of individual differences in demographics.
Table 1. Characteristics of Study Participants (N = 272).
| Characteristic | n | % |
|---|---|---|
| Race/ethnicity | ||
| African American | 157 | 57.7 |
| Hispanic | 67 | 24.7 |
| White | 30 | 11.0 |
| Mixed | 8 | 2.9 |
| Other | 10 | 3.7 |
| Sexual identity | ||
| Gay/homosexual | 130 | 47.8% |
| Straight/heterosexual | 109 | 40.1% |
| Bisexual | 33 | 12.1% |
| Relationship status | ||
| Single | 160 | 58.8% |
| Currently in a relationship | 112 | 41.2% |
| Employment status | ||
| Full-time | 14 | 5.1% |
| Part-time | 32 | 11.8% |
| Disabled—not working | 86 | 31.6% |
| Disabled—working off the books | 16 | 5.9% |
| Unemployed non-student | 110 | 40.4% |
| Unemployed student | 14 | 5.1% |
| Income | ||
| Less than $10,000 | 172 | 63.2% |
| $10,000–$19,999 | 69 | 25.4% |
| $20,000–$29,999 | 20 | 7.4% |
| More than $30,000 | 11 | 4.0% |
| Education | ||
| Did not complete high school | 60 | 22.0% |
| High school diploma/GED | 102 | 37.5% |
| Some college | 68 | 25.0% |
| Bachelor's degree | 32 | 11.9% |
| Graduate level training | 10 | 3.6% |
Note. Mean age = 43.7 years (SD = 7.23); mean CD4 counts = 418.41 (SD = 296.01); mean log10 HIV viral load = 3.29 copies/ml (SD = 1.47).
While all participants had at least a score of 8 on the AUDIT, indicating a level of problematic drinking (Maisto et al., 2000), the mean AUDIT score was 18.58 (SD = 7.26). On average, participants drank on 39.4% of days, and on those days the mean number of drinks consumed was 7.5 (SD = 8.09). Despite the severity of drinking in the sample, the majority (57.7%, n = 157) had never been treated for alcohol abuse. At least 95% adherence was reported by 43.0% (n = 118) of participants, and mean adherence for the past 14 days was 84.4%. Mean number of HAART medications was 2.82 (SD = .91), and the sample was on HIV medication for an average of 6.99 (SD = 4.15) years. On the basis of HIV polymerase chain reaction (PCR) analyses done at the baseline assessment and transformation into log10, the mean log10 HIV viral load was detected at an average of 3.29 copies/ml (SD = 1.47). The logarithmic transformation of the absolute number of copies has become the preferred unit of measurement for viral load. Mean CD4 counts were 418.41 (SD = 296.01).
Day in timeline did not show a significant linear association with medication adherence (OR = 1.01, 95% CI = .99 – 1.03). Similarly, day in timeline did not show a significant linear relationship with number of standard drinks reported (β = −0.01, SE = 0.02, p = .495). The lack of a significant relationship between day in timeline and reports of medication adherence or number of standard drinks suggests the lack of systematic error related to day in the TLFB.
Table 2 contains the results of the multilevel model fit to reports of HIV medication nonadherence and number of standard drinks consumed at Level 1, and the Cognitive, Alcohol, Side Effect, and Regimen Complexity factors at Level 2. The model allowed for main effects of each independent variable as well as cross-level interactions for which Level 2 variables moderated the relationship between alcohol consumption and medication adherence. At the top of Table 2, the parameter estimate 0.056 represents the mean odds of medication nonadherence on days in which no alcohol was consumed, holding constant all Level 2 predictors at their mean level. Moving down the table, the odds ratio 1.200 represents the effect of consuming alcohol on the likelihood of medication nonadherence. This represents a significant increase of 20% in the odds of medication nonadherence for each standard drink consumed.
