Abstract
Objective
To assess the efficacy of a behavioral intervention designed to improve HIV medication adherence and reduce alcohol consumption among HIV-positive men and women.
Design
A randomized controlled trial conducted between July 2002 and August 2005.
Setting
A behavioral research center in New York City.
Participants
HIV-positive men and women (n = 143) who were on HIV antiretroviral medication and met criteria for hazardous drinking.
Intervention
Participants were randomly assigned to an 8-session intervention based on motivational interviewing and cognitive-behavioral skills building or a time- and content-equivalent educational condition.
Outcome Measures
Viral load, CD4 cell count, and self-reported adherence and drinking behavior were assessed at baseline and at 3- and 6-month follow-ups.
Results
Relative to the education condition, participants in the intervention demonstrated significant decreases in viral load and increases in CD4 cell count at the 3-month follow-up and significantly greater improvement in percent dose adherence and percent day adherence. There were no significant intervention effects for alcohol use, however, and effects on viral load, CD4 cell count, and adherence were not sustained at 6 months.
Conclusions
An 8-session behavioral intervention can result in improvement in self-report and biologic markers of treatment adherence and disease progression. This type of intervention should be considered for dissemination and integration into HIV clinics providing comprehensive care for HIV-positive persons with alcohol problems. Although the effect was attenuated over time, future studies might test the added effectiveness of booster sessions or ongoing adherence counseling.
Keywords: alcohol consumption, behavioral intervention, CD4, hazardous drinking, medication adherence, viral load
Problem level alcohol consumption is a common issue in the treatment of individuals with HIV infection.1–4 In a national probability survey of HIV-positive adults,2 53% reported drinking in the past month and 8% (15% of those reporting drinking) could be classified as heavy drinkers, a rate twice that among the general population.5 Although the impact of alcohol on HIV disease is not fully defined, researchers believe alcohol consumption may have an impact on HIV viral replication, disease progression, and drug toxicity6 and may impair immune system function.7–10 Heavy consumption, or hazardous drinking, is likely to lead to increased risk for toxicity from antiretroviral therapy because it intensifies conditions that also place a strain on the liver, such as hepatitis C or chronic hepatitis B.6 In a series of recent studies, HIV-positive drinkers on highly active antiretroviral therapy (HAART) were twice as likely to have CD4 counts <500 cells/mL and 4 times less likely to achieve a positive virologic response to medication as compared with nondrinkers.11–13
Alcohol consumption has been linked to lower rates of adherence to HAART regimens.1,14 HIV-positive patients who drink have worse adherence than those who do not,15 with nonadherence increasing with level of drinking severity.16 Among HIV-positive persons with alcohol problems, alcohol was the most significant predictor of nonadherence,17 and problem drinkers are more likely than nondrinkers to report forgetting medication, taking medication off schedule, or running out of pills.1
Adherence to HAART regimens is of critical importance to all persons living with HIV, because nonadherence can result in inadequate viral suppression, immunologic failure, rapid disease progression, and drug resistance. Targeted interventions designed to increase adherence among HIV-positive individuals have met with only limited success.18–20 Although some interventions have demonstrated positive effects on self-reported adherence or adherence measured through pill counts or electronic bottle caps,21–27 few have demonstrated parallel improvements in clinical indicators, such as viral load.28,29 A recently published meta-analysis of 19 randomized controlled trials of HAART adherence interventions found that no single intervention had a significant effect on self-reported adherence, viral load, and CD4 count.20 Interventions focused on HIV-positive individuals with alcohol problems have proven even more challenging, failing to find significant effects.30,31 This present study was designed to evaluate the effectiveness of a theory-based intervention, Project PLUS (Positive Living Through Understanding and Support), intended to improve adherence and reduce alcohol use among HIV-positive individuals who report hazardous drinking.
Methods
Study Participants
From July 2002 through August 2005, potential participants were recruited through a variety of strategies, including flyers placed in clinic waiting rooms and active recruitment at HIV-related community events throughout the New York City area. Initial screening criteria required that participants were HIV-positive, at least 18 years of age, English speaking, currently taking a HAART regimen, and had scored 8 or greater on the Alcohol Use Disorder Identification Test (AUDIT).32 Because the intervention was specifically designed to target heavy or hazardous drinkers, the final requirements for enrollment included meeting criteria for hazardous drinking (>16 standard drinks per week for men or >12 standard drinks per week for women)33 and having alcohol problems greater than those associated with other drugs. All study participants gave written informed consent, and all study procedures were reviewed and approved by the Institutional Review Board of Hunter College.
Study Design and Procedures
Eligible participants were assigned to the 8-session intervention or to an 8-session educational comparison condition, using urn randomization procedures, which are systematically biased in favor of balancing groups. This procedure preserves randomization as the primary basis for assignment to condition, is less susceptible to experimenter bias or manipulation of the assignment process by staff, allows matching on several variables (in this study, viral load and AUDIT score), and most efficiently ensures multivariate equivalence of treatment groups.34 Although it is always possible to control statistically for differences between groups in later analyses, the potential removal of such differences in the randomization process increases power by reducing the number of covariates included in primary analyses.
