Abstract
p300 is a well known histone acetyltransferase and coactivator that plays pivotal roles in many physiological processes. Despite extensive research for the functions of p300 in embryogenesis and transcription regulation, its roles in regulating embryonic stem (ES) cell pluripotency are poorly understood. To address this issue, we investigated the self-renewal ability and early differentiation process in both wild-type mouse ES cells and ES cells derived from p300 knock-out (p300-/-) mice. We found that p300 ablation did not affect self-renewal capacity overtly when ES cells were maintained under undifferentiated conditions. However, the absence of p300 caused a significantly abnormal expression pattern of germ layer markers when differentiation was induced by embryoid body (EB) formation. Interestingly, the expression level of pluripotency marker Nanog but not Oct4 was markedly lower in EBs from p300-/- ES cells compared with that in EBs from wild-type ES cells. Exogenous expression of Nanog rescued abnormal expression of extra-embryonic endoderm marker partially but not mesoderm and ectoderm markers. Furthermore, we demonstrate that p300 was directly involved in modulating Nanog expression. Importantly, epigenetic modification of histone acetylation at the distal regulatory region of Nanog was found to be dependent on the presence of p300, which could contribute to the mechanism of regulating Nanog expression by p300. Collectively, our results show that p300 plays an important role in the differentiation process of ES cells and provide the first evidence for the involvement of p300 in regulating Nanog expression during differentiation, probably through epigenetic modification of histone on Nanog.
Mouse embryonic stem (ES)2 cells are self-renewing and pluripotent cell lines derived from the inner cell mass of the blastocyst of preimplantation embryos. The pluripotent state and self-renewing ability of ES cells are maintained by both extracellular and intracellular factors. Transcription factor Oct4 is a key regulator of the pluripotent state (1). Cooperatively with transcription factor Sox2, Oct4 activates expression of genes promoting self-renewal and pluripotency of ES cells but suppresses genes associated with differentiation (2, 3). Nanog is another important regulator specifically expressed in pluripotent cells in preimplantation mouse embryos and undifferentiated ES cells. In the absence of Nanog, mouse embryos die shortly after implantation, and ES cells in culture lose the ability to self-renew, differentiating into the extra-embryonic endoderm lineage (4, 5). Thus, both Oct4 and Nanog are absolutely required for the maintenance of ES cell characteristics. More recently, they were shown to be able to reprogram differentiated somatic cells, together with other factors such as Sox2, Klf4, c-Myc, and Lin28 (6, 7). Obviously, it is extremely important to understand how Oct4 and Nanog expression is controlled as a basis for novel strategies to maintain ES cell self-renewal or to direct their differentiation and perhaps to induce pluripotency in differentiated somatic cells.
Extensive research has been conducted to elucidate molecular mechanisms underlying Oct4 expression regulation since its discovery nearly two decades ago. However, less is known about Nanog expression regulation. Nevertheless, it is drawing increasing attention. Kuroda et al. (8) reported that Oct4 and Sox2 activate Nanog expression. In contrast, p53 and Tcf3 were reported to suppress Nanog expression in mouse ES cells, respectively (9, 10), suggesting that the steady-state level of Nanog expression in undifferentiated ES cells is controlled by the balance between its activators and repressors. In addition, regulation of Nanog expression by T (Brachyury) and Stat3 was investigated during an early stage of ES cell specification toward mesoderm lineage (11). The finding suggests that strict control of Nanog expression exists in differentiating as well as undifferentiated ES cells.
p300 is a well known transcriptional coactivator with histone acetyltransferase activity and plays pivotal roles in a wide range of biological and cellular processes (12–14). Mutagenesis studies in mice have demonstrated that p300 knock-out leads to embryonic lethality at or before embryonic day 11.5 with severe central nervous system and heart abnormalities (14). Further study showed that expression of p300 is regulated in a stage- and tissue-specific manner during mouse embryogenesis (15). Despite its important position in development and cellular functions, the role of p300 in regulation of self-renewal and cell fate decision of ES cells remains largely unexplored. Considering its essential role in embryogenesis and its close association with transcription regulation, understanding the functional contribution of p300 to maintaining ES cell identity and controlling expression of pluripotency genes should shed light on the molecular mechanisms governing the basic features of ES cells.
The purpose of this study was to determine whether p300 plays any role in the process of ES cell self-renewal and differentiation. With the availability of p300-/- ES cells, we studied the role of p300 in maintenance of self-renewal and further compared morphological features and molecular events between wild-type and p300-deficient ES cells during leukemia inhibitory factor (LIF) withdrawal and aggregation-induced differentiation. The results demonstrate that p300 is required for ES cells to undergo early differentiation appropriately, partially through its positively regulatory effect on Nanog expression.
EXPERIMENTAL PROCEDURES
ES Cell Culture—Mouse ES cell lines with genotypes of p300+/+ and p300-/- (gifts from Andrew Kung) were cultured in an undifferentiated state under standard conditions (16). The CGR8 ES cell line (a gift from Austin Smith and Ian Chambers) was cultured under feeder-free conditions as described previously (17).