Table 2. Multilevel Model Predicting HIV Medication Nonadherence From Number of Standard Drinks, Cognitive Factor, Alcohol Factor, Medication Side Effects, and Regimen Complexity.
| Variable | β | SE | Odds ratio | 95% confidence interval | p |
|---|---|---|---|---|---|
| Level 1 effects | |||||
| Mean nonadherence | 0.056 | 0.042–0.077 | |||
| Number of standard drinks | 0.182 | 0.007 | 1.200 | 1.182–1.218 | 0.000 |
| Level 2 effects | |||||
| Cognitive factor | −0.509 | 0.148 | 0.601 | 0.450–0.803 | 0.001 |
| Alcohol factor | 0.482 | 0.174 | 1.620 | 1.151–2.279 | 0.006 |
| Side effects | 0.062 | 0.083 | 1.064 | 0.905–1.252 | 0.450 |
| Regimen complexity | 0.041 | 0.090 | 1.042 | 0.873–1.245 | 0.646 |
| Cross-level interactions with number of standard drinks | |||||
| Cognitive factor | 0.023 | 0.008 | 1.023 | 1.006–1.040 | 0.007 |
| Alcohol factor | −0.052 | 0.008 | 0.949 | 0.934–0.965 | 0.000 |
| Side effects | 0.000 | 0.004 | 1.000 | 0.992–1.008 | 0.951 |
| Regimen complexity | 0.009 | 0.004 | 1.009 | 1.000–1.018 | 0.059 |
Note. Medication nonadherence was coded so that 0 = adherent and 1 = nonadherent. Level 2 variables are standardized.
The parameters listed under the Level 2 section of Table 2 represent the main effects of these variables on the average rate of medication nonadherence for each participant. These effects are conceptually similar to the results that would be achieved if each participant's medication nonadherence was averaged across their reports, and then the Cognitive, Alcohol, Side Effect, and Regimen Complexity factors were regressed onto these means. Under the Cognitive factor, the significant odds ratio of 0.601 indicates that a 1 standard deviation increase in the Cognitive factor resulted in a 40% decrease (i.e., 1–0.60) in the odds of medication nonadherence across all days. The Alcohol factor was also significantly related to medication nonadherence, with a 1 standard deviation increase in this factor associated with a 62% increase in the odds of medication nonadherence, holding all other predictors constant. The Side Effects and Regimen Complexity factors failed to show a significant main effect on medication adherence.
In the bottom third of Table 2 are the cross-level interactions, which allow for the Level 2 predictors to moderate the relationship between number of standard drinks and the odds of medication nonadherence. As is seen in the table, both the Cognitive and Alcohol factors significantly moderated the relationship between alcohol consumption and medication nonadherence. Figure 1a illustrates the moderating effect of the Cognitive factor. In this figure, the heavy line represents the relationship between number of standard drinks (x axis) and the probability of medication nonadherence (y axis) for participants 1 standard deviation above the mean on the Cognitive factor, whereas the thin line represents this relationship for participants 1 standard deviation below the mean. These lines significantly differ in both intercept and slope, as is indicated by the parameter estimates in Table 2. The difference in intercept indicates that participants higher on the Cognitive factor are less likely to be nonadherent to their HIV medication when no alcohol is being consumed. The small difference in slope indicates that participants who scored higher on the Cognitive factor were more strongly affected by alcohol consumption in regards to medication adherence. Figure 1b illustrates the moderating effect of the Alcohol factor on the relationship between alcohol consumption and medication nonadherence. Again, both the slopes and the intercepts are significantly different between those higher (heavy line) and lower (thin line) on the Alcohol factor. When no alcohol is being consumed, participants higher on the Alcohol factor are still less likely to be adherent to their HIV medications, as is indicated by the difference in intercept. However, the difference in slope between these two lines suggests that individuals higher on the Alcohol factor are less affected in their medication adherence by the consumption of alcohol. Neither the Side Effect nor the Regimen Complexity factors moderated the relationship between alcohol consumption and medication adherence.
Figure 1.