Each session in both conditions was 60 minutes in length and delivered individually in private offices. An 8 session intervention was selected based on input from HIV clinic directors (who indicated a willingness to deliver a more intensive intervention for heavy drinkers as these are some of their most challenging patients) and because the intervention was targeting 2 problematic and complicated behaviors that have typically not responded well to brief interventions. Sessions were designed to be completed weekly, but participants had 12 weeks to complete all 8 sessions. The first session was delivered immediately on completion of the baseline assessment, thereby ensuring that each participant had a minimum dose of 1 session.
Intervention Condition
The Project PLUS intervention was based on the Information-Motivation-Behavioral Skills (IMB) Model,35 which posits that information and motivation activate behavioral skills, resulting in behavior change. Two complementary techniques—motivational interviewing (MI)36 and cognitive-behavioral skills training (CBST)37—were integrated, allowing trained counselors to match targeted information and skill-building techniques to the particulars of each client's motivation for change. The integration of MI and CBST provides maximum flexibility to meet the individualized needs of clients38 and has been shown to be effective in previous substance use interventions.39,40 All sessions were delivered by master's degree–prepared counselors who completed significant training in MI and CBST and received individual and group supervision throughout the project.
Sessions 1 and 2 focused on the delivery of factual information and the use of MI techniques to enhance motivation, promote personal responsibility for improving adherence and reducing alcohol use, and develop individualized behavior change plans. Each participant received individualized feedback on both behaviors based on baseline data and was provided with a wallet-sized card to facilitate self-monitoring of HAART adherence and drinking behaviors, which were reviewed at each subsequent session. In session 3, counselors conducted a functional analysis of individual antecedents for both behaviors so as to select 4 individually tailored skills-building modules (2 related to improving adherence, 2 related to reducing alcohol use) for use in sessions 4 through 7. Each module (eg, “Managing HAART Side Effects,” “Drink Refusal Skills,” “Coping with Triggers,” and “Increasing Social Support”) included a didactic portion, a self-assessment, skills-building activities, opportunities to practice, and suggested take-home activities. Session 8 addressed termination issues and relapse prevention to reinforce skills that had been developed, gain insight about participant experiences, and facilitate access to community-based resources. Fidelity to the intervention was maintained through videotaped review of 10% of each therapist's sessions, using the Motivational Interviewing Treatment Integrity coding system.41
Education Condition
The education condition was matched to the intervention for time and content. Participants attended 8 sessions facilitated by a health educator focused on the provision of factual information through didactic methods and structured discussions about videotapes pertaining to HIV, HAART adherence, and alcohol. Health educators received initial training and ongoing individual and group supervision. All education sessions were videotaped and reviewed to ensure they did not include elements of MI or CBST.
Measures
The primary outcome measures were viral, immunologic, and self-report measures of adherence. All blood draws were conducted on-site by a certified phlebotomist and were analyzed by Specialty Laboratories. HIV viral load was measured by reverse transcriptase polymerase chain reaction (RT-PCR) using the HIV-1 Ultraquant assay (Specialty Laboratories, Santa Monica, CA), and results were log-transformed to adjust for skew. CD4 cell counts were measured by flow cytometry.
Adherence was assessed using a timeline follow-back interview42,43 to recall, day by day, all medication doses taken and missed during the past 2 weeks. A period of 14 days was used to capture 2 weeks of weekday and weekend activity. Before the timeline follow-back, participants explained their HAART regimen and reported the number of scheduled dosing times for each medication per day. To control for differences among participants' regimens, percent dose adherence was defined as the number of doses taken in the past 14 days divided by the number of scheduled doses during that period. For each participant, an adherent day was defined as a day in which the participant missed none of his or her scheduled doses. Percent day adherence was calculated as the percent of days with perfect adherence in the past 2 weeks.
Self-reported alcohol use was measured by the collection of data on standard drinks consumed, using the timeline follow-back interview.42,43 We calculated a measure of drinks per drinking day by dividing the total number of standard drinks in the past 14 days by the number of days during that period in which the participant had at least 1 alcoholic drink.
Data Analysis
An intent-to-treat analysis was used in which participants who completed the first follow-up assessment were analyzed according to their original assigned study condition irrespective of the number of sessions they attended.44 Analyses of intervention effects were conducted by comparing differences in means between the 2 conditions using a 2 (time period: baseline, follow-up) by 2 (condition: intervention, education) repeated measures analysis of variance (ANOVA). Repeated measures ANOVA controls for each participant's score at baseline and evaluates both the main effect of time (ie, whether all participants improve over time regardless of condition) and the time by condition interaction (ie, whether participants' improvement over time differs by experimental condition). Separate ANOVAs were conducted for each outcome measure and examined change from baseline to 3 months and from baseline to 6 months. For the primary outcome measures (log viral load and CD4 cell count), we also created dichotomous variables representing meaningful clinical improvement from baseline to after the intervention to examine the odds ratios (ORs) of improvement over time by condition.