Plasmid Construction—The pPyCAGIP vector and p300/pCMVβ were gifts from Austin Smith and Tony Kouzarides, respectively. Full-length cDNA fragments encoding Nanog and p300 were subcloned into the pPyCAGIP vector, yielding Nanog/pPyCAGIP and p300/pPyCAGIP plasmids. The Nanog reporter plasmid Nanog-4.8kb/pGL3, composed of the 5′ upstream -4.8 kb (-4,828/+190, relative to the transcription start site), was a gift from Da-Yong Wu (18). The Nanog-3.8kb reporter was generated by subcloning the 5′ upstream fragment (-3,794/+190) from Nanog-4.8kb/pGL3 into a pGL3-Basic vector.
EB Formation—p300+/+ and p300-/- ES cells were trypsinized to small clumps containing three to five cells. The cell suspension was applied to gelatin-coated dishes and kept in a 37 °C incubator for 45 min to let feeder cells attach to dishes. EBs were formed by suspending ES cells in ES cell culture medium without LIF and were harvested at the indicated time points.
Quantitative Real-time PCR (qPCR)—RNA was extracted according to the manufacturer's instructions for TRIzol reagent (Invitrogen) and was reverse-transcribed into cDNA and subjected to qPCR with SYBR Green PCR Master Mix (Applied Biosystems) in a thermal cycler (ABI PRISM 7900). The amount of target cDNA in different samples was calculated by dividing the glyceraldehyde-3-phosphate dehydrogenase cDNA amount in each sample for normalization. Primer sequences are shown in supplemental Table S1.
Western Blot Analysis—Western blot analysis was performed as described previously (19). For quantification, films were scanned using a photodensitometer (UMAX) and analyzed by Quantity One software (Bio-Rad). Primary antibodies used in this study included p300 monoclonal antibody (RW128, Upstate), affinity-purified rabbit polyclonal antibodies against Nanog and Oct4 (produced in our laboratory), and tubulin polyclonal antibody (Sigma).
Colony Formation Assay—p300+/+ and p300-/- ES cells were trypsinized into single cells. 2 × 103 cells were plated in 35-mm cell culture dishes with a feeder layer in the presence of LIF. After culture for 7 days, cells were stained using the alkaline phosphatase substrate kit III (Vector Laboratories). Colonies were counted and classified into three categories as undifferentiated, mixed, and differentiated.
Reporter Assays—p300-/- ES cells were plated in 24-well plates and transfected with Lipofectamine 2000 (Invitrogen). After 24 h, cells were harvested for luciferase activity measurement with the Dual-Luciferase reporter assay system (Promega) and a Lumat LB 9507 luminometer (Berthold Technologies) according to the manufacturer's instructions.
Chromatin Immunoprecipitation (ChIP) Assays—CGR8 ES cells or p300+/+ and p300-/- ES cells were cultured without LIF and feeder cells for 2 days and subjected to ChIP assay as described previously (20). Antibodies against p300 (Upstate), acetylated histone H3 lysine 9 (acetyl-H3K9; Abcam), acetylated histone H3 lysine 14 (acetyl-H3K14; Upstate), and acetylated histone H3 lysines 9 and 14 (acetyl-H3K9/14; Upstate) were used. The target sequences were amplified by specific primers shown in supplemental Table S2.
RESULTS
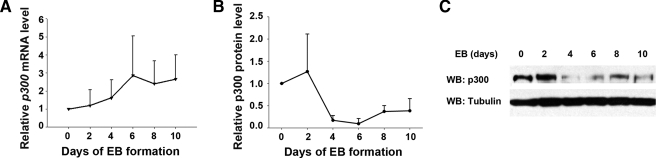
p300 Expression Level Changes Dynamically during ES Cell Differentiation—To determine the expression pattern of p300 in pluripotent and differentiated ES cells, we examined p300 mRNA and protein levels during EB formation with wild-type ES cells. qPCR analysis showed that the p300 mRNA level gradually increased with EB formation up to 10 days (Fig. 1A). The p300 protein level fluctuated during the same time course (Fig. 1, B and C). A high level of p300 protein was seen on EB days 0 and 2. Unexpectedly, it declined to a much lower level from days 4 to 10. The observed discrepancy between mRNA and protein levels of p300 during the EB formation process was probably due to post-translational modifications that occurred during differentiation (21). The distinct dynamic change in the p300 expression level may implicate the important role of p300 in different stages of ES cell differentiation.
FIGURE 1.
The p300 expression pattern during ES cell differentiation. A, mRNA level of p300 during EB formation by qPCR. EBs derived from wild-type ES cells were harvested on days 0, 2, 4, 6, 8, and 10. Data were from three independent experiments. B and C, protein level of p300 during EB formation by Western blot (WB) analysis. Quantitative data and representative results of three independent experiments are shown.
