Abstract
Increased polyamine synthesis and inflammation have long been associated with colon carcinogenesis in both preclinical models and in humans. Recent experimental studies suggest that polyamines may be mechanistically involved in colonic inflammatory processes. Genetic epidemiology results indicate that a single nucleotide polymorphism influencing the expression of a polyamine biosynthetic gene is associated with both risk of colon polyp occurrence and recurrence, and the response to aspirin as a polyp preventive agent. A prospective, randomized, placebo-controlled clinical trial of combination difluoromethylornithine (DFMO), a selective inhibitor of polyamine synthesis, and sulindac, a non-steroidal anti-inflammatory drug (NSAID), found that the three year treatment was associated with a 70% reduction recurrence of all adenomas, and over a 90% reduction in recurrence of advanced and/or multiple adenomas, without evidence of serious toxicities. This proof-of-principle trial indicates that targeting polyamine synthesis and inflammation can be an effective strategy for preventing the occurrence of the advanced and/or multiple adenomas that are most closely associated with the development of colon cancers in humans.
BACKGROUND
Virchow speculated about the role of chronic inflammation in cancer in the 1860’s; this topic has been more recently reviewed (1). A century later, Russell and Snyder were among the first to document high levels of ornithine decarboxylase (ODC) enzyme activity in proliferating cells and tissues, including those derived from various tumor types (2). The role of ODC, the first enzyme in the synthesis of the ubiquitous polyamines which are involved in growth, development and cancer has been reviewed elsewhere (3).
Evidence for the efficacy of targeting inflammation and polyamine synthesis for cancer chemoprevention began to accumulate over thirty years ago. Chemoprevention of cancer is a strategy that employs treatments during the stages of carcinogenesis prior to the development of invasive cancer (4). Sporn (5) was among the first to propose combinations of agents for cancer chemoprevention. The rationale for this proposal followed after the success of combination chemotherapy for certain types of cancer (6), and offered the prospect of reduced toxicities by lowering doses of individual agents. Representative studies targeting polyamine synthesis and inflammation for chemoprevention of colon and intestinal carcinogenesis evaluated several rodent models treated with the selective ODC inhibitor D,L-α-difluoromethylornithine (DFMO) alone and in combination with several non-steroidal anti-inflammatory drugs (NSAIDS), including piroxicam (7), aspirin (8), celecoxib (9) and sulindac (10). These combinations have proved to be potent inhibitors of colon and intestinal polyps in all models and, in the case of carcinogen-treated rats, invasive colon cancers.
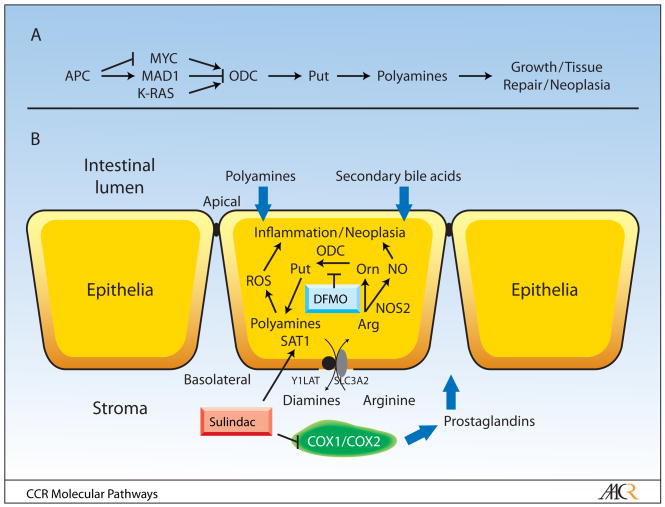
Risk factors for colon cancer include both genetic and intestinal luminal factors. One heritable genetic risk factor, which conveys risk of colon cancer, is the adenomatous polyposis coli (APC) tumor suppressor gene (11). APC and the K-RAS oncogene are two of the most commonly mutated genes found in human colon cancers (12). As depicted in Figure 1A, APC and K-RAS are both activators of polyamine synthesis, albeit by different mechanisms (3). Polyamines, which derive from the amino acid ornithine and its precursor arginine, are also intestinal luminal risk factors. Dietary polyamines enhance intestinal and colonic tumorigenesis (13, 14). Colonic luminal polyamines are also produced by enteric bacteria; both diet and enteric bacteria provide sources of potential tumor-promoting polyamines for colonic epithelial cells (see Figure 1B).
Fig. 1.