Results
Study Participation
Figure 1 shows the flow of participants through each time point in the study. A total of 1545 potential participants completed an initial prescreen by telephone (n = 925) or in person (n = 620). Of these, 495 met initial screening criteria and 359 (74%) presented for final screening. Of these, 49 were deemed ineligible because of having drug-related problems more severe than their alcohol-related problems and/or active psychosis and 167 failed to meet criteria for hazardous drinking. In total, 143 met all criteria, completed baseline assessments, and were randomized to 1 of 2 study conditions.
FIGURE 1.
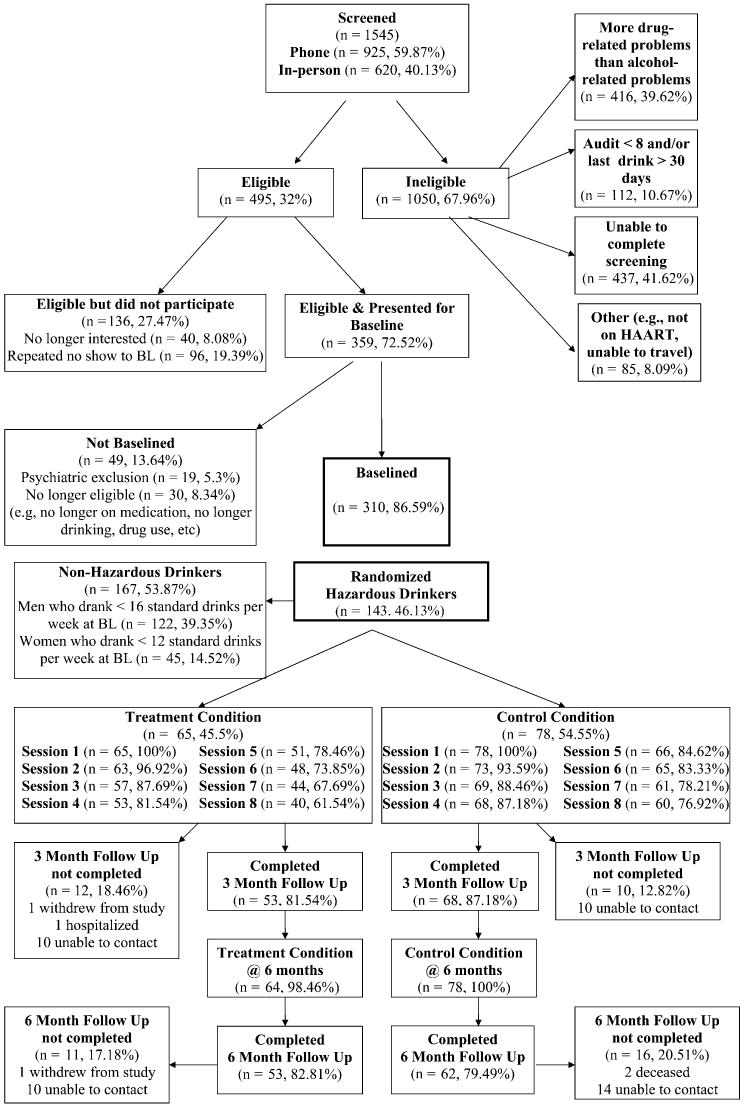
Flow of participants through study.
Table 1 presents background characteristics of the final sample of 130 (91%) participants who returned for their 3-month follow-up visit. The attrition rate was the same (10%) for the 6-month follow-up, and there was no significant difference in attrition between the 2 conditions at 3 or 6 months (χ2 = 0.86; P = 0.35 and χ2 = 0.08; P = 0.75, respectively). As such, retention rates were comparable to or better than those of similar randomized controlled trials of HIV adherence interventions.20 The number of sessions completed did not significantly differ between the intervention and comparison conditions (mean [M] = 7.04, SD = 1.79 and M = 7.08, SD = 2.05, respectively).
TABLE 1.