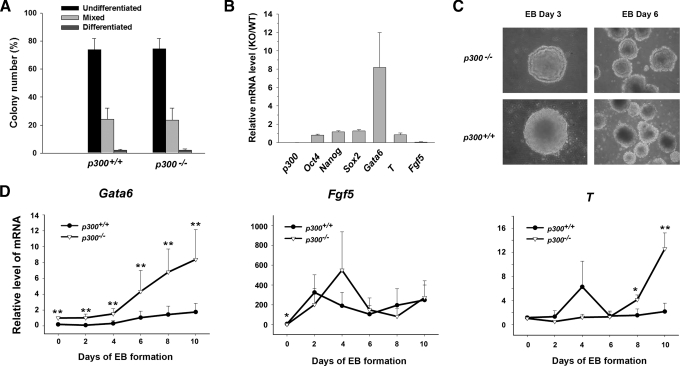
p300 Is Required for Proper Differentiation Process of ES Cells—To find out the function of p300 in maintaining self-renewal of ES cells, a colony formation assay was performed (Fig. 2A and supplemental Fig. S1). There was little difference in the calculative percentage of the three kinds of colonies (undifferentiated, mixed, and differentiated) between p300+/+ and p300-/- ES cells. Moreover, our data show that deletion of p300 did not affect the cell cycle profile (supplemental Table S3) and the cell growth rate (data not shown). These findings suggest that p300 is dispensable for ES cell self-renewal in an undifferentiated state. We further examined the molecular markers in undifferentiated p300+/+ and p300-/- ES cells by qPCR (Fig. 2B). Consistent with the result of colony formation assays, pluripotency markers Oct4, Nanog, and Sox2 were expressed at similar levels in p300+/+ and p300-/- ES cells. Surprisingly, the endoderm marker Gata6 was found to be markedly higher in p300-/- ES cells. In contrast, the ectoderm marker Fgf5 was expressed at a lower level in p300-/- ES cells than in wild-type ones.
FIGURE 2.
p300 is required for ES cells to differentiate normally in vitro. A, colony formation efficiency of p300+/+ and p300-/- ES cells. B, mRNA levels of pluripotency markers Oct4, Nanog, and Sox2 and differentiation markers Gata6, T, and Fgf5 in undifferentiated p300+/+ and p300-/- ES cells by qPCR. The mRNA level of each marker in p300-/- ES cells was divided by that in p300+/+ ES cells. KO, knock-out; WT, wild-type. C, morphology of EBs derived from p300+/+ and p300-/- ES cells. EBs were formed and photographed on day 3 (×200 magnification) and on day 6 (×100 magnification), respectively. D, mRNA levels of germ layer markers in EBs derived from p300+/+ and p300-/- ES cells. EBs were harvested every 2 days from days 0 to 10 and subjected to qPCR. Data are shown as mean ± S.D. (n = 10 for Gata6; n = 3 for Fgf5 and T). *, p < 0.05; **, p < 0.01.
Subsequently, the role of p300 during spontaneous EB formation was examined. We found that EBs formed from p300-/- ES cells were smaller during the first 2 or 3 days, as had been reported by other researchers (16, 22). Moreover, no apparent cell death was observed in EBs of p300-/- ES cells as in those of p300+/+ ES cells. Notably, multiple stacks of cell layers outlining the surface of EBs from p300-/- ES cells were observed (Fig. 2C, upper panel). Normally, only one or two layers of extra-embryonic endoderm cells exist in the outer layer of EBs (23). To investigate the nature of abnormal EBs from p300-/- ES cells, the mRNA level of three embryonic germ layer markers, including Gata6 (endoderm), Brachyury (T) (mesoderm), and Fgf5 (ectoderm), was examined during the EB formation process by qPCR. As shown in Fig. 2D, dynamic expression patterns of all three marker genes in EBs of p300-/- ES cells were different from those of p300+/+ ES cells. For example, the Gata6 expression level was significantly higher in EBs of p300-/- ES cells at all time points examined. This result agrees with the observation of overgrowth of extra-embryonic endoderm-like cells in the outer layer of their EBs because it is well known that Gata6 promotes extra-embryonic endoderm differentiation in mouse ES cells (24). The results indicate that ablation of p300 could lead to abnormal expression of several germ layer markers, implying that p300 is required for maintaining an orderly differentiation process during ES cell in vitro differentiation.
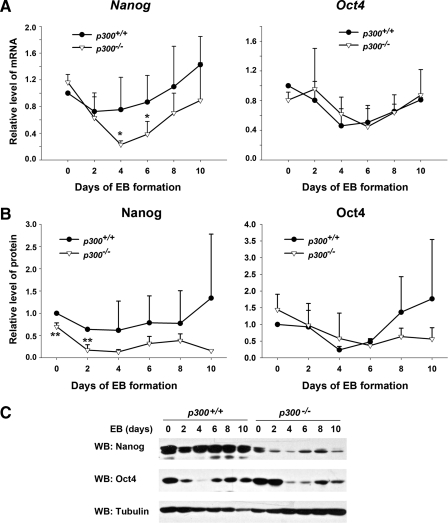
Nanog Expression Is Reduced in the Absence of p300 during ES Cell Differentiation—Because the absence of p300 affected proper expression of molecular markers of multiple germ layers, we wanted to know whether Oct4 and Nanog, well known key regulators of ES cells, were involved in the process. To this end, we measured mRNA and protein levels of Oct4 and Nanog during EB formation. qPCR analysis showed that in wild-type ES cells the Nanog transcript level was down-regulated markedly on EB day 2 and gradually rose up until day 10, which is in agreement with a previous report (9). Of note, an evidently lower level of Nanog transcript in EBs of p300-/- ES cells was detected from EB day 2 to 10 compared with that in EBs of wild-type ES cells, although the general expression trend along EB formation was similar in these two ES cell types (Fig. 3A, left panel). Similarly, the Nanog protein level in EBs of p300-/- ES cells was evidently lower than that in EBs of wild-type ES cells (Fig. 3, B and C). In contrast, we did not find a significant difference in the Oct4 mRNA level between the two ES cell types (Fig. 3A, right panel), although the protein level of Oct4 seemed lower in EBs of p300-/- ES cells than in EBs of p300+/+ ES cells after EB day 6 (Fig. 3, B, right panel, and C). Taken together, the findings demonstrate that transcription of Nanog during differentiation is specifically dependent on the presence of p300.