(panel A) ODC expression is regulated by a number of signaling pathways. The APC tumor suppressor gene influences the expression of the MYC oncogene and the MYC antagonist MAD1(34). These E-box transcription factors, in turn, bind to consensus elements, including two E-boxes flanking a SNP which has functional consequences for transcription factor binding, to regulate ODC transcription (27). ODC expression is also regulated by the K-RAS oncogene (35). ODC converts ornithine to the diamine putrescine. This diamine is the precursor for the longer chain amines spermidine and spermine. Genetic and pharmacological evidence shows that this pathway is essential for normal growth, development, tissue repair and neoplasia (3). (panel B) The relationship between polyamine metabolism and inflammatory pathways in the colonic mucosa are depicted in this panel. Abbreviations include: ROS (reactive oxygen species), NO (nitric oxide), Arg (arginine), Orn (ornithine), Put (putrescine), COX (cyclooygenease), NOS (nitric oxide synthase), ODC (ornithine decarboxylase), SAT1 (spermidine/spermine N1-acetyltransferase). Diamines, including putrescine, monoacetylspermidine and diacetylspermidine are exported by a common transporter (36), which was recently identified by us to including the solute carrier protein SLC3A2 (23). SLC3A2 partners with members of the Y+LAT family to form an arginine transporter. The roles of luminal factors, including polyamines and secondary bile acids, and stromal factors, such as prostaglandins, are shown. DFMO and sulindac lower polyamine contents in colonic epithelial cells by suppressing polyamine synthesis and activating polyamine catabolism and export. Sulindac also inhibits mucosal cyclooxygenases, which are predominately expressed in the colonic stroma prior to the formation of epithelial neoplasia.
Colonic bacteria provide sources of other luminal risk factors for colon cancer. These bacteria metabolize primary bile acids to secondary bile acids, which have been associated with colon cancers in humans (15) and are capable of promoting colon carcinogenesis in rodent models (16). Dietary administration of the secondary bile acid deoxycholate induces a colitis-like phenotype in mice (17). This bile acid-induced inflammatory response is suppressed by loss of nitric oxide synthase 2 (NOS2) alleles in genetically engineered mice (GEM) (18), which imply a role for the NOS2 substrate arginine (see Figure 1B). Other studies have provided a direct linkage between dietary arginine and intestinal and colonic tumorigenesis. These studies indicate that loss of NOS2 alleles (19), or treatment with DFMO (20), can suppress arginine-induced intestinal carcinogenesis. Together with the studies of Bernstein et al (17,18), these latter studies suggest a linkage between polyamines and inflammation and polyamines, inflammation and colon cancer.
This linkage may be more than simply an association. Microarray analysis of human colon cancer-derived cells identified the spermidine/spermine acetyltransferase (SAT1) as a target of the NSAID sulindac (21). Sulindac and other NSAIDS act by distinct transcriptional mechanisms to induce SAT1 and promote the export of diamines and acetylpolyamines (the products of SAT1) in both cell and mouse models (14, 22). Acetylation and export work in concert with inhibition of polyamine synthesis to lower cell and tissue polyamine contents. A diamine and acetylpolyamine exporter has recently been identified as a component of the solute carrier and arginine transporter containing SLC3A2 and Y+LAT subunits. SAT1 physically associates with SLC3A2 (23) (Figure 1B). Since this complex works as an arginine/diamine (or acetylpolyamine) antiporter, increased production of the diamine putrescine as a consequence of increased ODC activity would enhance, while decreased production of putrescine would suppress, the activity of the SLC3A2/Y+LAT transporter. In certain cases, increased polyamine synthesis may be anti-inflammatory by channeling arginine metabolism away from nitric oxide production (24). In this model, treatment with DFMO exacerbated the colitis, presumably by increasing NO levels. It is unknown whether DFMO would have a similar effect on inflammation-associated carcinogenesis in this model. However, these data suggest that DFMO combinations with NO inhibitors might be useful in this context. We have obtained evidence for channeling of polyamine metabolism with the unexpected finding that ODC, SAT1 and SLC3A2 are physically associated in colon-derived cells (23).
Polyamines contribute to inflammatory responses by mechanisms in addition to those affecting tissue arginine levels. Polyamines are oxidized by several amine oxidases to produce reactive oxygen species and aldehydes (25) (Figure 1B). Polyamines can also influence the expression of the pro-inflammatory gene cyclooxygenase 2 (COX2) by a posttranscriptional mechanism (26).
CLINICAL-TRANSLATIONAL ADVANCES
The hypothesis that increased arginine might be associated with colon carcinogenesis in humans was evaluated in a genetic and dietary epidemiology study of patients in a cancer registry (9). Increased survival in colon cancer patients with a family history of this disease was associated with low consumption of red meat, which was used as a surrogate for arginine consumption.