Background Characteristics of Study Sample at Baseline
| Characteristic | All Participants (n = 130) |
|---|---|
| Sociodemographic data | |
| Age, mean (SD) | 43.6 (6.9) |
| Male, % (n) | 79% (103) |
| Race, % (n) | |
| African American | 65.9% (83) |
| Hispanic | 17.5% (22) |
| White | 5.6% (7) |
| Other | 11.1% (14) |
| Sexual identity, % (n) | |
| Gay, queer, or homosexual | 40% (52) |
| Straight or heterosexual | 46.9% (61) |
| Bisexual | 13.1% (17) |
| High school equivalent or less, % (n) | 65.4% (51) |
| Unemployed, % (n) | 47% (62) |
| Annual income ≤$10,000, % (n) | 69.3% (88) |
| HIV related, mean (SD) | |
| Years since diagnosis | 10.6 (5.1) |
| Years on HAART | 7.3 (4.3) |
| Dose times per day | 4.4 (1.6) |
Table 2 presents the baseline, 3-month, and 6-month follow-up data for each of the primary outcomes by study condition. An examination of our fidelity measures indicated that 92% of coded sessions met or exceeded standardized criteria for MI.
TABLE 2.
Group Means at Baseline, 3-Month, and 6-Month Follow-Up
| Variable | Baseline | 3 Months* | T ηp2 | T × C ηp2 | 6 Months† | T ηp2 | T × C ηp2 |
|---|---|---|---|---|---|---|---|
| Log viral load | 0.00 | 0.05§ | 0.03 | 0.01 | |||
| Intervention | 3.3 | 3.0 | 3.4 | ||||
| Education | 3.3 | 3.6 | 3.6 | ||||
| CD4 cell count | 0.00 | 0.05§ | 0.01 | 0.01 | |||
| Intervention | 431.2 | 481.1 | 463.5 | ||||
| Education | 443.2 | 410.6 | 424.0 | ||||
| Percent dose adherence | 0.11‡ | 0.04‖ | 0.07§ | 0.01 | |||
| Intervention | 78% | 92.7% | 90.6% | ||||
| Education | 85.1% | 89.4% | 88.2% | ||||
| Percent day adherence | 0.17‡ | 0.04‖ | 0.07§ | 0.02 | |||
| Intervention | 74.1% | 91.7% | 89.4% | ||||
| Education | 80.4% | 87.3% | 82.6% | ||||
| Standard drinks | 0.16‡ | 0.00 | 0.30‡ | 0.00 | |||
| Intervention | 81.4 | 22.5 | 22.4 | ||||
| Education | 69.9 | 29.8 | 31.2 | ||||
| Drinks per drinking day | 0.24‡ | 0.01 | 0.03‡ | 0.01 | |||
| Intervention | 11.2 | 5.4 | 4.5 | ||||
| Education | 10.4 | 6.2 | 5.5 |
n = 130.
n = 116.
P < 0.001;
P < 0.02;
P < 0.05.
T ηp2 indicates partial η2 for main effect of time; T×C ηp2, partial η2 for time by condition interaction effect.
All P values are calculated using repeated measures ANOVA.
Intervention Effects on Virologic and Immunologic Outcomes
Results from repeated-measures ANOVA revealed a significant time × condition interaction effect at the 3-month follow-up, indicating that the log viral load of participants in the intervention condition decreased from baseline, whereas the log viral load of those in the education condition increased [F(1, 116) = 6.09; P < 0.02]. To estimate the clinical significance of this reduction, we created dichotomous variables for a viral load improvement of 0.5 log or greater and a viral load improvement of 1.0 log or greater. Each 0.5-log reduction in viral load has been associated with a 30% reduction in the risk of clinical progression,45 and the most recent treatment guidelines report that effective regimens and high levels of adherence should result in 1.0-log decreases in viral load.46 A χ2 analysis of viral load improvement between intervention and comparison conditions is presented in Table 3. Compared with individuals in the education condition, intervention participants were significantly more likely to demonstrate a 0.5-log reduction in viral load (OR = 2.7; P = 0.02) and a 1.0-log reduction in viral load (OR = 2.7; P = 0.03) at the 3-month follow-up. There were no significant differences between conditions in log viral load at the 6-month visit.
TABLE 3.
Effects of Intervention on Indicators of Clinical Progression at 3 Months
| Intervention % (n) | Education % (n) | OR (95% Confidence Interval) | P | |
|---|---|---|---|---|
| 0.5-log reduction in viral load | 38.8% (19) | 18.8% (13) | 2.7 (1.2 to 6.3) | 0.02 |
| 1.0-log reduction in viral load | 28.6% (14) | 13% (9) | 2.7 (1.1 to 6.8) | 0.03 |
| 10% increase in CD4 cell count | 30.6% (15) | 11.9% (8) | 3.3 (1.3 to 8.5) | 0.01 |
A significant time × condition interaction effect at the 3-month follow-up was also found for CD4 cell counts, such that CD4 cell counts of participants in the intervention condition increased from baseline, whereas CD4 cell counts of education participants declined [F(1, 115) = 6.44; P < 0.02]. To estimate the clinical significance of this reduction, we created a dichotomous variable that defined CD4 “improvement” as an increase in CD4 cell count of 10% or greater. Each 10% increase in CD4 cell count has been associated with a 15% reduction in risk of clinical progression.45 A χ2 analysis of this comparison is presented in Table 3. Compared with individuals in the education condition, intervention participants were significantly more likely to demonstrate a 10% or greater increase in CD4 cell count at the 3-month follow-up (OR = 3.4; P = 0.013). At the 6-month follow-up, CD4 cell counts of participants in the intervention condition remained higher than those in the education condition, but the interaction effect did not achieve statistical significance.