FIGURE 3.
Reduction in the expression level of Nanog in the absence of p300 during ES cell differentiation. A, transcript levels of pluripotency markers Nanog and Oct4 during EB formation from p300+/+ and p300-/- ES cells by qPCR. B, protein levels of Nanog and Oct4 during EB formation from p300+/+ and p300-/- ES cells. Results from EBs of p300+/+ and p300-/- ES cells were quantified and compared. C, representative data of Nanog and Oct4 protein levels during EB formation from p300+/+ and p300-/- ES cells by Western blot (WB) analysis. Data are shown as mean ± S.D. (n = 4). *, p < 0.05; **, p < 0.01.
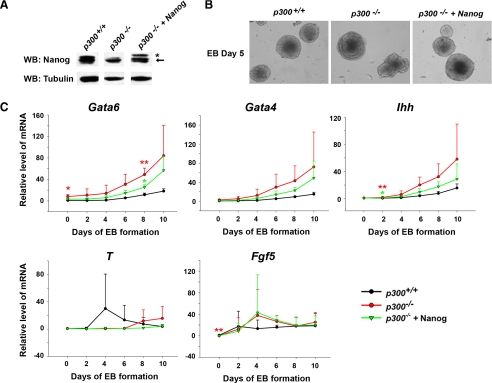
Overexpression of Nanog Rescues Phenotypes in EBs of p300-/- ES Cells—We were interested in knowing whether the reduction in Nanog expression was responsible for phenotypes detected in the EBs of p300-/- ES cells because it was reported that down-regulation of Nanog expression could lead to ES cell differentiation (4, 25). We overexpressed Nanog in p300-/- ES cells to test whether this could rescue the phenotypes (Fig. 4A). When EB formation assays were performed with the three ES cell lines (wild-type, p300-/-, and p300-/- +Nanog ES cells), the stacks of endoderm-like cells in the outer layer of EBs of p300-/- ES cells disappeared with Nanog overexpression (Fig. 4B), suggesting that Nanog could, at least in part, rescue the abnormal morphology of EBs seen in the absence of p300. To further define the rescuing ability of Nanog at a molecular level, we quantified the transcript levels of several germ layer markers in EBs from all three ES cell types at different stages (Fig. 4C). Consistent with our morphological observation, significantly high levels of the extra-embryonic endoderm markers Gata6, Gata4, and Ihh detected in EBs of p300-/- ES cells were obviously corrected by overexpression of Nanog, although their expression levels did not recover completely to those in EBs of wild-type ES cells. In contrast, aberrant expression of other marker genes, T and Fgf5, in EBs of p300-/- ES cells was not affected by overexpression of Nanog. These results indicate that improper regulation of Nanog expression during ES cell differentiation is probably one of the causes of the phenotypes seen in EBs of p300-/- ES cells.
FIGURE 4.
Overexpression of Nanog partially rescues the phenotype of EBs derived from p300-/- ES cells. A, verification of exogenous Nanog overexpression in p300-/- ES cells by Western blot (WB) analysis. The asterisk and arrow indicate exogenous and endogenous Nanog protein, respectively, in p300-/-+Nanog ES cells (right lane). B, morphology of EBs derived from p300+/+, p300-/-, and p300-/-+Nanog ES cells. EBs of day 5 were photographed at ×200 magnification. C, exogenous Nanog overexpression rescues the abnormal expression of extra-embryonic endoderm markers but not those of other germ layers in EBs from p300-/-+Nanog ES cells. EBs from p300+/+, p300-/-, and p300-/-+Nanog ES cell lines were harvested every 2 days from days 0 to 10. Differentiation markers of Gata6, Gata4, Ihh, T, and Fgf5 were examined by qPCR. Data are shown as mean ± S.D. (n = 3). *, p < 0.05; **, p < 0.01.