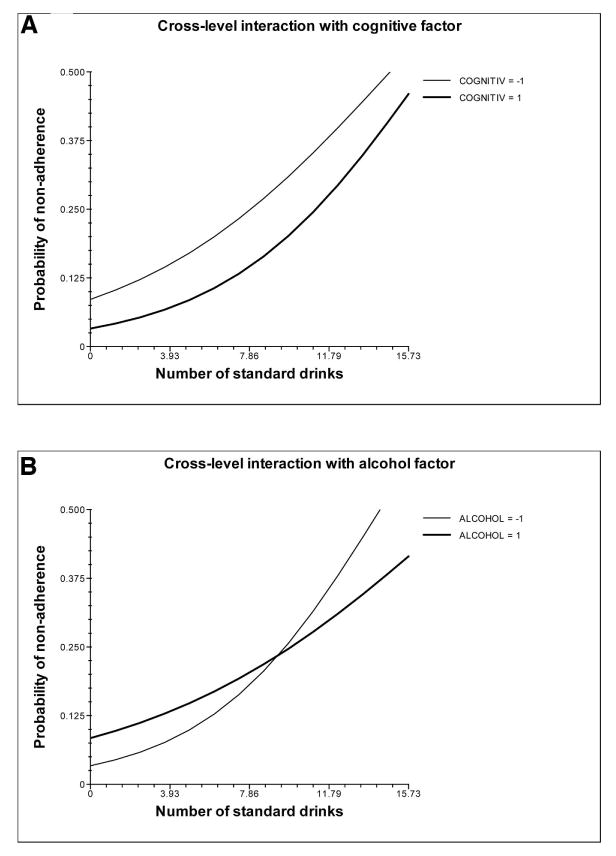
(A) Cognitive factors moderate the relationship between number of standard drinks and medication adherence. (B) Alcohol factors moderate the relationship between number of standard drinks and medication adherence. Medication nonadherence was coded so that 0 = adherent and 1 = nonadherent. COGNITIV represents the standardized Cognitive factor score. ALCOHOL represents the standardized Alcohol factor score.
Table 3 contains the results of the HLM analysis treating alcohol consumption as a dichotomous variable. In this model, having one or more drinks increased the odds of nonadherence by 8.78 times. As before, the Alcohol and Cognitive factors had significant main effects on individual differences in medication adherence. In this model there was a difference in the moderators of the association between alcohol consumption and medication adherence in comparison with the model that treated alcohol consumption as a continuous variable. Here, neither the Alcohol or Cognitive factorshowed significant moderation, but the Regimen Complexity factor did. As is shown in Figure 2, participants who had more complex regimens (bar with cross-hatch) were more affected by having one or more drinks than did participants who had less complex regimens (open bar).
Table 3. Multilevel Model Predicting HIV Medication Nonadherence From Dichotomous Alcohol Consumption, Cognitive Factor, Alcohol Factor, Medication Side Effects, and Regimen Complexity.
| Variable | β | SE | Odds ratio | 95% confidence interval | p |
|---|---|---|---|---|---|
| Level 1 effects | |||||
| Mean nonadherence | 0.036 | 0.026–0.049 | |||
| Dichotomous alcohol use | 2.173 | 0.104 | 8.782 | 7.160–10.771 | 0.000 |
| Level 2 effects | |||||
| Cognitive factor | −0.442 | 0.149 | 0.642 | 0.479–0.862 | 0.004 |
| Alcohol factor | 0.407 | 0.181 | 1.502 | 1.054–2.143 | 0.025 |
| Side effects | 0.055 | 0.084 | 1.057 | 0.897–1.245 | 0.510 |
| Regimen complexity | 0.033 | 0.089 | 1.033 | 0.868–1.230 | 0.712 |
| Cross-level interactions with dichotomous alcohol use | |||||
| Cognitive factor | 0.136 | 0.116 | 1.146 | 0.913–1.438 | 0.239 |
| Alcohol factor | 0.037 | 0.149 | 1.038 | 0.775–1.390 | 0.805 |
| Side effects | 0.049 | 0.055 | 1.050 | 0.943–1.169 | 0.377 |
| Regimen complexity | 0.124 | 0.058 | 1.132 | 1.009–1.269 | 0.034 |
Note. Medication nonadherence was coded so that 0 = adherent and 1 = nonadherent. Level 1 alcohol use was coded so that 0 = no alcohol use and 1 = alcohol use. Level 2 variables are standardized.
Figure 2.