The hypothesis that polyamines, inflammation and carcinogenesis are linked is strengthened by several genetic epidemiology studies. Our group first reported that a single nucleotide polymorphism (SNP) in the ODC promoter, which displayed functional consequences for E-Box activator (e.g. MYC) and repressor (e.g. MAD1) binding (see Figure 1A), was associated with risk of recurrence of colon polyps in a clinical cancer prevention trial (27). Six hundred eighty-eight individuals in the Wheat Bran Fiber prevention trial were genotyped for the ODC G316A SNP. The ODC 316AA genotype was associated with approximately a 50% reduction in risk of polyp recurrence, compared to those individuals with the ODC 316GG genotype (odds ratio 0.48). In reported aspirin users, the ODC 316AA genotype was associated with a 90% reduction in risk of polyp recurrence, compared to non-aspirin use reporters with the ODC 316GG genotype. Hubner and co-workers (28) genotyped 546 participants for the ODC G316A SNP in the United Kingdom Colorectal Adenoma (CRA) Prevention trial of aspirin for CRA recurrence prevention. They found a similar reduction in risk of adenoma recurrence in people with the ODC 316AA genotype, compared to those with the ODC 316GG genotype (relative risk of 0.43). The risk of polyp recurrence in this trial was further decreased in the ODC 316AA group, compared to the ODC 316GG group, by aspirin use. A third group provided independent corroboration of an association between the ODC G316A SNP and aspirin use. Barry and co-workers genotyped participants in a prospective, randomized study of aspirin for prevention of CRA conducted by the Polyp Prevention Study Group. The ODC G316A SNP was not an independent prognostic factor for adenoma recurrence, but was a statistically significant predictor of response to aspirin for prevention of CRA recurrence in this study (29).
A model for the interaction between the ODC SNP and NSAID action in colon carcinogenesis (3), which includes both cyclooxygenase-dependent and –independent actions of NSAIDS, is depicted in Figure 1B.
This model has been tested over the past decade in a prospective, randomized placebo-controlled trial of combination DFMO and sulindac for prevention of recurrence in patients with prior CRA (30). Entry criteria for this trial included removal of a CRA within one year of study entry. Patients with genetic risk of colon cancer, such as individuals with familial adenomatous polyposis (FAP) and other polyposis syndromes, were excluded. Participants with current or prior colon or other cancers were also excluded. Participants received the combination of DFMO (one 500 mg pill daily) and sulindac (one 150 mg pill daily), or placebo pills, for 3 years. Primary endpoints included adenoma recurrence and toxicity assessment. Treatment with DFMO and sulindac was associated with a 70% reduction in total polyps, and over a 90% reduction in both advanced adenomas and in patients with multiple recurrent adenomas, at the end of three years. The only statistically significant toxicity noted in this trial was a hearing loss of uncertain clinical significance, which appears to be limited to a small subset of participants (31).
The clinical trial of combination DFMO and sulindac serves as an important proof of the principle that targeting polyamine synthesis and inflammation is an effective method for prevention of recurrent colon polyps. The challenge facing us and other workers in this field is to bring this advance into clinical practice for the management of patients with high risk for colon, and potentially other, cancers. It is clear that this drug combination has some toxicities, and initial applications should be limited to those individuals where a clear positive benefit to risk ratio can be established, such as people with high risk of developing colon cancer. High risk groups would include those with genetic risk factors, prior advanced and/or multiple colon adenomas and prior colon cancer. Future clinical trials in all three of these risk groups are in the planning stages.
Drug availability is another serious challenge. Both DFMO and sulindac are old drugs, and DFMO has not been commercially available for some time. We have recently established a company to produce good medical practice (GMP) quality DFMO for future clinical cancer prevention and treatment trials in humans.
The time to completion of clinical trials that might support an approval by the Food and Drug Administration (FDA) for a cancer prevention indication is a very serious problem that must be solved. Most epithelial cancers have long natural histories, with years often separating the development of invasive cancers after appearance of precancerous intraepithelial neoplasia (32). As evidenced by the approval of celecoxib for treatment of patients with the genetic syndrome familial adenomatous polyposis (FAP), which confers risk of colon cancer, the FDA has not accepted reduction in number of colon polyps as an indication of clinical benefit in this patient population. In the approval of celecoxib for FAP, the FDA expressed concerns regarding both clinical benefit and long term safety of the drug.