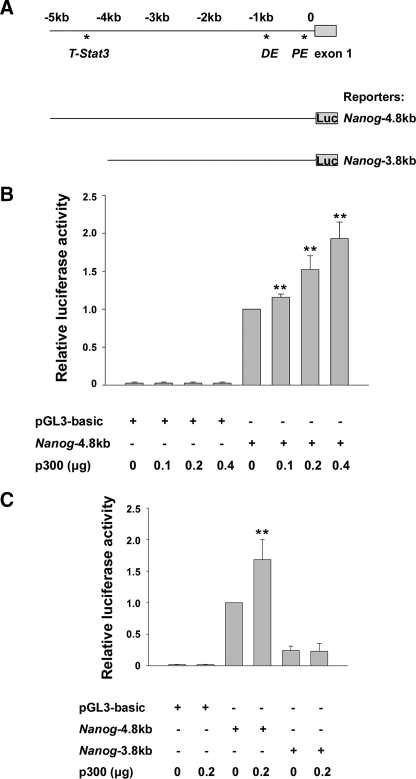
p300 Participates in Regulation of Nanog Expression—On the basis of the above results, we hypothesized that p300 might participate in regulating Nanog expression. To verify this idea, we evaluated the effect of p300 on activity of the reporter containing the 5′ upstream regulatory region of Nanog (Nanog-4.8kb) in p300-/- ES cells (Fig. 5A). As shown in Fig. 5B, p300 enhanced the Nanog-4.8kb reporter activity in a dose-dependent manner, suggesting that p300 was able to promote Nanog expression at a transcriptional level. Moreover, stimulation of p300 upon Nanog transcription was consistently observed regardless of the presence or absence of LIF in the culture medium (data not shown). To further define in which region of the Nanog regulatory sequence p300 performed its activation function, we repeated the reporter assay with a truncated form of the Nanog reporter, Nanog-3.8kb (Fig. 5, A and C). With reporter plasmids alone, the luciferase activity of the Nanog-3.8kb reporter was lower than that of the Nanog-4.8kb reporter, consistent with a previous report (18). Importantly, addition of p300 did not enhance the activity of the Nanog-3.8kb reporter, whereas the same dose of p300 activated the Nanog-4.8kb reporter significantly. The results suggest that the region from -3.8 to -4.8 kb may contain an important regulatory element for Nanog expression in ES cells and that p300 probably regulates Nanog expression through its action in this region, which has been reported to harbor an early mesoderm progenitor enhancer regulated by Stat3 and T (11).
FIGURE 5.
p300 regulates Nanog expression directly. A, schematic structure of the Nanog 5′ upstream regulatory region and reporter plasmids. Upper panel, the structure of the 5′ upstream regulatory region of Nanog and the positions of the T-Stat3-binding site, distal enhancer (DE), proximal enhancer (PE), and exon 1. Lower panel, structure of the Nanog-4.8kb and Nanog-3.8kb reporter plasmids. B, p300 activates the Nanog reporter dose-dependently. The Nanog-4.8kb reporter was cotransfected with p300 into p300-/- ES cells cultured in the presence of LIF. C, p300 specifically functions at the 5′ distal regulatory region of Nanog from -4.8 to -3.8 kb. The Nanog-4.8kb and Nanog-3.8kb reporters were cotransfected with p300 into p300-/- ES cells cultured with LIF, and luciferase (Luc) activities were measured. Data are shown as mean ± S.D. (n = 4 for B and C). **, p < 0.01.
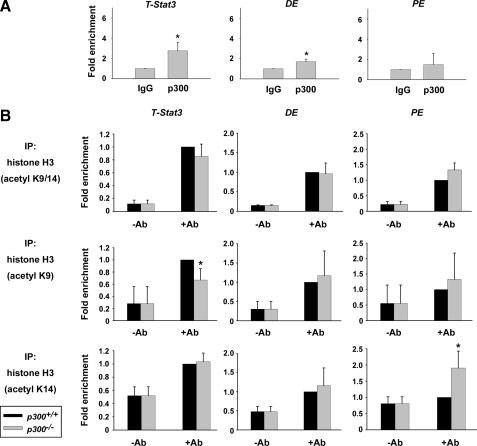
p300 May Regulate Nanog Expression by Epigenetic Modification of Histone Acetylation—To elucidate the mechanism by which p300 regulates Nanog expression, we first determined whether p300 was directly associated with the regulatory region of Nanog by ChIP assays. In line with the results of reporter assays, we detected significant recruitment of p300 protein to the distal regulatory region of Nanog containing the T- and Stat3-binding site (Nanog T-Stat3) but not to the proximal enhancer (Nanog PE) (Fig. 6A). In addition, weak association of p300 with the distal enhancer (Nanog DE) was also observed. Thus, it appears that p300 directly regulates Nanog expression primarily through its binding to the distal regulatory region during ES cell differentiation. We next focused on the acetylation modification state of histone at the regulatory region of Nanog, considering the histone acetyltransferase activity of p300. Having known that the 9th (H3K9) and 14th (H3K14) lysine residues of histone H3 are substrates of p300 and that their acetylation usually serves as markers of transcriptionally active chromatin (26), ChIP assays were conducted with specific antibodies against acetyl-H3K9, acetyl-H3K14, or both. When p300+/+ and p300-/- ES cells were induced into differentiation by withdrawal of LIF for 2 days, which was sufficient to trigger differential Nanog expression in p300-/- ES cells (data not shown), acetylated histone H3 at the Nanog T-Stat3-binding site, detected by antibodies against acetyl-H3K9 and acetyl-H3K9/14, decreased significantly in p300-/- ES cells compared with that in p300+/+ ES cells (Fig. 6B). In contrast, there was no difference in the signal detected by an acetyl-H3K14 antibody, implying that p300 plays a role in H3K9 acetylation but not H3K14 acetylation at this particular site. Furthermore, deletion of p300 did not compromise H3K9 or H3K14 acetylation at the Nanog DE or Nanog PE regions. Instead, an increase in acetyl-H3K14 associated with Nanog PE was detected. The result corresponds well with our earlier finding that p300 was predominantly recruited to the Nanog T-Stat3 region (Fig. 6A). Thus, our data imply that transcriptional regulation of Nanog by p300 may be mediated through epigenetic modification of histone acetylation at the T-Stat3-binding site of the Nanog distal regulatory region during ES cell differentiation.