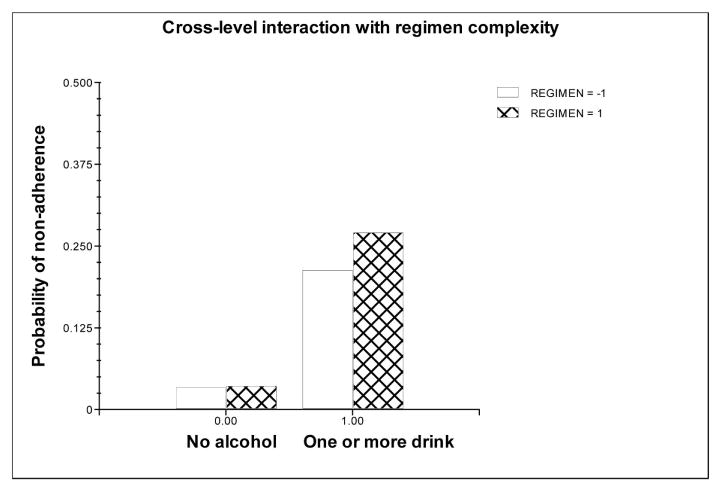
Regimen complexity moderated the relationship between dichotomous alcohol consumption and medication adherence. Medication nonadherence was coded so that 0 = adherent and 1 = nonadherent. REGIMEN represents the sum of total number of dosing times and the total number of HAART pills taken per day.
Discussion
This sample of urban HIV-positive men and women dealing with alcohol-related problems and the need for HIV medication adherence allowed a close examination of the relationship between these two behaviors in unprecedented ways. We found consumption of alcohol significantly and substantially increased the odds of medication nonadherence. Moreover, with each additional drink the odds of nonadherence continued to increase. This is a within-person relationship localized in time so that alcohol consumption was linked to reports of medication adherence on the same day.
Consistent with our previous reports (Parsons et al., 2007, in press) using regression analyses and structural equation modeling, both the Alcohol (a composite score of recent problems related to alcohol use) and Cognitive (a composite score of attitudes regarding positive expectations for taking HIV medications and self-efficacy to adhere) factors had significant between-persons effects on medication adherence. The Cognitive and Alcohol factors also had small but significant moderating effects on the within-person relationship between alcohol consumption and medication nonadherence. Specifically, people with higher scores on the Cognitive factor were less likely to be nonadherent when no alcohol was consumed but more quickly affected in terms of their adherence than did those with lower Cognitive factor scores. This suggests that people who are cognitively focused on their health (in terms of viewing positive benefits of taking HIV medications and with high self-confidence in their ability to adhere) are actually more negatively impacted by alcohol use than those who see few benefits or have little self-efficacy for medication adherence.
The Health Belief Model (Rosenstock, 1974), which posits that a cost-benefit analysis precedes a decision to take action, offers some explanation for why those with a higher cognitive approach to their adherence were more impacted by alcohol, since intoxication is known to impair executive cognitive functioning such as planning, organization, self-monitoring, and attention. Alcohol may have a greater effect on their ability to think through health-related decisions because they exercise a more conscientious approach in the first place. A second explanation may lie in the particular beliefs participants hold regarding negative interactions between medication and alcohol (Sankar, Wunderlich, Neufeld, & Luborsky, 2007). It may be that participants made a purposeful decision to not take their HIV medications during drinking episodes because of erroneous fears that it is actually worse to take medications while drinking than it is to skip doses. It is not clear why alcohol had less of an impact on people with lower Cognitive factor scores. Perhaps for them, adherence is more of a habitual response, with less room for behavioral disruption resulting from drinking. More research is necessary to fully understand the link between cognitive factors and alcohol consumption.
For those with higher scores on the Alcohol factor (more severe alcohol-related problems), alcohol use had less of an effect on adherence than for those with fewer alcohol- related problems. These results are consistent with findings that light and moderate drinkers skip medication when drinking more frequently than do heavy drinkers (Sankar et al., 2007). One explanation is that people with more alcohol problems presumably drink more and may have established a consistent drinking routine that may be less disruptive to behaviors such as medication taking. However, for those who have lower scores on the Alcohol factor, presumably the less frequent drinkers, adherence behaviors may be more disrupted by the break in routine resulting from drinking.
Regimen complexity did not directly impact medication adherence, but it did moderate the relationship between consuming any alcohol and nonadherence. Those with more complex regimens were the most negatively affected in their adherence when consuming even one alcoholic beverage. While previous findings have not directly shown this, one study did find that cognitively compromised participants on more complex regimens had the greatest difficulty with adherence (Hinkin et al., 2002). Once the stressor of alcohol is introduced, and the associated cognitive impairment, regimen complexity then appears to become an issue. Fortunately, regimen complexity will continue to become less of an issue as HAART dosing becomes more simplified. People with more stressors in their lives may benefit the most from a reduction in regimen complexity.