Intervention Effects on Adherence
Repeated measures ANOVAs revealed significant main effects of time, such that participants in both conditions reported significant increases in percent dose adherence [F(1, 107) = 13.5; P < 0.001] and percent day adherence [F(1, 111) = 21.9; P < 0.001] from baseline to 3 months. The time × condition interactions were also significant, indicating that participants in the intervention condition reported a significantly larger increase in percent dose adherence [F(1, 107) = 4.0; P < 0.05] and in percent day adherence [F(1, 111) = 4.1; P < 0.05] compared with participants in the education condition. On average, percent dose adherence for individuals in the intervention condition increased 14.6% (SD = 26.3%), whereas percent dose adherence for individuals in the education condition increased only 4.3% (SD = 26.5%). Percent day adherence for participants in the intervention condition improved an average of 17.6% (SD = 17.5%) compared with only an average of 6.9% (SD = 27.8%) for those in the control condition. At 6 months, participants in both conditions reported significant improvements in percent dose adherence (M = 8.2%, SD = 29.4%) and percent day adherence (M = 8.7%, SD = 33.7%) from baseline. Improvement in percent dose adherence remained greater for individuals in the intervention condition (M = 11.1%, SD = 40%) compared with those in the education condition (M = 5.8%, SD = 27.1%), but this difference did not achieve statistical significance in the repeated measures ANOVA. The same was found for percent day adherence, with improvement remaining greater for those in the intervention condition (M = 13.5%, SD = 32.2%) compared with those in the education condition (M = 4.6%, SD = 34.7%); however, again, this difference did not achieve statistical significance.
Intervention Effects on Drinking
Repeated measures ANOVA revealed a significant main effect of time, such that participants in both conditions reported significant decreases in the number of standard drinks from baseline to 3 months [F(1, 112) = 62.7; P < 0.001]. This significant main effect of time was sustained at the 6-month follow-up [F(1, 93) = 48.7; P < 0.001]. Similarly, all participants reported significant decreases in number of drinks per drinking day from baseline to 3 months [F(1, 112) = 35.1; P < 0.001] and from baseline to 6 months [F(1, 93) = 43.7; P < 0.001]. There were no significant time × condition interaction effects for these variables at either time point.
Discussion
The results of this randomized controlled trial of an IMB-based intervention designed to improve HAART adherence among HIV-positive hazardous drinkers demonstrated that the intervention led to significant short-term improvement in viral and immunologic outcomes and improvements in self-reported adherence behavior compared with the education comparison condition. At the 3-month follow-up, participants in the intervention condition demonstrated significantly greater decreases in viral load and increases in CD4 cell count and self-reported adherence behavior compared with participants in the comparison condition. Project PLUS is the first behavioral adherence intervention to demonstrate such improvements in all 3 measures (viral load, CD4 cell count, and percent adherence) and is the first intervention for HIV-positive individuals with alcohol-related problems to demonstrate any significant effects.
The efficacy of the Project PLUS intervention may be attributable to several factors. First, the intervention was designed to incorporate the full IMB model of behavior change, including the provision of information, utilization of MI techniques, and selection of skills-training modules. The combination of MI with CBST seems to confer greater benefit than MI alone.21 Second, the Project PLUS intervention manual includes >15 different skill-building modules, such that the skills training is targeted to specific deficits identified by each participant. Other HAART adherence interventions have used motivational and cognitive-behavioral components25 but failed to find significant effects on viral load or CD4 cell count, perhaps because they did not individually tailor modules, provide ongoing self-monitoring of adherence, and utilize personalized feedback on factors related to nonadherence. The comparison group never achieved at least 90% adherence at follow-up, unlike the intervention group, which may account for the fact that viral loads increased and CD4 cell counts declined in the comparison group.
It is important to note that participants enrolled in the Project PLUS intervention did not have to report difficulties with adherence at baseline, as has been common in other adherence interventions. At baseline, 38% of participants reported that they were already at least 95% adherent. As such, the population enrolled mirrors the broad spectrum of HIV-positive clients with alcohol problems seen in clinical settings. One meta-analytic review of HIV adherence studies found that intervention effects were significantly stronger in trials in which adherence problems were a criterion for eligibility.18 This finding suggests that the impact of the Project PLUS intervention might be even greater were it restricted to those individuals with the most significant adherence problems.