FIGURE 6.
Epigenetic modification of histone is involved in the regulation of Nanog expression by p300. A, the direct association of p300 with the distal regulatory region of Nanog in CGR8 ES cells was detected by ChIP assays. p300-associated DNA fragments were detected by qPCR. -Fold enrichment is the relative abundance of the indicated DNA fragments over the glyceraldehyde-3-phosphate dehydrogenase fragment. n = 3. *, p < 0.05. DE, distal enhancer; PE, proximal enhancer. B, the acetylation state of histone H3 lysines 9 and 14 was examined in p300+/+ and p300-/- ES cells after LIF withdrawal for 2 days by ChIP assays. Acetyl-H3K9 and acetyl-H3K14-associated genomic regions were amplified by qPCR. Data are presented as in A. n = 3. IP, immunoprecipitation; Ab, antibody.
DISCUSSION
In this study, we investigated the roles of p300 in keeping ES cell self-renewal and differentiation running properly. We show that deletion of p300 in ES cells markedly disturbs normal differentiation processes, although the self-renewal capacity appears not to be affected overtly. Moreover, our data indicate that reduced Nanog expression in the absence of p300 is responsible for the abnormal differentiation toward the extra-embryonic endoderm lineage during EB formation. Finally, we demonstrate that p300 positively participates in Nanog transcriptional regulation probably by acetylation of histone at the distal regulatory region of Nanog. The study provides the first evidence for the role and potential mechanism of p300 in the control of ES cell fate decision during early differentiation.
The roles of p300 in cell differentiation and signal transduction pathways have been investigated previously (27). Particularly, p300 was demonstrated to be essential for ES cells to differentiate into hematopoietic and myogenic cells in vitro, respectively (16, 22). However, the defects caused by p300 ablation in these studies were detected after the ES cells had undergone considerable extensive differentiation. So far, little is known about what roles p300 plays in ES cell self-renewal and differentiation into three primitive germ layers during the early differentiation process in vitro, which mimic postimplantation embryonic tissues in vivo (28). In this study, we consistently observed a difference in expression of endoderm and ectoderm markers between wild-type and p300-deficient ES cells when they were cultured in an undifferentiated state. However, what keeps p300-/- ES cells in an undifferentiated state even in the presence of aberrant expression of certain differentiation genes is unknown. Although p300-/- ES cells still possessed the ability to undergo self-renewal, they seemed predisposed toward differentiation. Indeed, p300-/- ES cells responded to differentiation-inducing cues differently from wild-type ES cells. Thus, we have demonstrated for the first time that p300 may regulate the expression of certain early differentiation genes and that it is required for ES cells to maintain a normal early differentiation process. The finding may provide new insights into molecular mechanisms underlying the impaired hematopoietic and myogenic differentiation detected in later stages of differentiation processes reported in previous studies (16, 22).
The finding that down-regulation of Nanog is responsible for abnormal extra-embryonic endoderm differentiation in EBs of p300-/- ES cells is consistent with previous studies that demonstrated the essential role of Nanog in suppression of extra-embryonic endoderm differentiation (4, 25). The results point out that maintenance of pluripotency genes at an appropriate level is critical not only in the undifferentiated state but also during the differentiation of ES cells. However, the inability of Nanog overexpression to rescue the aberrant expression of other lineage markers implies that factors other than Nanog may mediate p300 deficiency-dependent phenotypes, which needs further analysis. In addition, we discovered that Nanog expression is under direct regulation of p300, which adds another factor to the list of the known regulators of Nanog expression, including Oct4 and Sox2 (8, 29), p53 (10), Tcf3 (9), and T and Stat3 (11, 30).