Cancer researchers should consider the example followed by investigators working to prevent deaths due to cardiovascular disease. It has been estimated that the dramatic decrease in deaths due to heart disease over the past 30 years are roughly equally divided between efforts to reduce risk factors, including use of chemoprevention methods, and mechanism-based therapies (33). Public and private sector interests involving academic research, professional medical and commercial pharmaceutical groups, aided by patient advocacy groups, need to work with the FDA to identify paths to implement cancer chemoprevention strategies into the standard practice of managing patients with high risk of colon and other cancers.
Acknowledgments
This work has been supported by a series of grants and contracts from the National Institutes of Health/National Cancer Institute, including CA23074, CA47396, CA59024, CN75019, CA72008, CA88078, CA95060 and CA123065.
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell D, Snyder SH. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968;60:1420–7. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerner EW, Meyskens FL., Jr Polyamines and cancer: old molecules, new understanding. Nature Reviews Cancer. 2004;4:781–92. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 4.Sporn MB, Dunlop NM, Newton DL, Smith JM. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids) Fed Proc. 1976;35:1332–8. [PubMed] [Google Scholar]
- 5.Sporn MB. Combination chemoprevention of cancer. Nature. 1980;287:107–8. doi: 10.1038/287107a0. [DOI] [PubMed] [Google Scholar]
- 6.Frei E., 3rd Combination cancer therapy: Presidential address. Cancer Res. 1972;32:2593–607. [PubMed] [Google Scholar]
- 7.Nigro ND, Bull AW, Boyd ME. Inhibition of intestinal carcinogenesis in rats: effect of difluoromethylornithine with piroxicam or fish oil. J Natl Cancer Inst. 1986;77:1309–13. [PubMed] [Google Scholar]
- 8.Li H, Schut HA, Conran P, et al. Prevention by aspirin and its combination with alpha-difluoromethylornithine of azoxymethane-induced tumors, aberrant crypt foci and prostaglandin E2 levels in rat colon. Carcinogenesis. 1999;20:425–30. doi: 10.1093/carcin/20.3.425. [DOI] [PubMed] [Google Scholar]
- 9.Zell JA, Ignatenko NA, Yerushalmi HF, et al. Risk and risk reduction involving arginine intake and meat consumption in colorectal tumorigenesis and survival. Int J Cancer. 2007;120:459–68. doi: 10.1002/ijc.22311. [DOI] [PubMed] [Google Scholar]
- 10.Ignatenko NAea. Nutrition and Cancer. 2008 doi: 10.1080/01635580802401317. in press. [DOI] [PubMed] [Google Scholar]
- 11.Groden J, Thliveris A, Samowitz W, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 12.Sjoblom T, Jones S, Wood LD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–74. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 13.Duranton B, Nsi-Emvo E, Schleiffer R, Gosse F, Galluser M, Raul F. Suppression of preneoplastic changes in the intestine of rats fed low levels of polyamines. Cancer Res. 1997;57:573–5. [PubMed] [Google Scholar]
- 14.Ignatenko NA, Besselsen DG, Roy UK, et al. Dietary putrescine reduces the intestinal anticarcinogenic activity of sulindac in a murine model of familial adenomatous polyposis. Nutr Cancer. 2006;56:172–81. doi: 10.1207/s15327914nc5602_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy BS, Wynder EL. Metabolic epidemiology of colon cancer. Fecal bile acids and neutral sterols in colon cancer patients and patients with adenomatous polyps. Cancer. 1977;39:2533–9. doi: 10.1002/1097-0142(197706)39:6<2533::aid-cncr2820390634>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.Reddy BS, Narasawa T, Weisburger JH, Wynder EL. Promoting effect of sodium deoxycholate on colon adenocarcinomas in germfree rats. J Natl Cancer Inst. 1976;56:441–2. doi: 10.1093/jnci/56.2.441. [DOI] [PubMed] [Google Scholar]
- 17.Bernstein H, Holubec H, Bernstein C, et al. Unique dietary-related mouse model of colitis. Inflamm Bowel Dis. 2006;12:278–93. doi: 10.1097/01.MIB.0000209789.14114.63. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein H, Holubec H, Bernstein C, et al. Deoxycholate-induced colitis is markedly attenuated in Nos2 knockout mice in association with modulation of gene expression profiles. Dig Dis Sci. 2007;52:628–42. doi: 10.1007/s10620-006-9608-0. [DOI] [PubMed] [Google Scholar]