Unlike most published HIV intervention studies,20 our comparison condition was neither a “standard care” condition nor an attention control focused on different behaviors than those in the treatment condition (eg, exercise, diet/nutrition). The comparison condition was time- and content-equivalent to the intervention. Although delivered by health educators rather than by counselors and focused on didactic information and discussion of videotapes rather than on motivation and skills building, the education condition specifically addressed HAART adherence and drinking. For this reason, it is not surprising that the education condition also improved self-reported adherence and drinking behaviors. Although adherence improvement among participants in the education control condition was smaller relative to those in the intervention condition, improvement in self-reported drinking behavior was of similar magnitude, and self-reported improvement in both conditions was sustained through to the 6-month visit. These findings suggest that education alone might be sufficient to change self-reported behavior, but a more individualized and sophisticated intervention is necessary to influence virologic and immunologic outcomes. It is also possible that improvements over time in both conditions reflect a reporting bias, whereas changes in viral load and CD4 cell count reflect true changes in adherence behavior as a result of the intervention.
The intervention, however, did not seem to result in greater changes in drinking behavior compared with the education condition. Because participants were required to have alcohol problems as part of the eligibility criteria, it is possible that perceived demands to report decreased alcohol use were stronger than those for reporting adherence changes. It is also possible that for HIV-positive persons, information alone is enough to reduce alcohol use but that the individualized and tailored motivation and skills-building modules are essential to affect medication adherence. This difference may be attributable to the fact that the information provided for alcohol use focused heavily on the impact that such use has on the health and immune functioning of HIV-positive persons, perhaps making this education more salient than has traditionally been the case when education-based interventions have failed to demonstrate efficacy.
The study failed to maintain significant interaction effects at the 6-month visit, most likely because participants were no longer receiving the intervention content. Other adherence interventions have also failed to find significant effects at 6 months,25,47 and the absence of significant intervention effects for viral load and CD4 cell count at the 6-month follow-up could be attributable to a host of other clinical and biologic factors, including the development of resistance, preexisting resistance, or length of time on HAART. In addition, given the sample size at the 6-month follow-up (n = 116), the study had sufficient power (α = 0.05, 2-tailed; β = 0.20) to detect only a medium effect size or greater (Cohen d ≥ 0.51). Mean scores at the 6-month follow-up are all in the hypothesized direction (ie, the intervention group demonstrating better clinical outcomes, higher levels of adherence, and less drinking compared with the education group). It is possible that the Project PLUS intervention would benefit from “booster visits” to reinforce the intervention components and to help participants sustain the positive effects impact on adherence and virologic and immunologic functioning. Future studies should consider the inclusion of booster sessions to examine their impact on long-term outcomes.
Because of its flexibility in tailoring intervention components to the specific needs of individual patients, the Project PLUS intervention is a perfect model for integration into HIV clinical care settings. Although an “intensive” intervention by some standards, the success of Project PLUS in improving clinical outcomes suggests that it might be a cost-effective investment, especially if delivered to the patients at highest risk for nonadherence. The intervention could be delivered by many different clinic professionals, including nurses, social workers, or case managers. If integrated within an HIV clinic setting, individual CBST modules could be delivered as “booster” sessions during routine care visits or when a client presented with treatment failure because of nonadherence. Many HIV clinics struggle with providing adherence interventions for their substance-using clients, including hazardous drinkers, and Project PLUS provides a model that allows providers to target their intersecting needs and barriers to adherence. It is also possible that although designed for hazardous drinkers, the adherence components of Project PLUS could prove effective even for those without substance use problems. Because all participants were hazardous drinkers, the extent to which the intervention would work effectively with those with fewer drinking problems is unknown, but it is also possible that such persons might require a less lengthy and intensive intervention. More research is needed to develop and replicate the Project PLUS model within HIV care settings and to modify this promising intervention for use with other HIV-positive populations. Adaptations of the Project PLUS intervention, including contracted versions, should be examined for effectiveness.
Acknowledgments
The authors acknowledge the contributions of the other members of the Project PLUS team: Joseph C. Punzalan, Dr. Jose E. Nanin, Dr. Bradley Thomason, Michael Adams, Dr. Christian Grov, James Kelleher, Juline Koken, Brooke Wells, and Chris Hietikko. We would also like to thank Kendall Bryant for his support of the project, and all of the clinics and sites that provided access to potential participants.
Supported by a grant from the National Institute of Alcohol Abuse and Alcoholism (RO1 AA13556, Jeffrey T. Parsons, Principal Investigator).