Currently, it is not clear how p300 exerts its control over Nanog expression during early events of ES cell differentiation. The transcriptional regulation functions of p300 are known to be exerted through multiple mechanisms. First, it regulates transcription through its interaction with a wide range of DNA-binding transcriptional factors, such as p53, E2F, AP1, MyoD, and NF-κB (13), and it functions in both acetyltransferase activity-dependent and -independent manners. Recent genome-wide mapping of p300-binding sequences in ES cells revealed that most p300-binding sites were associated with three to six other transcription factors, including Nanog, Oct4, and Sox2, followed by Smad1, Esrrb, Klf4, and Stat3 at a lower probability (31). In this study, we found that p300 associated with a distal regulatory region of Nanog that contains binding sites for T and Stat3, supporting the possibility that p300 may activate Nanog expression through association with T or Stat3. As a matter of fact, it was reported that p300-mediated Stat3 acetylation could activate Stat3 sequence-specific DNA binding and transcription (32). Further investigation is under way to address this issue. In addition, p300 has the ability to influence chromatin state by modulating nucleosomal histones. Our finding that acetylation of H3K9 in the distal regulatory region of Nanog was reduced in p300-/- ES cells suggests that epigenetic modification of the core histone may be involved in Nanog expression regulation by p300 during ES cell differentiation. Interestingly, we observed an increase in acetyl-histone H3K14 associated with Nanog PE, whereas association of p300 with the site was not detected. This phenomenon could be brought about by an indirect influence of p300 knock-out. Growing evidence demonstrates that ES cell proliferation and differentiation are controlled by epigenetic factors that affect transcription of key regulators for ES cell self-renewal and pluripotency (33). For instance, DNA methyltransferase was shown to methylate the promoters of Oct4 and Nanog (34). In addition, the Jmjd2c histone H3K9 demethylase has been shown to be a positive regulator for Nanog expression (35). Furthermore, ChIP assays revealed that histones H3 and H4 were highly acetylated at the Nanog locus in ES cells (36). Thus, it appears that Nanog is regulated by epigenetic mechanisms involving both DNA methylation and histone modifications. Despite these findings, the acetyltransferase(s) associated with Nanog expression in ES cells has not yet been identified. p300 is the first acetyltransferase demonstrated to have the capacity to modulate Nanog expression. However, whether the histone acetyltransferase activity is required for its role in the regulation of Nanog expression is not known yet.
Supplementary Material
Acknowledgments
We thank Andrew Kung for generously sharing p300+/+ and p300-/- ES cells as well as Austin Smith and Ian Chambers for CGR8 ES cells and the pPyCAGIP plasmid. We also thank Tony Kouzarides and Da-Yong Wu for the p300/pCMVβ and Nanog reporter plasmids, respectively, and thank Erbo Xu for help with preparation of the manuscript.
This work was supported by National High Technology Research and Development Program of China Grants 2006CB943901 and 2007CB947904, National Natural Science Foundation Grants 3057907 and 30730051, and Shanghai Leading Academic Discipline Project Grant S30201. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1, Tables S1–S3, and Refs. 1 and 2.
Footnotes
The abbreviations used are: ES, embryonic stem; EB, embryoid body; ChIP, chromatin immunoprecipitation; LIF, leukemia inhibitory factor; qPCR, quantitative real-time PCR.
References
- 1.Niwa, H., Miyazaki, J., and Smith, A. G. (2000) Nat. Genet. 24 372-376 [DOI] [PubMed] [Google Scholar]
- 2.Niwa, H. (2007) Development (Camb.) 134 635-646 [DOI] [PubMed] [Google Scholar]
- 3.Masui, S., Nakatake, Y., Toyooka, Y., Shimosato, D., Yagi, R., Takahashi, K., Okochi, H., Okuda, A., Matoba, R., Sharov, A. A., Ko, M. S., and Niwa, H. (2007) Nat. Cell Biol. 9 625-635 [DOI] [PubMed] [Google Scholar]
- 4.Chambers, I., Colby, D., Robertson, M., Nichols, J., Lee, S., Tweedie, S., and Smith, A. (2003) Cell 113 643-655 [DOI] [PubMed] [Google Scholar]
- 5.Mitsui, K., Tokuzawa, Y., Itoh, H., Segawa, K., Murakami, M., Takahashi, K., Maruyama, M., Maeda, M., and Yamanaka, S. (2003) Cell 113 631-642 [DOI] [PubMed] [Google Scholar]
- 6.Takahashi, K., and Yamanaka, S. (2006) Cell 126 663-676 [DOI] [PubMed] [Google Scholar]
- 7.Yu, J., Vodyanik, M. A., Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, J. L., Tian, S., Nie, J., Jonsdottir, G. A., Ruotti, V., Stewart, R., Slukvin, I. I., and Thomson, J. A. (2007) Science 318 1917-1920 [DOI] [PubMed] [Google Scholar]