- 19.Yerushalmi HF, Besselsen DG, Ignatenko NA, et al. The role of NO synthases in arginine-dependent small intestinal and colonic carcinogenesis. Molecular Carcinogenesis. 2006;45:93–105. doi: 10.1002/mc.20168. [DOI] [PubMed] [Google Scholar]
- 20.Yerushalmi HF, Besselsen DG, Ignatenko NA, et al. Role of polyamines in arginine-dependent colon carcinogenesis in Apc(Min) (/+) mice. Mol Carcinog. 2006;45:764–73. doi: 10.1002/mc.20246. [DOI] [PubMed] [Google Scholar]
- 21.Babbar N, Ignatenko NA, Casero RA, Jr, Gerner EW. Cyclooxygenase-independent induction of apoptosis by sulindac sulfone is mediated by polyamines in colon cancer. J Biol Chem. 2003;278:47762–75. doi: 10.1074/jbc.M307265200. [DOI] [PubMed] [Google Scholar]
- 22.Babbar N, Gerner EW, Casero RA., Jr Induction of spermidine/spermine N1-acetyltransferase (SSAT) by aspirin in Caco-2 colon cancer cells. Biochemical Journal. 2006;394:317–24. doi: 10.1042/BJ20051298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uemura T, Yerushalmi HF, Tsaprailis G, et al. Identification and characterization of a diamine exporter in colon epithelial cells. J Biol Chem. 2008 doi: 10.1074/jbc.M804714200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gobert AP, Cheng Y, Akhtar M, et al. Protective role of arginase in a mouse model of colitis. J Immunol. 2004;173:2109–17. doi: 10.4049/jimmunol.173.3.2109. [DOI] [PubMed] [Google Scholar]
- 25.Babbar N, Murray-Stewart T, Casero RA., Jr Inflammation and polyamine catabolism: the good, the bad and the ugly. Biochem Soc Trans. 2007;35:300–4. doi: 10.1042/BST0350300. [DOI] [PubMed] [Google Scholar]
- 26.Parker MT, Gerner EW. Polyamine-mediated post-transcriptional regulation of COX-2. Biochimie. 2002;84:815–9. doi: 10.1016/s0300-9084(02)01439-6. [DOI] [PubMed] [Google Scholar]
- 27.Martinez ME, O’Brien TG, Fultz KE, et al. Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene. Proc Natl Acad Sci U S A. 2003;100:7859–64. doi: 10.1073/pnas.1332465100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hubner RA, Muir KR, Liu JF, Logan RF, Grainge MJ, Houlston RS. Ornithine decarboxylase G316A genotype is prognostic for colorectal adenoma recurrence and predicts efficacy of aspirin chemoprevention. Clin Cancer Res. 2008;14:2303–9. doi: 10.1158/1078-0432.CCR-07-4599. [DOI] [PubMed] [Google Scholar]
- 29.Barry EL, Baron JA, Bhat S, et al. Ornithine decarboxylase polymorphism modification of response to aspirin treatment for colorectal adenoma prevention. Journal of the National Cancer Institute. 2006;98:1494–500. doi: 10.1093/jnci/djj398. [DOI] [PubMed] [Google Scholar]
- 30.Meyskens FLJ, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, Lawson MJ, Kidao J, McCracken J, Albers CG, Ahnen DJ, Turgeon DK, Goldschmid S, Lance P, Hagedorn CH, Gillen DL, Gerner EW. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: A randomized placebo controlled, double-blind trial. Cancer Prevention Research. 2008;1:32–8. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLaren CE, Fujikawa-Brooks S, Chen WP, Gillen DL, Pelot D, Gerner EW, Meyskens FL., Jr Longitudinal assessment of air conduction audiograms in a phase III clinical trial of difluoromethyornithine and sulindac for prevention of sporadic colorectal adenomas. Cancer Prevention Research. 2008 doi: 10.1158/1940-6207.CAPR-08-0074. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Shaughnessy JA, Kelloff G, Gordon GB, et al. Treatment and prevention of intraepithelial neoplasia: An important target for accelerated new agent development. Clin Cancer Res. 2002;8:314–46. [PubMed] [Google Scholar]
- 33.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–98. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 34.Fultz KE, Gerner EW. APC-dependent regulation of ornithine decarboxylase in human colon tumor cells. Mol Carcinog. 2002;34:10–8. doi: 10.1002/mc.10043. [DOI] [PubMed] [Google Scholar]
- 35.Ignatenko NA, Babbar N, Mehta D, Casero RA, Jr, Gerner EW. Suppression of polyamine catabolism by activated Ki-ras in human colon cancer cells. Mol Carcinog. 2004;39:91–102. doi: 10.1002/mc.10166. [DOI] [PubMed] [Google Scholar]
- 36.Xie X, Gillies RJ, Gerner EW. Characterization of a diamine exporter in Chinese hamster ovary cells and identification of specific polyamine substrates. J Biol Chem. 1997;272:20484–9. doi: 10.1074/jbc.272.33.20484. [DOI] [PubMed] [Google Scholar]