References
- 1.Cook RL, Sereika SM, Hunt SC, et al. Problem drinking and medication adherence among persons with HIV infection. J Gen Intern Med. 2001;16:83–86. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galvan FH, Bing EG, Fleishman JA, et al. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol. 2002;63:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- 3.Lefevre F, O'Leary B, Moran M, et al. Alcohol consumption among HIV-infected patients. J Gen Intern Med. 1995;10:458–460. doi: 10.1007/BF02599920. [DOI] [PubMed] [Google Scholar]
- 4.Samet JH, Phillips SJ, Horton NJ, et al. Detecting alcohol problems in HIV-infected patients: use of the CAGE questionnaire. AIDS Res Hum Retroviruses. 2004;20:151–155. doi: 10.1089/088922204773004860. [DOI] [PubMed] [Google Scholar]
- 5.Greenfield TK, Midanik LT, Rogers JD. A 10-year national trend study of alcohol consumption, 1984–1995: is the period of declining drinking over? Am J Public Health. 2000;90:47–52. doi: 10.2105/ajph.90.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant KJ. Expanding research on the role of alcohol consumption and related risks in the prevention and treatment of HIV/AIDS. Subst Use Misuse. 2006;41:1465–1507. doi: 10.1080/10826080600846250. [DOI] [PubMed] [Google Scholar]
- 7.Brodie C, Domenico J, Gelfand EW. Ethanol inhibits early events in T-lymphocyte activation. Clin Immunol Immunopathol. 1994;70:129–136. doi: 10.1006/clin.1994.1020. [DOI] [PubMed] [Google Scholar]
- 8.Cook RT. Alcohol abuse, alcoholism, and damage to the immune system: a review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- 9.Szabo G. Alcohol's contribution to compromised immunity. Alcohol Health Res World. 1997;21:30–38. [PMC free article] [PubMed] [Google Scholar]
- 10.Bagby GJ, Stoltz DA, Zhang P, et al. The effect of chronic binge ethanol consumption on the primary stage of SIV infection in rhesus macaques. Alcohol Clin Exp Res. 2003;27:495–502. doi: 10.1097/01.ALC.0000057947.57330.BE. [DOI] [PubMed] [Google Scholar]
- 11.Miguez MJ, Shor-Posner G, Fishman J, et al. The overlooked impact of alcohol use on thymic volume in HIV-infected subjects receiving HAART and HIV negative controls. Presented at: Radiological Society of North America 91st Scientific Assembly and Annual Meeting; 2005; Chicago. [Google Scholar]
- 12.Miguez MJ, Shor-Posner G, Morales G, et al. Alcohol and HIV infection in the HAART era. Am Clin Lab. 2001;20:20–22. [PubMed] [Google Scholar]
- 13.Miguez MJ, Shor-Posner G, Morales G, et al. HIV treatment in drug abusers: impact of alcohol use. Addict Biol. 2003;8:33–37. doi: 10.1080/1355621031000069855. [DOI] [PubMed] [Google Scholar]
- 14.Halkitis PN, Parsons JT, Wolitski RJ, et al. Characteristics of HIV antiretroviral treatments, access and adherence in an ethnically diverse sample of men who have sex with men. AIDS Care. 2003;15:89–102. doi: 10.1080/095401221000039798. [DOI] [PubMed] [Google Scholar]
- 15.Palepu A, Horton NJ, Tibbetts N, et al. Uptake and adherence to highly active antiretroviral therapy among HIV-infected people with alcohol and other substance use problems: the impact of substance abuse treatment. Addiction. 2004;99:361–368. doi: 10.1111/j.1360-0443.2003.00670.x. [DOI] [PubMed] [Google Scholar]
- 16.Tucker JS, Burnam MA, Sherbourne CD, et al. Substance use and mental health correlates of nonadherence to antiretroviral medication in a sample of patients with Human Immunodeficiency Virus Infection. Am J Med. 2003;114:573–580. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 17.Samet JH, Horton NJ, Meli S, et al. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- 18.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996–2004. J Acquir Immune Defic Syndr. 2006;41:285–297. doi: 10.1097/01.qai.0000197870.99196.ea. [DOI] [PubMed] [Google Scholar]
- 19.Rueda S, Park-Wyllie LY, Bayourni AM, et al. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database Syst Rev. 2006;3:CD001442. doi: 10.1002/14651858.CD001442.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simoni JM, Pearson CR, Pantalone DW, et al. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load: a meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S23–S35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golin CE, Earp J, Tien HC, et al. A 2-arm, randomized, controlled trial of a motivational interviewing based intervention to improve adherence to antiretroviral therapy (ART) among patients failing or initiating ART. J Acquir Immune Defic Syndr. 2006;42:42–51. doi: 10.1097/01.qai.0000219771.97303.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goujard C, Baernard N, Sohier N, et al. Impact of a patient education program on adherence to HIV medication: a randomized clinical trial. J Acquir Immune Defic Syndr. 2003;34:191–194. doi: 10.1097/00126334-200310010-00009. [DOI] [PubMed] [Google Scholar]