Although previous studies have identified side effects as a factor related to both HIV medication nonadherence as well as overall reductions in quality of life among seropositive persons (Ammassari et al., 2001; Johnson et al., 2005), we did not find that side effects moderated the relationship between alcohol use and medication adherence. It is possible that, among a sample composed entirely of those with problematic levels of alcohol use, that participants were unable to distinguish between side effects attributed to HIV medications and those attributed to alcohol abuse. In addition, participants in the study had been on HIV medications, on average, for almost 7 years. As a result, participants have had considerable time to adjust to side effects and develop strategies to cope with them, such that the side effects do not negatively impact their adherence.
While we found no evidence of systematic error in reports of alcohol consumption and medication adherence across days of reporting using the TLFB approach, it is important to acknowledge that another type of measurement error is possible—participants may be less accurate in their reports of behaviors as they go further back in their timeline. To the extent that this occurs, it will result an in underestimate of the true relationship between alcohol consumption and medication adherence. Because it is not possible to statistically correct for this potential error in our design, the significant association between alcohol consumption and medication adherence that we report should be considered the lower bound of the true relationship. Fortunately, previous studies designed to test for this type of error among substance- abusing populations suggest that the TLFB approach has good reliability and validity (Demarce et al., 2007; Fals-Stewart et al., 2000; Rice, 2007). Of course, it is also possible that the self-report data, in general, were subject to difficulties with recall among our participants. Future studies should consider the use of multiple measures, self-report and biological, in assessing both drinking behaviors and HIV medication adherence.
Implications of this research point to the importance of cognitive–behavioral interventions when addressing medication adherence. Those with a more cognitively driven approach to HIV medication adherence suffered more in their adherence when drinking alcohol, suggesting more of a disruption in cognitive processes. Behavioral interventions designed to help patients solidify behavioral patterns around adherence behavior (particularly in the presence of alcohol use) could offer a buffer against the interruption effect that alcohol appears to have. This could be paired with motivational interviewing, which would help to highlight the discrepancy in these patients between their general conscientious approach to adherence and their behavior when drinking, as well as to enhance their self-efficacy to adhere in general, even while drinking. Enhancing positive expectations for taking HIV medications, while at the same time providing factual and accurate information regarding the lack of any real danger from mixing alcohol with HIV medications, could further help to promote adherence during drinking episodes.
Additionally, interventions that work to decrease quantity of, frequency of, and related alcohol problems may increase medication adherence, even on days when a patient drinks. This speaks to the relevance of harm reduction and therapies that consider reduction in alcohol use as an acceptable goal. This has been seen most recently in much of the motivational interviewing philosophy that asks clients to participate in goal setting, including nonabstinence goals. As shown in our data, even reducing one or two drinks per drinking event can positively increase HIV medication adherence, and as such, moderated drinking goals should be considered for those patients not interested in abstinence.
A final implication of these findings is that less complex medication regimes are associated with greater adherence when alcohol is being consumed. This is important information for healthcare providers who may then consider alcohol use as a factor in determining medication regimen. Providers should make special efforts to simplify the HIV medication regimens of their patients who use alcohol. These results also call to attention the need for screening for problem drinking in HIV-positive patients so that proper referrals to treatment can be made, thereby potentially increasing medication adherence.
Acknowledgments
Project PLUS was supported by a grant from the National Institute of Alcohol Abuse and Alcoholism (RO1 AA13556) to Jeffrey T. Parsons. The contributions of Elana Rosof were supported through a postdoctoral fellowship in the Behavioral Sciences Training in Drug Abuse Research program sponsored by Medical and Health Research Association of New York City, Inc., and the National Development and Research Institutes with funding from the National Institute on Drug Abuse (5T32 DA07233). The authors acknowledge the contributions of the other members of the Project PLUS team: Catherine Holder, Jose Nanin, Bradley Thomason, Michael Adams, Christian Grov, James Kelleher, Juline Koken, and Chris Hietikko. We would also like to thank Kendall Bryant for his support of the project and all of the clinics and sites that provided access to potential participants.
Contributor Information
Jeffrey T. Parsons, Hunter College and the Graduate Center of the City University of New York, Center for HIV/AIDS Educational Studies and Training
Elana Rosof, Medical and Health Research Association of New York City, Inc..