- 8.Kuroda, T., Tada, M., Kubota, H., Kimura, H., Hatano, S. Y., Suemori, H., Nakatsuji, N., and Tada, T. (2005) Mol. Cell. Biol. 25 2475-2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira, L., Yi, F., and Merrill, B. J. (2006) Mol. Cell. Biol. 26 7479-7491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin, T., Chao, C., Saito, S., Mazur, S. J., Murphy, M. E., Appella, E., and Xu, Y. (2005) Nat. Cell Biol. 7 165-171 [DOI] [PubMed] [Google Scholar]
- 11.Suzuki, A., Raya, A., Kawakami, Y., Morita, M., Matsui, T., Nakashima, K., Gage, F. H., Rodriguez-Esteban, C., and Izpisua Belmonte, J. C. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 10294-10299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman, R. H., and Smolik, S. (2000) Genes Dev. 14 1553-1577 [PubMed] [Google Scholar]
- 13.Chan, H. M., and La Thangue, N. B. (2001) J. Cell Sci. 114 2363-2373 [DOI] [PubMed] [Google Scholar]
- 14.Yao, T. P., Oh, S. P., Fuchs, M., Zhou, N. D., Ch'ng, L. E., Newsome, D., Bronson, R. T., Li, E., Livingston, D. M., and Eckner, R. (1998) Cell 93 361-372 [DOI] [PubMed] [Google Scholar]
- 15.Partanen, A., Motoyama, J., and Hui, C. C. (1999) Int. J. Dev. Biol. 43 487-494 [PubMed] [Google Scholar]
- 16.Roth, J. F., Shikama, N., Henzen, C., Desbaillets, I., Lutz, W., Marino, S., Wittwer, J., Schorle, H., Gassmann, M., and Eckner, R. (2003) EMBO J. 22 5186-5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith, A. G., Heath, J. K., Donaldson, D. D., Wong, G. G., Moreau, J., Stahl, M., and Rogers, D. (1988) Nature 336 688-690 [DOI] [PubMed] [Google Scholar]
- 18.Wu, D. Y., and Yao, Z. (2005) Cell Res. 15 317-324 [DOI] [PubMed] [Google Scholar]
- 19.Xu, H. M., Liao, B., Zhang, Q. J., Wang, B. B., Li, H., Zhong, X. M., Sheng, H. Z., Zhao, Y. X., Zhao, Y. M., and Jin, Y. (2004) J. Biol. Chem. 279 23495-23503 [DOI] [PubMed] [Google Scholar]
- 20.Zhang, Z., Liao, B., Xu, M., and Jin, Y. (2007) FASEB J. 21 3042-3051 [DOI] [PubMed] [Google Scholar]
- 21.Brouillard, F., and Cremisi, C. E. (2003) J. Biol. Chem. 278 39509-39516 [DOI] [PubMed] [Google Scholar]
- 22.Rebel, V. I., Kung, A. L., Tanner, E. A., Yang, H., Bronson, R. T., and Livingston, D. M. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 14789-14794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimoda, M., Kanai-Azuma, M., Hara, K., Miyazaki, S., Kanai, Y., Monden, M., and Miyazaki, J. (2007) J. Cell Sci. 120 3859-3869 [DOI] [PubMed] [Google Scholar]
- 24.Fujikura, J., Yamato, E., Yonemura, S., Hosoda, K., Masui, S., Nakao, K., Miyazaki Ji, J., and Niwa, H. (2002) Genes Dev. 16 784-789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hough, S. R., Clements, I., Welch, P. J., and Wiederholt, K. A. (2006) Stem Cells (Durham) 24 1467-1475 [DOI] [PubMed] [Google Scholar]
- 26.McManus, K. J., and Hendzel, M. J. (2003) Mol. Cell. Biol. 23 7611-7627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawasaki, H., Eckner, R., Yao, T. P., Taira, K., Chiu, R., Livingston, D. M., and Yokoyama, K. K. (1998) Nature 393 284-289 [DOI] [PubMed] [Google Scholar]
- 28.Desbaillets, I., Ziegler, U., Groscurth, P., and Gassmann, M. (2000) Exp. Physiol. 85 645-651 [PubMed] [Google Scholar]
- 29.Rodda, D. J., Chew, J. L., Lim, L. H., Loh, Y. H., Wang, B., Ng, H. H., and Robson, P. (2005) J. Biol. Chem. 280 24731-24737 [DOI] [PubMed] [Google Scholar]
- 30.Suzuki, A., Raya, A., Kawakami, Y., Morita, M., Matsui, T., Nakashima, K., Gage, F. H., Rodriguez-Esteban, C., and Belmonte, J. C. (2006) Nat. Clin. Pract. Cardiovasc. Med. 3 Suppl. 1, S114-S122 [DOI] [PubMed] [Google Scholar]
- 31.Chen, X., Xu, H., Yuan, P., Fang, F., Huss, M., Vega, V. B., Wong, E., Orlov, Y. L., Zhang, W., Jiang, J., Loh, Y. H., Yeo, H. C., Yeo, Z. X., Narang, V., Govindarajan, K. R., Leong, B., Shahab, A., Ruan, Y., Bourque, G., Sung, W. K., Clarke, N. D., Wei, C. L., and Ng, H. H. (2008) Cell 133 1106-1117 [DOI] [PubMed] [Google Scholar]
- 32.Wang, R., Cherukuri, P., and Luo, J. (2005) J. Biol. Chem. 280 11528-11534 [DOI] [PubMed] [Google Scholar]
- 33.Bibikova, M., Laurent, L. C., Ren, B., Loring, J. F., and Fan, J. B. (2008) Cell Stem Cell 2 123-134 [DOI] [PubMed] [Google Scholar]
- 34.Li, J. Y., Pu, M. T., Hirasawa, R., Li, B. Z., Huang, Y. N., Zeng, R., Jing, N. H., Chen, T., Li, E., Sasaki, H., and Xu, G. L. (2007) Mol. Cell. Biol. 27 8748-8759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loh, Y. H., Zhang, W., Chen, X., George, J., and Ng, H. H. (2007) Genes Dev. 21 2545-2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hattori, N., Imao, Y., Nishino, K., Hattori, N., Ohgane, J., Yagi, S., Tanaka, S., and Shiota, K. (2007) Genes Cells 12 387-396 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.