- 23.Safren SA, Hendriksen ES, DeSousa N, et al. Use of an on-line pager system to increase adherence to antiretroviral medications. AIDS Care. 2003;15:787–793. doi: 10.1080/09540120310001618630. [DOI] [PubMed] [Google Scholar]
- 24.Tuldra A, Fumaz CR, Ferrer MJ, et al. Prospective randomized two-arm controlled study to determine the efficacy of a specific intervention to improve long-term adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;25:221–228. doi: 10.1097/00126334-200011010-00003. [DOI] [PubMed] [Google Scholar]
- 25.Wagner GJ, Kanouse DE, Golinelli D, et al. Cognitive-behavioral intervention to enhance adherence to antiretroviral therapy: a randomized controlled trial (CCTG 578) AIDS. 2006;20:1295–1302. doi: 10.1097/01.aids.0000232238.28415.d2. [DOI] [PubMed] [Google Scholar]
- 26.Weber R, Christen L, Christen S, et al. Effect of individual cognitive behavior intervention on adherence to antiretroviral therapy: prospective randomized trial. Antivir Ther. 2004;9:85–95. [PubMed] [Google Scholar]
- 27.Williams AB, Fennie KP, Bova CA, et al. Home visits to improve adherence to highly active antiretroviral therapy: a randomized controlled trial. J Acquir Immune Defic Syndr. 2006;42:314–321. doi: 10.1097/01.qai.0000221681.60187.88. [DOI] [PubMed] [Google Scholar]
- 28.Chiou PY, Kuo BI, Lee MB, et al. A programme of symptom management for improving quality of life and drug adherence in AIDS/HIV patients. J Adv Nurs. 2006;55:169–179. doi: 10.1111/j.1365-2648.2006.03902.x. [DOI] [PubMed] [Google Scholar]
- 29.Pradier C, Bentz L, Spire B, et al. Efficacy of an educational and counseling intervention on adherence to highly active antiretroviral therapy: French prospective controlled study. HIV Clin Trials. 2003;4:121–131. doi: 10.1310/brbv-3941-h1pp-ndry. [DOI] [PubMed] [Google Scholar]
- 30.Chander G, Himelhoch S, Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients: epidemiology and impact on antiretroviral therapy. Drugs. 2006;66:769–789. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]
- 31.Samet J, Horton N, Meli S, et al. A randomized controlled trial to enhance antiretroviral therapy adherence in patients with a history of alcohol problems. Antivir Ther. 2005;10:83–93. doi: 10.1177/135965350501000106. [DOI] [PubMed] [Google Scholar]
- 32.Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423–432. doi: 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- 33.Gordon AJ, Maisto SA, McNeil M, et al. Three questions can detect hazardous drinkers. J Fam Pract. 2001;50:313–320. [PubMed] [Google Scholar]
- 34.Stout R, Wirtz P, Carbonari J. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol. 1994;2(Suppl):70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- 35.Fisher JD, Fisher WA, Amico KR, et al. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25:462–473. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- 36.Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change. 2nd. New York: Guilford Press; 2002. [Google Scholar]
- 37.Morgenstern J, Longabaugh R. Cognitive-behavioral treatment for alcohol dependence: a review of evidence for its hypothesized mechanisms of action. Addiction. 2000;95:1475–1490. doi: 10.1046/j.1360-0443.2000.951014753.x. [DOI] [PubMed] [Google Scholar]
- 38.Baer JS, Kivlahan DR, Donovan DM. Integrating skills training and motivational therapies: implications for the treatment of substance dependence. J Subst Abuse Treat. 1999;17:15–23. doi: 10.1016/s0740-5472(98)00072-5. [DOI] [PubMed] [Google Scholar]
- 39.Annis HM, Schober R, Kelly E. Matching addiction outpatient counseling to client readiness for change: the role of structured relapse prevention counseling. Exp Clin Psychopharmacol. 1996;4:37–45. [Google Scholar]
- 40.Allsop S, Saunders B, Phillips M, et al. A trial of relapse prevention with severely dependent male problem drinkers. Addiction. 1997;92:61–73. [PubMed] [Google Scholar]
- 41.Moyers TB, Martin T, Manuel JK, et al. Assessing competence in the use of motivational interviewing. J Subst Abuse Treat. 2005;28:19–26. doi: 10.1016/j.jsat.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Humana Press; Totowa, NJ: pp. 41–72. [Google Scholar]
- 43.Parsons JT, Rosof E, Mustanski B. Patient-related factors predicting HIV medication adherence among men and women with alcohol problems. J Health Psychol. 2007;12:357–370. doi: 10.1177/1359105307074298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piantadosi S. Clinical Trials: A Methodological Perspective. New York: John Wiley and Sons; 1997. [Google Scholar]
- 45.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 46.Hammer SM, Saag MS, Schechter M, et al. International AIDS Society-USA panel. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006;296:827–843. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 47.Remien RH, Stirratt MJ, Dolezal C, et al. Couple-focused support to improve HIV medication adherence: a randomized controlled trial. AIDS. 2005;19:807–814. doi: 10.1097/01.aids.0000168975.44219.45. [DOI] [PubMed] [Google Scholar]