Brian Mustanski, University of Illinois—Chicago.
References
- Ammassari A, Murri R, Pezzotti P, Trotta MP, Ravasio L, De Longis P, et al. Self-reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. Journal of Acquired Immune Deficiency Syndromes. 2001;28:445–449. doi: 10.1097/00042560-200112150-00006. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clinical Infectious Diseases. 2006a;43(7):939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR. Monitoring adherence to HIV antiretroviral therapy in routine clinical practice: The past, the present, and the future. AIDS and Behavior. 2006b;10(3):249–251. doi: 10.1007/s10461-006-9121-7. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Acosta E, Gupta R, Guzman D, Riley ED, Harrigan PR. Differences in protease and non-nucleoside reverse transcriptase inhibitor adherence-resistance relationships are explained by virologic fitness. AIDS. 2005;20(2):223–231. doi: 10.1097/01.aids.0000199825.34241.49. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): Validation of a screening instrument for use in medical settings. Journal of Studies on Alcohol. 1995;56:423–432. doi: 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- Bollen KA. Structural equations with latent variables. New York: Wiley; 1989. [Google Scholar]
- Braithwaite RS, Conigliaro J, Roberts MS, Schecher S, Schaefer A, McGinnis K, et al. Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care. 2007;19(4):459–466. doi: 10.1080/09540120601095734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcoholism: Clinical and Experimental Research. 2005;29:1190–1197. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- Carney MA, Tennen H, Affleck G, DelBoca FK, Kranzler HR. Levels and patterns of alcohol consumption using timeline followback, daily diaries and real-time “electronic interviews”. Journal of Studies on Alcohol. 1998;59(4):447–454. doi: 10.15288/jsa.1998.59.447. [DOI] [PubMed] [Google Scholar]
- Chandler G, Lau B, Moore RD. Hazardous alcohol use: A risk factor for non-adherence and lack of suppression in HIV infection. Journal of Acquired Immune Deficiency Syndromes. 2006;43(4):411–417. doi: 10.1097/01.qai.0000243121.44659.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RL, Sereika SM, Hunt SC, Woodard WC, Erlen JA, Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. Journal of General and Internal Medicine. 2001;16:83–86. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarce JM, Burden JL, Lash SJ, Stephens RS, Brambow SC. Convergent validity of the timeline followback for persons with comorbid psychiatric disorders engaged in residential substance use treatment. Addictive Behaviors. 2007;32(8):1582–1592. doi: 10.1016/j.addbeh.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, O'Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: Psychometric properties. Journal of Consulting and Clinical Psychology. 2000;68(1):134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, et al. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States. Journal of Studies on Alcohol. 2002;63:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- Greenfield TK, Mdanik LT, Rogers JD. A 10-year national trend study of alcohol consumption, 1984-1995: Is the period of declining drinking over? American Journal of Public Health. 2000;90:47–52. doi: 10.2105/ajph.90.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble JN, Miller HG, Rogers SM, Turner CF. Interview mode and measurement of sexual behaviors: Methodological issues. Journal of Sexual Research. 1999;36:16–25. doi: 10.1080/00224499909551963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkitis PN, Kutnick AH, Slater S. The social realities of adherence to protease inhibitor regimens: Substance use, health care, and psychological states. Journal of Health Psychology. 2005;10:545–558. doi: 10.1177/1359105305053422. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, et al. Medication adherence among HIV+ adults: Effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59(12):1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin TW, Morgenstern J, Parsons JT, Wainberg M, Labouvie E. Alcohol and sexual HIV risk behavior among problem drinking men who have sex with men: An event level analysis of timeline followback data. AIDS & Behavior. 2006;10(3):299–07. doi: 10.1007/s10461-005-9045-7. [DOI] [PubMed] [Google Scholar]
- Johnson MO, Charlebois E, Morin SF, Catz SL, Goldstein RB, Remien RH, et al. Perceived adverse effects of antiretroviral therapy. Journal of Pain & Symptom Management. 2005;29:193–205. doi: 10.1016/j.jpainsymman.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Lefevre F, O'Leary B, Moran M, Mossar M, Yarnold PR, Martin GJ, et al. Alcohol consumption among HIV-infected patients. Journal of General Internal Medicine. 1995;10:458–460. doi: 10.1007/BF02599920. [DOI] [PubMed] [Google Scholar]
- Maisto SA, Carey MP, Carey KB, Gordon CM, Gleason JR. Use of the AUDIT and the DAST-10 to identify alcohol and drug use disorders among adults with a severe and persistent mental illness. Psychological Assessment. 2000;12:186–192. doi: 10.1037//1040-3590.12.2.186. [DOI] [PubMed] [Google Scholar]
- Miller WR, Tonigan JS. Project MATCH Monograph Series. Vol. 4. Rockville, MD: NIAAA; 1995. The Drinker Inventory of Consequences (DrInC) DHHS Publication No. 95-3911. [Google Scholar]
- Parks KA, Fals-Stewart W. The temporal relationship between college women's alcohol consumption and victimization experiences. Alcoholism: Clinical and Experimental Research. 2004;28(4):625–629. doi: 10.1097/01.alc.0000122105.56109.70. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Rosof E, Mustanski B. Patient related factors predicting HIV medication adherence among men and women with alcohol problems. Journal of Health Psychology. 2007;12:357–370. doi: 10.1177/1359105307074298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Rosof E, Mustanski B. Medication adherence mediates the relationship between adherence self-efficacy and biological assessments of HIV health among those with alcohol use disorders. AIDS & Behavior. 2008;12:95–103. doi: 10.1007/s10461-007-9241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Rosof E, Punzalan JC, DiMaria L. Integration of motivational interviewing and cognitive behavioral therapy to improve HIV medication adherence and reduce substance use among HIV-positive men and women: Results of a pilot project. AIDS Patient Care and STDs. 2005;19:31–39. doi: 10.1089/apc.2005.19.31. [DOI] [PubMed] [Google Scholar]
- Pearson CR, Simoni JM, Hoff P, Kurth AE, Martin DP. Assessing antiretroviral adherence via electronic drug monitoring and self-report: An examination of key methodological issues. AIDS & Behavior. 2007;11(2):161–173. doi: 10.1007/s10461-006-9133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd. Newbury Park, CA: Sage; 2002. [Google Scholar]
- Rice C. Retest reliability of self-reported daily drinking: Form 90. Journal of Studies on Alcohol and Drugs. 2007;68(4):615. doi: 10.15288/jsad.2007.68.615. [DOI] [PubMed] [Google Scholar]
- Rosenstock IM. The health belief model and preventive health behavior. Health Education Monographs. 1974;2:354–386. doi: 10.1177/109019817800600406. [DOI] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcoholism: Clinical and Experimental Research. 2004;28:72–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- Sankar A, Wunderlich T, Neufeld S, Luborsky M. Seropositive African Americans' beliefs about alcohol and their impact on anti-retroviral adherence. AIDS & Behavior. 2007;11(2):195–203. doi: 10.1007/s10461-006-9144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS & Behavior. 2006;10(3):227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A technique for assessing self-reported alcohol consumption. In: Litton R, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. [Google Scholar]
- Toll BA, Cooney NL, McKee SA, O'Malley SS. Correspondence between interactive voice response (IVR) and timeline followback (TLFB) reports of drinking behavior. Addictive Behaviors. 2006;31(4):726–731. doi: 10.1016/j.addbeh.2005.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medication in a sample of patients with human immunodeficiency virus infection. The Journal of American Medicine. 2003;114:573–580. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- Turner CF, Ku L, Rogers SM, Linberg LD, Pleck JH, Sonenstein FL. Adolescent sexual behavior, drug use, and violence: Increased reporting with computer survey technology. Science. 1998;280:867–874. doi: 10.1126/science.280.5365.867. [DOI] [PubMed] [Google Scholar]
- Velicer WF, DiClemente CC, Prochaska JO, Brandenburg N. Decisional balance measure for assessing and predicting smoking status. Journal of Personality and Social Psychology. 1985;48:1279–1289. doi: 10.1037//0022-3514.48.5.1279. [DOI] [PubMed] [Google Scholar]
- Weinhardt LS, Carey MP, Carey KB, Maisto SA, Gordon CM. The relation of alcohol use to HIV-risk sexual behavior among adults with a severe and persistent mental illness. Journal of Consulting and Clinical Psychology. 2001;69(1):77–84. doi: 10.1037//0022-006x.69.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]


